UPLC-Q/TOF-MS-Based Serum Metabolomics Reveals Hypoglycemic Effects of Rehmannia glutinosa, Coptis chinensis and Their Combination on High-Fat-Diet-Induced Diabetes in KK-Ay Mice
Abstract
1. Introduction
2. Results
2.1. RG, CC, and Their Combination Decreased Fasting Blood Glucose Levels in HFD-Induced Diabetic Mice
2.2. RG, CC, and Their Combination Improved Glucose Tolerance of Diabetic Mice
2.3. RG, CC, and Their Combination Similarly Modulated Serum Lipid Levels of Diabetic Mice
2.4. Effects of RG, CC, and Their Combination on Serum Metabolomics of Diabetic Mice
2.4.1. UPLC-Q/TOF-MS Method Validation
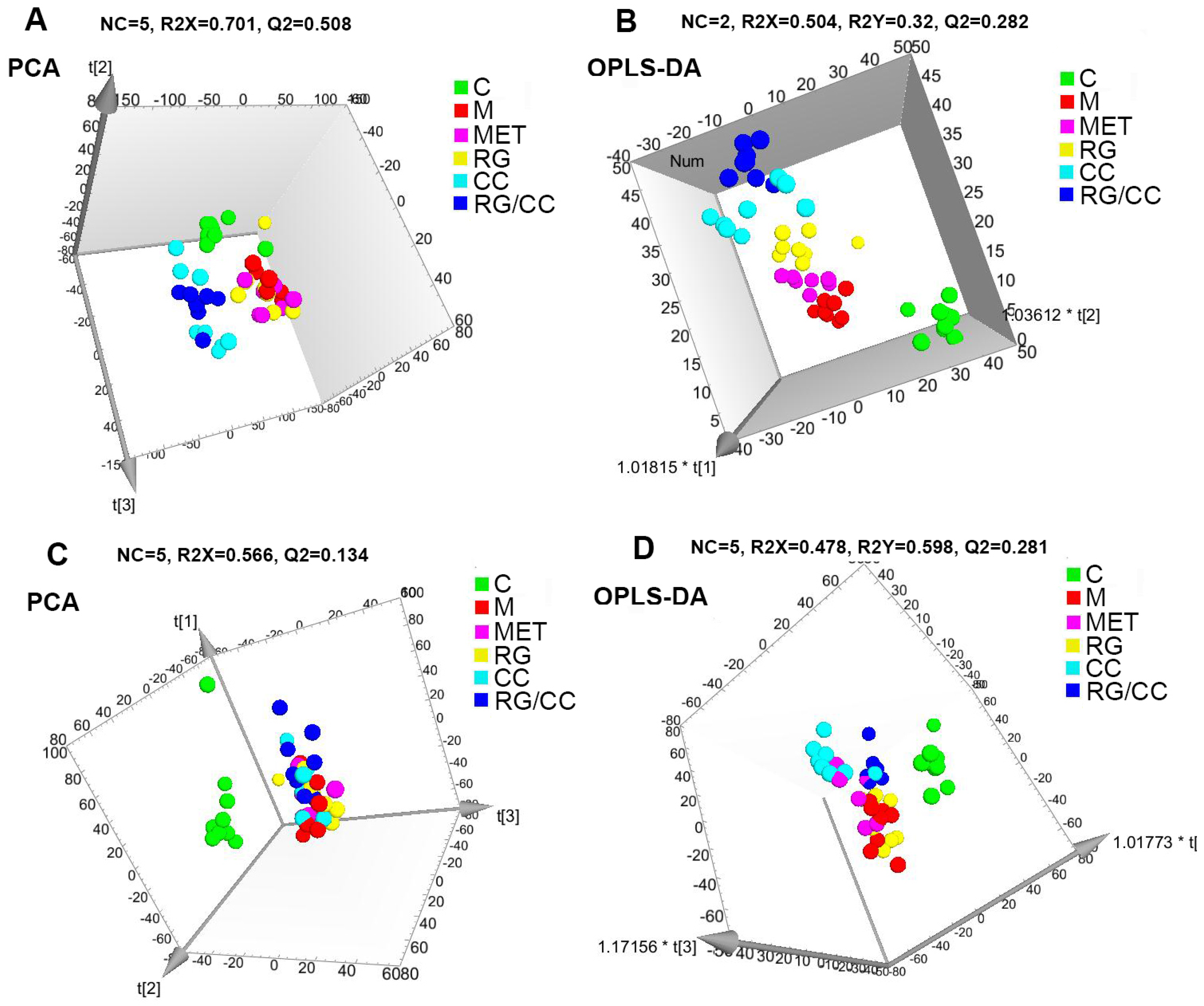
2.4.2. Global Multivariate Analysis of Serum Metabolomics
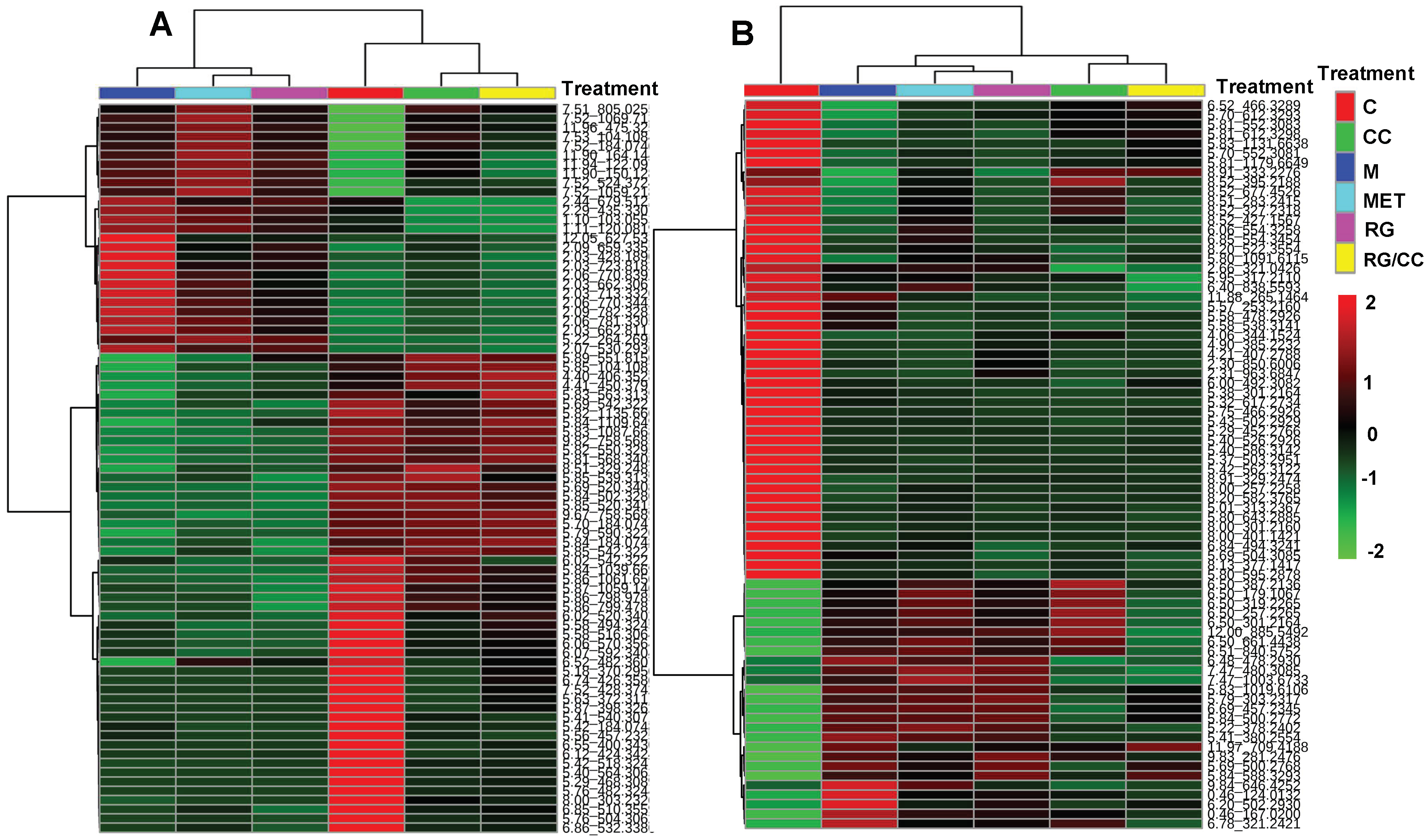
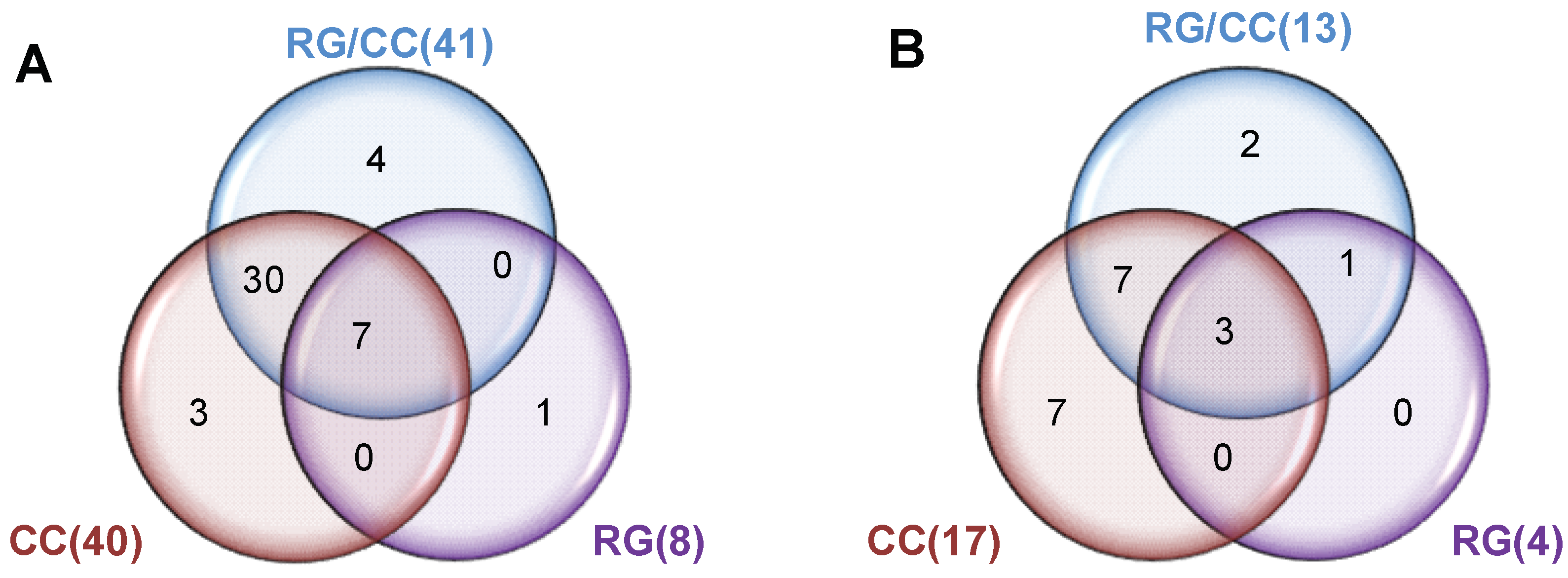
2.4.3. Modulation of RG, CC, and Their Combination on HFD-Induced Diabetic Biomarkers
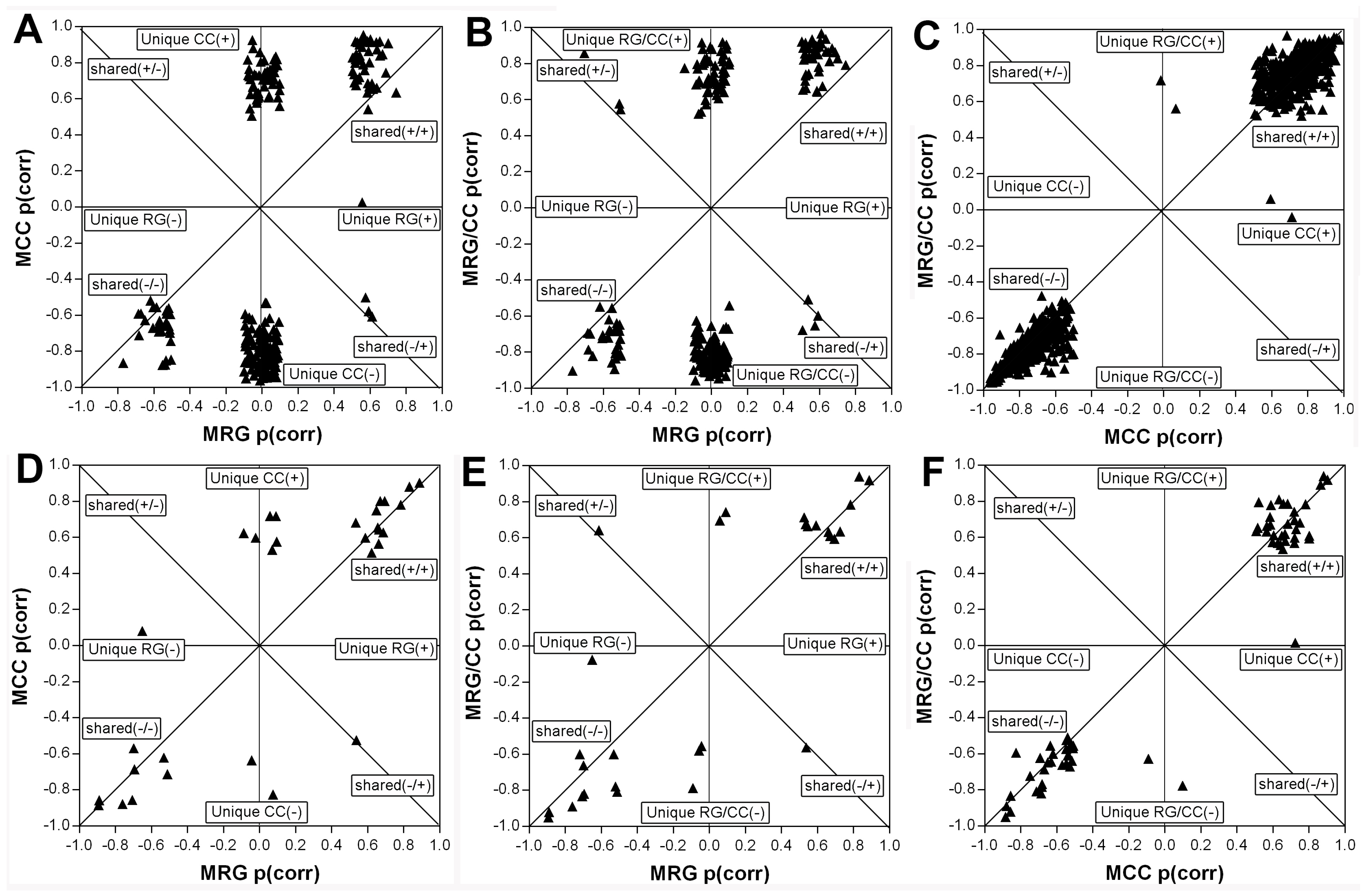
2.4.4. RG and CC Showed the Same Restoring Trends on Metabolites in Diabetic Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of RG, CC, and RG/CC Decoction
4.3. Animals and Treatments
4.4. Evaluation of Antidiabetic Activities of RG, CC, and Their Combination
4.4.1. Determination of Fast Blood Glucose and Glucose Tolerance
4.4.2. Biochemical Assays
4.5. Serum Metabolomic Analysis
4.5.1. Preparation of Serum Samples and QC Samples
4.5.2. UPLC-Q/TOF-MS Analysis
4.5.3. Data Processing and Multivariate Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar] [PubMed]
- Alvarez, C.A.; Lingvay, I.; Vuylsteke, V.; Koffarnus, R.L.; McGuire, D.K. Cardiovascular risk in diabetes mellitus: Complication of the disease or of antihyperglycemic medications. Clin. Pharmacol. Ther. 2015, 98, 145–161. [Google Scholar] [CrossRef]
- Dong, Y.H.; Gao, W.G.; Zhang, L.; Wei, J.; Hammar, N.; Cabrera, C.S.; Wu, X.L.; Qiao, Q. Patient characteristics related to metabolic disorders and chronic complications in type 2 diabetes mellitus patients hospitalized at the Qingdao Endocrine and Diabetes Hospital from 2006 to 2012 in China. Diab. Vasc. Dis. Res. 2017, 14, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Volmer-Thole, M.; Lobmann, R. Neuropathy and diabetic foot syndrome. Int. J. Mol. Sci. 2016, 17, 917. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, S.; Kuwabara, T. Diabetes-induced dysfunction of mitochondria and stem cells in skeletal muscle and the nervous system. Int. J. Mol. Sci. 2017, 18, 2147. [Google Scholar] [CrossRef] [PubMed]
