The Role of Tumor Necrosis Factor α in the Biology of Uterine Fibroids and the Related Symptoms
Abstract
1. Introduction
1.1. Uterine Fibroids—An Overview
1.2. Uterine Fibroids—Growth Factors and Steroid Control
1.3. Uterine Fibroids and the Extracellular Matrix
1.4. Uterine Fibroids and Cytokines
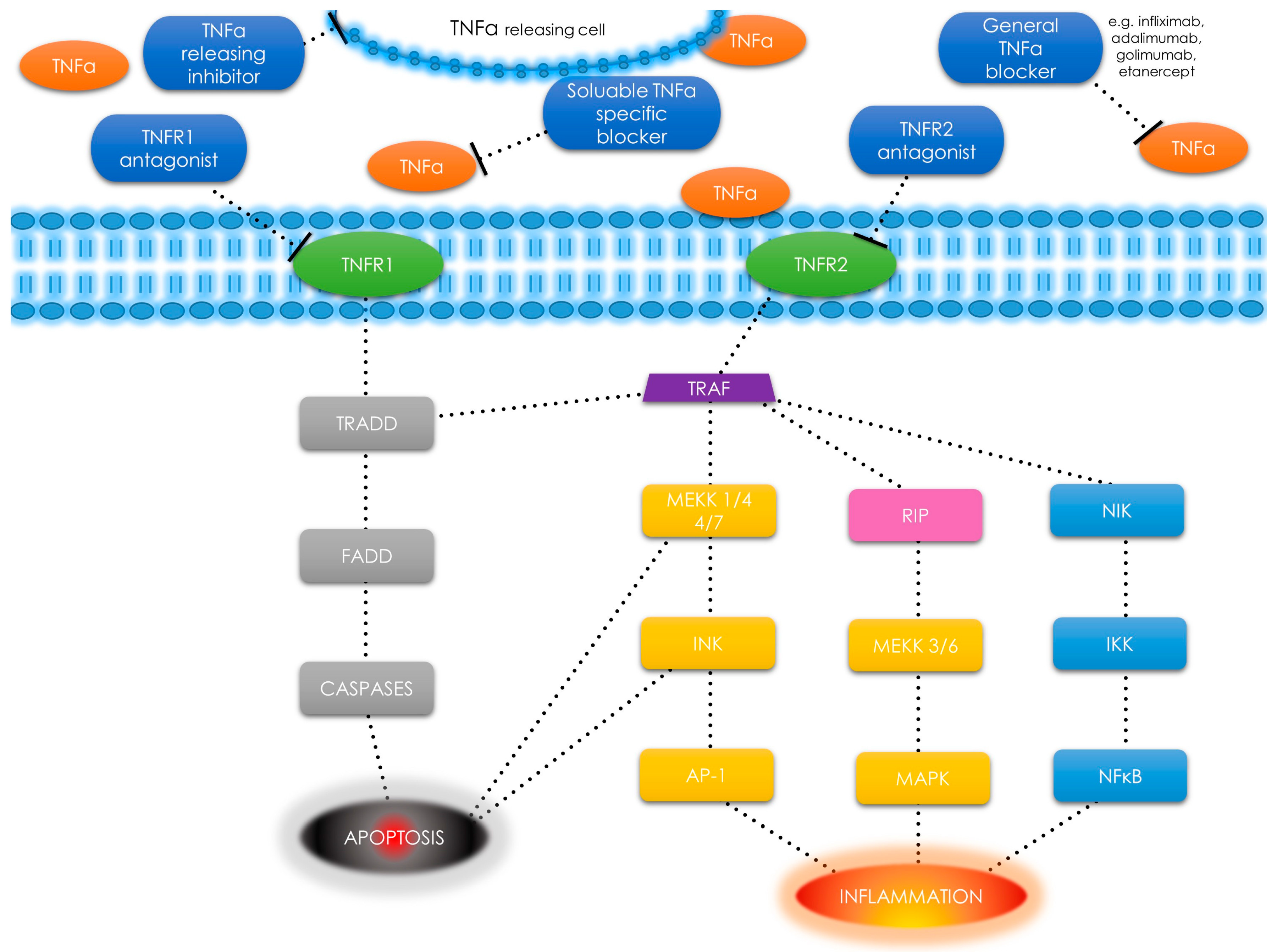
1.5. Tumor Necrosis Factor α—An Overview and Pathways
2. Material and Methods
3. Discussion
3.1. Uterine Fibroids and Inflammation
3.2. ECM and Inflammation in Uterine Fibroids
3.3. Tumor Necrosis Factor α in Uterine Fibroids
3.4. Obesity, Inflammation and Tumor Necrosis Factor α in Uterine Fibroids
3.5. Tumor Necrosis Factor α, Uterine Fibroids and the Related Symptoms—Overview
3.5.1. Pain
3.5.2. Infertility
3.5.3. Gastrointestinal Issues
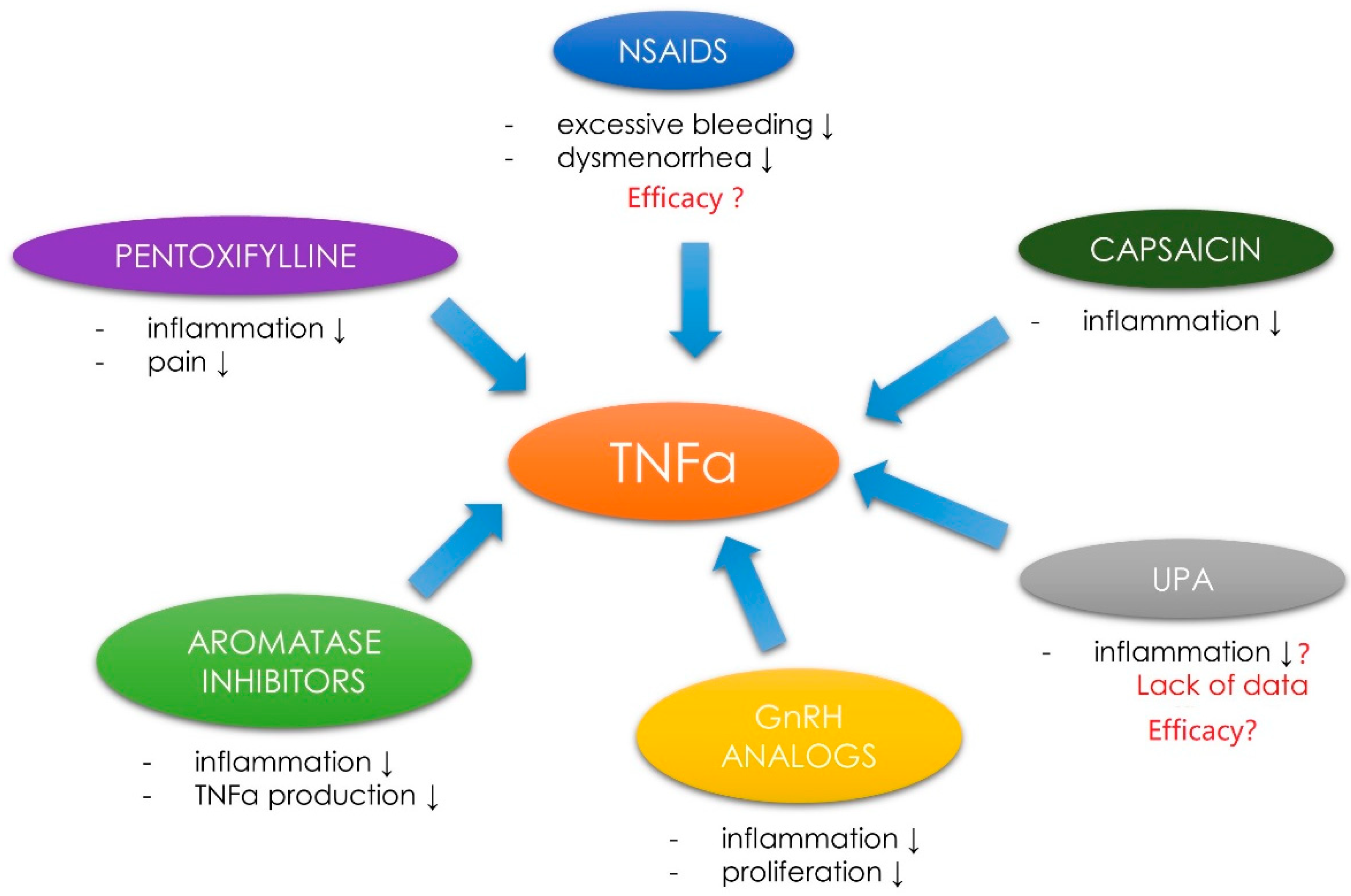
3.6. Tumor Necrosis Factor α, Uterine Fibroids and the Related Symptoms—Management
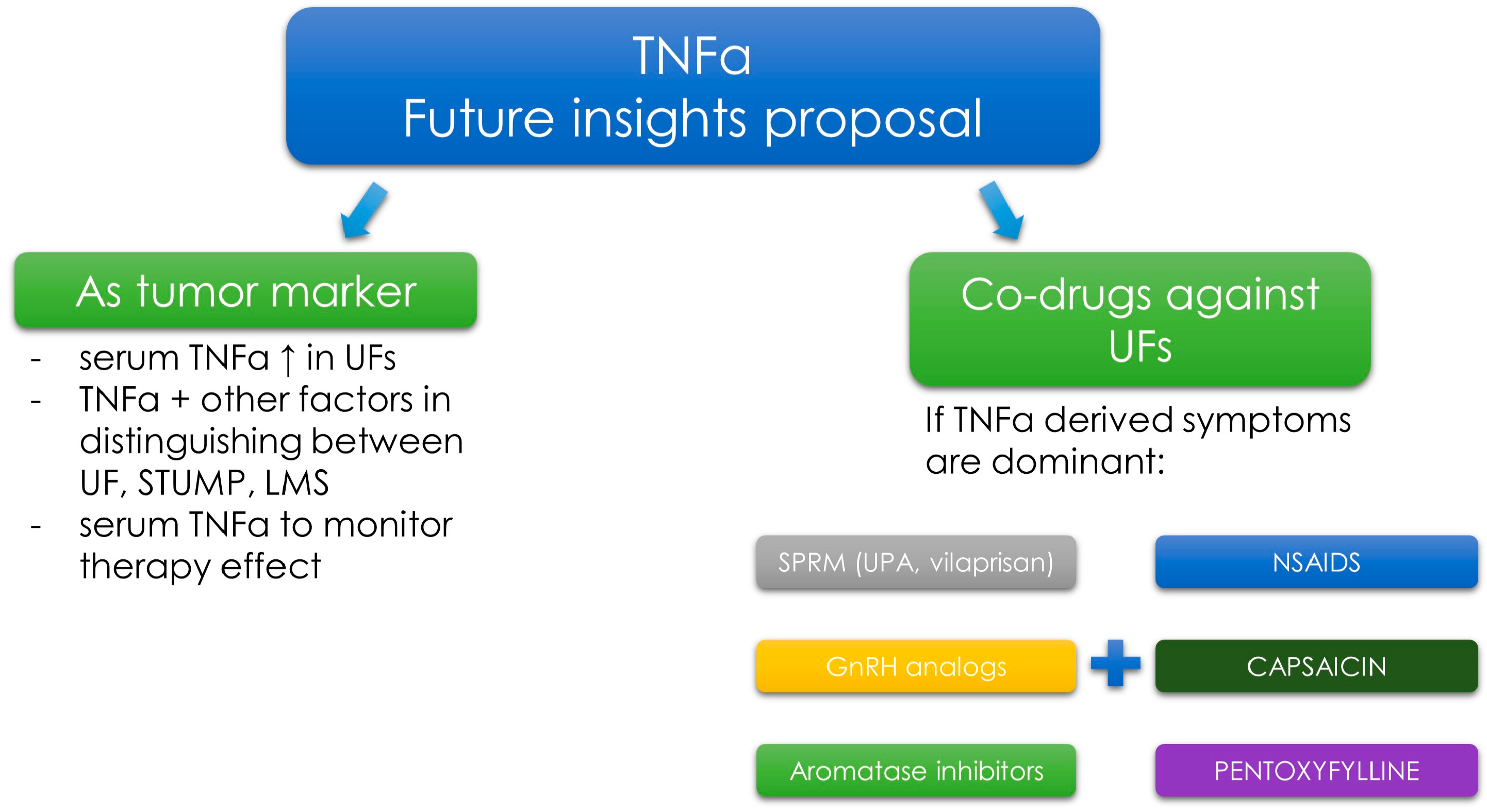
3.7. Tumor Necrosis Factor α—Novel Concepts in Diagnosis and Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADAM17 | ADAM metallopeptidase domain 17 |
| AMH | anti-Müllerian hormone |
| AP-1 | activator protein 1 |
| BMP | bone morphogenetic protein |
| COX-2 | cyclooxygenase 2 |
| ECM | extracellular matrix |
| EGF | endothelial growth factor |
| ER | estrogen receptor |
| ERK | extracellular signal–regulated kinase |
| FADD | Fas-associated protein with death domain |
| FAK | focal adhesion kinase |
| FGF | fibroblast growth factor |
| GnRH | gonadotropin-releasing hormone |
| IFN | Interferon |
| IGF-1 | insulin-like growth factor 1 |
| IKK | I kappa B kinase |
| IL | Interleukin |
| JNK | c-jun N-terminal kinase |
| LMS | leiomyosarcoma |
| MAPK | mitogen-activated protein kinases |
| MEKK | mitogen-activated protein kinase kinase kinase |
| MMP | matrix metalloproteinase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIK | NF-kappa-B-inducing kinase |
| NK | natural killer |
| NSAID | nonsteroidal anti-inflammatory drug |
| PCOS | polycystic ovary syndrome |
| PDGF | platelet-derived growth factor |
| PG | Prostaglandin |
| PR | progesterone receptor |
| RIP | receptor interacting protein |
| ROS | reactive oxygen species |
| SHBG | sex hormone-binding globulin |
| SODD | silencer of death domains |
| SPRM | selective progesterone receptor modulator |
| STUMP | smooth muscle tumor of uncertain malignant potential |
| TAK | TGF-β-activated kinase |
| TGF-β | transforming growth factor β |
| TIMP | tissue inhibitor of metalloproteinase |
| TNFR | TNF-α receptor |
| TNF-α | tumor necrosis factor α |
| TRADD | tumor necrosis factor receptor type 1-associated death domain |
| TRAF | tumor necrosis factor receptor-associated factor |
| UF | uterine fibroid |
| UPA | ulipristal acetate |
| VEGF | vascular endothelial growth factor |
References
- Stewart, E.A. Uterine fibroids. Lancet 2001, 357, 293–298. [Google Scholar] [CrossRef]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG 2017, 124, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007, 87, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.; Farquhar, C.M.; Li, T.C. Is another meta-analysis on the effects of intramural fibroids on reproductive outcomes needed? Reprod. Biomed. Online 2011, 23, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Soave, I.; Marci, R. Uterine leiomyomata: The snowball effect. Curr. Med. Res. Opin 2017, 33, 1909–1911. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Islam, M.S.; Reis, F.M.; Gray, P.C.; Bloise, E.; Petraglia, F.; Vale, W.; Castellucci, M. Growth factors and myometrium: Biological effects in uterine fibroid and possible clinical implications. Hum. Reprod. Update 2011, 17, 772–790. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Myers, E.R.; Stewart, E. Uterine fibroids: Burden and unmet medical need. Semin. Reprod. Med. 2017, 35, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Margolis, M.K.; Castelli-Haley, J.; Fuldeore, M.J.; Owens, C.D.; Coyne, K.S. Impact of uterine fibroid symptoms on health-related quality of life of us women: Evidence from a cross-sectional survey. Curr. Med. Res. Opin 2017, 33, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, E.R.; Clark, A.D.; Banks, N.K.; Henne, M.B.; Stegmann, B.J.; Segars, J.H. The estimated annual cost of uterine leiomyomata in the united states. Am. J. Obstet. Gynecol. 2012, 206, e211–e219. [Google Scholar] [CrossRef]
- Soliman, A.M.; Yang, H.; Du, E.X.; Kelkar, S.S.; Winkel, C. The direct and indirect costs of uterine fibroid tumors: A systematic review of the literature between 2000 and 2013. Am. J. Obstet. Gynecol. 2015, 213, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Uterine fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Sozen, I.; Arici, A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril 2002, 78, 1–12. [Google Scholar] [CrossRef]
