AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling
Abstract
1. Introduction
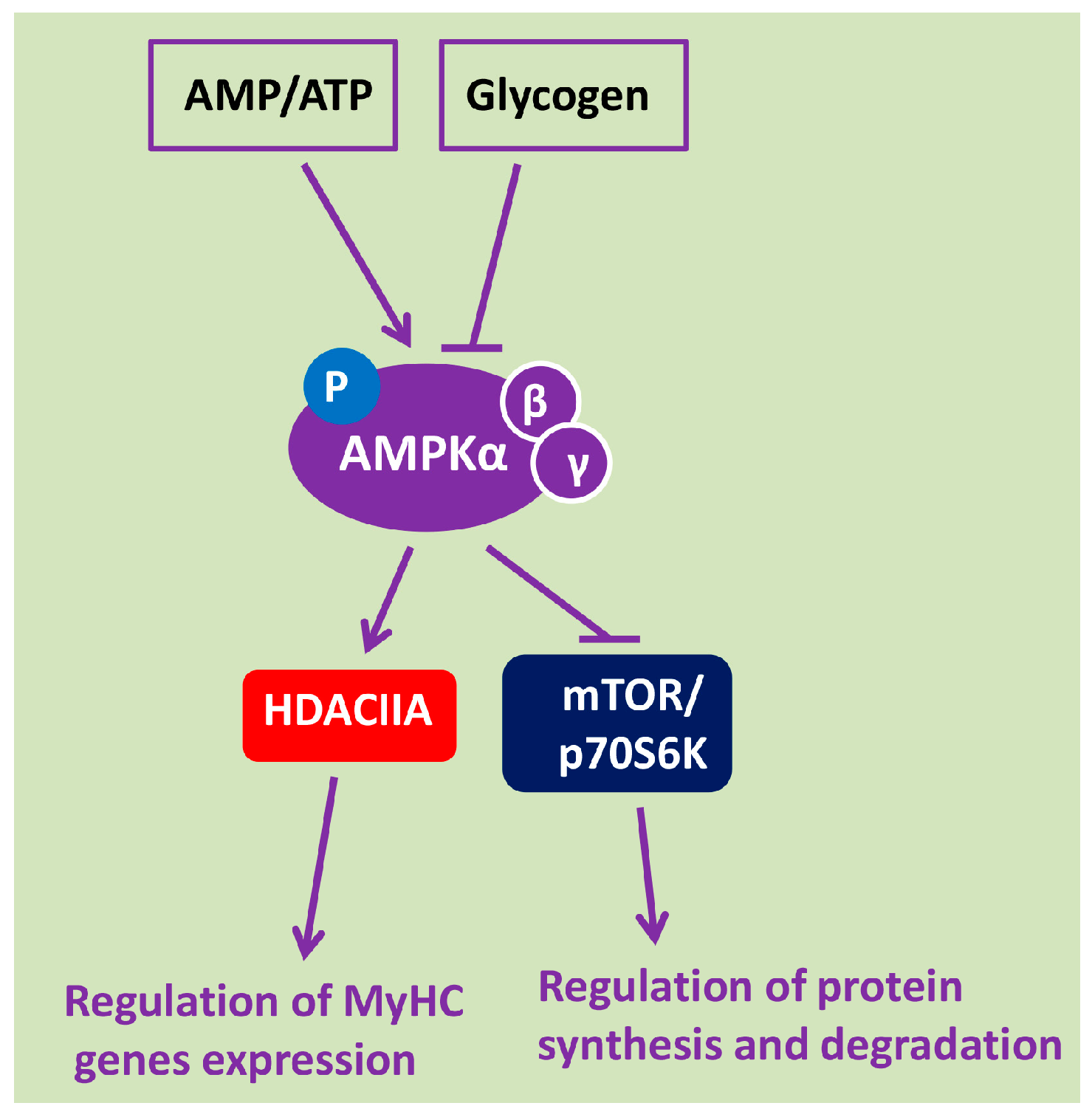
2. AMPK Is a Key Energy Sensor and Metabolic Regulator of Signaling Pathways in Skeletal Muscle Fibers
3. AMPK Activity under Conditions of Mechanical Unloading
4. The Role of AMPK in the Regulation of mTOR/p70S6K and Akt/FOXO3/MuRF-1/MAFbx/Atrogin-1 Signaling Pathways under Gravitational Unloading
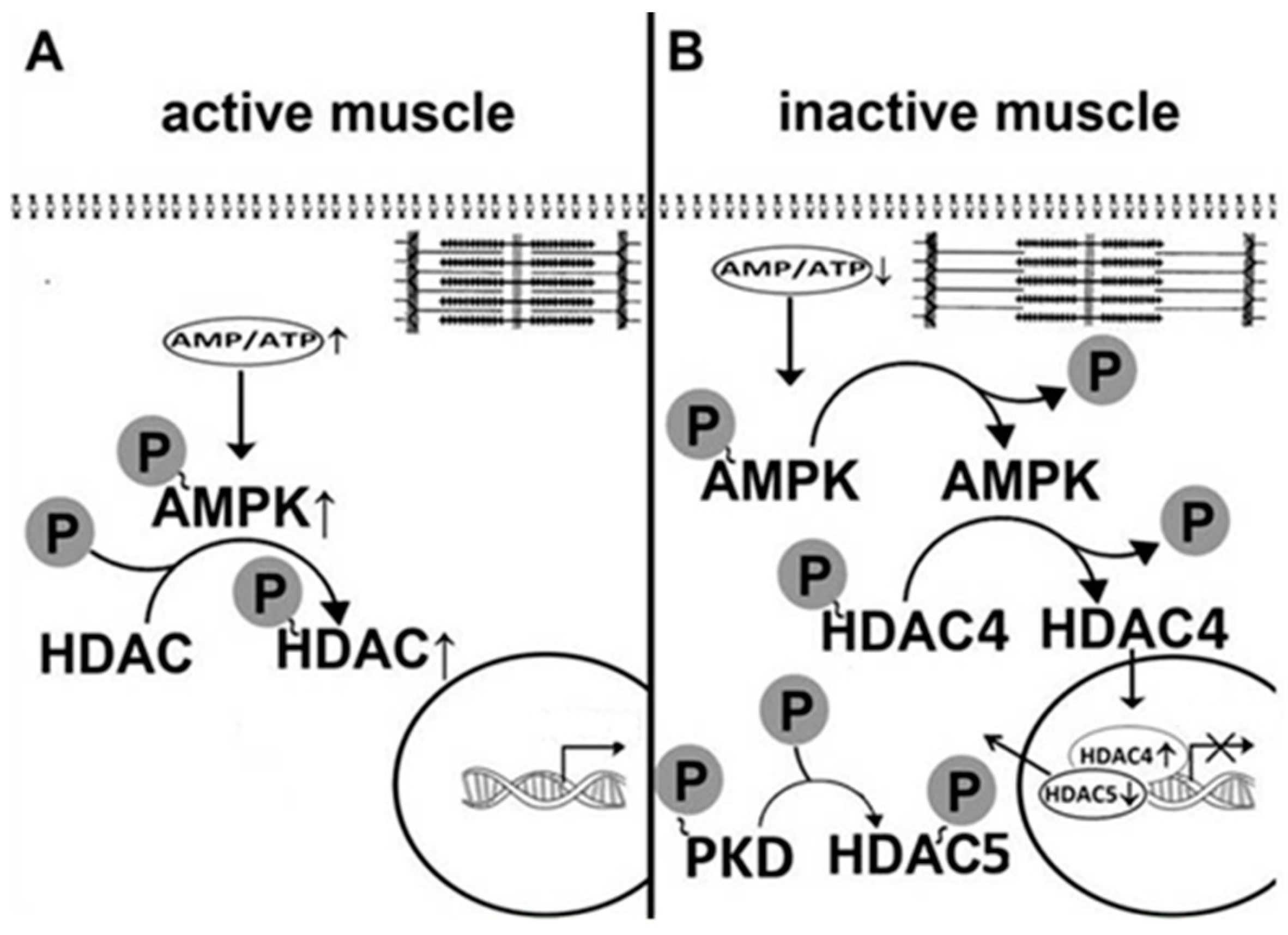
5. AMPK Is Involved in the Regulation of Myosin Phenotype under Mechanical Unloading
6. AMPK and Disuse-Induced Motor Endplate Remodeling
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | 5′ adenosine monophosphate -activated protein kinase |
| AICAR | 5-aminoimidazole-4-carboxamide ribonucleotide |
| BGPA | β-Guanidinopropionic acid |
| HS | hindlimb suspension |
| mTOR | mammalian/mechanistic target of rapamycin |
| p70S6K | ribosomal protein S6 kinase beta-1 |
| CaMKKβ | Ca(2+)/calmodulin-dependent protein kinase kinase β |
| LKB1 | liver kinase B1 |
| BDNF | brain-derived neurotrophic factor |
| MuRF-1 | Muscle RING-finger protein-1 |
| FOXO | forkhead box proteins |
| IRS-1 | insulin receptor substrate 1 |
| MyHC | myosin heavy chain isoforms |
| nAChRs | nicotinic acetylcholine receptors |
| ULK-1 | Unc-51-like kinase |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
References
- Grigor’ev, A.I.; Kozlovskaia, I.B.; Shenkman, B.S. The role of support afferents in organisation of the tonic muscle system. Rossiiskii fiziologicheskii zhurnal imeni IM Sechenova 2004, 90, 508–521. [Google Scholar]
- Kozlovskaia, I.B.; Grigor’eva, L.S.; Gevlich, G.I. Comparative analysis of the effect of weightlessness and its model on the velocity-strength properties and tonus of human skeletal muscles. Kosm. Biol. Aviakosm. Med. 1984, 18, 22–26. [Google Scholar] [PubMed]
- Oganov, V.S.; Skuratova, S.A.; Murashko, L.M.; Guba, F.; Takach, O. Effect of short-term space flights on physiological properties and composition of myofibrillar proteins of the skeletal muscles of rats. Kosm. Biol. Aviakosm. Med. 1988, 22, 50–54. [Google Scholar] [PubMed]
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef] [PubMed]
- Kandarian, S.C.; Stevenson, E.J. Molecular events in skeletal muscle during disuse atrophy. Exerc. Sport Sci. Rev. 2002, 30, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.R.; Baldwin, K.M. Age dependence of myosin heavy chain transitions induced by creatine depletion in rat skeletal muscle. J. Appl. Physiol. 1995, 78, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S. From Slow to Fast: Hypogravity-Induced Remodeling of Muscle Fiber Myosin Phenotype. Acta Nat. 2016, 8, 47–59. [Google Scholar]
- Chopard, A.; Hillock, S.; Jasmin, B.J. Molecular events and signalling pathways involved in skeletal muscle disuse-induced atrophy and the impact of countermeasures. J. Cell Mol. Med. 2009, 13, 3032–3050. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C. Disuse-induced muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Kachaeva, E.V.; Shenkman, B.S. Various jobs of proteolytic enzymes in skeletal muscle during unloading: Facts and speculations. J. Biomed. Biotechnol. 2011, 2012, 493618. [Google Scholar] [CrossRef] [PubMed]
- Mirzoev, T.; Tyganov, S.; Vilchinskaya, N.; Lomonosova, Y.; Shenkman, B. Key Markers of mTORC1-Dependent and mTORC1-Independent Signaling Pathways Regulating Protein Synthesis in Rat Soleus Muscle during Early Stages of Hindlimb Unloading. Cell. Physiol. Biochem. 2016, 39, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.; Sultan, K.R.; Peuker, H.; Gohlsch, B.; Mounier, Y.; Pette, D. Time dependent changes in myosin heavy chain mRNA and protein isoforms 94 in unloaded soleus muscle of rat. Am. J. Physiol. Cell. Physiol. 1999, 277, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Giger, J.M.; Bodell, P.W.; Zeng, M.; Baldwin, K.M.; Haddad, F. Rapid muscle atrophy response to unloading: Pretranslational processes involving MHC and actin. J. Appl. Physiol. 2009, 107, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Lomonosova, Y.N.; Turtikova, O.V.; Shenkman, B.S. Reduced expression of MyHC slow isoform in rat soleus during unloading is accompanied by alterations of endogenous inhibitors of calcineurin/NFAT signaling pathway. J. Muscle Res. Cell Motil. 2016, 37, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Alford, E.K.; Roy, R.R.; Hodgson, J.A.; Edgerton, V.R. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hind limb suspension. Exp. Neurol. 1987, 96, 635–649. [Google Scholar] [CrossRef]
