Relative Contribution of PIN-Containing Secretory Vesicles and Plasma Membrane PINs to the Directed Auxin Transport: Theoretical Estimation
Abstract
1. Introduction
2. Results
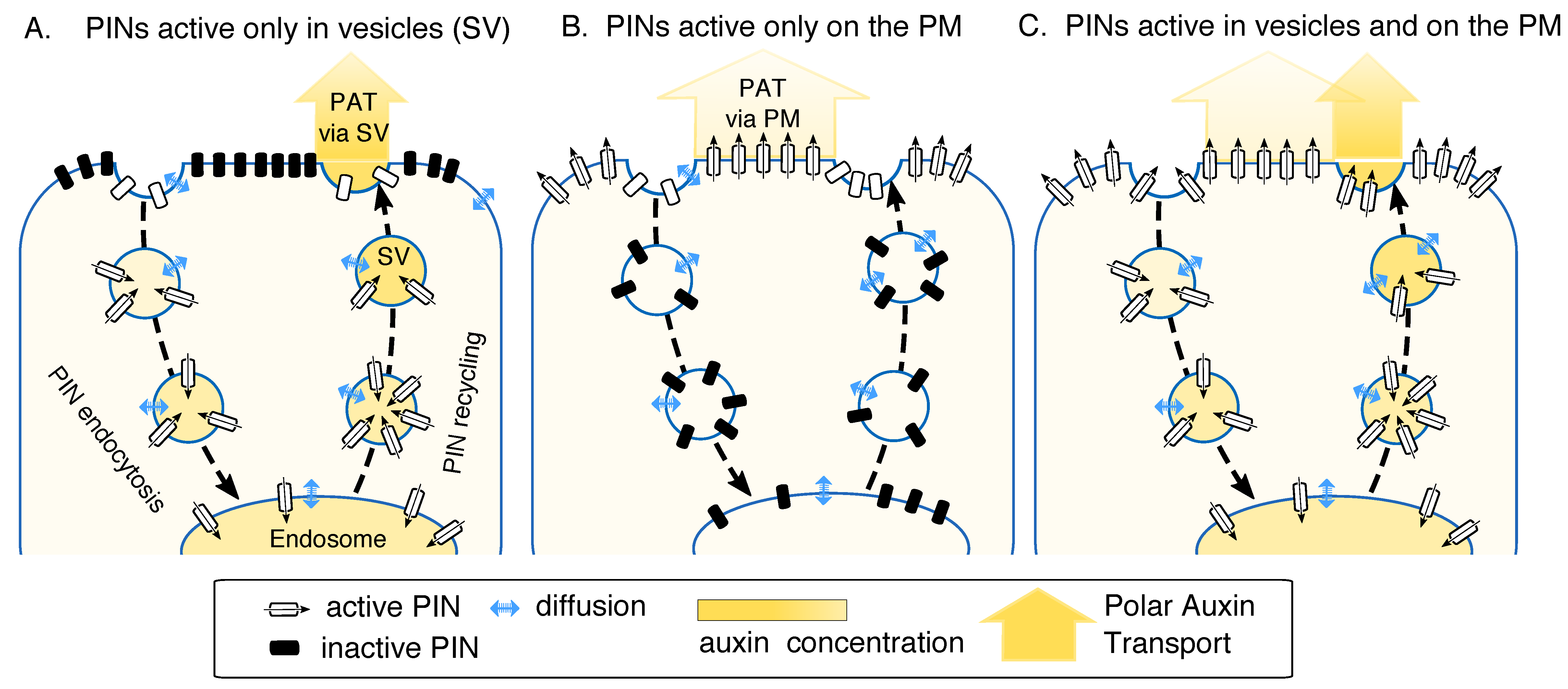
2.1. Model Assumptions: PINs Can Be Active on the Endomembrane Vesicles Exclusively or in Addition to Their Activity on the Plasma Membrane (PM)