- Dixon, D.; He, H.; Haseman, J.K. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ. Health Perspect. 2000, 108 (Suppl. 5), 795–802. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Protic, O.; Stortoni, P.; Grechi, G.; Lamanna, P.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil Steril 2013, 100, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.S.; Malik, M.; Nurudeen, S.; Catherino, W.H. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril 2010, 93, 1500–1508. [Google Scholar] [CrossRef]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen receptors and signaling in fibroids: Role in pathobiology and therapeutic implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef]
- Nierth-Simpson, E.N.; Martin, M.M.; Chiang, T.C.; Melnik, L.I.; Rhodes, L.V.; Muir, S.E.; Burow, M.E.; McLachlan, J.A. Human uterine smooth muscle and leiomyoma cells differ in their rapid 17beta-estradiol signaling: Implications for proliferation. Endocrinology 2009, 150, 2436–2445. [Google Scholar] [CrossRef]
- Chegini, N. Proinflammatory and profibrotic mediators: Principal effectors of leiomyoma development as a fibrotic disorder. Semin. Reprod. Med. 2010, 28, 180–203. [Google Scholar] [CrossRef]
- Maruo, T.; Ohara, N.; Wang, J.; Matsuo, H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Update 2004, 10, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Barbarisi, A.; Petillo, O.; Di Lieto, A.; Melone, M.A.; Margarucci, S.; Cannas, M.; Peluso, G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: Evidence for a stimulation of protein kinase-dependent pathway. J. Cell. Physiol. 2001, 186, 414–424. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Chill, H.H.; Safrai, M.; Reuveni Salzman, A.; Shushan, A. The rising phoenix-progesterone as the main target of the medical therapy for leiomyoma. Biomed. Res. Int. 2017, 2017, 4705164. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.L.; Stewart, E.A. Uterine fibroids: The elephant in the room. Science 2005, 308, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Wlodarczyk, M.; Slabuszewska-Jozwiak, A.; Nowicka, G.; Jakiel, G. Influence of vitamin D and transforming growth factor beta3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil Steril 2016, 106, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Slabuszewska-Jozwiak, A.; Nowicka, G.; Jakiel, G. Ulipristal acetate decreases transforming growth factor beta3 serum and tumor tissue concentrations in patients with uterine fibroids. Fertil Steril 2018, 109, 501–507. [Google Scholar] [CrossRef]
- Halder, S.; Al-Hendy, A. Hypovitaminosis D and high serum transforming growth factor beta-3: Important biomarkers for uterine fibroids risk. Fertil Steril 2016, 106, 1648–1649. [Google Scholar] [CrossRef]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Meczekalski, B.; Nowicka, G.; Lukaszuk, K.; Ciebiera, M.; Slabuszewska-Jozwiak, A.; Jakiel, G. Role of transforming growth factor beta in uterine fibroid biology. Int J. Mol. Sci. 2017, 18, 2435. [Google Scholar] [CrossRef]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Wojtyla, C.; Blazej, M.; Nowicka, G.; Lukaszuk, K.; Jakiel, G. TNF-alpha serum levels are elevated in women with clinically symptomatic uterine fibroids. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418779461. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.Y.; Shin, J.H.; Kim, H.; Ku, S.Y.; Suh, C.S. Variation in microRNA expression profile of uterine leiomyoma with endometrial cavity distortion and endometrial cavity non-distortion. Int. J. Mol. Sci. 2018, 19, 2524. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Jayes, F.L.; Segars, J.H. The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet. Gynecol. Int. 2014, 2014, 783289. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Norian, J.; McCarthy-Keith, D.; Britten, J.; Catherino, W.H. Why leiomyomas are called fibroids: The central role of extracellular matrix in symptomatic women. Semin. Reprod. Med. 2010, 28, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Segars, J.H.; Leppert, P.C. Mechanical signaling and extracellular matrix in uterine fibroids. Semin. Reprod. Med. 2017, 35, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Protic, O.; Toti, P.; Islam, M.S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D.A. Mechanisms of fibrogenesis. Exp. Biol. Med. 2008, 233, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Arici, A.; Sozen, I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril 2000, 73, 1006–1011. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Wolanska, M.; Taudul, E.; Bankowska-Guszczyn, E.; Kinalski, M. Tumor necrosis factor in uterine leiomyomas at various stages of tumor growth. Ginekol. Pol. 2010, 81, 431–434. [Google Scholar]
