Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells
Abstract
1. Introduction
2. Results
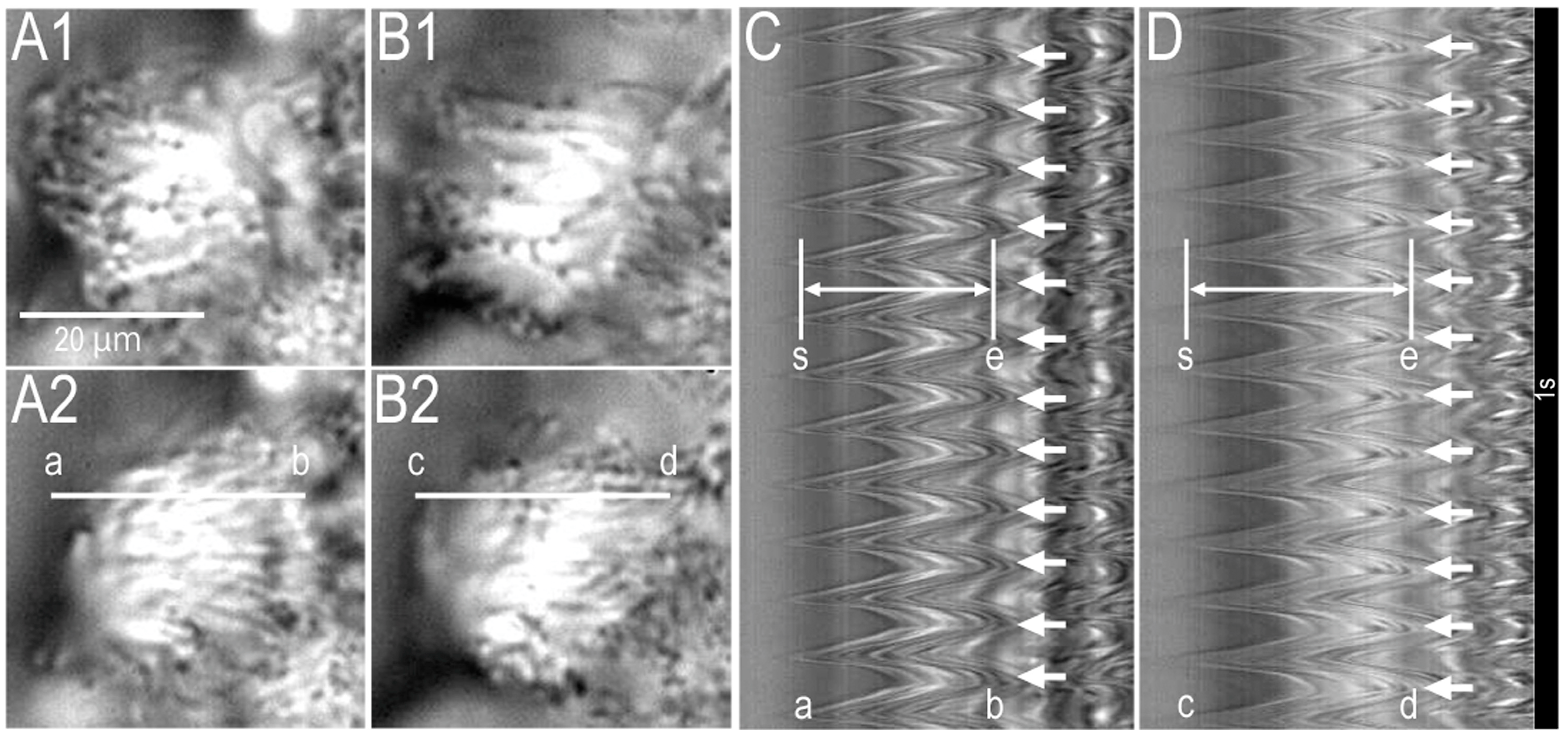
2.1. Video Images of cHNECs Activated by Daidzein
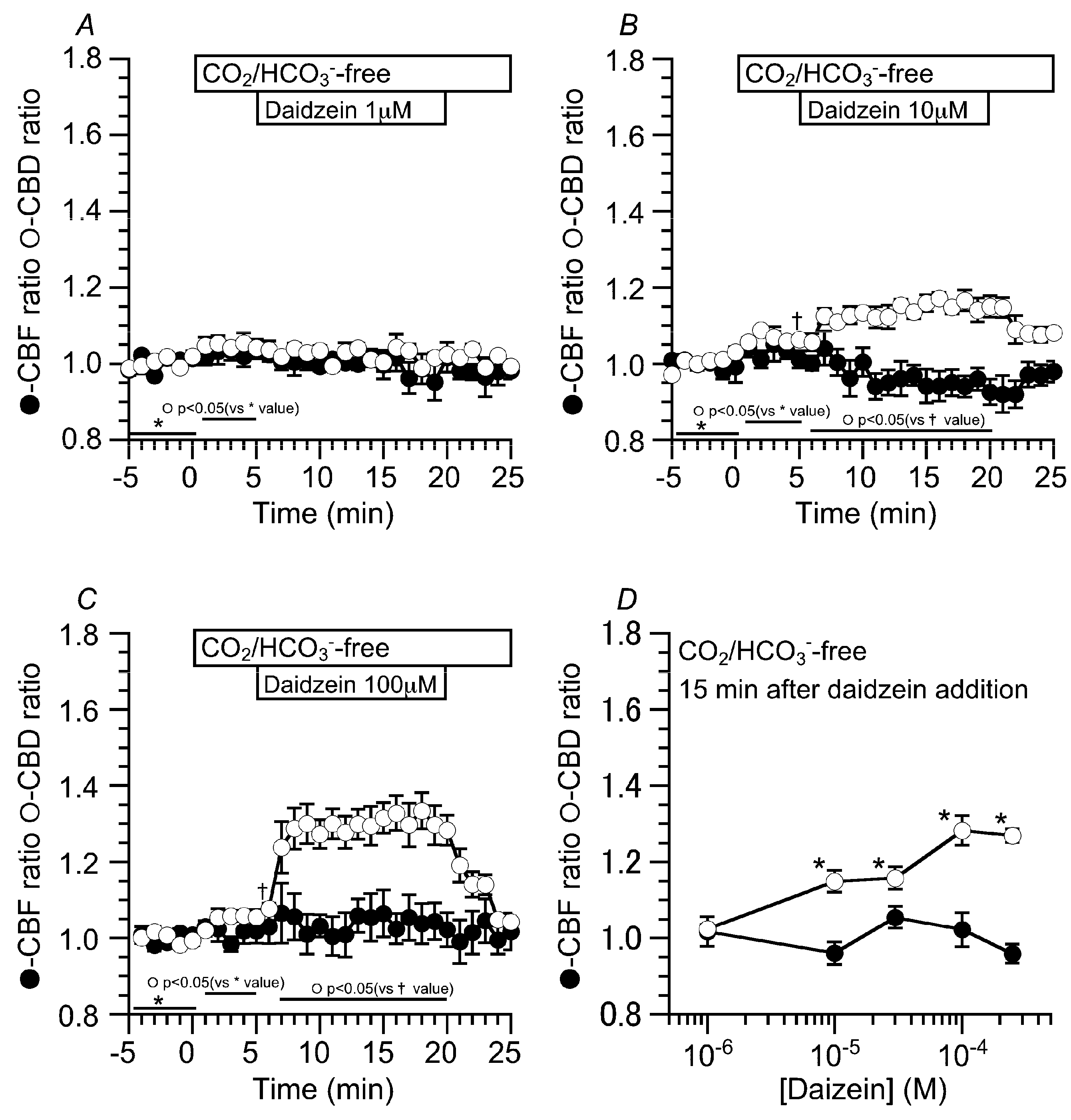
2.2. Effects of Daidzein on CBD and CBF
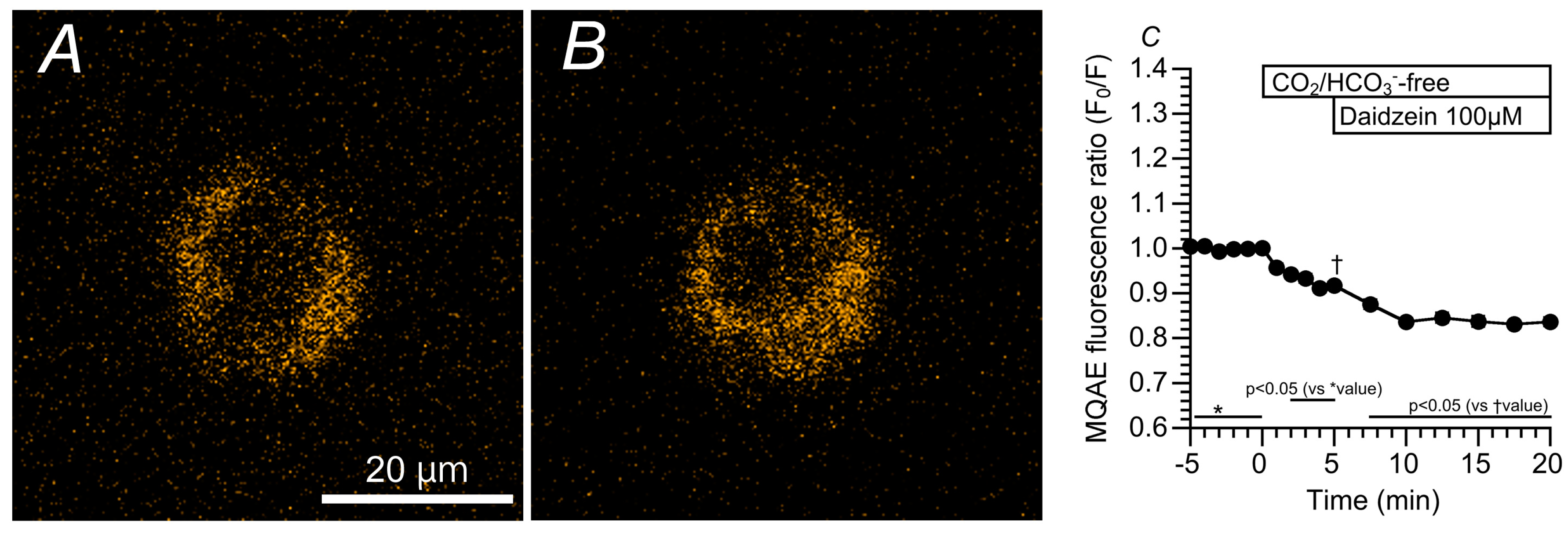
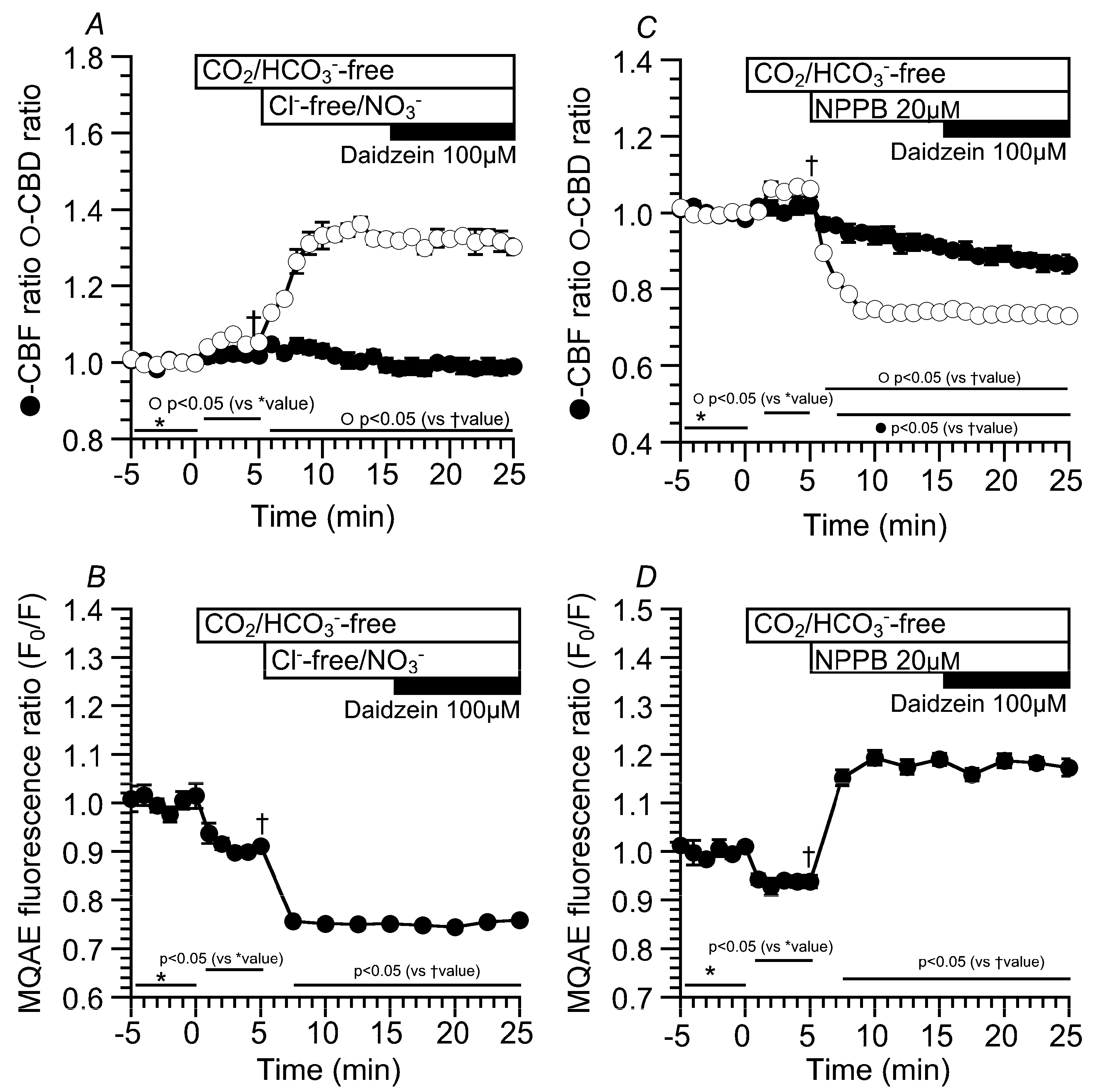
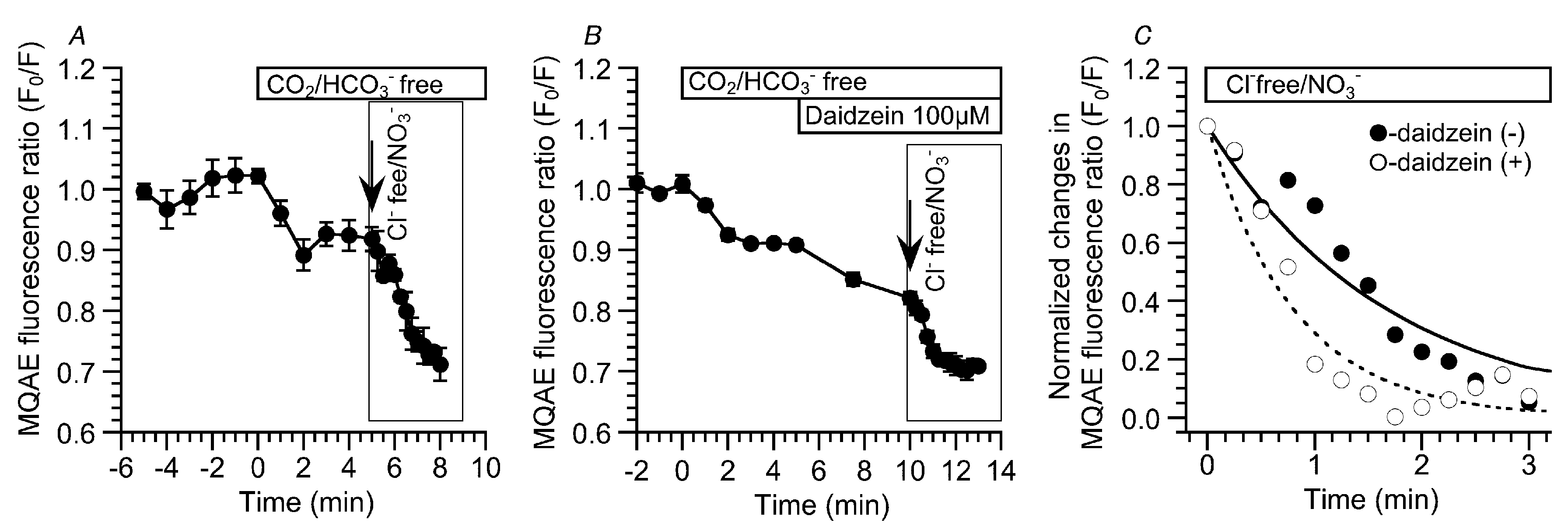
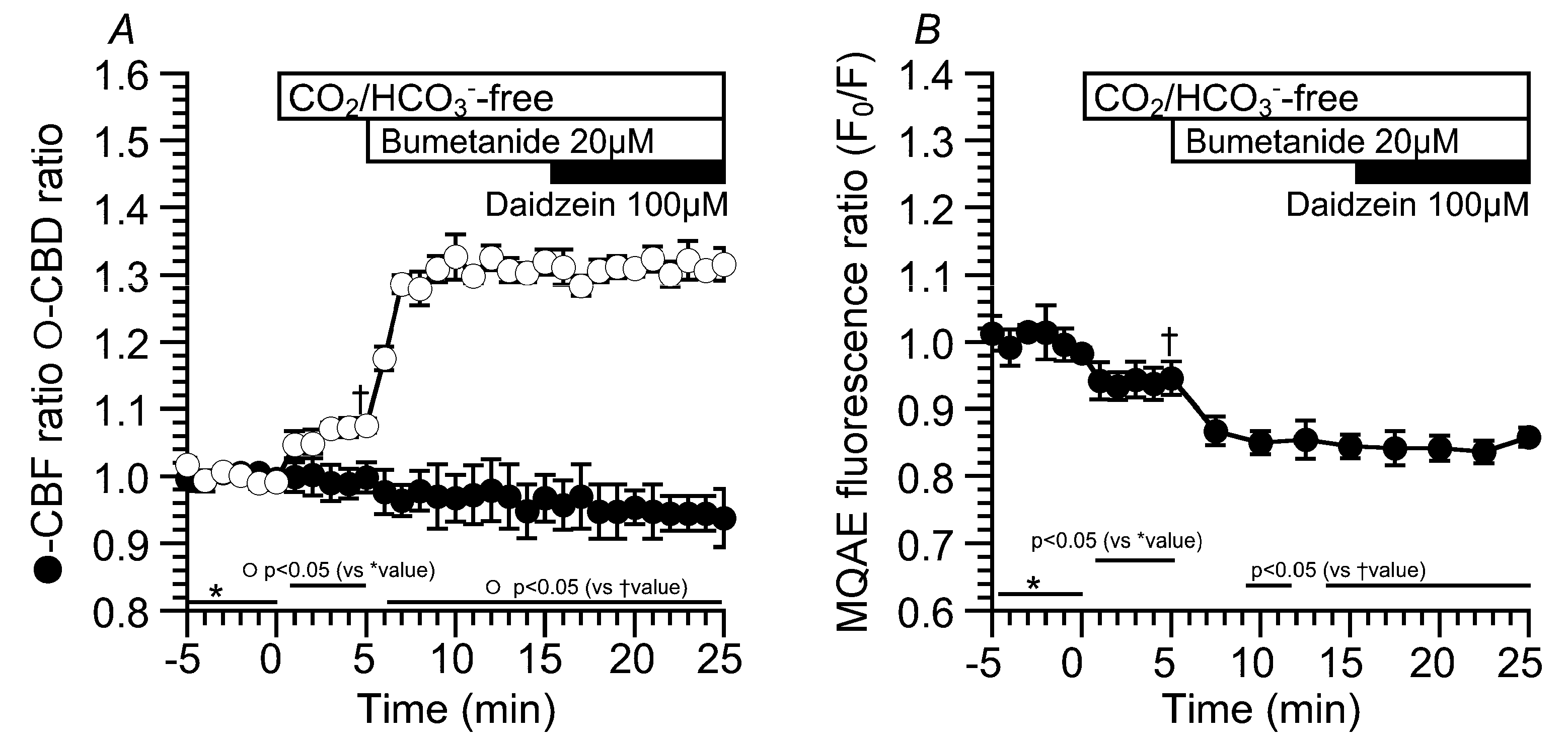
2.3. Effects of Daidzein on [Cl−]i, CBD, and CBF
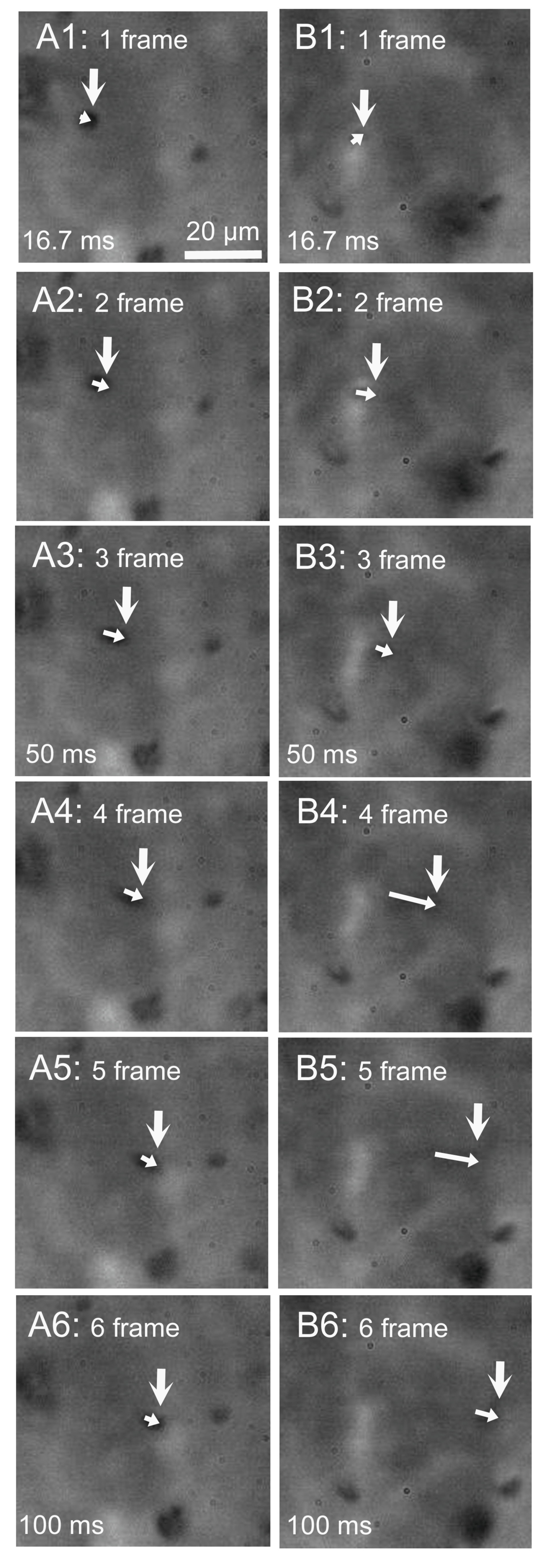
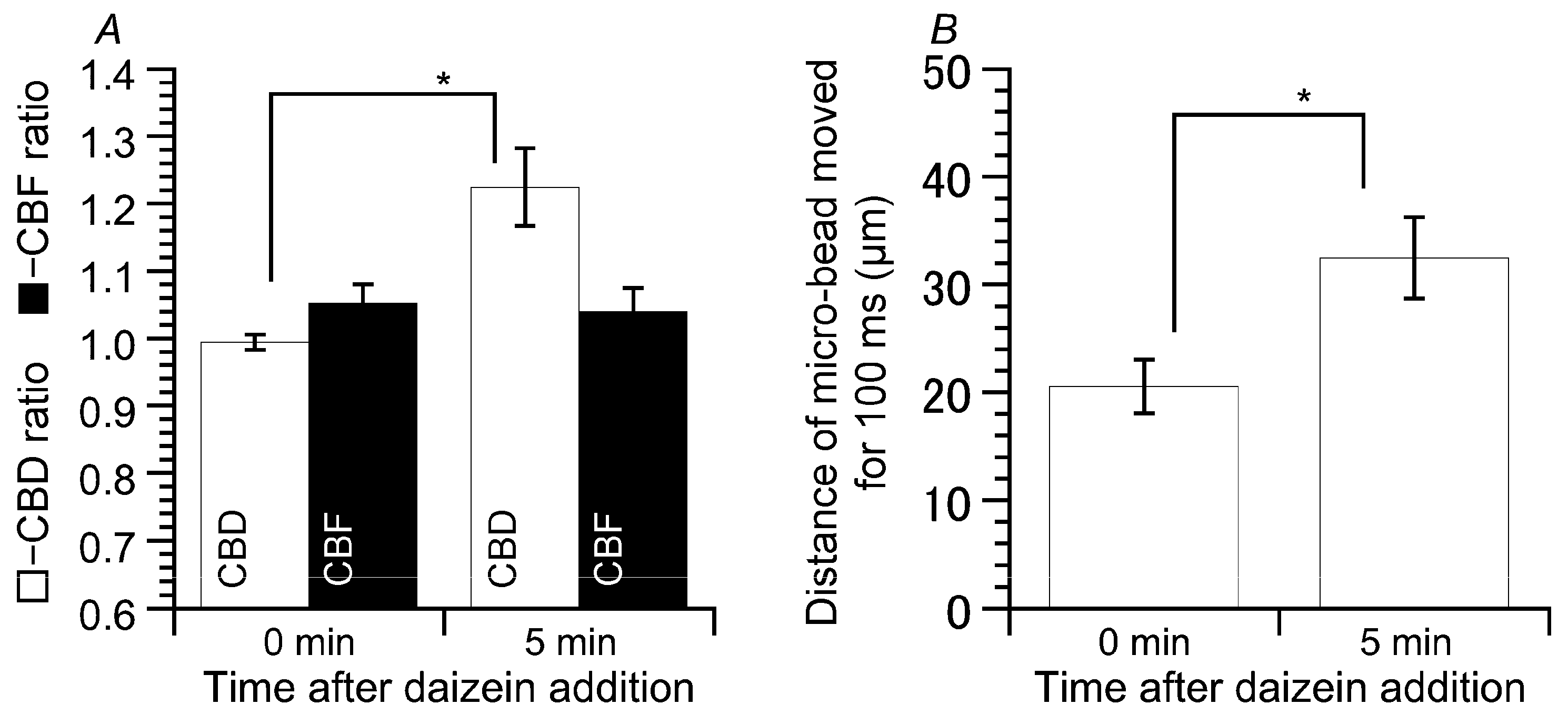
2.4. Latex Microbeads Movement Driven by the Beating Cilia of cHNECs
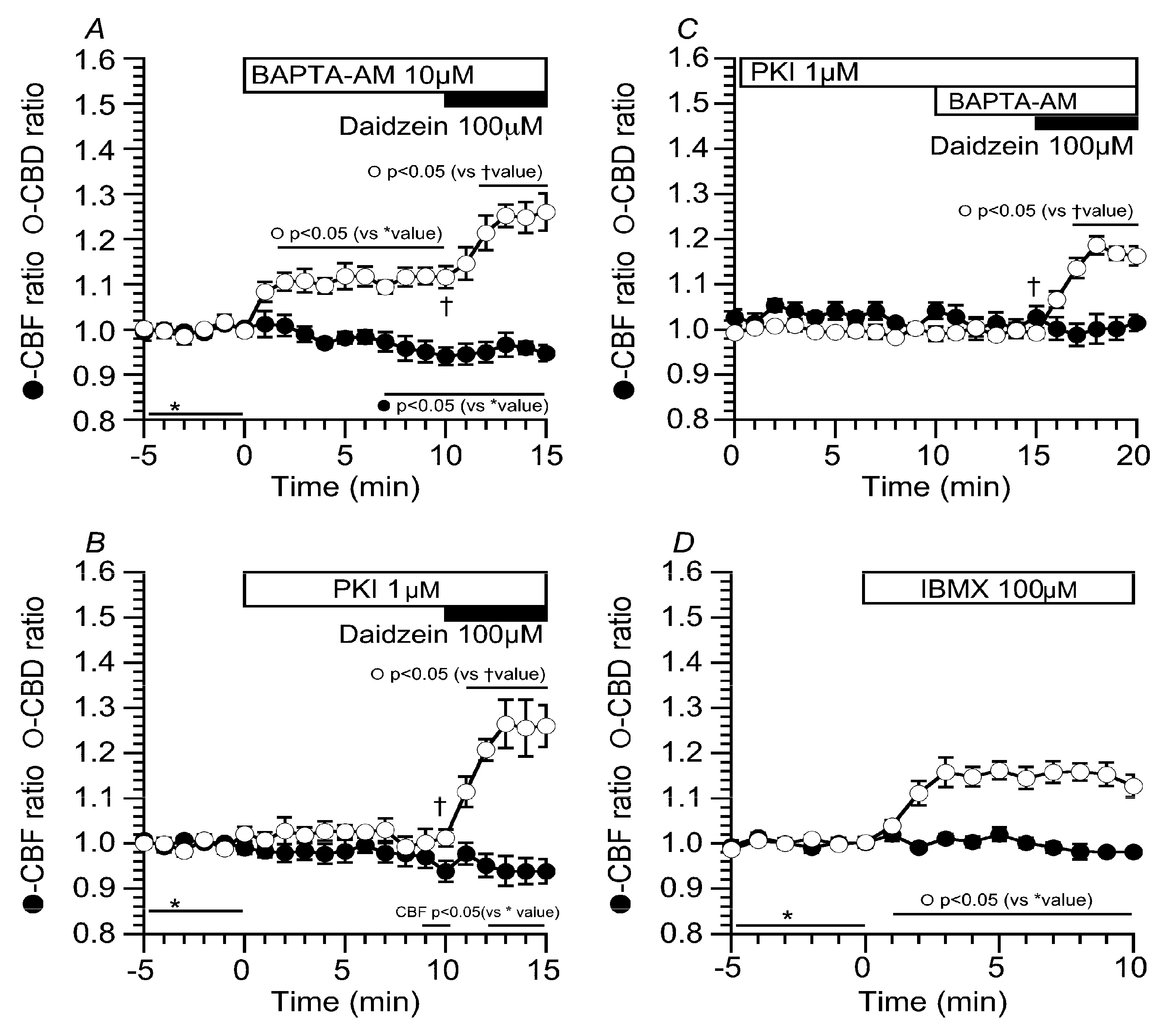
2.5. Effects of Ca2+ and cAMP on CBD and CBF Stimulated by Daidzein
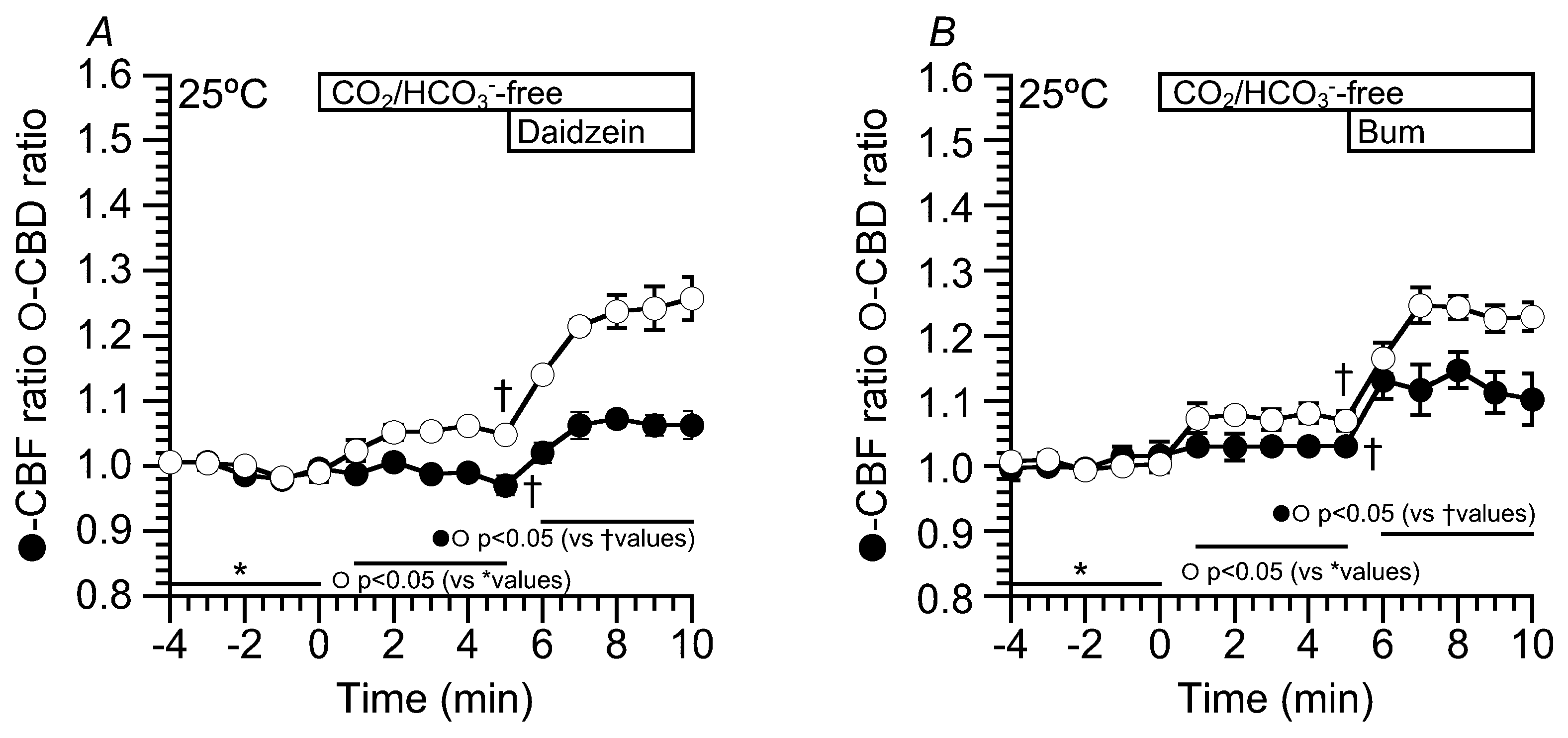
2.6. Effects of Daidzein on CBD and CBF at 25 °C
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Solution and Chemicals
4.3. Cell Preparation and Culture
4.4. CBD and CBF Measurements
4.5. Measurement of [Cl−]i
4.6. Observation of Latex Microbead Transport in cHNECs
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afzelius, B.A. Cilia-related diseases. J. Pathol. 2004, 204, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Wanner, A.; Salathe, M.; O’Riordan, T.G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 1996, 154, 1868–1902. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Daniels, L.A.; Davis, S.D.; Zariwala, M.A.; Leigh, M.W. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am. J. Respir. Crit. Care Med. 2013, 188, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Komatani-Tamiya, N.; Daikoku, E.; Takemura, Y.; Shimamoto, C.; Nakano, T.; Iwasaki, Y.; Kohda, Y.; Matsumura, H.; Marunaka, Y.; Nakahari, T. Procaterol-stimulated increases in ciliary bend amplitude and ciliary beat frequency in mouse bronchioles. Cell. Physiol. Biochem. 2012, 29, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Nakano, T.; Sano, K.I.; Inui, T.; Marunaka, Y.; et al. A low [Ca2+]i-induced enhancement of cAMP-activated ciliary beating by PDE1A inhibition in mouse airway cilia. Pflugers Arch. 2017, 469, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Inui, T.; Marunaka, Y.; Nakahari, T. [Ca(2+)]i modulation of cAMP-stimulated ciliary beat frequency via PDE1 in airway ciliary cells of mice. Exp. Physiol. 2018, 103, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, H.; Ikeuchi, Y.; Sumiya, M.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Inui, T.; Marunaka, Y.; Nakahari, T. Seihai-to (TJ-90)-Induced Activation of Airway Ciliary Beatings of Mice: Ca(2+) Modulation of cAMP-Stimulated Ciliary Beatings via PDE1. Int. J. Mol. Sci. 2018, 19, 658. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Tanaka, S.; Shimamto, C.; Matsumura, H.; Inui, T.; Marunaka, Y.; Nakahari, T. Carbocisteine stimulated an increase in ciliary bend angle via a decrease in [Cl-]i in mouse airway cilia. Pflugers Arch. 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Foskett, J.K. [Ca2+]i modulation of Cl- content controls cell volume in single salivary acinar cells during fluid secretion. Am. J. Physiol. 1990, 259, C998–C1004. [Google Scholar] [CrossRef] [PubMed]
- Treharne, K.J.; Marshall, L.J.; Mehta, A. A novel chloride-dependent GTP-utilizing protein kinase in plasma membranes from human respiratory epithelium. Am. J. Physiol. 1994, 267, L592–L601. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, C.; Umegaki, E.; Katsu, K.; Kato, M.; Fujiwara, S.; Kubota, T.; Nakahari, T. [Cl−]i modulation of Ca2+-regulated exocytosis in ACh-stimulated antral mucous cells of guinea pig. Am. J. Physiol. 2007, 293, G824–G837. [Google Scholar] [CrossRef] [PubMed]
- Conger, B.T.; Zhang, S.; Skinner, D.; Hicks, S.B.; Sorscher, E.J.; Rowe, S.M.; Woodworth, B.A. Comparison of cystic fibrosis transmembrane conductance regulator (CFTR) and ciliary beat frequency activation by the CFTR Modulators Genistein, VRT-532, and UCCF-152 in primary sinonasal epithelial cultures. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Niisato, N.; Ito, Y.; Marunaka, Y. Activation of Cl− channel and Na+/K+/2Cl− cotransporter in renal epithelial A6 cells by flavonoids: Genistein, daidzein, and apigenin. Biochem. Biophys. Res. Commun. 1999, 254, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, Y. Actions of quercetin, a flavonoid, on ion transporters: Its physiological roles. Ann. N. Y. Acad. Sci. 2017, 1398, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, A.; Dolovich, M.B. Airway Epithelial Cell Cilia and Obstructive Lung Disease. Cells 2016, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Inui, T.; Nakahari, T.; Marunaka, Y. Measurement of [Cl(−)]i unaffected by the cell volume change using MQAE-based two-photon microscopy in airway ciliary cells of mice. J. Physiol. Sci. 2018, 68, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Munkonge, F.; Alton, E.W.; Andersson, C.; Davidson, H.; Dragomir, A.; Edelman, A.; Farley, R.; Hjelte, L.; McLachlan, G.; Stern, M.; et al. Measurement of halide efflux from cultured and primary airway epithelial cells using fluorescence indicators. J. Cyst. Fibros 2004, 3, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, Y. Hormonal and osmotic regulation of NaCl transport in renal distal nephron epithelium. Jpn. J. Physiol. 1997, 47, 499–511. [Google Scholar] [CrossRef]