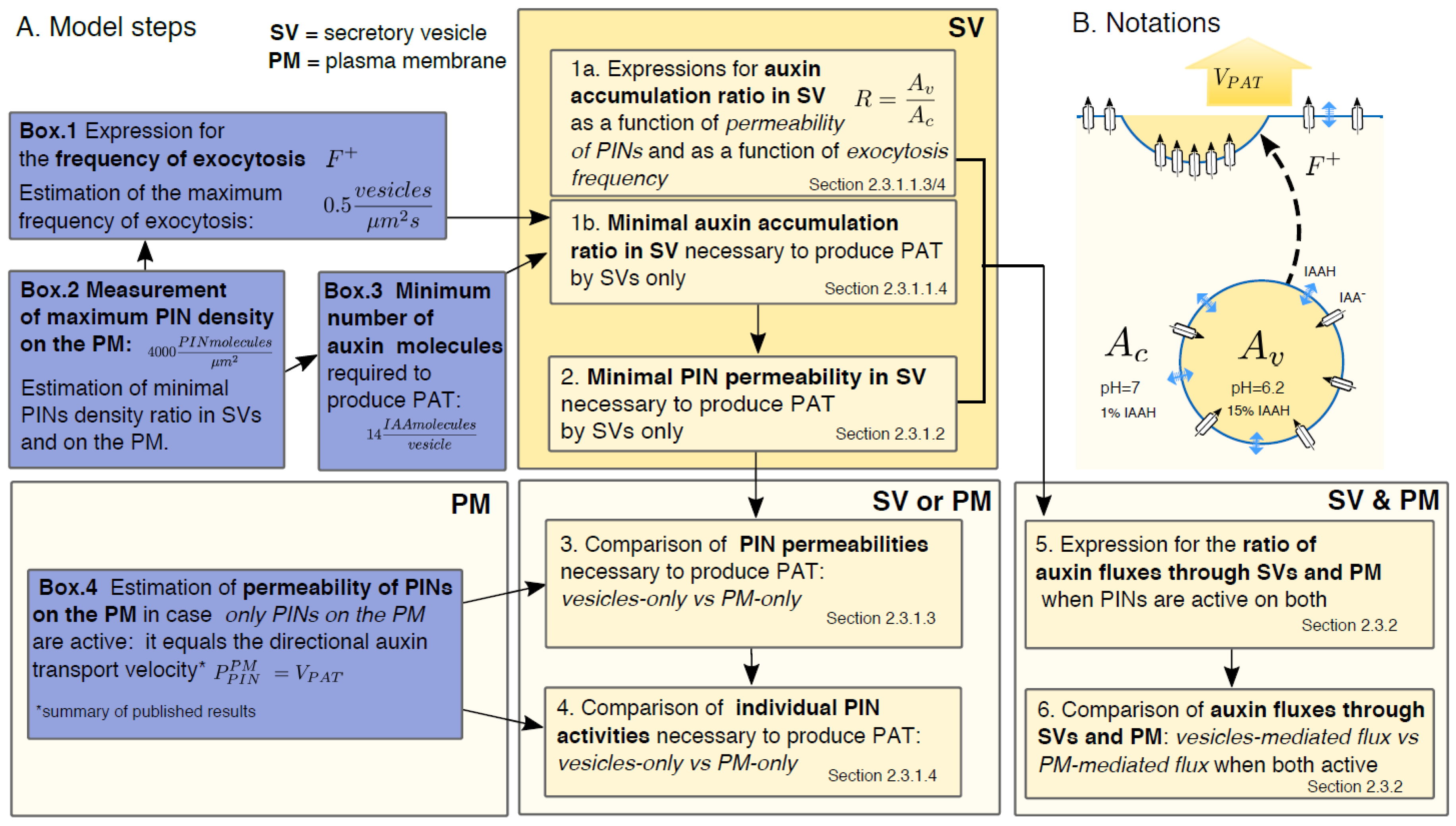
2.2. Short Summary of Model Results
2.3. Detailed Model Description
2.3.1. PINs Active Only in Vesicles. Comparison to the Case When PINs are Active Only on PM
2.3.1.1. How Much Auxin Should a Vesicle with Active PIN Contain to Produce the PAT?
2.3.1.2. Minimal Permeability of PINs in Vesicles Necessary for Vesicular Auxin Transport
2.3.1.3. Comparison to the Case when PINs are Active Only on the PM: Permeability due to PINs has to be Much Greater on the Vesicles than on PM to Produce the Same Auxin Transport Velocity
2.3.1.4. Individual Activity of PINs Has to Be Much Higher on the Vesicles than on the PM to Produce the Same Auxin Transport Velocity
2.3.2. PINs Active Both in Vesicles and on the PM
- PINs half-life on the PM is higher;
- pH in the vesicle is lower;
- vesicles do not stay in the cytoplasm for long enough before fusing with the PM to be fully filled with IAA;
- the size of vesicles is smaller.
3. Discussion
4. Materials and Methods
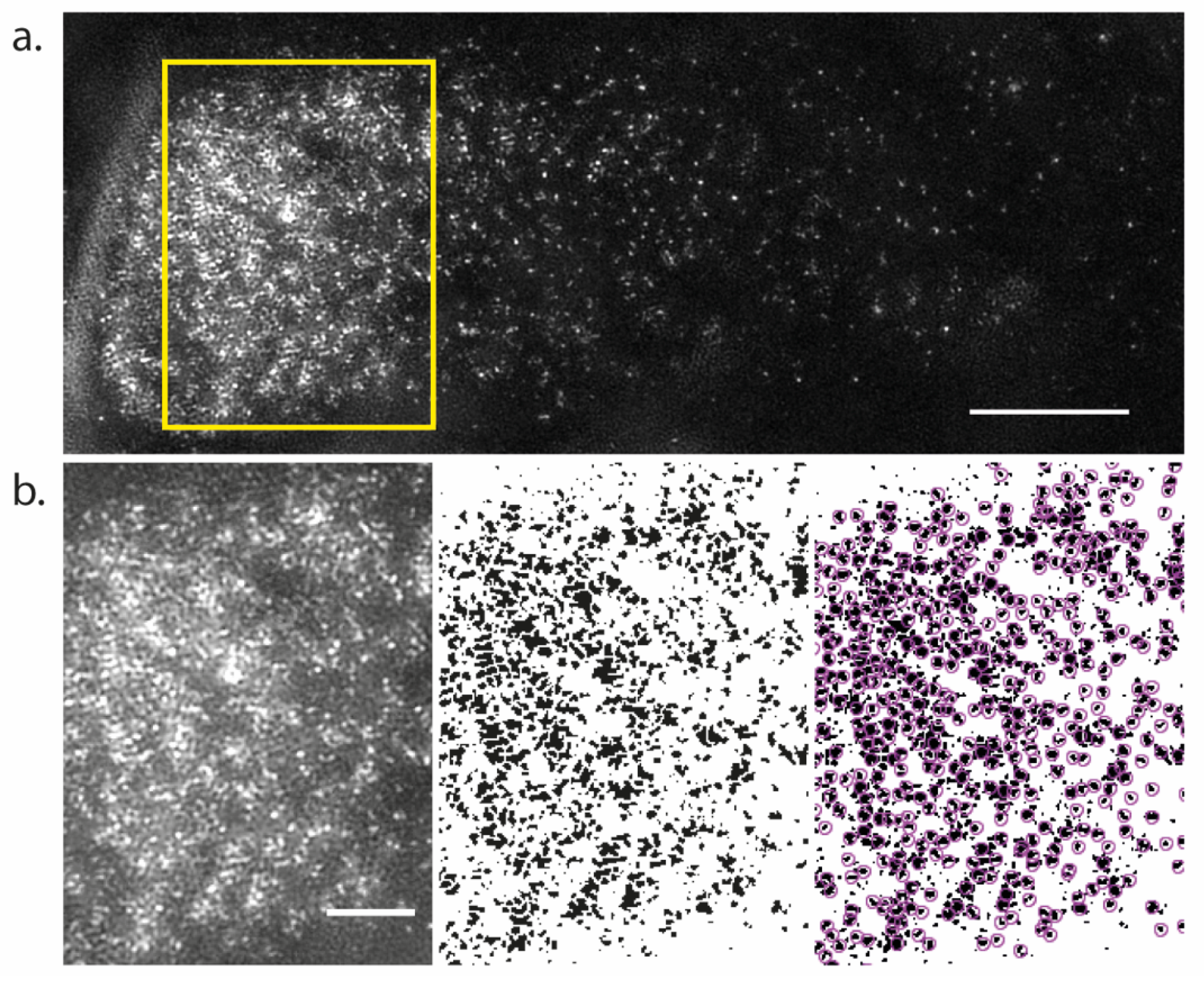
Measurement of PIN Density Using Structured Illumination Microscopy (SIM)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PM | Plasma membrane |
| SV | Secretory vesicle |
| IAA | Indole-3-acetic acid = auxin |
| PINs | PIN-formed transporters |
| TGN/EE | Trans-Golgi network/ early endosome |
References
- Adamowski, M.; Friml, J. PIN-Dependent Auxin Transport: Action, Regulation, and Evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Vehn, J.; Langowski, L.; Wisniewska, J.; Dhonukshe, P.; Brewer, P.B.; Friml, J. Cellular and Molecular Requirements for Polar PIN Targeting and Transcytosis in Plants. Mol. Plant 2008, 1, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-J.; Luo, J. The PIN-FORMED Auxin Efflux Carriers in Plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef] [PubMed]
- Geldner, N.; Friml, J.; Stierhof, Y.-D.D.; Jürgens, G.; Palme, K. Auxin Transport Inhibitors Block PIN1 Cycling and Vesicle Trafficking. Nature 2001, 413, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Dhonukshe, P.; Aniento, F.; Hwang, I.; Robinson, D.G.; Mravec, J.; Stierhof, Y.