- Kawano, F.; Matsuoka, Y.; Oke, Y.; Higo, Y.; Terada, M.; Wang, X.D.; Nakai, N.; Fukuda, H.; Imajoh-Ohmi, S.; Ohira, Y. Role(s) of nucleoli and phosphorylation of ribosomal protein S6 and/or HSP27 in the regulation of muscle mass. Am. J. Physiol. Cell Physiol. 2007, 293, 35–44. [Google Scholar] [CrossRef] [PubMed]
- De-Doncker, L.; Kasri, M.; Picquet, F.; Falempin, M. Physiologically adaptive changes of the L5afferent neurogram and of the rat soleus EMG activity during 14 days of hindlimb unloading and recovery. J. Exp. Biol. 2005, 208, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, I.; Dmitrieva, I.; Grigorieva, L.; Kirenskaya, A.; Kreidich, Y. Gravitational mechanisms in the motor system studies in real and simulated weightlessness. In Stance and Motion Facts and Concepts; Gurfinkel, V.S., Loffe, M.E., Massion, J., Eds.; Springer: New York, NY, USA, 1988; ISBN 978-1-4899-0823-0. [Google Scholar]
- Navasiolava, N.M.; Custaud, M.A.; Tomilovskaya, E.S.; Larina, I.M.; Mano, T.; Gauquelin-Koch, G.; Gharib, C.; Kozlovskaya, I.B. Long-term dry immersion: Review and prospects. Eur. J. Appl. Physiol. 2011, 111, 1235–1260. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, V.V.; Matchkov, V.V.; Bouzinova, E.V.; Vasiliev, A.N.; Razgovorova, I.A.; Heiny, J.A.; Krivoi, I.I. Isoform-specific Na,K-ATPase alterations precede disuse-induced atrophy of rat soleus muscle. Biomed. Res. Int. 2015, 720172. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, V.V.; Petrov, A.M.; Matchkov, V.V.; Bouzinova, E.V.; Vasiliev, A.N.; Benziane, B.; Zefirov, A.L.; Chibalin, A.V.; Heiny, J.A.; Krivoi, I.I. Distinct α2 Na,K-ATPase membrane pools are differently involved in early skeletal muscle remodeling during disuse. J. Gen. Physiol. 2016, 147, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.M.; Kravtsova, V.V.; Matchkov, V.V.; Vasiliev, A.N.; Zefirov, A.L.; Chibalin, A.V.; Heiny, J.A.; Krivoi, I.I. Membrane lipid rafts are disturbed in the response of rat skeletal muscle to short-term disuse. Am. J. Physiol. Cell Physiol. 2017, 312, 627–C637. [Google Scholar] [CrossRef] [PubMed]
- Lechado, I.; Terradas, A.; Vitadello, M.; Traini, L.; Namuduri, A.V.; Gastaldello, S.; Gorza, L. Sarcolemmal loss of active nNOS (Nos1) is an oxidative stress-dependent, early event driving disuse atrophy. J. Pathol. 2018. [Google Scholar] [CrossRef]
- Gordon, S.E.; Flück, M.; Booth, F.W. Selected Contribution: Skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J. Appl. Physiol. 2001, 90, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Ohira, Y.; Yasui, W.; Kariya, F.; Wakatsuki, T.; Nakamura, K.; Asakura, T.; Edgerton, V.R. Metabolic adaptation of skeletal muscles to gravitational unloading. Acta Astronaut. 1994, 33, 113–117. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Ohira, Y.; Yasui, W.; Nakamura, K.; Asakura, T.; Ohno, H.; Yamamoto, M. Responses of contractile properties in rat soleus to high-energy phosphates and/or unloading. Jpn. J. Physiol. 1994, 44, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J.; Tischler, M.E. Time Course of the Response of Carbohydrate Metabolism to Unloading of the Soleus. Metabolism 1988, 37, 201–208. [Google Scholar] [CrossRef]
- Witczak, C.A.; Sharoff, C.G.; Goodyear, L.J. AMP-activated protein kinase in skeletal muscle: From structure and localization to its role as a master regulator of cellular metabolism. Cell Mol. Life Sci. 2008, 65, 3737–3755. [Google Scholar] [CrossRef] [PubMed]
- Mounier, R.; Théret, M.; Lantier, L.; Foretz, M.; Viollet, B. Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol. Metab. 2015, 26, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Aström, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Glund, S.; Treebak, J.T.; Long, Y.C.; Barres, R.; Viollet, B.; Wojtaszewski, J.F.; Zierath, J.R. Role of adenosine 5′-monophosphate-activated protein kinase in interleukin-6 release from isolated mouse skeletal muscle. Endocrinology 2009, 150, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Soltow, Q.A.; Long, J.H.; Betters, J.L.; Sellman, J.E.; Criswell, D.S. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Brown, D.L.; Lira, A.K.; Kavazis, A.N.; Soltow, Q.A.; Zeanah, E.H.; Criswell, D.S. Nitric oxide and AMPK cooperatively regulate PGC-1α in skeletal muscle. J. Physiol. 2010, 588, 3551–3566. [Google Scholar] [CrossRef] [PubMed]