- Lee, J.W.; Juliano, R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol. Cells 2004, 17, 188–202. [Google Scholar]
- Dinarello, C.A. Historical insights into cytokines. Eur. J. Immunol. 2007, 37 (Suppl. 1), S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Markowska, A.; Mardas, M.; Gajdzik, E.; Zagrodzki, P.; Markowska, J. Oxidative stress markers in uterine fibroids tissue in pre- and postmenopausal women. Clin. Exp. Obstet. Gynecol. 2015, 42, 725–729. [Google Scholar] [PubMed]
- Sivarajasingam, S.P.; Imami, N.; Johnson, M.R. Myometrial cytokines and their role in the onset of labour. J. Endocrinol. 2016, 231, R101–R119. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, CD012179. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Balkwill, F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002, 13, 135–141. [Google Scholar] [CrossRef]
- Kurachi, O.; Matsuo, H.; Samoto, T.; Maruo, T. Tumor necrosis factor-alpha expression in human uterine leiomyoma and its down-regulation by progesterone. J. Clin. Endocrinol. Metab. 2001, 86, 2275–2280. [Google Scholar] [CrossRef]
- Postal, M.; Lapa, A.T.; Sinicato, N.A.; de Oliveira Pelicari, K.; Peres, F.A.; Costallat, L.T.; Fernandes, P.T.; Marini, R.; Appenzeller, S. Depressive symptoms are associated with tumor necrosis factor alpha in systemic lupus erythematosus. J. Neuroinflamm. 2016, 13, 5. [Google Scholar] [CrossRef]
- Victor, F.C.; Gottlieb, A.B. TNF-alpha and apoptosis: Implications for the pathogenesis and treatment of psoriasis. J. Drugs Dermatol. 2002, 1, 264–275. [Google Scholar] [PubMed]
- Brynskov, J.; Foegh, P.; Pedersen, G.; Ellervik, C.; Kirkegaard, T.; Bingham, A.; Saermark, T. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut 2002, 51, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Decourt, B.; Lahiri, D.K.; Sabbagh, M.N. Targeting tumor necrosis factor alpha for Alzheimer’s disease. Curr. Alzheimer Res. 2017, 14, 412–425. [Google Scholar] [CrossRef]
- Chuang, M.J.; Sun, K.H.; Tang, S.J.; Deng, M.W.; Wu, Y.H.; Sung, J.S.; Cha, T.L.; Sun, G.H. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 2008, 99, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Victor, F.C.; Gottlieb, A.B.; Menter, A. Changing paradigms in dermatology: Tumor necrosis factor alpha (TNF-alpha) blockade in psoriasis and psoriatic arthritis. Clin. Dermatol. 2003, 21, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, L.M.; Birnbaum, A.D.; Goldstein, D.A. TNF inhibition for ophthalmic indications: Current status and outlook. BioDrugs 2013, 27, 347–357. [Google Scholar] [CrossRef]
- Matsuno, H.; Yudoh, K.; Katayama, R.; Nakazawa, F.; Uzuki, M.; Sawai, T.; Yonezawa, T.; Saeki, Y.; Panayi, G.S.; Pitzalis, C.; et al. The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): A study using a human ra/scid mouse chimera. Rheumatology 2002, 41, 329–337. [Google Scholar] [CrossRef]
- Gupta, M.; Babic, A.; Beck, A.H.; Terry, K. TNF-alpha expression, risk factors, and inflammatory exposures in ovarian cancer: Evidence for an inflammatory pathway of ovarian carcinogenesis? Hum. Pathol. 2016, 54, 82–91. [Google Scholar] [CrossRef]
- Olszewski, M.B.; Groot, A.J.; Dastych, J.; Knol, E.F. TNF trafficking to human mast cell granules: Mature chain-dependent endocytosis. J. Immunol. 2007, 178, 5701–5709. [Google Scholar] [CrossRef]
- Tang, P.; Hung, M.C.; Klostergaard, J. Human pro-tumor necrosis factor is a homotrimer. Biochemistry 1996, 35, 8216–8225. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.L.; Jin, S.L.; Milla, M.E.; Bickett, D.M.; Burkhart, W.; Carter, H.L.; Chen, W.J.; Clay, W.C.; Didsbury, J.R.; Hassler, D.; et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 1997, 385, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Palladino, M.A.; Bahjat, F.R.; Theodorakis, E.A.; Moldawer, L.L. Anti-TNF-alpha therapies: The next generation. Nat. Rev. Drug Discov. 2003, 2, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Dubravec, D.B.; Spriggs, D.R.; Mannick, J.A.; Rodrick, M.L. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA 1990, 87, 6758–6761. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Goeddel, D.V. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef]
- Reactome. TNF Signaling. Available online: http://www.reactome.org/content/detail/R-HSA-75893 (accessed on 7 October 2018).