-D.D.; Friml, J.J. Clathrin-Mediated Constitutive Endocytosis of PIN Auxin Efflux Carriers in Arabidopsis. Curr. Biol. 2007, 17, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Adamowski, M.; Narasimhan, M.; Kania, U.; Glanc, M.; De Jaeger, G.; Friml, J. A Functional Study of AUXILIN-LIKE1 and 2, Two Putative Clathrin Uncoating Factors in Arabidopsis. Plant Cell 2018. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Palme, K. Polar Auxin Transport—Old Questions and New Concepts? Plant Mol. Biol. 2002, 49, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Baluška, F.; Šamaj, J.; Menzel, D. Polar Transport of Auxin: Carrier-Mediated Flux across the Plasma Membrane or Neurotransmitter-like Secretion? Trends Cell Biol. 2003, 13, 282–285. [Google Scholar] [CrossRef]
- Mancuso, S.; Marras, A.M.; Mugnai, S.; Schlicht, M.; Zársky, V.; Li, G.; Song, L.; Xue, H.-W.; Baluska, F. Phospholipase Dzeta2 Drives Vesicular Secretion of Auxin for Its Polar Cell-Cell Transport in the Transition Zone of the Root Apex. Plant Signal. Behav. 2007, 2, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Mettbach, U.; Strnad, M.; Mancuso, S.; Baluška, F. Immunogold-EM Analysis Reveal Brefeldin a-Sensitive Clusters of Auxin in Arabidopsis Root Apex Cells. Commun. Integr. Biol. 2017, 10, e1327105. [Google Scholar] [CrossRef] [PubMed]
- Schlicht, M.; Strnad, M.; Scanlon, M.J.; Mancuso, S.; Hochholdinger, F.; Palme, K.; Volkmann, D.; Menzel, D.; Baluska, F. Auxin Immunolocalization Implicates Vesicular Neurotransmitter-like Mode of Polar Auxin Transport in Root Apices. Plant Signal. Behav. 2006, 1, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Alpi, A.; Amrhein, N.; Bertl, A.; Blatt, M.R.; Blumwald, E.; Cervone, F.; Dainty, J.; De Michelis, M.I.; Epstein, E.; Galston, A.W.; et al. Plant Neurobiology: No Brain, No Gain? Trends Plant Sci. 2007, 12, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.G.; Hawes, C.; Hillmer, S.; Jürgens, G.; Schwechheimer, C.; Stierhof, Y.-D.; Viotti, C. Auxin and Vesicle Traffic. Plant Physiol. 2018, 176, 1884–1888. [Google Scholar] [CrossRef] [PubMed]
- Kramer, E.M.; Rutschow, H.L.; Mabie, S.S. AuxV: A Database of Auxin Transport Velocities. Trends Plant Sci. 2011, 16, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Petrášek, J.; Friml, J. Auxin Transport Routes in Plant Development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef] [PubMed]