- Garbincius, J.F.; Michele, D.E. Dystrophin-glycoprotein complex regulates muscle nitric oxide production through mechanoregulation of AMPK signaling. Proc. Natl. Acad. Sci. USA 2015, 112, 13663–13668. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, K.A.; Valentine, R.J.; Sudit, B.S.; Allen, K.; Dagon, Y.; Kahn, B.B.; Ruderman, N.B.; Saha, A.K. PKD1 Inhibits AMPKα2 through Phosphorylation of Serine 491 and Impairs Insulin Signaling in Skeletal Muscle Cells. Biochem. J. 2016, 473, 4681–4697. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMP-activated protein kinase: A target for drugs both ancient and modern. Chem. Biol. 2012, 19, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Koay, A.; Woodcroft, B.; Petrie, E.J.; Yue, H.; Emanuelle, S.; Bieri, M.; Bailey, M.F.; Hargreaves, M.; Park, J.T.; Park, K.H.; et al. AMPK beta subunits display isoform specific affinities for carbohydrates. FEBS Lett. 2010, 584, 3499–3503. [Google Scholar] [CrossRef] [PubMed]
- Ingwersen, M.S.; Kristensen, M.; Pilegaard, H.; Wojtaszewski, J.F.; Richter, E.A.; Juel, C. Na, K-ATPase activity in mouse muscle is regulated by AMPK and PGC-1α. J. Membr. Biol. 2011, 242, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benziane, B.; Bjornholm, M.; Pirkmajer, S.; Austin, R.L.; Kotova, O.; Viollet, B.; Zierath, J.R.; Chibalin, A.V. Activation of AMP-activated protein kinase stimulates Na+,K+-ATPase activity in skeletal muscle cells. J. Biol. Chem. 2012, 287, 23451–23463. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; McConell, G.K.; Belinda, J.; Snow, R.J.; Canny, B.J.; Kemp, B.E. AMPK signaling in contracting human skeletal muscle: Acetyl-CoA carboxylase and NO synthase phosphorylation. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1202–E1206. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, G.J.; Lang, C.H. Sepsis and AMPK Activation by AICAR Differentially Regulate FoxO-1, -3 and -4 mRNA in Striated Muscle. Int. J. Clin. Exp. Med. 2008, 1, 50–63. [Google Scholar] [PubMed]
- Nakashima, K.; Yakabe, Y.; Ishida, A.; Katsumata, M. Effects of orally administered glycine on myofibrillar proteolysis and expression of proteolytic-related genes of skeletal muscle in chicks. Amino Acids 2008, 35, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K. AMPK and mTOR regulate autophagy via direct phosphorylation of ULK1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.A.; Tee, A.R. The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem. Soc. Trans. 2013, 41, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Röckl, K.S.; Hirshman, M.F.; Brandauer, J.; Fujii, N.; Witters, L.A.; Goodyear, L.J. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 2007, 56, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. AMPK-mediated regulation of transcription in skeletal muscle. Clin. Sci. (London) 2010, 118, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Meng, J.; Tang, Y.; Wang, T.; Wei, B.; Feng, R.; Gong, B.; Wang, H.; Ji, G.; Lu, Z. AMP-activated kinase α2 deficiency protects mice from denervation-induced skeletal muscle atrophy. Arch. Biochem. Biophys. 2016, 600, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, Y.F. Distinct signal transductions in fast- and slow- twitch muscles upon denervation. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Kostovski, E.; Boon, H.; Hjeltnes, N.; Lundell, L.S.; Ahlsén, M.; Chibalin, A.V.; Krook, A.; Iversen, P.O.; Widegren, U. Altered content of AMP-activated protein kinase isoforms in skeletal muscle from spinal cord injured subjects. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1071–E1080. [Google Scholar] [CrossRef] [PubMed]
- Mirzoev, T.M.; Vil’chinskaia, N.A.; Lomonosova, I.N.; Nemirovskaia, T.L.; Shenkman, B.S. Effect of 30-day space flight and subsequent readaptation on the signaling processes in m. longissimus dorsi of mice. Aviakosm. Ekolog. Med. 2014, 48, 12–15. [Google Scholar] [PubMed]