- Sun, S.C. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011, 21, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Delhase, M. The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signalling. Semin. Immunol. 2000, 12, 85–98. [Google Scholar] [CrossRef]
- Oltmanns, U.; Issa, R.; Sukkar, M.B.; John, M.; Chung, K.F. Role of c-Jun N-terminal kinase in the induced release of GM-CSF, RANTES and Il-8 from human airway smooth muscle cells. Br. J. Pharmacol. 2003, 139, 1228–1234. [Google Scholar] [CrossRef]
- Festjens, N.; Vanden Berghe, T.; Cornelis, S.; Vandenabeele, P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell. Death Differ. 2007, 14, 400–410. [Google Scholar] [CrossRef]
- Riches, D.W.; Chan, E.D.; Winston, B.W. TNF-alpha-induced regulation and signalling in macrophages. Immunobiology 1996, 195, 477–490. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Swat, W.; Zhang, S.; Zhang, Z.Y.; Neel, B.G.; Flavell, R.A.; Davis, R.J. TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Genes Dev. 2011, 25, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Scheurich, P. Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in tnf signaling. Int. J. Biochem. Cell Biol. 2001, 33, 19–32. [Google Scholar] [CrossRef]
- Takada, H.; Chen, N.J.; Mirtsos, C.; Suzuki, S.; Suzuki, N.; Wakeham, A.; Mak, T.W.; Yeh, W.C. Role of sodd in regulation of tumor necrosis factor responses. Mol. Cell. Biol. 2003, 23, 4026–4033. [Google Scholar] [CrossRef] [PubMed]
- Pobezinskaya, Y.L.; Liu, Z. The role of TRADD in death receptor signaling. Cell Cycle 2012, 11, 871–876. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, D.; Sheng, J.; Luo, L.; Zhang, W. Identification of TRADD as a potential biomarker in human uterine leiomyoma through Itraq based proteomic profiling. Mol. Cell. Probes 2017, 36, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Jacobi, C.; Feige, J.N.; Glass, D.J. Tak-1/p38/NFkappaB signaling inhibits myoblast differentiation by increasing levels of Activin A. Skelet Muscle 2012, 2, 3. [Google Scholar] [CrossRef]
- Protic, O.; Islam, M.S.; Greco, S.; Giannubilo, S.R.; Lamanna, P.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; Hinz, B.; Ciarmela, P. Activin A in inflammation, tissue repair, and fibrosis: Possible role as inflammatory and fibrotic mediator of uterine fibroid development and growth. Semin. Reprod. Med. 2017, 35, 499–509. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Vlahopoulos, S.; Zoumpourlis, V.C. JNK: A key modulator of intracellular signaling. Biochemistry 2004, 69, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.T.; Davis, R.J. Signal transduction by the c-Jun N-terminal kinase (JNK)—From inflammation to development. Curr. Opin Cell Biol. 1998, 10, 205–219. [Google Scholar] [CrossRef]
- Wagner, E.F.; Nebreda, A.R. Signal integration by jnk and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Gaur, U.; Aggarwal, B.B. Regulation of proliferation, survival and apoptosis by members of the tnf superfamily. Biochem. Pharmacol. 2003, 66, 1403–1408. [Google Scholar] [CrossRef]
- Chow, M.T.; Moller, A.; Smyth, M.J. Inflammation and immune surveillance in cancer. Semin. Cancer Biol. 2012, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wegienka, G. Are uterine leiomyoma a consequence of a chronically inflammatory immune system? Med. Hypotheses 2012, 79, 226–231. [Google Scholar] [CrossRef]
- Wegienka, G.; Baird, D.D.; Cooper, T.; Woodcroft, K.J.; Havstad, S. Cytokine patterns differ seasonally between women with and without uterine leiomyomata. Am. J. Reprod. Immunol. 2013, 70, 327–335. [Google Scholar] [CrossRef]
- Miura, S.; Khan, K.N.; Kitajima, M.; Hiraki, K.; Moriyama, S.; Masuzaki, H.; Samejima, T.; Fujishita, A.; Ishimaru, T. Differential infiltration of macrophages and prostaglandin production by different uterine leiomyomas. Hum. Reprod. 2006, 21, 2545–2554. [Google Scholar] [CrossRef]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef]
- Leppert, P.C.; Baginski, T.; Prupas, C.; Catherino, W.H.; Pletcher, S.; Segars, J.H. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril 2004, 82 (Suppl. 3), 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, C.; Castellot, J.J., Jr. Matrix production and remodeling as therapeutic targets for uterine leiomyoma. J. Cell Commun. Signal. 2014, 8, 179–194. [Google Scholar] [CrossRef]
- Feng, L.; Jayes, F.L.; Johnson, L.N.C.; Schomberg, D.W.; Leppert, P.C. Biochemical pathways and myometrial cell differentiation leading to nodule formation containing collagen and fibronectin. Curr. Protein Pept. Sci. 2017, 18, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Makinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. Med12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, H.R.; Sarvilinna, N.S.; Sjoberg, J.; Kampjarvi, K.; Pitkanen, E.; Vahteristo, P.; Makinen, N.; Aaltonen, L.A. Med12 mutation frequency in unselected sporadic uterine leiomyomas. Fertil Steril 2014, 102, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Makinen, N.; Heinonen, H.R.; Moore, S.; Tomlinson, I.P.; van der Spuy, Z.M.; Aaltonen, L.A. MED12 exon 2 mutations are common in uterine leiomyomas from South African patients. Oncotarget 2011, 2, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Laknaur, A.; Miller, J.; Layman, L.C.; Diamond, M.; Al-Hendy, A. Novel MED12 gene somatic mutations in women from the southern United States with symptomatic uterine fibroids. Mol. Genet. Genom. 2015, 290, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Shin, Y.H.; Yatsenko, S.A.; Castro, C.A.; Surti, U.; Rajkovic, A. MED12 gain-of-function mutation causes leiomyomas and genomic instability. J. Clin. Investig. 2015, 125, 3280–3284. [Google Scholar] [CrossRef] [PubMed]
- Elkafas, H.; Qiwei, Y.; Al-Hendy, A. Origin of uterine fibroids: Conversion of myometrial stem cells to tumor-initiating cells. Semin. Reprod. Med. 2017, 35, 481–486. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, A.; Chaudhry, Z.; Al-Hendy, A.; Ismail, N. Uterine fibroids: Bridging genomic defects and chronic inflammation. Semin. Reprod. Med. 2017, 35, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Segars, J.H. The role of angiogenic factors in fibroid pathogenesis: Potential implications for future therapy. Hum. Reprod. Update 2014, 20, 194–216. [Google Scholar] [CrossRef]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef]
- Islam, M.S.; Catherino, W.H.; Protic, O.; Janjusevic, M.; Gray, P.C.; Giannubilo, S.R.; Ciavattini, A.; Lamanna, P.; Tranquilli, A.L.; Petraglia, F.; et al. Role of Activin-A and myostatin and their signaling pathway in human myometrial and leiomyoma cell function. J. Clin. Endocrinol. Metab. 2014, 99, E775–E785. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Alzheimer, C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev. 2006, 17, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Chan, S.Y.; Lim, I.J.; Phillips, D.J.; Phan, T.T. The role of the activin system in keloid pathogenesis. Am. J. Physiol. Cell Physiol. 2007, 292, C1331–C1338. [Google Scholar] [CrossRef] [PubMed]