- Rashotte, A.M.; Poupart, J.; Waddell, C.S.; Muday, G.K. Transport of the Two Natural Auxins, Indole-3-Butyric Acid and Indole-3-Acetic Acid, in Arabidopsis. Plant Physiol. 2003, 133, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, G.J. The Dynamics of Auxin Transport. Proc. R. Soc. B Biol. Sci. 1980, 209, 489–511. [Google Scholar] [CrossRef]
- Rutschow, H.L.; Baskin, T.I.; Kramer, E.M. The Carrier AUXIN RESISTANT (AUX1) Dominates Auxin Flux into Arabidopsis Protoplasts. New Phytol. 2014, 204, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.H.; Goldsmith, T.H.; Martin, M.H. Mathematical Analysis of the Chemosmotic Polar Diffusion of Auxin through Plant Tissues. Proc. Natl. Acad. Sci. USA 1981, 78, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Pimpl, P.; Movafeghi, A.; Coughlan, S.; Denecke, J.; Hillmer, S.; Robinson, D.G. In Situ Localization and in Vitro Induction of Plant COPI-Coated Vesicles. Plant Cell 2000, 12, 2219–2236. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Scholl, S.; Doering, A.; Zhang, Y.; Irani, N.G.; Di Rubbo, S.; Neumetzler, L.; Krishnamoorthy, P.; Van Houtte, I.; Mylle, E.; et al. V-ATPase Activity in the TGN/EE Is Required for Exocytosis and Recycling in Arabidopsis. Nat. Plants 2015, 1, 15094. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zeng, Y.; Zhuang, X.; Sun, L.; Yao, X.; Pimpl, P.; Jiang, L. Organelle PH in the Arabidopsis Endomembrane System. Mol. Plant 2013, 6, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Jásik, J.; Boggetti, B.; Baluška, F.; Volkmann, D.; Gensch, T.; Rutten, T.; Altmann, T.; Schmelzer, E. PIN2 Turnover in Arabidopsis Root Epidermal Cells Explored by the Photoconvertible Protein Dendra2. PLoS ONE 2013, 8, e61403. [Google Scholar] [CrossRef] [PubMed]
- Delbarre, A.; Muller, P.; Imhoff, V.; Guern, J. Comparison of Mechanisms Controlling Uptake and Accumulation of 2,4-Dichlorophenoxy Acetic Acid, Naphthalene-1-Acetic Acid, and Indole-3-Acetic Acid in Suspension-Cultured Tobacco Cells. Planta 1996, 198, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Kramer, E.M.; Perry, P.; Knox, K.; Leyser, H.M.O.; Haseloff, J.; Beemster, G.T.S.S.; Bhalerao, R.; Bennett, M.J.; Leyser, O.; et