- Augusto, V.; Padovani, R.C.; Campos, G.E.R. Skeletal muscle fiber types in C57BL6J mice. Braz. J. Morphol. Sci. 2004, 21, 89–94. [Google Scholar]
- Vilchinskaya, N.A.; Mirzoev, T.M.; Lomonosova, Y.N.; Kozlovskaya, I.B.; Shenkman, B.S. Human muscle signaling responses to 3-day head-out dry immersion. J. Musculoskelet. Neuronal Interact. 2015, 15, 286–293. [Google Scholar] [PubMed]
- Hawley, S.A.; Boudeau, J.; Reid, J.L.; Mustard, K.J.; Udd, L.; Mäkelä, T.P.; Alessi, D.R.; Hardie, D.G. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Vertommen, D.; Neumann, D.; Turk, R.; Bayliss, J.; Schlattner, U.; Wallimann, T.; Carling, D.; Rider, M.H. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol. Chem. 2003, 278, 28434–28442. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhu, M.J.; Ma, C.; Du, M. Rat hindlimb unloading down-regulates insulin like growth factor-1 signaling and AMP-activated protein kinase, and leads to severe atrophy of the soleus muscle. Appl. Physiol. Nutr. Metab. 2007, 32, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Hilder, T.L.; Baer, L.A.; Fuller, P.M.; Fuller, C.A.; Grindeland, R.E.; Wade, C.E.; Graves, L.M. Insulinindependent pathways mediating glucose uptake in hindlimbsuspended skeletal muscle. J. Appl. Physiol. 2005, 99, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Goto, A.; Ohno, Y.; Yokoyama, S.; Ikuta, A.; Suzuki, M.; Sugiura, T.; Ohira, Y.; Yoshioka, T.; Hayashi, T.; et al. Involvement of AMPK in regulating slow-twitch muscle atrophy during hindlimb unloading in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 309, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Ohno, Y.; Goto, A.; Yokoyama, S.; Hayashi, T.; Goto, K. AMPK Mediates Muscle Mass Change But Not the Transition of Myosin Heavy Chain Isoforms during Unloading and Reloading of Skeletal Muscles in Mice. Int. J. Mol. Sci. 2018, 19, 2954. [Google Scholar] [CrossRef] [PubMed]
- Chibalin, A.V.; Benziane, B.; Zakyrjanova, G.F.; Kravtsova, V.V.; Krivoi, I.I. Early endplate remodeling and skeletal muscle signaling events following rat hindlimb suspension. J. Cell. Physiol. 2018, 233, 6329–6336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.F.; Zhang, Y.; Li, B.; Chen, N. Physical inactivity induces the atrophy of skeletal muscle of rats through activating AMPK/FoxO3 signal pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 199–209. [Google Scholar] [PubMed]
- Grano, M.; Mori, G.; Minielli, V.; Barou, O.; Colucci, S.; Giannelli, G.; Alexandre, C.; Zallone, A.Z.; Vico, L. Rat hindlimb unloading by tail suspension reduces osteoblast differentiation, induces IL-6 secretion, and increases bone resorption in ex vivo cultures. Calcif. Tissue Int. 2002, 70, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Yakabe, M.; Ogawa, S.; Ota, H.; Iijima, K.; Eto, M.; Ouchi, Y.; Akishita, M. Inhibition of interleukin-6 decreases atrogene expression and ameliorates tail suspension-induced skeletal muscle atrophy. PLoS ONE 2018, 13, e0191318. [Google Scholar] [CrossRef] [PubMed]
- Mutin-Carnino, M.; Carnino, A.; Roffino, S.; Chopard, A. Effect of muscle unloading, reloading and exercise on inflammation during a head-down bed rest. Int. J. Sports Med. 2014, 35, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Keller, C.; Richard, A.M.; Saha, A.K.; Luo, Z.; Xiang, X.; Giralt, M.; Ritov, V.B.; Menshikova, E.V.; Kelley, D.E.; et al. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes 2006, 55, S48–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, H. Effects of hindlimb unloading on neurotrophins in the rat spinal cord and soleus muscle. Brain Res. 2016, 1630, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dupont, E.; Cieniewski-Bernard, C.; Bastide, B.; Stevens, L. Electrostimulation during hindlimb unloading modulates PI3K-AKT downstream targets without preventing soleus atrophy and restores slow phenotype through ERK. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Bajotto, G.; Sato, Y.; Kitaura, Y.; Shimomura, Y. Effect of branched-chain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles. Eur. J. Appl. Physiol. 