- Blicklé, J.F. The place of thiazolidinediones in the treatment of type 2 diabetes. Presse Med. 2004, 33, 1034–1040. [Google Scholar] [CrossRef]
- Wang, G.S.; Hoyte, C. Review of biguanide (metformin) toxicity. J. Intensive Care Med. 2018. [Google Scholar] [CrossRef]
- Liou, Y.S.; Yang, F.Y.; Chen, H.Y.; Jong, G.P. Antihyperglycemic drugs use and new-onset atrial fibrillation: A population-based nested case control study. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Qi, L.W.; Liu, E.H.; Chu, C.; Peng, Y.B.; Cai, H.X.; Li, P. Anti-diabetic agents from natural products—An update from 2004 to 2009. Curr. Top. Med. Chem. 2010, 10, 434–457. [Google Scholar] [CrossRef]
- Gao, Z.Z.; Li, Q.W.; Wu, X.M.; Zhao, X.M.; Zhao, L.H.; Tong, X.L. New Insights into the mechanisms of chinese herbal products on diabetes: A focus on the “bacteria-mucosal immunity-inflammation-diabetes” axis. J. Immunol. Res. 2017. [Google Scholar] [CrossRef]
- Marles, R.J.; Farnsworth, N.R. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–189. [Google Scholar] [CrossRef]
- Liu, J.P.; Zhang, M.; Wang, W.Y.; Grimsgaard, S. Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2004, CD003642. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.M.; Wang, X.Y.; Jia, W. Using Chinese natural products for diabetes mellitus drug discovery and development. Expert Opin. Drug Discov. 2007, 2, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Deng, Y.X.; Shi, Q.Z.; He, M.Y.; Chen, B.; Qiu, X.M. Hypolipidemic effect of the Chinese polyherbal Huanglian Jiedu decoction in type 2 diabetic rats and its possible mechanism. Phytomedicine 2014, 21, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Waisundara, V.Y.; Huang, M.; Hsu, A.; Huang, D.; Tan, B.K. Characterization of the anti-diabetic and antioxidant effects of Rehmannia glutinosa in streptozotocin-induced diabetic Wistar rats. Am. J. Chin. Med. 2008, 36, 1083–1104. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Xu, Y.P.; Qin, G.J.; Liu, C.; Wang, S.J. Effects of water extracts of Rehmannia glutinosa on antioxidant system of Nrf2 in paraquat-induced insulin resistance diabetic rat model. Exp. Ther. Med. 2017, 14, 5847–5850. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Wang, L. Effects of Coptis chinensis decoction on blood glucose and lipid levels of diabetic mice. Fujian Med. J. 2011, 33, 90–92. [Google Scholar]
- Guo, Y.Z.; Du, J.; Li, X.M.; Feng, X.Z.; Guo, W. Effect of Coptis Chinensis on expression of NF-κB and PPAR-γ in kidney of diabetic nephropathy rats. World Chin. Med. 2017, 12, 884–887. [Google Scholar]
- Wang, J.; Yuan, Z.M.; Li, Y.X.; Kong, H.W.; Xu, G.W. Study on mechanism of combined administration of Coptidis Rhizoma and Rehmanniae Radix in treating type II diabetes mellitus. China J. Chin. Mater. Med. 2014, 39, 526–530. [Google Scholar]
- Zao, Y.Q.; Yi, D.W. Experimental study of Qianjin HuangLian bolus and its separated recipes on insulin resistance of type 2 diabetes. Xinjiang J. Tradit. Chin. Med. 2012, 30, 33–35. [Google Scholar]
- Wang, J.; Yuan, Z.M.; Kong, H.W.; Li, Y.; Lu, X.; Xu, G.W. Exploring the mechanism of Rhizoma Coptidis in treating type II diabetes mellitus based on metabolomics. Chin. J. Chromatogr. 2012, 30, 8–13. [Google Scholar] [CrossRef]
- Yang, M.W.; Wang, Z.W.; Lu, F.E. Effect of rhizoma Coptidis and radix Rehmanniae and their combination on the secreting of adiponectin and leptin in insulin resistant 3T3-L1 adipocytes. Res. Integr. Tradit. Chin. West. Med. 2014, 6, 293–296. [Google Scholar]
- Cheng, B.; Zheng, H.; Wu, F.; Wu, J.X.; Liu, X.W.; Tang, C.L.; Lu, S.Y.; Chen, Z.N.; Song, F.M.; Ruan, J.X.; et al. Metabolomics analysis of Danggui Sini decoction on treatment of collagen-induced arthritis in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061–1062, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.M.; Zhu, B.J.; Cao, H.T.; Zhang, X.Y.; Zhang, Q.C.; Xin, G.Z.; Pan, L.M.; Liu, L.F.; Zhu, H.X. Explore the effects of Huang-Lian-Jie-Du-Tang on Alzheimer’s disease by UPLC-QTOF/MS-based plasma metabolomics study. J. Pharm. Biomed. Anal. 2018, 151, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yue, X.F.; Chen, Y.X.; Liu, X.M.; Wang, L.S.; Cao, F.R.; Wang, Q.; Liao, Y.H.; Pan, R.L.; Chang, Q. LC/MS-based metabolomics strategy to assess the amelioration effects of ginseng total saponins on memory deficiency induced by simulated microgravity. J. Pharm. Biomed. Anal. 2016, 125, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Hou, X.; Li, X.; Qu, M.; Tong, C.; Li, W. Metabolomics analysis of alloxan-induced diabetes in mice using UPLC-Q-TOF-MS after Crassostrea gigas polysaccharide treatment. Int. J. Biol. Macromol. 2018, 108, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Cao, H.; Sun, L.; Li, B.; Guo, L.; Duan, J.; Zhu, H.; Zhang, Q. Metabolomics-based mechanisms exploration of Huang-Lian Jie-Du decoction on cerebral ischemia via UPLC-Q-TOF/MS analysis on rat serum. J. Ethnopharmacol. 2018, 216, 147–156. [Google Scholar] [CrossRef]
- Duan, W.N.; Zhang, Z.L.; Kong, Y.Y.; Chen, Z.Q.; Li, M.L.Z. Comparison in treatment of hypoglycemia and hyperlipemia by zengye tang prescription with Rehmanniae radix processed by different method in diabetes rats. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 187–191. [Google Scholar]
- Liu, Q.; Liu, S.N.; Gao, L.H.; Sun, S.J.; Huan, Y.; Li, C.N.; Wang, Y.; Guo, N.; Shen, Z.F. Anti-diabetic effects and mechanisms of action of a Chinese herbal medicine preparation JQ-R in vitro and in diabetic KK-Ay mice. Acta Pharm. Sin. B 2017, 7, 461–469. [Google Scholar] [CrossRef]
- Wu, J.R.; Wang, K.H.; Ji, K.; Zhang, D.; Zhang, X.M.; Yang, B.; Feng, R.B. Medication Rules for treating diabetes based on data mining. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 214–217. [Google Scholar]
- Zhao, L.; Mu, Y.N.; Liang, M.X. Comparison of application rules of Chinese herbal medicines on wasting thirst disease in ancient and present China. J. Beijing Univ. Tradit. Chin. Med. 2016, 39, 769–773. [Google Scholar]
- Lu, S.T.; Chen, Q.G.; Xu, P.Y.; Tao, F.; Yao, Z.; Lu, H.; Ding, X.P. Analysis on Professor DING Xue-ping’s herbal administration experience for diabetes by using traditional chinese medicine inheritance support system. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 198–205. [Google Scholar]