al. Root Gravitropism Requires Lateral Root Cap and Epidermal Cells for Transport and Response to a Mobile Auxin Signal. Nat. Cell Biol. 2005, 7, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Vert, G. Single Event Resolution of Plant Plasma Membrane Protein Endocytosis by TIRF Microscopy. Front. Plant Sci. 2017, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Konopka, C.A.; Bednarek, S.Y. Comparison of the Dynamics and Functional Redundancy of the Arabidopsis Dynamin-Related Isoforms DRP1A and DRP1C during Plant Development. Plant Physiol. 2008, 147, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Konopka, C.A.; Backues, S.K.; Bednarek, S.Y. Dynamics of Arabidopsis Dynamin-Related Protein 1C and a Clathrin Light Chain at the Plasma Membrane. Plant Cell Online 2008, 20, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Fendrych, M.; Akhmanova, M.; Merrin, J.; Glanc, M.; Hagihara, S.; Takahashi, K.; Uchida, N.; Torii, K.U.; Friml, J. Rapid and Reversible Root Growth Inhibition by TIR1 Auxin Signalling. Nat. Plants 2018, 4, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Zourelidou, M.; Absmanner, B.; Weller, B.; Barbosa, I.C.; Willige, B.C.; Fastner, A.; Streit, V.; Port, S.A.; Colcombet, J.; de la Fuente van Bentem, S.; et al. Auxin Efflux by PIN-FORMED Proteins Is Activated by Two Different Protein Kinases, D6 PROTEIN KINASE and PINOID. Elife 2014, 3, e02860. [Google Scholar] [CrossRef] [PubMed]
- Paciorek, T.; Zazímalová, E.; Ruthardt, N.; Petrásek, J.; Stierhof, Y.-D.; Kleine-Vehn, J.; Morris, D.A.; Emans, N.; Jürgens, G.; Geldner, N.; et al. Auxin Inhibits Endocytosis and Promotes Its Own Efflux from Cells. Nature 2005, 435, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Kubeš, M.; Baster, P.; Robert, S.; Dobrev, P.I.; Friml, J.; Petrášek, J.; Zažímalová, E. Defining the Selectivity of Processes along the Auxin Response Chain: A Study Using Auxin Analogues. New Phytol. 2013, 200, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Tinevez, J.-Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An Open and Extensible Platform for Single-Particle Tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Description | Value/Range | Ref. |
|---|---|---|---|