2011, 111, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Childs, T.E.; Spangenburg, E.E.; Vyas, D.R.; Booth, F.W. Temporal alterations in protein signaling cascades during recovery from muscle atrophy. Am. J. Physiol. Cell. Physiol. 2003, 285, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Abe, N.; Nagano, M.; Goto, K.; Sakuma, K.; Naito, H.; Yoshioka, T.; Powers, S.K. Changes in PKB/Akt and calcineurin signaling during recovery in atrophied soleus muscle induced by unloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Gwag, T.; Lee, K.; Ju, H.; Shin, H.; Lee, J.W.; Choi, I. Stress and signaling responses of rat skeletal muscle to brief endurance exercise during hindlimb unloading: A catch-up process for atrophied muscle. Cell Physiol. Biochem. 2009, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, E.A.; Turtikova, O.V.; Kachaeva, E.V.; Ushakov, I.B.; Shenkman, B.S. Time course of ribosomal kinase activity during hindlimb unloading. Dokl. Biochem. Biophys. 2010, 434, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.L.; Philp, A.; MacKenzie, M.G.; Patton, A.; Towler, M.C.; Gallagher, I.J.; Bodine, S.C.; Baar, K. Molecular brakes regulating mTORC1 activation in skeletal muscle following synergist ablation. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E365–E373. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ji, J.; Yan, X.H. Cross-talk between AMPK and mTOR in regulating energy balance. Crit. Rev. Food. Sci. Nutr. 2012, 52, 373–381. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Mustard, K.J.; Hardie, D.G.; Baar, K. Normal hypertrophy accompanied by phosphoryation and activation of AMP-activated protein kinase alpha1 following overload in LKB1 knockout mice. J. Physiol. 2008, 586, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Mounier, R.; Lantier, L.; Leclerc, J.; Sotiropoulos, A.; Pende, M.; Daegelen, D.; Sakamoto, K.; Foretz, M.; Viollet, B. Important role for AMPKalpha1 in limiting skeletal muscle cell hypertrophy. FASEB J. 2009, 23, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, T.A.; Hunter, R.B.; Kandarian, S.C.; Esser, K.A. Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats. Am. J. Physiol. Cell Physiol. 2001, 281, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Krawiec, B.J.; Nystrom, G.J.; Frost, R.A.; Jefferson, L.S.; Lang, C.H. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1555–E1567. [Google Scholar] [CrossRef] [PubMed]
- Vilchinskaya, N.A.; Mochalova, E.P.; Belova, S.P.; Shenkman, B.S. Dephosphorylation of AMP-activated protein kinase in a postural muscle: A key signaling event on the first day of functional unloading. Biophysics 2016, 61, 1019–1025. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Chuang, J.H.; Yang, W.C.; Yin, Y.; Lin, Y. Ceramide inhibits insulin-stimulated Akt phosphorylation through activation of Rheb/mTORC1/S6K signaling in skeletal muscle. Cell. Signal. 2014, 26, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.A.; Smith, M.E.; Anthonymuthu, T.S.; Evanson, M.J.; Brassfield, E.S.; Hodson, A.E.; Bressler, M.A.; Tucker, B.J.; Thatcher, M.O.; Prince, J.T.; et al. AICAR inhibits ceramide biosynthesis in skeletal muscle. Diabetol. Metab. Syndr. 2012, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Bryndina, I.G.; Shalagina, M.N.; Ovechkin, S.V.; Ovchinina, N.G. Sphingolipids in skeletal muscles of C57B1/6 mice after short-term simulated microgravity. Rossiiskii fiziologicheskii zhurnal imeni IM Sechenova 2014, 100, 1280–1286. [Google Scholar]
- Salaun, E.; Lefeuvre-Orfila, L.; Cavey, T.; Martin, B.; Turlin, B.; Ropert, M.; Loreal, O.; Derbré, F. Myriocin prevents muscle ceramide accumulation but not muscle fiber atrophy during short-term mechanical unloading. J. Appl. Physiol. 2016, 120, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.; Vilchinskaya, N.; Mochalova, E.; Belova, S.; Nemirovskaya, T. Signaling events at the early stage of muscle disuse. FEBS J. 2017, 284, 367. [Google Scholar] [CrossRef]