- Miyazaki, H.; Shiozaki, A.; Niisato, N.; Marunaka, Y. Physiological significance of hypotonicity-induced regulatory volume decrease: Reduction in intracellular Cl- concentration acting as an intracellular signaling. Am. J. Physiol. Renal Physiol. 2007, 292, F1411–F1417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Smith, N.; Schuster, D.; Azbell, C.; Sorscher, E.J.; Rowe, S.M.; Woodworth, B.A. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: Therapeutic implications for chronic rhinosinusitis. Am. J. Rhinol. Allergy 2011, 25, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Skinner, D.; Hicks, S.B.; Bevensee, M.O.; Sorscher, E.J.; Lazrak, A.; Matalon, S.; McNicholas, C.M.; Woodworth, B.A. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS ONE 2014, 9, e104090. [Google Scholar] [CrossRef] [PubMed]
- Azbell, C.; Zhang, S.; Skinner, D.; Fortenberry, J.; Sorscher, E.J.; Woodworth, B.A. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol. Head Neck Surg. 2010, 143, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Shiima-Kinoshita, C.; Min, K.Y.; Hanafusa, T.; Mori, H.; Nakahari, T. Beta 2-adrenergic regulation of ciliary beat frequency in rat bronchiolar epithelium: Potentiation by isosmotic cell shrinkage. J. Physiol. 