| Typical diameter of a vesicle | [20] | ||
| πd2 | Surface area of a vesicle | ||
| πd3 | Volume of a vesicle | ||
| pHv | pH in the lumen of the trans-Golgi network/early endosome (TGN/EE)-derived vesicle | 5.5–6.5 | [21,22] |
| pHc | pH in the cytoplasm | 7 | [21,22] |
| Speed of directional plant hormone auxin (IAA) transport in epidermal cells of Arabidopsis root tip | [14,16] | ||
| Half-life of PINs on the plasma membrane (PM) | [23] | ||
| Minimum PIN density in a vesicle | One molecule per vesicle | ||
| Maximum PIN density on a plasma membrane | Close-packing of equal spheres of 4 nm radius.Estimate based on experimental data, this study. | ||
| Diffusional permeability of IAA through membrane | [18,24] |
| Assumption | Effect on Efficiency of SVs to Transport Auxin | Effect on Efficiency of PM to Transport Auxin | |
|---|---|---|---|
| 1 | Steady state/homogeneity: auxin fluxes and parameters that influence auxin fluxes are constant in time. Note, that experimentally measured values of parameters used in the model were also assumed to be constant when measured (Table 1): size of SV, pH in the cell and in SV, and listed below: | ||
| 1.1 | PINs density on the PM equals an average density of PINs, being constant and homogenous along the whole polar side of the PM. | ||
| 1.2 | PINs density on SVs is constant, all SV are identical and contain the same density of PINs. | ||
| 1.3 | Intracellular auxin concentration near the PM is constant and homogenous. | ||
| 1.4 | Intravesicular auxin concentration is homogenous. | ||
| 1.5 | Removal of PINs from the PM is proportional to the PINs concentration on the PM and is a constant process in time, characterized by constant half-life of PINs on the PM. It doesn’t depend on the exocytosis rate. | Less efficient | No effect |
| 2 | SVs fuse with the PM and deliver all PIN proteins that they contain to the PM. | Less efficient | No effect |
| 3 | SV fills with auxin to its maximum concentration and then all auxin inside the SV is released outside the cell. | More efficient | No effect |
| 4 | Diffusion of anion form (IAA−) through the membrane (PM and SV) is negligible. | More efficient | - |