- Wada, W.; Kuwano, H.; Hasegawa, Y.; Kojima, I. The dependence of transforming growth factor-beta-induced collagen production on autocrine factor Activin A in hepatic stellate cells. Endocrinology 2004, 145, 2753–2759. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Puig-Kroger, A.; Blanco, F.J.; Nieto, C.; Bragado, R.; Palomero, M.I.; Bernabeu, C.; Vega, M.A.; Corbi, A.L. Activin a skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 2011, 117, 5092–5101. [Google Scholar] [CrossRef]
- Ciarmela, P.; Bloise, E.; Gray, P.C.; Carrarelli, P.; Islam, M.S.; De Pascalis, F.; Severi, F.M.; Vale, W.; Castellucci, M.; Petraglia, F. Activin-A and myostatin response and steroid regulation in human myometrium: Disruption of their signalling in uterine fibroid. J. Clin. Endocrinol. Metab. 2011, 96, 755–765. [Google Scholar] [CrossRef]
- Norian, J.M.; Malik, M.; Parker, C.Y.; Joseph, D.; Leppert, P.C.; Segars, J.H.; Catherino, W.H. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod. Sci. 2009, 16, 1153–1164. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and QSARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Dou, Q.; Tarnuzzer, R.W.; Williams, R.S.; Schultz, G.S.; Chegini, N. Differential expression of matrix metalloproteinases and their tissue inhibitors in leiomyomata: A mechanism for gonadotrophin releasing hormone agonist-induced tumour regression. Mol. Hum. Reprod. 1997, 3, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.; Wagih, M.; Kilic, G.S.; Diaz-Arrastia, C.R.; Baraka, M.A.; Salama, S.A. Overhydroxylation of lysine of collagen increases uterine fibroids proliferation: Roles of lysyl hydroxylases, lysyl oxidases, and matrix metalloproteinases. Biomed. Res. Int. 2017, 2017, 5316845. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol. Reprod. 2013, 89, 150. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chegini, N. Regulation of matrix metalloproteinases (MMPs) and their tissue inhibitors in human myometrial smooth muscle cells by TGF-beta1. Mol. Hum. Reprod. 1999, 5, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum. Reprod. 2013, 28, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Al-Hendy, A. Adipocytes enhance the proliferation of human leiomyoma cells via TNF-alpha proinflammatory cytokine. Reprod. Sci. 2011, 18, 1186–1192. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Chang, C.C.; Tsai, F.J.; Lin, C.C.; Yeh, L.S.; Tsai, C.H. Tumor necrosis factor-alpha-308 promoter and p53 codon 72 gene polymorphisms in women with leiomyomas. Fertil Steril 2004, 82 (Suppl. 3), 1177–1181. [Google Scholar] [CrossRef]
- Litovkin, K.V.; Domenyuk, V.P.; Bubnov, V.V.; Zaporozhan, V.N. Interleukin-6 -174g/c polymorphism in breast cancer and uterine leiomyoma patients: A population-based case control study. Exp. Oncol. 2007, 29, 295–298. [Google Scholar]
- Pietrowski, D.; Thewes, R.; Sator, M.; Denschlag, D.; Keck, C.; Tempfer, C. Uterine leiomyoma is associated with a polymorphism in the interleukin 1-beta gene. Am. J. Reprod. Immunol. 2009, 62, 112–117. [Google Scholar] [CrossRef]
- Martel, K.M.; Ko, A.C.; Christman, G.M.; Stribley, J.M. Apoptosis in human uterine leiomyomas. Semin. Reprod. Med. 2004, 22, 91–103. [Google Scholar] [CrossRef]
- Plewka, A.; Madej, P.; Plewka, D.; Kowalczyk, A.; Miskiewicz, A.; Wittek, P.; Leks, T.; Bilski, R. Immunohistochemical localization of selected pro-inflammatory factors in uterine myomas and myometrium in women of various ages. Folia Histochem. Cytobiol. 2013, 51, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Matsuo, H.; Shimomura, Y.; Kurachi, O.; Gao, Z.; Nakago, S.; Yamada, T.; Chen, W.; Wang, J. Effects of progesterone on growth factor expression in human uterine leiomyoma. Steroids 2003, 68, 817–824. [Google Scholar] [CrossRef]
- Manta, L.; Suciu, N.; Toader, O.; Purcarea, R.M.; Constantin, A.; Popa, F. The etiopathogenesis of uterine fibromatosis. J. Med. Life 2016, 9, 39–45. [Google Scholar] [PubMed]
- Wilkens, J.; Male, V.; Ghazal, P.; Forster, T.; Gibson, D.A.; Williams, A.R.; Brito-Mutunayagam, S.L.; Craigon, M.; Lourenco, P.; Cameron, I.T.; et al. Uterine nk cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J. Immunol. 2013, 191, 2226–2235. [Google Scholar] [CrossRef]
- Guo, W.; Li, P.; Zhao, G.; Fan, H.; Hu, Y.; Hou, Y. Glucocorticoid receptor mediates the effect of progesterone on uterine natural killer cells. Am. J. Reprod. Immunol. 2012, 67, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, F.; Wang, D.; Duncan, L.J.; Purohit, A.; Ghilchick, M.W.; Reed, M.J. Stimulation of aromatase activity in breast fibroblasts by tumor necrosis factor alpha. Mol. Cell. Endocrinol. 1994, 106, 17–21. [Google Scholar] [CrossRef]
- Kamel, M.; Shouman, S.; El-Merzebany, M.; Kilic, G.; Veenstra, T.; Saeed, M.; Wagih, M.; Diaz-Arrastia, C.; Patel, D.; Salama, S. Effect of tumour necrosis factor-alpha on estrogen metabolic pathways in breast cancer cells. J. Cancer 2012, 3, 310–321. [Google Scholar] [CrossRef]
- Hubner, G.; Werner, S. Serum growth factors and proinflammatory cytokines are potent inducers of activin expression in cultured fibroblasts and keratinocytes. Exp. Cell Res. 1996, 228, 106–113. [Google Scholar] [CrossRef]
- Shao, L.E.; Frigon, N.L., Jr.; Yu, A.; Palyash, J.; Yu, J. Contrasting effects of inflammatory cytokines and glucocorticoids on the production of activin a in human marrow stromal cells and their implications. Cytokine 1998, 10, 227–235. [Google Scholar] [CrossRef]
- Hillier, S.G.; Miro, F. Inhibin, activin, and follistatin. Potential roles in ovarian physiology. Ann. N. Y. Acad. Sci. 1993, 687, 29–38. [Google Scholar] [CrossRef]
- Shukovski, L.; Findlay, J.K. Activin-a inhibits oxytocin and progesterone production by preovulatory bovine granulosa cells in vitro. Endocrinology 1990, 126, 2222–2224. [Google Scholar] [CrossRef]
- Ali, M.; Al-Hendy, A. Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biol. Reprod. 2017, 97, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ohara, N.; Xu, Q.; Chen, W.; Wang, J.; Nakabayashi, K.; Sasaki, H.; Morikawa, A.; Maruo, T. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin. Reprod. Med. 2010, 28, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Carrarelli, P.; Islam, M.S.; Janjusevic, M.; Zupi, E.; Tosti, C.; Castellucci, M.; Petraglia, F. Ulipristal acetate modulates the expression and functions of activin a in leiomyoma cells. Reprod. Sci. 2014, 21, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, G.; Wang, J.; Zhou, Y.; Liu, Y.; Shi, Y.; Zhu, Y.; Lin, W.; Xu, Y.; Li, Z. Differential effects of tumor necrosis factor-alpha on matrix metalloproteinase-2 expression in human myometrial and uterine leiomyoma smooth muscle cells. Hum. Reprod. 2015, 30, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Erk1/2 map kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef] [PubMed]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Albuquerque, D.; Nobrega, C.; Manco, L.; Padez, C. The contribution of genetics and environment to obesity. Br. Med. Bull. 2017, 123, 159–173. [Google Scholar] [CrossRef]