- Nakao, R.; Hirasaka, K.; Goto, J.; Ishidoh, K.; Yamada, C.; Ohno, A.; Okumura, Y.; Nonaka, I.; Yasutomo, K.; Baldwin, K.M.; et al. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol. Cell. Biol. 2009, 29, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S.; Nemirovskaya, T.L. Calcium-dependent signaling mechanisms and soleus fiber remodeling under gravitational unloading. J. Muscle Res. Cell Motil. 2008, 29, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Physiology of a microgravity environment invited review: Microgravity and skeletal muscle. J. Appl. Physiol. 2000, 89, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Templeton, G.H.; Sweeney, H.L.; Timson, B.F.; Padalino, M.; Dudenhoeffer, G.A. Changes in fiber composition of soleus muscle during rat hindlimb suspension. J. Appl. Physiol. 1988, 65, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Desplanches, D.; Mayet, M.H.; Sempore, B.; Flandrois, R. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J. Appl. Physiol. 1987, 63, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Desplanches, D.; Kayar, S.R.; Sempore, B.; Flandrois, R.; Hoppeler, H. Rat soleus muscle ultrastructure after hindlimb suspension. J. Appl. Physiol. 1990, 69, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.A.; Slocum, G.R.; Bain, J.L.; Sedlak, F.R.; Sedlak, F.R.; Sowa, T.E.; Mellender, J.W. Rat hindlimb unloading: Soleus histochemistry, ultrastructure, and electromyography. J. Appl. Physiol. 1990, 69, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Trappe, S.; Costill, D.; Gallagher, P.; Creer, A.; Peters, J.R.; Evans, H.; Riley, D.A.; Fitts, R.H. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. J. Appl. Physiol. 2009, 106, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Randall, W.R.; Martin, F.; Schneider, M.F. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J. Cell Biol. 2005, 168, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Vilchinskaya, N.A.; Mochalova, E.P.; Nemirovskaya, T.L.; Mirzoev, T.M.; Turtikova, O.V.; Shenkman, B.S. Rapid decline in MyHC I(β) mRNA expression in rat soleus during hindlimb unloading is associated with AMPK dephosphorylation. J. Physiol. 2017, 595, 7123–7134. [Google Scholar] [CrossRef] [PubMed]
- Pandorf, C.E.; Haddad, F.; Wright, C.; Bodell, P.W.; Baldwin, K.M. Differential epigenetic modifications of histones at the myosin heavy chain genes in fast and slow skeletal muscle fibers and in response to muscle unloading. Am. J. Physiol. Cell Physiol. 2009, 297, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.J.; Choi, M.C.; Kapur, M.; Lira, V.A.; Lira, V.A.; Yan, Z.; Yao, T.P. HDAC4 regulates muscle fiber type-specific gene expression programs. Mol. Cells. 2015, 38, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Vilchinskaya, N.A.; Turtikova, O.V.; Shenkman, B.S. Regulation of the Nuclear–Cytoplasmic Traffic of Class IIa Histone Deacethylases in Rat Soleus Muscle at the Early Stage of Gravitational Unloading. Biol. Membr. 2017, 34, 109–115. [Google Scholar] [CrossRef]
- Yoshihara, T.; Machida, S.; Kurosaka, Y.; Kakigi, R.; Sugiura, T.; Naito, H. Immobilization induces nuclear accumulation of HDAC4 in rat skeletal muscle. J. Physiol. Sci. 2016, 66, 337–343. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Swinton, C.; Morrison, S.; Gaur, V.; Gaur, V.; Campbell, D.E.; Jorgensen, S.B.; Kemp, B.E.; Baar, K.; Steinberg, G.R.; Hargreaves, M. Compensatory regulation of HDAC5 in muscle maintains metabolic adaptive responses and metabolism in response to energetic stress. Faseb, J. 2014, 28, 3384–3395. [Google Scholar] [CrossRef] [PubMed]
- Ya, F.; Rubin, C.S. Protein kinase D: Coupling extracellular stimuli to the regulation of cell physiology. EMBO Rep. 2011, 12, 785–796. [Google Scholar]
- Gan, Z.; Rumsey, J.; Hazen, B.C.; Lai, L.; Leone, T.C.; Vega, R.B.; Xie, H.; Conley, KE.; Auwerx, J.; Smith, S.R.; et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J. Clin. Investig. 2013, 123, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Cannavino, J.; Brocca, L.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. PGC1-α over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J. Physiol. 2014, 592, 4575–4589. [Google Scholar] [CrossRef] [PubMed]
- Sharlo, C. A.; Lomonosova, Y. N.; Turtikova, O.V.; Mitrofanova, O.V.; Kalamkarov, G.R.; Bugrova, A.E.; Shevchenko, T.F. The Role of GSK-3β Phosphorylation in the Regulation of Slow Myosin Expression in Soleus Muscle during Functional Unloading. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2018, 1, 85–91. [Google Scholar] [CrossRef]