- Wu, J.H.; Gu, H.Y.; La, X.J.; Lv, J.D.; Wang, Y.; La, W.Y. Effects of Rehmanniae radix and Rehmanniae radix praeparata on blood glucose and lipid in diabetic mice. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 161–163. [Google Scholar]
- Duan, W.N.; Zhang, Z.L.; Kong, Y.Y.; Chen, Z.Q.; Li, M.L. Comparison of hypoglycemic effects of Zengye Decoction with different processed products of Rehmanniae in diabetes rats. China J. Tradit. Chin. Med. Pharm. 2014, 29, 266–268. [Google Scholar]
- Li, J.C.; Meng, X.L.; Cui, R.; Lai, X.R.; Fan, X.J.; Zeng, Y. Comparison in pharmacodynamic effects of different types of processed rhizoma Coptis on mouse diabetes. Chin. Tradit. Pat. Med. 2010, 32, 1922–1925. [Google Scholar]
- Tong, X.L. Syndrome-based usages of classical prescriptions containing the primary herb Coptis Chinensis in T2DM treatment. J. Tradit. Chin. Med. 2013, 54, 209–211. [Google Scholar]
- Liu, W.; Liu, P.; Tao, S.; Deng, Y.; Li, X.; Lan, T.; Zhang, X.; Guo, F.; Huang, W.; Chen, F.; et al. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch. Biochem. Biophys. 2008, 475, 128–134. [Google Scholar] [CrossRef]
- Jiang, S.; Du, P.G.; An, L.P.; Yuan, G.X.; Sun, Z.W. Anti-diabetic effect of Coptis Chinensis polysaccharide in high-fat diet with STZ-induced diabetic mice. Int. J. Biol. Macromol. 2013, 55, 118–122. [Google Scholar] [CrossRef]
- Ma, H.; Hu, Y.R.; Zou, Z.Y.; Feng, M.; Ye, X.L.; Li, X.G. Antihyperglycemia and antihyperlipidemia effect of protoberberine alkaloids from rhizoma Coptidis in HepG2 Cell and diabetic KK-Ay mice. Drug Dev. Res. 2016, 77, 163–170. [Google Scholar] [CrossRef]
- Yue, S.J.; Liu, J.; Feng, W.W.; Zhang, F.L.; Chen, J.X.; Xin, L.T.; Peng, C.; Guan, H.S.; Wang, C.Y.; Yan, D. System pharmacology-based dissection of the synergistic mechanism of Huangqi and Huanglian for diabetes mellitus. Front. Pharmacol. 2017, 8, 694. [Google Scholar] [CrossRef]