- Baird, D.D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. Association of physical activity with development of uterine leiomyoma. Am. J. Epidemiol. 2007, 165, 157–163. [Google Scholar] [CrossRef]
- Shikora, S.A.; Niloff, J.M.; Bistrian, B.R.; Forse, R.A.; Blackburn, G.L. Relationship between obesity and uterine leiomyomata. Nutrition 1991, 7, 251–255. [Google Scholar]
- Shozu, M.; Murakami, K.; Inoue, M. Aromatase and leiomyoma of the uterus. Semin. Reprod. Med. 2004, 22, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sumitani, H.; Shozu, M.; Segawa, T.; Murakami, K.; Yang, H.J.; Shimada, K.; Inoue, M. In situ estrogen synthesized by aromatase p450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 2000, 141, 3852–3861. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.D.; Jirikowski, G.F. Sex hormone binding globulin and aging. Horm. Metab. Res. 2009, 41, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Saez-Lopez, C.; Barbosa-Desongles, A.; Hernandez, C.; Selva, D.M. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol. Metab. 2015, 26, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Alpay, Z.; Saed, G.M.; Diamond, M.P. Female infertility and free radicals: Potential role in adhesions and endometriosis. J. Soc. Gynecol. Investig. 2006, 13, 390–398. [Google Scholar] [CrossRef]
- Ilaria, S.; Marci, R. From obesity to uterine fibroids: An intricate network. Curr. Med. Res. Opin 2018, 1–3. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Lafontan, M. Fat cells: Afferent and efferent messages define new approaches to treat obesity. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 119–146. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, H.; Simental-Mendia, L.E.; Rodriguez-Ramirez, G.; Reyes-Romero, M.A. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Zaragosi, L.E.; Wdziekonski, B.; Villageois, P.; Keophiphath, M.; Maumus, M.; Tchkonia, T.; Bourlier, V.; Mohsen-Kanson, T.; Ladoux, A.; Elabd, C.; et al. Activin A plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes 2010, 59, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.M. Endometriosis and mechanisms of pelvic pain. J. Minim. Invasive Gynecol. 2009, 16, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Eisermann, J.; Gast, M.J.; Pineda, J.; Odem, R.R.; Collins, J.L. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertil Steril 1988, 50, 573–579. [Google Scholar] [CrossRef]
- Calhaz-Jorge, C.; Costa, A.P.; Barata, M.; Santos, M.C.; Melo, A.; Palma-Carlos, M.L. Tumour necrosis factor alpha concentrations in the peritoneal fluid of infertile women with minimal or mild endometriosis are lower in patients with red lesions only than in patients without red lesions. Hum. Reprod. 2000, 15, 1256–1260. [Google Scholar] [CrossRef]
- Sommer, C.; Kress, M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef]
- Dogru, H.Y.; Ozsoy, A.Z.; Karakus, N.; Delibas, I.B.; Isguder, C.K.; Yigit, S. Association of genetic polymorphisms in tnf and mif gene with the risk of primary dysmenorrhea. Biochem. Genet. 2016, 54, 457–466. [Google Scholar] [CrossRef]
- Chen, D.B.; Yang, Z.M.; Hilsenrath, R.; Le, S.P.; Harper, M.J. Stimulation of prostaglandin (PG) F2 alpha and PGE2 release by tumour necrosis factor-alpha and interleukin-1 alpha in cultured human luteal phase endometrial cells. Hum. Reprod. 1995, 10, 2773–2780. [Google Scholar] [CrossRef]
- Wiemer, A.J.; Hegde, S.; Gumperz, J.E.; Huttenlocher, A. A live imaging cell motility screen identifies prostaglandin E2 as a T cell stop signal antagonist. J. Immunol. 2011, 187, 3663–3670. [Google Scholar] [CrossRef]
- Ceyhan, S.T.; Onguru, O.; Fidan, U.; Ide, T.; Yaman, H.; Kilic, S.; Baser, I. Comparison of aromatase inhibitor (letrozole) and immunomodulators (infliximab and etanercept) on the regression of endometriotic implants in a rat model. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 154, 100–104. [Google Scholar] [CrossRef]
- Gurunath, S.; Pandian, Z.; Anderson, R.A.; Bhattacharya, S. Defining infertility—A systematic review of prevalence studies. Hum. Reprod. Update 2011, 17, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Iwabe, T.; Harada, T.; Terakawa, N. Role of cytokines in endometriosis-associated infertility. Gynecol. Obstet. Investig. 2002, 53 (Suppl. 1), 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ng, S.C.; Kwak-Kim, J.; Gilman-Sachs, A.; Beer, A.; Beaman, K. Increased tumor necrosis factor-alpha level in infertility patient. Clin. Appl. Immunol. Rev. 2002, 3, 6. [Google Scholar] [CrossRef]
- Falconer, H.; Sundqvist, J.; Gemzell-Danielsson, K.; von Schoultz, B.; D’Hooghe, T.M.; Fried, G. Ivf outcome in women with endometriosis in relation to tumour necrosis factor and anti-mullerian hormone. Reprod. Biomed. Online 2009, 18, 582–588. [Google Scholar] [CrossRef]
- Haider, S.; Knofler, M. Human tumour necrosis factor: Physiological and pathological roles in placenta and endometrium. Placenta 2009, 30, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Giannubilo, S.R.; Landi, B.; Pozzi, V.; Sartini, D.; Cecati, M.; Stortoni, P.; Corradetti, A.; Saccucci, F.; Tranquilli, A.L.; Emanuelli, M. The involvement of inflammatory cytokines in the pathogenesis of recurrent miscarriage. Cytokine 2012, 58, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Azizieh, F.Y.; Raghupathy, R.G. Tumor necrosis factor-alpha and pregnancy complications: A prospective study. Med. Princ. Pract. 2015, 24, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Malamitsi-Puchner, A. Reviews: Adipocytokines in normal and complicated pregnancies. Reprod. Sci. 2009, 16, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gu, Y.; Yin, X. High serum tumor necrosis factor-alpha levels in women with polycystic ovary syndrome: A meta-analysis. PLoS ONE 2016, 11, e0164021. [Google Scholar] [CrossRef] [PubMed]
- Pritts, E.A.; Parker, W.H.; Olive, D.L. Fibroids and infertility: An updated systematic review of the evidence. Fertil Steril 2009, 91, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Van Heertum, K.; Barmat, L. Uterine fibroids associated with infertility. Womens Health 2014, 10, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Purohit, P.; Vigneswaran, K. Fibroids and infertility. Curr. Obstet. Gynecol. Rep. 2016, 5, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Lukaszuk, K.; Meczekalski, B.; Ciebiera, M.; Wojtyla, C.; Slabuszewska-Jozwiak, A.; Jakiel, G. Alternative oral agents in prophylaxis and therapy of uterine fibroids-an up-to-date review. Int. J. Mol. Sci. 2017, 18, 2586. [Google Scholar] [CrossRef] [PubMed]
- Association Pour Le Developpement En Fecondation In Vitro. Impact of Esmya on Fertility to Infertile Women with Fibroids Managed with Assisted Reproduction Techniques (NACRE). Available online: https://clinicaltrials.gov/ct2/show/NCT03349190 (accessed on 8 October 2018).