| 5 | PINs transport auxin (IAA−) only in one direction (inside the vesicle, outside the cell on the PM). | More efficient | More efficient |
| 6 | Non-polar auxin transport is neglected when equating auxin flux through PM or via SVs and directional auxin transport rate | More efficient, magnitude of correction is the same for PM and SVs | |
| R * | |||||
|---|---|---|---|---|---|
| 5.5 | 0.833 | 0.167 | ** | ||
| 6.2 | 0.962 | 0.038 | ** | ||
| 6.5 | 0.980 | 0.020 | ** | ||
| 7.0 | 0.994 = | 0.006 | ** |
| Endocytic Marker | DRP1C-GFP (Dynamin-Related Protein 1C-GFP) | CLC-GFP (Clathrin Light Chain-GFP) |
| foci per µm2 | 3.54 | 3.48 |
| standard deviation (SD) | 0.62 | 0.55 |
| average lifetime (s) | 17.7 | 19.7 |
| SD | 8.8 | 6.8 |
| foci per model cell (15 × 15 micron) | 796.5 | 783 |
| SD | 139.5 | 123.75 |
| Endocytosis events per cell per second | DRP1C-GFP | CLC-GFP |
| Average | 45.0 | 39.7 |
| max | 105.2 | 70.3 |
| min | 24.8 | 24.9 |
| Maximum rate of endocytosis per second per µm2 | 0.467 | 0.312 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hille, S.; Akhmanova, M.; Glanc, M.; Johnson, A.; Friml, J. Relative Contribution of PIN-Containing Secretory Vesicles and Plasma Membrane PINs to the Directed Auxin Transport: Theoretical Estimation. Int. J. Mol. Sci. 2018, 19, 3566. https://doi.org/10.3390/ijms19113566
Hille S, Akhmanova M, Glanc M, Johnson A, Friml J. Relative Contribution of PIN-Containing Secretory Vesicles and Plasma Membrane PINs to the Directed Auxin Transport: Theoretical Estimation. International Journal of Molecular Sciences. 2018; 19(11):3566. https://doi.org/10.3390/ijms19113566
Chicago/Turabian StyleHille, Sander, Maria Akhmanova, Matouš Glanc, Alexander Johnson, and Jiří Friml. 2018. "Relative Contribution of PIN-Containing Secretory Vesicles and Plasma Membrane PINs to the Directed Auxin Transport: Theoretical Estimation" International Journal of Molecular Sciences 19, no. 11: 3566. https://doi.org/10.3390/ijms19113566
APA StyleHille, S., Akhmanova, M., Glanc, M., Johnson, A., & Friml, J. (2018). Relative Contribution of PIN-Containing Secretory Vesicles and Plasma Membrane PINs to the Directed Auxin Transport: Theoretical Estimation. International Journal of Molecular Sciences, 19(11), 3566. https://doi.org/10.3390/ijms19113566