- Zhang, R.X.; Zhou, J.H.; Jia, Z.P.; Zhang, Y.X.; Gu, G.M. Hypoglycemic effect of Rehmannia glutinosa oligosaccharide in hyperglycemic and alloxan-induced diabetic rats and its mechanism. J Ethnopharmacol. 2004, 90, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.P.; Cheng, K.C.; Chung, H.H.; Kerh, Y.F.; Yeh, C.H.; Cheng, J.T. Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2011, 59, 3747–3753. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, G.; Ma, S.; Li, F.; Yuan, M.; Xu, H.; Huang, K. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-κB pathways. Biochem. Biophys. Res. Commun. 2015, 467, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.F.; Wang, Y.; Liu, Z.Q.; Wang, J.H.; Wan, D.; Feng, S.; Yang, X.; Wang, T. Antidiabetic and antioxidant effects of catalpol extracted from Rehmannia glutinosa (Di Huang) on rat diabetes induced by streptozotocin and high-fat, high-sugar feed. Chin. Med. 2016, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, H.D.; Song, J.; Wang, J.; Zhao, L.H.; Tong, X. Syndrome differentiation of diabetes by the traditional Chinese medicine according to evidence-based medicine and expert consensus opinion. Evid. Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.H.; Yu, R.; Liu, X.; Wu, Y.J.; Qin, Y.H. Discussion on the medication and compatibility characteristics of Dilian pill. Guid. J. Tradit. Chin. Med. Pharmacol. 2018, 24, 54–56. [Google Scholar]
- Xu, W.J.; Zhang, L.X.; Huang, Y.H.; Yang, Q.X.; Xiao, H.B.; Zhang, D.Q. Discrimination of type 2 diabetes mellitus corresponding to different traditional Chinese medicine syndromes based on plasma fatty acid profiles and chemometric methods. J Ethnopharmacol. 2012, 143, 463–468. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhou, J.; Zhang, L.; Ma, J.H.; Sun, Y.N.; Zhao, Y.W. Characteristics of blood glucose excursions in type 2 diabetes mellitus patients with three different traditional Chinese medicine syndromes. J. Tradit. Chin. Med. 2015, 35, 537–545. [Google Scholar]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
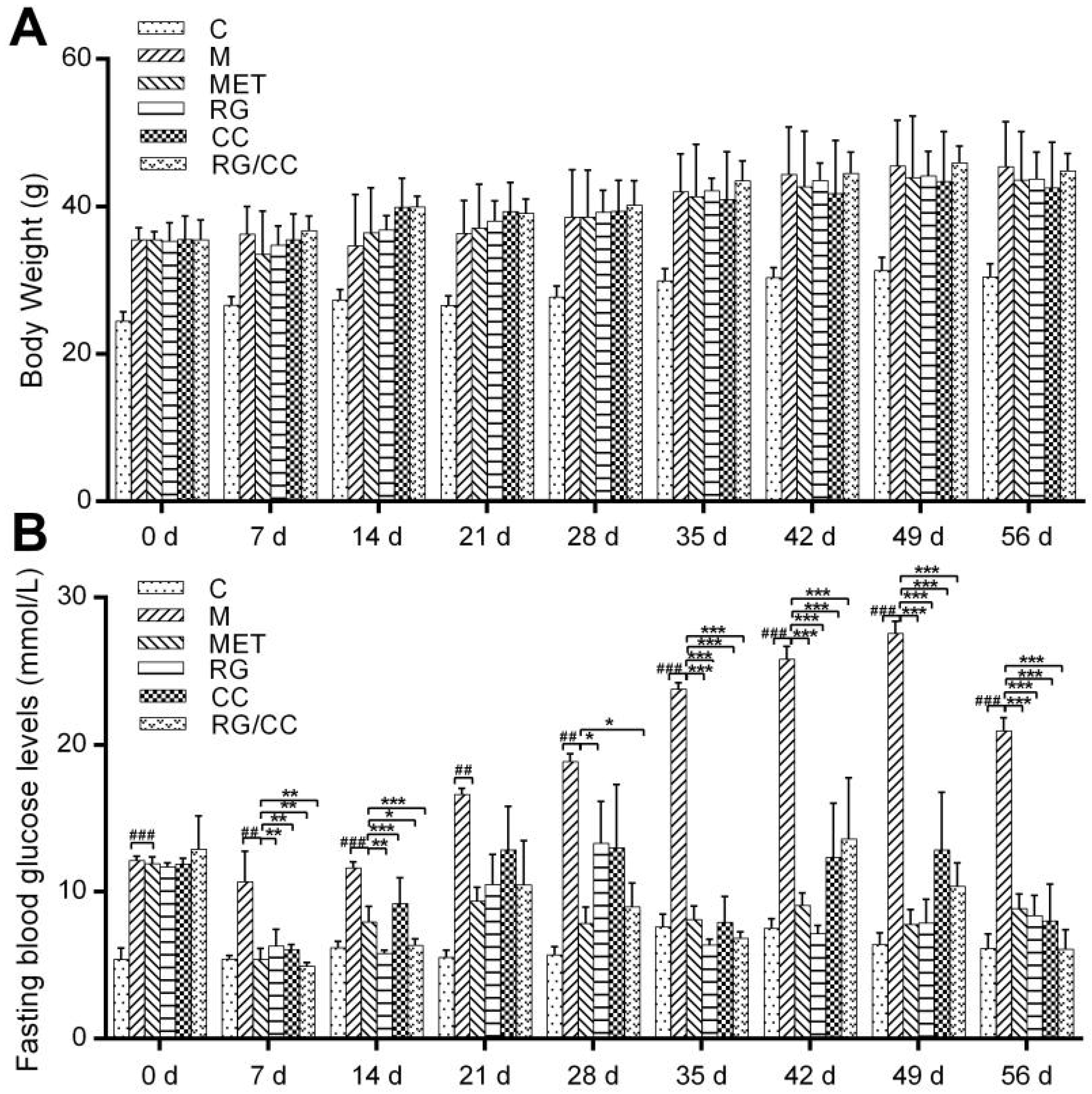
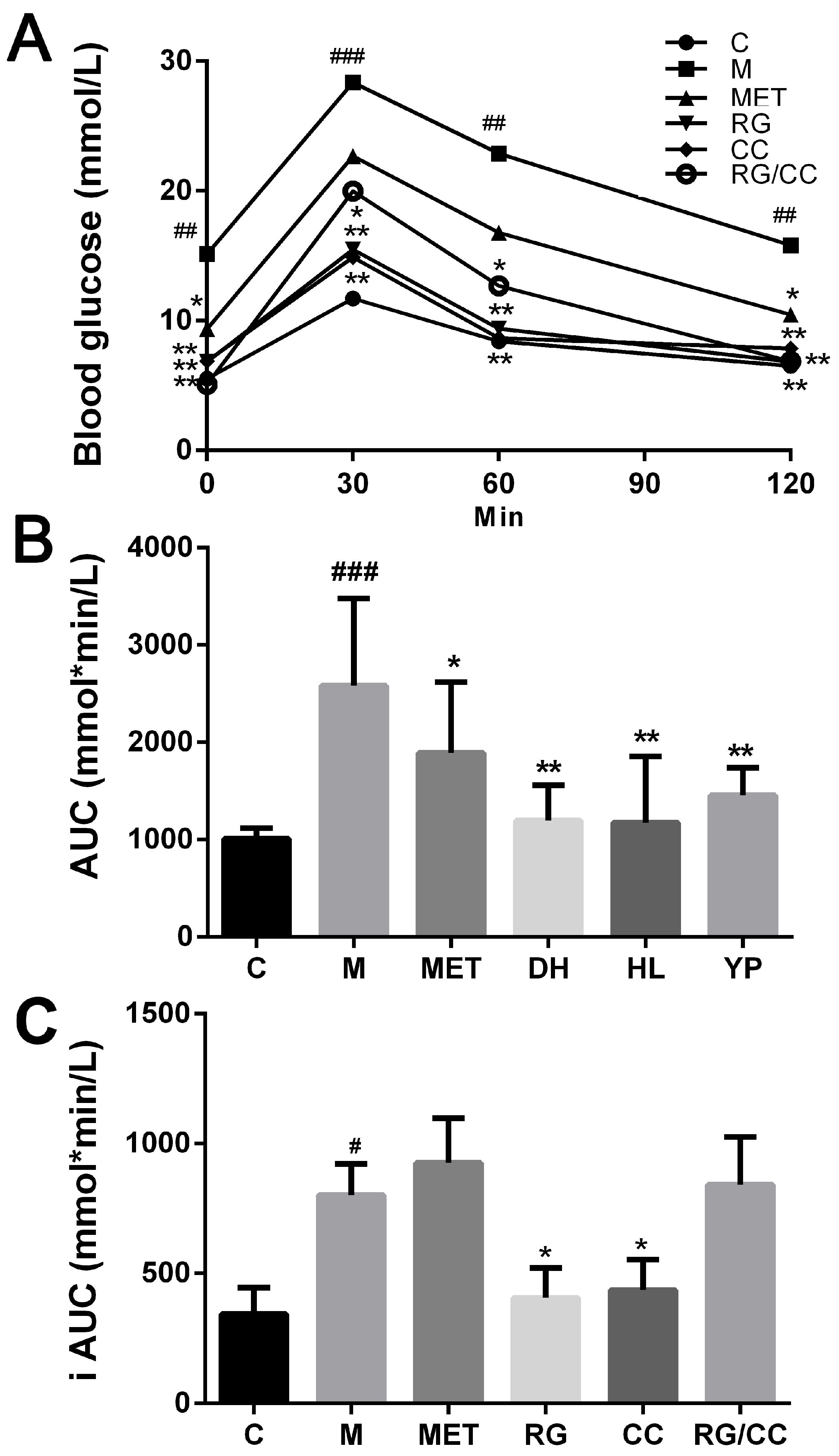
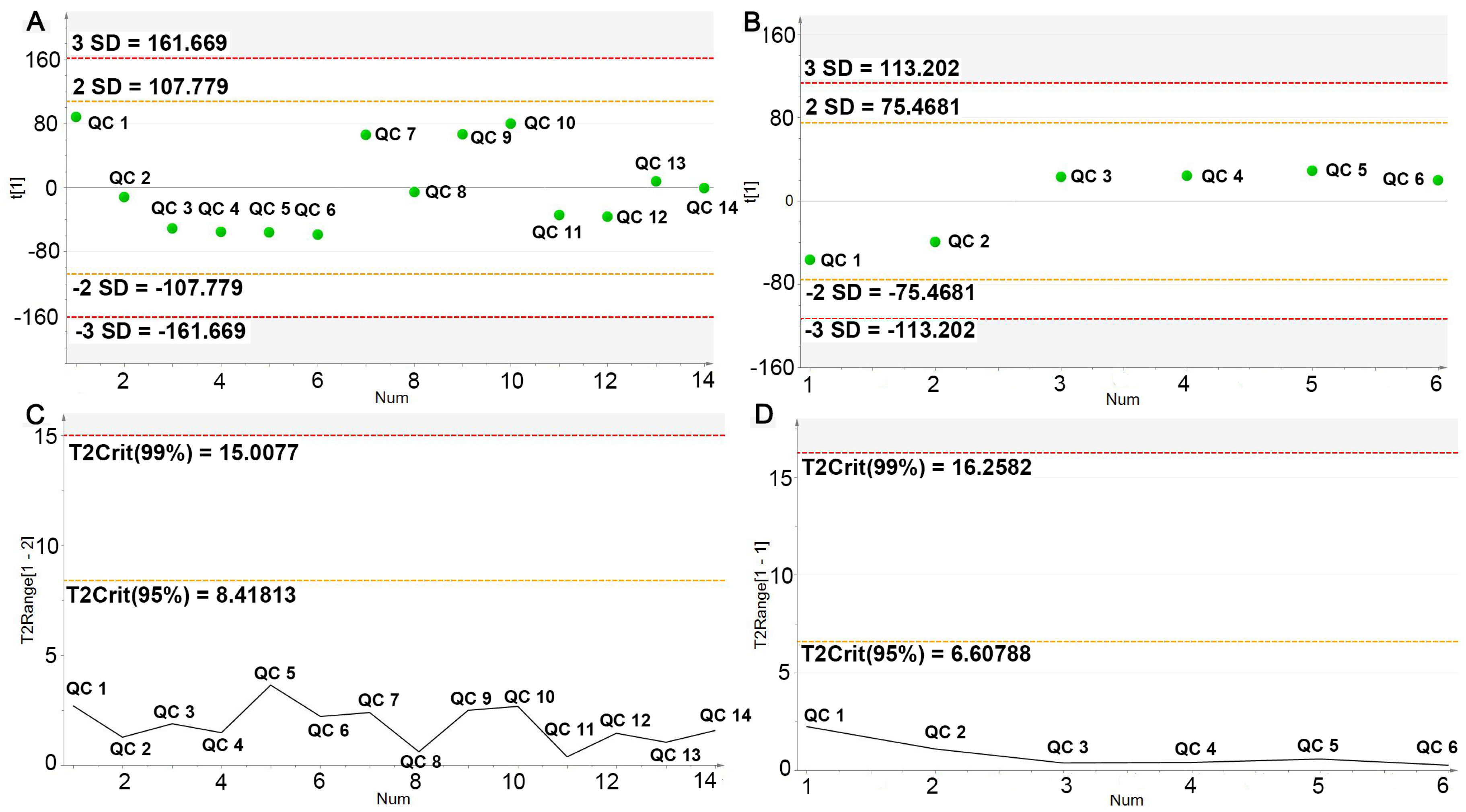
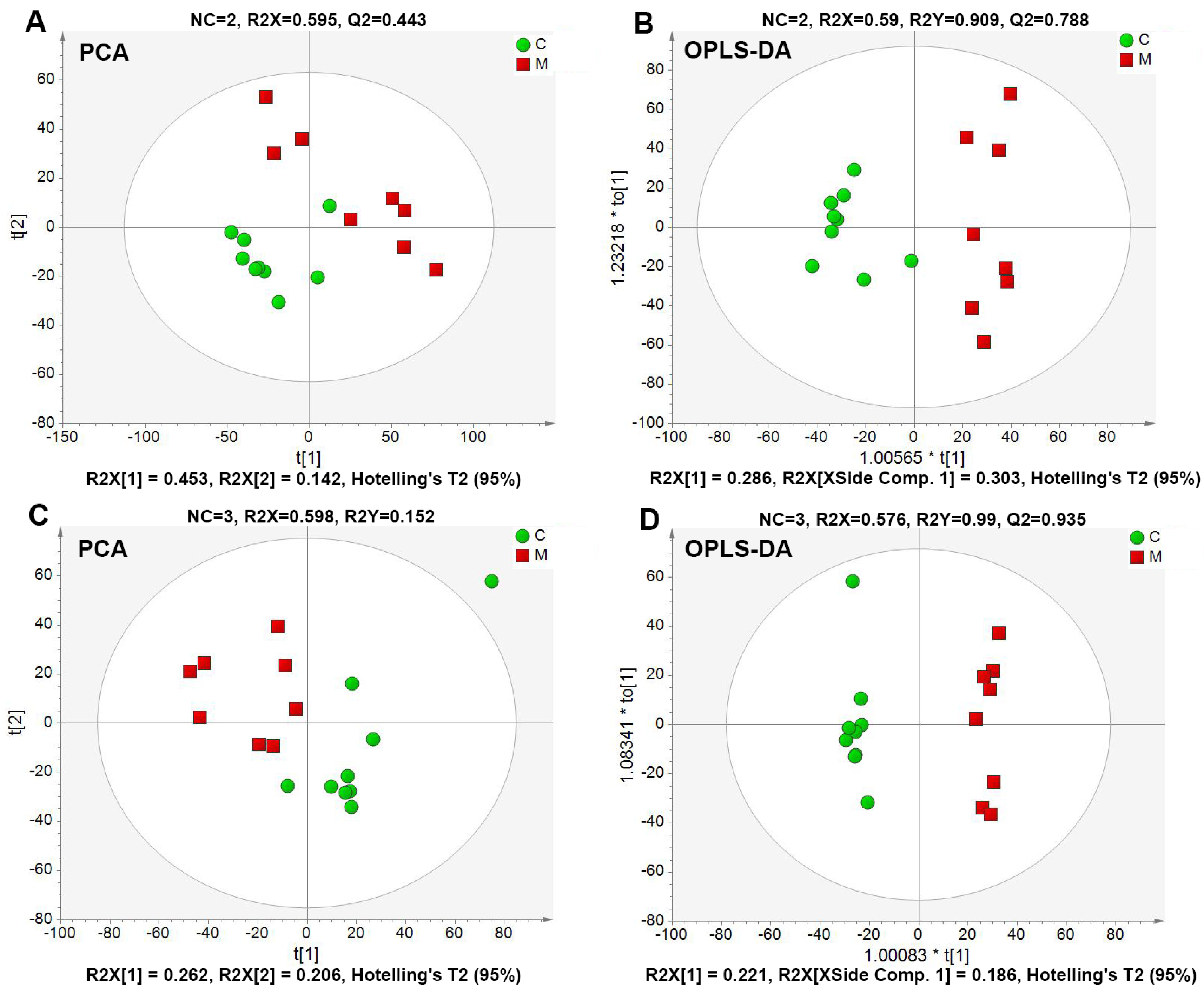
| Treatment | TC (mmol·L−1) | TG (mmol·L−1) | HDL-C (mmol·L−1) | LDL-C (mmol·L−1) |
|---|---|---|---|---|
| C | 2.26 ± 0.16 | 0.89 ± 0.16 | 2.88 ± 0.32 | 0.25 ± 0.07 |