- Tansey, M.G.; Szymkowski, D.E. The TNF superfamily in 2009: New pathways, new indications, and new drugs. Drug Discov. Today 2009, 14, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Reinecker, H.C.; Steffen, M.; Witthoeft, T.; Pflueger, I.; Schreiber, S.; MacDermott, R.P.; Raedler, A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 1993, 94, 174–181. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kita, M.; Kodama, T.; Sawai, N.; Kashima, K.; Imanishi, J. Induction of various cytokines and development of severe mucosal inflammation by caga gene positive helicobacter pylori strains. Gut 1997, 41, 442–451. [Google Scholar] [CrossRef]
- Wolfe, M.M.; Nompleggi, D.J. Cytokine inhibition of gastric acid secretion—A little goes a long way. Gastroenterology 1992, 102, 2177–2178. [Google Scholar] [CrossRef]
- Tahara, T.; Shibata, T.; Okubo, M.; Ishizuka, T.; Kawamura, T.; Yamashita, H.; Nakamura, M.; Nakagawa, Y.; Nagasaka, M.; Arisawa, T.; et al. Association between interleukin-1beta and tumor necrosis factor-alpha polymorphisms and symptoms of dyspepsia. Mol. Med. Rep. 2015, 11, 3888–3893. [Google Scholar] [CrossRef]
- Berns, M.; Hommes, D.W. Anti-tnf-alpha therapies for the treatment of crohn’s disease: The past, present and future. Expert Opin. Investig. Drugs 2016, 25, 129–143. [Google Scholar] [CrossRef]
- Ungar, B.; Levy, I.; Yavne, Y.; Yavzori, M.; Picard, O.; Fudim, E.; Loebstein, R.; Chowers, Y.; Eliakim, R.; Kopylov, U.; et al. Optimizing anti-TNF-alpha therapy: Serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2016, 14, 550–557. [Google Scholar] [CrossRef]
- Kawalec, P.; Mikrut, A.; Wisniewska, N.; Pilc, A. Tumor necrosis factor-alpha antibodies (infliximab, adalimumab and certolizumab) in Crohn’s disease: Systematic review and meta-analysis. Arch. Med. Sci. 2013, 9, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Etanercept: A review of its use in autoimmune inflammatory diseases. Drugs 2014, 74, 1379–1410. [Google Scholar] [CrossRef] [PubMed]
- Korneev, K.V.; Atretkhany, K.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. Tlr-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Belland, L.; Leyland, N.; von Riedemann, S.; Murji, A. The past, present, and future of selective progesterone receptor modulators in the management of uterine fibroids. Am. J. Obstet. Gynecol. 2018, 218, 563–572. [Google Scholar] [CrossRef]
- Murji, A.; Whitaker, L.; Chow, T.L.; Sobel, M.L. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst. Rev. 2017, 4, CD010770. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Craessaerts, M.; Timmerman, D.; Cornillie, F.; Kennedy, S. Anti-TNF-alpha treatment for deep endometriosis-associated pain: A randomized placebo-controlled trial. Hum. Reprod. 2008, 23, 2017–2023. [Google Scholar] [CrossRef]
- Lu, D.; Song, H.; Shi, G. Anti-TNF-alpha treatment for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2013, CD008088. [Google Scholar] [CrossRef]
- Essayan, D.M. Cyclic nucleotide phosphodiesterases. J. Allergy Clin. Immunol. 2001, 108, 671–680. [Google Scholar] [CrossRef]
- Deree, J.; Martins, J.O.; Melbostad, H.; Loomis, W.H.; Coimbra, R. Insights into the regulation of tnf-alpha production in human mononuclear cells: The effects of non-specific phosphodiesterase inhibition. Clinics 2008, 63, 321–328. [Google Scholar] [CrossRef]
- Kamencic, H.; Thiel, J.A. Pentoxifylline after conservative surgery for endometriosis: A randomized, controlled trial. J. Minim. Invasive Gynecol. 2008, 15, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Crawford, T.J.; Allen, C.; Hopewell, S.; Prentice, A. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst. Rev. 2017, 1, CD004753. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Evangelisti, G.; Barra, F. Current and emerging treatment options for endometriosis. Expert Opin. Pharmacother. 2018, 19, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Chwalisz, K.; Taylor, H. Current and emerging medical treatments for uterine fibroids. Semin. Reprod. Med. 2017, 35, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Meena, A.; Marler, L.E.; Kondratyuk, T.P.; Pezzuto, J.M. Suppression of tumor necrosis factor-alpha-induced nuclear factor kappaB activation and aromatase activity by capsaicin and its analog capsazepine. J. Med. Food 2011, 14, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Reierstad, S.; Demura, M.; Rademaker, A.W.; Kasai, T.; Inoue, M.; Usui, H.; Shozu, M.; Bulun, S.E. High aromatase expression in uterine leiomyoma tissues of african-american women. J. Clin. Endocrinol. Metab 2009, 94, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Singh, A.; Ghilchik, M.W.; Reed, M.J. Inhibition of tumor necrosis factor alpha-stimulated aromatase activity by microtubule-stabilizing agents, paclitaxel and 2-methoxyestradiol. Biochem. Biophys. Res. Commun. 1999, 261, 214–217. [Google Scholar] [CrossRef] [PubMed]