2004, 554, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, K.; Niisato, N.; Taruno, A.; Marunaka, Y. Simulation of Cl(−) Secretion in Epithelial Tissues: New Methodology Estimating Activity of Electro-Neutral Cl(−) Transporter. Front Physiol. 2015, 6, 370. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J.; Kamiya, R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskel. 1987, 8, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J. Control of flagellar bending: A new agenda based on dynein diversity. Cell Motil. Cytoskel. 1994, 28, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, A.; Miyazaki, H.; Niisato, N.; Nakahari, T.; Iwasaki, Y.; Itoi, H.; Ueda, Y.; Yamagishi, H.; Marunaka, Y. Furosemide, a blocker of Na+/K+/2Cl− cotransporter, diminishes proliferation of poorly differentiated human gastric cancer cells by affecting G0/G1 state. J. Physiol. Sci. 2006, 56, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Shpetner, H.S.; Paschal, B.M.; Vallee, R.B. Characterization of the microtubule-activated ATPase of brain cytoplasmic dynein (MAP 1C). J. Cell Biol. 1988, 107, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Foskett, J.K.; Wong, D. Calcium oscillations in parotid acinar cells induced by microsomal Ca(2+)-ATPase inhibition. J. Cell Biol. 1992, 262, C656–C663. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Shinohara, M.; Nagai, T.; Konishi, Y. Transport mechanisms for soy isoflavones and microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Biosci. Biotechnol. Biochem. 2013, 77, 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Kuremoto, T.; Kogiso, H.; Yasuda, M.; Inui, T.; Murakami, K.; Hirano, S.; Ikeuchi, Y.; Hosigi, S.; Inui, T.; Marunaka, Y.; et al. Spontaneous oscillation of the ciliary beat frequency regulated by release of Ca2+ from intracellular stores in mouse nasal epithelia. Biochem. Biophys. Res. Commun. 2018, 507, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Brighton, L.E.; Carson, J.L.; Fischer, W.A., 2nd; Jaspers, I. Culturing of human nasal epithelial cells at the air liquid interface. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Niisato, N.; Miyazaki, H.; Hama, T.; Dejima, K.; Hisa, Y.; Marunaka, Y. Epithelial ion transport of human nasal polyp and paranasal sinus mucosa. Am. J. Respir. Cell Mol. Biol. 2007, 36, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Niisato, N.; Miyazaki, H.; Iwasaki, Y.; Hama, T.; Dejima, K.; Hisa, Y.; Marunaka, Y. Epithelial Na+ channel and ion transport in human nasal polyp and paranasal sinus mucosa. Biochem. Biophys. Res. Commun. 2007, 362, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, P.; Sanderson, M.J. Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices. Am. J. Respir. Cell Mol. Biol. 2006, 35, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, I.M.; Liedtke, W.; Sanderson, M.J.; Valverde, M.A. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc. Natl. Acad. Sci. USA 2008, 105, 12611–12616. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inui, T.-a.; Yasuda, M.; Hirano, S.; Ikeuchi, Y.; Kogiso, H.; Inui, T.; Marunaka, Y.; Nakahari, T. Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells. Int. J. Mol. Sci. 2018, 19, 3754. https://doi.org/10.3390/ijms19123754
Inui T-a, Yasuda M, Hirano S, Ikeuchi Y, Kogiso H, Inui T, Marunaka Y, Nakahari T. Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells. International Journal of Molecular Sciences. 2018; 19(12):3754. https://doi.org/10.3390/ijms19123754
Chicago/Turabian StyleInui, Taka-aki, Makoto Yasuda, Shigeru Hirano, Yukiko Ikeuchi, Haruka Kogiso, Toshio Inui, Yoshinori Marunaka, and Takashi Nakahari. 2018. "Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells" International Journal of Molecular Sciences 19, no. 12: 3754. https://doi.org/10.3390/ijms19123754
APA StyleInui, T.-a., Yasuda, M., Hirano, S., Ikeuchi, Y., Kogiso, H., Inui, T., Marunaka, Y., & Nakahari, T. (2018). Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells. International Journal of Molecular Sciences, 19(12), 3754. https://doi.org/10.3390/ijms19123754