| M | 4.17 ± 0.48 ### | 1.98 ± 0.27 ### | 1.91 ± 0.16 ### | 0.90 ± 0.25 ### |
| MET | 3.24 ± 0.47 *** | 1.50 ± 0.35 *** | 2.53 ± 0.41 ** | 0.58 ± 0.11 *** |
| RG | 3.05 ± 0.45 *** | 1.35 ± 0.15 *** | 2.12 ± 0.35 | 0.59 ± 0.06 *** |
| CC | 3.64 ± 0.24 * | 0.99 ± 0.15 *** | 2.49 ± 0.15 ** | 0.52 ± 0.10 *** |
| RG/CC | 2.84 ± 0.41 *** | 1.25 ± 0.21 *** | 2.09 ± 0.30 | 0.52 ± 0.12 *** |
| VIP | RT (min) | Quasi-Molecular Ion (m/z) | Cal. m/z | Mass Error (ppm) | HMDB ID | Metabolite | Formula | Metabolic Pathway | Fold Change | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-M | M-MET | M-RG | M-CC | M-RG/CC | |||||||||
| 1.36 | 5.86 | 799.4781 [M + H]+ | 799.4838 | −7.1339 | HMDB40655 | Mabioside D | C42H70O14 | Lipid metabolism | 2.04 c | 1.00 | 0.70 | 1.58 b | 1.35 |
| 3.59 | 5.69 | 542.3221 [M + H]+ | 542.3241 | −3.6900 | HMDB10397 | LysoPC(20:5) | C28H48NO7P | Lipid metabolism | 1.86 b | 1.18 | 1.01 | 1.55 | 1.75 a |
| 4.02 | 5.70 | 184.0747 [M + H]+ | 184.0739 | 4.3478 | HMDB01565 | Phosphorylcholine | C5H15NO4P | Phospholipid biosynthesis | 1.90 b | 1.20 | 1.08 | 1.99 b | 2.02 b |
| 10.23 | 9.67 | 758.5689 [M + H]+ | 758.5694 | −6.5963 | HMDB08296 | PC(20:1/14:1) | C42H80NO8P | Lipid metabolism | 4.42 b | 0.11 | 0.72 | 4.67 a | 5.10 a |
| 15.35 | 5.85 | 520.3415 [M + H]+ | 520.3398 | 3.2692 | HMDB10386 | LysoPC(18:2) | C26H50NO7P | Lipid metabolism | 1.66 c | 1.07 | 1.00 | 1.68 c | 1.57 b |
| 11.37 | 5.81 | 568.3408 [M + H]+ | 568.3398 | 1.7606 | HMDB10404 | LysoPC(22:6) | C30H50NO7P | Lipid metabolism | 3.05 c | 1.37 | 1.43 | 2.78 c | 3.12 c |
| 3.28 | 8.52 | 327.2318 [M − H]− | 327.233 | −3.6697 | HMDB02183 | Docosahexaenoic acid | C22H32O2 | Lipid metabolism | 1.72 c | 1.28 | 1.10 | 1.41 a | 1.08 |
| 3.81 | 5.58 | 478.2926 [M − H]− | 478.2939 | −2.7197 | HMDB11476 | LysoPE(0:0/18:1) | C23H46NO7P | Fatty acid metabolism | 1.71 c | 0.65 | 0.73 | 0.59 a | 0.73 |
| 2.06 | 6.20 | 502.2930 [M − H]− | 502.2939 | −1.7928 | HMDB11515 | LysoPE(20:3/0:0) | C25H46NO7P | Lipid metabolism | 0.41 b | 0.61 | 0.69 | 0.53 a | 0.63 |
| 2.43 | 12.00 | 885.5492 [M − H]− | 885.5499 | −7.9096 | HMDB09900 | PI(20:4/18:0) | C47H83O13P | Lipid metabolism | 0.17 c | 1.05 | 1.19 | 1.43 | 0.35 b |