- To, S.Q.; Knower, K.C.; Clyne, C.D. Origins and actions of tumor necrosis factor alpha in postmenopausal breast cancer. J. Interferon Cytokine Res. 2013, 33, 335–345. [Google Scholar] [CrossRef] [PubMed]
- To, S.Q.; Knower, K.C.; Clyne, C.D. NFkappaB and MAPK signalling pathways mediate tnfalpha-induced early growth response gene transcription leading to aromatase expression. Biochem. Biophys. Res. Commun. 2013, 433, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Nakashima, M.; Ishimaru, T.; Masuzaki, H. Cell proliferation effect of gnrh agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum. Reprod. 2010, 25, 2878–2890. [Google Scholar] [CrossRef]
- Taniguchi, F.; Higaki, H.; Azuma, Y.; Deura, I.; Iwabe, T.; Harada, T.; Terakawa, N. Gonadotropin-releasing hormone analogues reduce the proliferation of endometrial stromal cells but not endometriotic cells. Gynecol. Obstet. Investig. 2013, 75, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Harada, T.; Horie, S.; Iba, Y.; Taniguchi, F.; Yoshida, S.; Iwabe, T.; Terakawa, N. Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: Gonadotropin-releasing hormone agonist treatment reduced il-8 expression. J. Clin. Endocrinol. Metab. 2003, 88, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Soto, I.; Salinas, E.; Quintanar, J.L. Leuprolide acetate inhibits spinal cord inflammatory response in experimental autoimmune encephalomyelitis by suppressing NF-kappaB activation. Neuroimmunomodulation 2016, 23, 33–40. [Google Scholar] [CrossRef]
- Courtoy, G.E.; Donnez, J.; Marbaix, E.; Dolmans, M.M. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil Steril 2015, 104, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Courtoy, G.E.; Donnez, J.; Ambroise, J.; Arriagada, P.; Luyckx, M.; Marbaix, E.; Dolmans, M.M. Gene expression changes in uterine myomas in response to ulipristal acetate treatment. Reprod. Biomed. Online 2018, 37, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, L.H.; Murray, A.A.; Matthews, R.; Shaw, G.; Williams, A.R.; Saunders, P.T.; Critchley, H.O. Selective progesterone receptor modulator (sprm) ulipristal acetate (upa) and its effects on the human endometrium. Hum. Reprod. 2017, 32, 531–543. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Falcone, T.; Sharma, R.K.; Goldberg, J.M.; Attaran, M.; Nelson, D.R.; Agarwal, A. Prediction of endometriosis with serum and peritoneal fluid markers: A prospective controlled trial. Hum. Reprod. 2002, 17, 426–431. [Google Scholar] [CrossRef]
- Parker, W.; Berek, J.S.; Pritts, E.; Olive, D.; Kaunitz, A.M.; Chalas, E.; Clarke-Pearson, D.; Goff, B.; Bristow, R.; Taylor, H.S.; et al. An open letter to the food and drug administration regarding the use of morcellation procedures in women having surgery for presumed uterine myomas. J. Minim. Invasive Gynecol. 2016, 23, 303–308. [Google Scholar] [CrossRef]
- Brakta, S.; Diamond, J.S.; Al-Hendy, A.; Diamond, M.P.; Halder, S.K. Role of vitamin d in uterine fibroid biology. Fertil Steril 2015, 104, 698–706. [Google Scholar] [CrossRef]
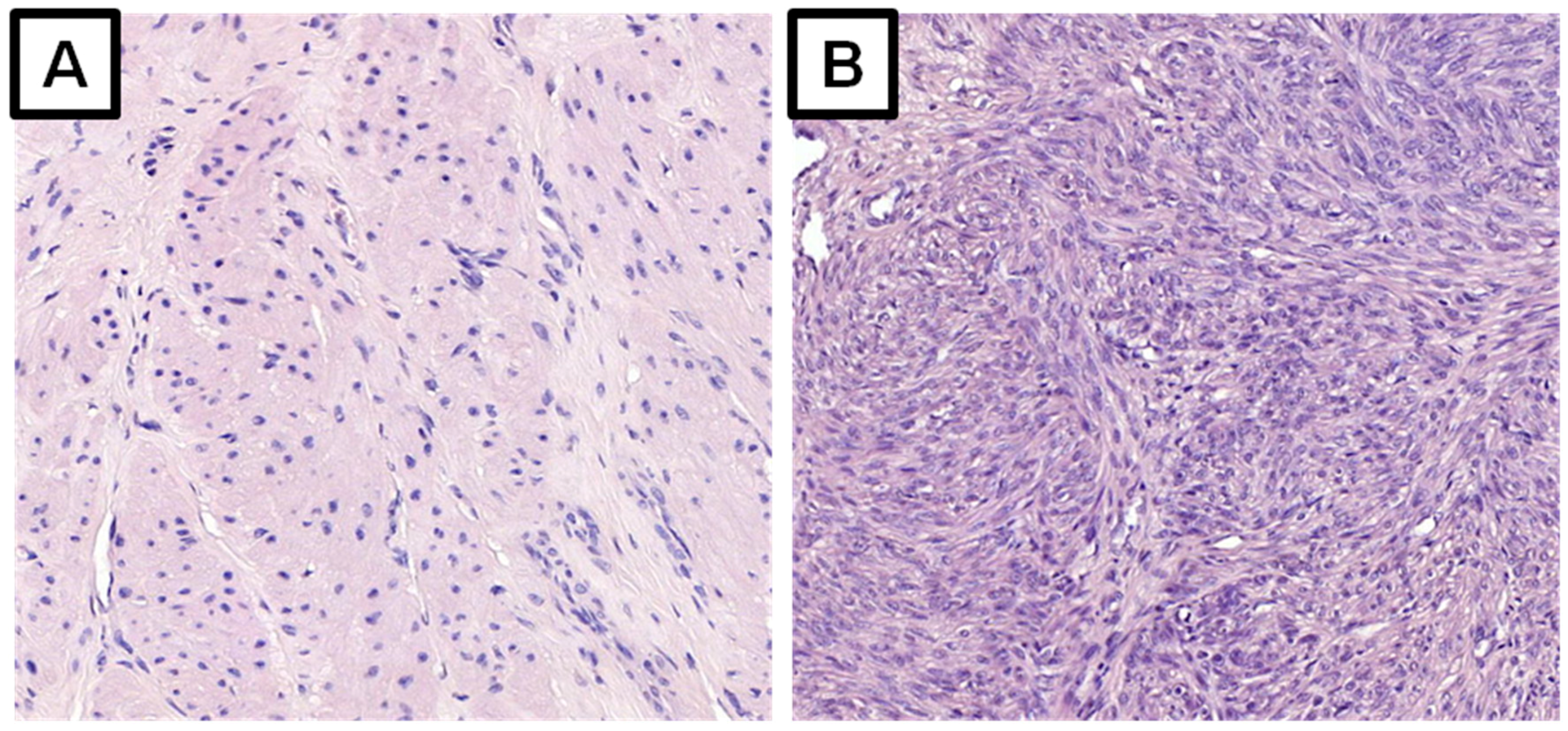

| Field | Examples |
|---|---|
| Rheumatology | Rheumatoid arthritis Psoriatic Arthritis Ankylosing Spondylitis |
| Dermatology | Plaque psoriasis |
| Ophtalmology | Uveitis |
| Psychiatry | Depression |
| Gastroenterology | Crohn’s Disease Ulcerative Colitis |
| Urology | Renal cell carcinoma |
| Gynecology | Ovarian cancer Uterine fibroids |
| Neurology | Alzheimer’s Disease |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciebiera, M.; Włodarczyk, M.; Zgliczyńska, M.; Łukaszuk, K.; Męczekalski, B.; Kobierzycki, C.; Łoziński, T.; Jakiel, G. The Role of Tumor Necrosis Factor α in the Biology of Uterine Fibroids and the Related Symptoms. Int. J. Mol. Sci. 2018, 19, 3869. https://doi.org/10.3390/ijms19123869
Ciebiera M, Włodarczyk M, Zgliczyńska M, Łukaszuk K, Męczekalski B, Kobierzycki C, Łoziński T, Jakiel G. The Role of Tumor Necrosis Factor α in the Biology of Uterine Fibroids and the Related Symptoms. International Journal of Molecular Sciences. 2018; 19(12):3869. https://doi.org/10.3390/ijms19123869
Chicago/Turabian StyleCiebiera, Michał, Marta Włodarczyk, Magdalena Zgliczyńska, Krzysztof Łukaszuk, Błażej Męczekalski, Christopher Kobierzycki, Tomasz Łoziński, and Grzegorz Jakiel. 2018. "The Role of Tumor Necrosis Factor α in the Biology of Uterine Fibroids and the Related Symptoms" International Journal of Molecular Sciences 19, no. 12: 3869. https://doi.org/10.3390/ijms19123869
APA StyleCiebiera, M., Włodarczyk, M., Zgliczyńska, M., Łukaszuk, K., Męczekalski, B., Kobierzycki, C., Łoziński, T., & Jakiel, G. (2018). The Role of Tumor Necrosis Factor α in the Biology of Uterine Fibroids and the Related Symptoms. International Journal of Molecular Sciences, 19(12), 3869. https://doi.org/10.3390/ijms19123869