| 2.07 | 6.78 | 321.2421 [M − H]− | 321.2435 | −4.3614 | HMDB62747 | 12(S)-HETrE | C20H34O3 | Lipid metabolism | 0.28 c | 0.63 a | 0.66 a | 0.77 | 0.45 c |
| 1.75 | 5.41 | 380.2554 [M − H]− | 380.2571 | −4.4737 | HMDB01383 | Sphinganine 1-phosphate | C18H40NO5P | Lipid metabolism | 0.20 c | 0.87 | 0.83 | 0.52 b | 0.55 a |
| 3.56 | 7.47 | 480.3085 [M − H]− | 480.3096 | −2.2917 | HMDB10381 | LysoPC(15:0) | C23H48NO7P | Lipid metabolism | 0.56 b | 1.11 | 1.04 | 0.65 b | 0.55 b |
| 1.36 | 5.37 | 503.2951 [M − H]− | 503.3014 | −12.5249 | HMDB33709 | Desglucocoroloside | C29H44O7 | Lipid metabolism | 16.26 c | 0 | 0.93 | 0.73 | 0.85 |
| 1.54 | 11.88 | 265.1464 [M − H]− | 265.1445 | 7.1698 | HMDB35210 | 10-Hydroxymyoporone | C15H22O4 | Lipid metabolism | 1.36 c | 0.58 c | 0.43 c | 0.44 c | 0.33 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.; Wang, W.; Liao, D.; Wu, X.; Li, X. UPLC-Q/TOF-MS-Based Serum Metabolomics Reveals Hypoglycemic Effects of Rehmannia glutinosa, Coptis chinensis and Their Combination on High-Fat-Diet-Induced Diabetes in KK-Ay Mice. Int. J. Mol. Sci. 2018, 19, 3984. https://doi.org/10.3390/ijms19123984
Qin Z, Wang W, Liao D, Wu X, Li X. UPLC-Q/TOF-MS-Based Serum Metabolomics Reveals Hypoglycemic Effects of Rehmannia glutinosa, Coptis chinensis and Their Combination on High-Fat-Diet-Induced Diabetes in KK-Ay Mice. International Journal of Molecular Sciences. 2018; 19(12):3984. https://doi.org/10.3390/ijms19123984
Chicago/Turabian StyleQin, Zhenxian, Wei Wang, Dengqun Liao, Xiaoying Wu, and Xian’en Li. 2018. "UPLC-Q/TOF-MS-Based Serum Metabolomics Reveals Hypoglycemic Effects of Rehmannia glutinosa, Coptis chinensis and Their Combination on High-Fat-Diet-Induced Diabetes in KK-Ay Mice" International Journal of Molecular Sciences 19, no. 12: 3984. https://doi.org/10.3390/ijms19123984
APA StyleQin, Z., Wang, W., Liao, D., Wu, X., & Li, X. (2018). UPLC-Q/TOF-MS-Based Serum Metabolomics Reveals Hypoglycemic Effects of Rehmannia glutinosa, Coptis chinensis and Their Combination on High-Fat-Diet-Induced Diabetes in KK-Ay Mice. International Journal of Molecular Sciences, 19(12), 3984. https://doi.org/10.3390/ijms19123984