- Wood, S.J.; Slater, C.R. Safety factor at the neuromuscular junction. Prog. Neurobiol. 2001, 64, 393–429. [Google Scholar] [CrossRef]
- Ruff, R.L. Endplate contributions to the safety factor for neuromuscular transmission. Muscle Nerve 2011, 44, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Morsch, M.; Murata, Y.; Ghazanfari, N.; Reddel, S.W.; Phillips, W.D. Sequence of age-associated changes to the mouse neuromuscular junction and the protective effects of voluntary exercise. PLoS ONE 2013, 8, e67970. [Google Scholar] [CrossRef] [PubMed]
- Carnio, S.; LoVerso, F.; Baraibar, M.A.; Longa, E.; Khan, M.M.; Maffei, M.; Reischl, M.; Canepari, M.; Loefler, S.; Kern, H.; et al. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep. 2014, 8, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Willadt, S.; Nash, M.; Slater, C.R. Age-related fragmentation of the motor endplate is not associated with impaired neuromuscular transmission in the mouse diaphragm. Sci. Rep. 2016, 6, 24849. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Chand, K.K.; Hammond, L.A.; Lavidis, N.A.; Noakes, P.G. Functional decline at the aging neuromuscular junction is associated with altered laminin-α4 expression. Aging 2017, 9, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Marcotte, G.R.; Marshall, A.G.; West, D.W.D.; Baehr, L.M.; Wallace, M.A.; Saleh, P.M.; Bodine, S.C.; Baar, K. Age-related differences in dystrophin: Impact on force transfer proteins, membrane integrity, and neuromuscular junction stability. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, R.; Khan, M.M.; Labeit, S.; Deschenes, M.R. Degeneration of neuromuscular junction in age and dystrophy. Front. Aging Neurosci. 2014, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Pratt, S.J.; Valencia, A.P.; Le, G.K.; Shah, S.B.; Lovering, R.M. Pre- and postsynaptic changes in the neuromuscular junction in dystrophic mice. Front. Physiol. 2015, 6, 252. [Google Scholar] [CrossRef] [PubMed]
- van der Pijl, E.M.; van Putten, M.; Niks, E.H.; Verschuuren, J.J.; Aartsma-Rus, A.; Plomp, J.J. Characterization of neuromuscular synapse function abnormalities in multiple Duchenne muscular dystrophy mouse models. Eur. J. Neurosci. 2016, 43, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Falk, D.J.; Todd, A.G.; Lee, S.; Soustek, M.S.; ElMallah, M.K.; Fuller, D.D.; Notterpek, L.; Byrne, B.J. Peripheral nerve and neuromuscular junction pathology in Pompe disease. Hum. Mol. Genet. 2015, 24, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Zhang, D.; Corrick, R.M.; Muelleman, R.L.; Wadman, M.C.; Li, Y.L. Morphological regeneration and functional recovery of neuromuscular junctions after tourniquet-induced injuries in mouse hindlimb. Front. Physiol. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Yampolsky, P.; Pacifici, P.G.; Witzemann, V. Differential muscle-driven synaptic remodeling in the neuromuscular junction after denervation. Eur. J. Neurosci. 2010, 31, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R.; Wilson, M.H. Age-related differences in synaptic plasticity following muscle unloading. J. Neurobiol. 2003, 57, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Nishimune, H.; Stanford, J.A.; Mori, Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve 2014, 49, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The neuromuscular junction: Aging at the crossroad between nerves and muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Tintignac, L.A.; Brenner, H.R.; Rüegg, M.A. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Zhang, P.; Liu, H.; He, J.; Zhang, C.; Fan, M.; Chen, X. PGC-1α over-expression suppresses the skeletal muscle atrophy and myofiber-type composition during hindlimb unloading. Biosci. Biotechnol. Biochem. 2017, 81, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rincon, M.; Morales-Alamo, D.; Calbet, J.A.L. Exercise-mediated modulation of autophagy in skeletal muscle. Scand. J. Med. Sci. Sports 2018, 28, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Strack, S.; Wild, F.; Hanashima, A.; Gasch, A.; Brohm, K.; Reischl, M.; Carnio, S.; Labeit, D.; Sandri, M.; et al. Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of nicotinic acetylcholine receptors. Autophagy 2014, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Cerveró, C.; Montull, N.; Tarabal, O.; Piedrafita, L.; Esquerda, J.E.; Calderó, J. Chronic treatment with the AMPK agonist AICAR prevents skeletal muscle pathology but fails to improve clinical outcome in a mouse model of severe spinal muscular atrophy. Neurotherapeutics 2016, 13, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Daussin, F.; Burelle, Y.; Li, T.; Godin, R.; Fauconnier, J.; Koechlin-Ramonatxo, C.; Hugon, G.; Lacampagne, A.; Coisy-Quivy, M.; et al. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am. J. Pathol. 2012, 181, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Ljubicic, V.; Jasmin, B.J. AMP-activated protein kinase at the nexus of therapeutic skeletal muscle plasticity in Duchenne muscular dystrophy. Trends Mol. Med. 2013, 19, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Habegger, K.M.; Hoffman, N.J.; Ridenour, C.M.; Brozinick, J.T.; Elmendorf, J.S. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology 2012, 153, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- Ambery, A.G.; Tackett, L.; Penque, B.A.; Brozinick, J.T.; Elmendorf, J.S. Exercise training prevents skeletal muscle plasma membrane cholesterol accumulation, cortical actin filament loss, and insulin resistance in C57BL/6J mice fed a western-style high-fat diet. Physiol. Rep. 2017, 5, e13363. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, G.; Hénin, J.; Law, R.; Eckenhoff, R.; Klein, M.L. Embedded cholesterol in the nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 14418–14423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Xiong, W.C.; Mei, L. Lipid rafts serve as a signaling platform for nicotinic acetylcholine receptor clustering. J. Neurosci. 2006, 26, 4841–4851. [Google Scholar] [CrossRef] [PubMed]
- Willmann, R.; Pun, S.; Stallmach, L.; Sadasivam, G.; Santos, A.F.; Caroni, P.; Fuhrer, C. Cholesterol and lipid microdomains stabilize the postsynapse at the neuromuscular junction. EMBO J. 2006, 25, 4050–4060. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Ye, Q.; Tian, J.; Jing, R.; Xie, Z. Regulation of α1 Na/K-ATPase expression by cholesterol. J. Biol. Chem. 2011, 286, 15517–15524. [Google Scholar] [CrossRef] [PubMed]
- Kapri-Pardes, E.; Katz, A.; Haviv, H.; Mahmmoud, Y.; Ilan, M.; Khalfin-Penigel, I.; Carmeli, S.; Yarden, O.; Karlish, S.J.D. Stabilization of the α2 isoform of Na,K-ATPase by mutations in a phospholipid binding pocket. J. Biol. Chem. 2011, 286, 42888–42899. [Google Scholar] [CrossRef] [PubMed]
- Haviv, H.; Habeck, M.; Kanai, R.; Toyoshima, C.; Karlish, S.J. Neutral phospholipids stimulate Na,K-ATPase activity: A specific lipid-protein interaction. J. Biol. Chem. 2013, 288, 10073–10081. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, F.; Habeck, M.; Kanai, R.; Toyoshima, C.; Karlish, S.J. General and specific lipid-protein interactions in Na,K-ATPase. Biochim. Biophys. Acta 2015, 1848, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, V.V.; Petrov, A.M.; Vasiliev, A.N.; Zefirov, A.L.; Krivoi, I.I. Role of cholesterol in the maintenance of endplate electrogenesis in rat diaphragm. Bull. Exp. Biol. Med. 2015, 158, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Heiny, J.A.; Kravtsova, V.V.; Mandel, F.; Radzyukevich, T.L.; Benziane, B.; Prokofiev, A.V.; Pedersen, S.E.; Chibalin, A.V.; Krivoi, I.I. The nicotinic acetylcholine receptor and the Na,K-ATPase α2 isoform interact to regulate membrane electrogenesis in skeletal muscle. J. Biol. Chem. 2010, 285, 28614–28626. [Google Scholar] [CrossRef] [PubMed]
- Chibalin, A.V.; Heiny, J.A.; Benziane, B.; Prokofiev, A.V.; Vasiliev, A.N.; Kravtsova, V.V.; Krivoi, I.I. Chronic nicotine exposure modifies skeletal muscle Na,K-ATPase activity through its interaction with the nicotinic acetylcholine receptor and phospholemman. PLoS ONE 2012, 7, e33719. [Google Scholar] [CrossRef] [PubMed]
- Matchkov, V.V.; Krivoi, I.I. Specialized functional diversity and interactions of the Na,K-ATPase. Front. Physiol. 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilchinskaya, N.A.; Krivoi, I.I.; Shenkman, B.S. AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling. Int. J. Mol. Sci. 2018, 19, 3558. https://doi.org/10.3390/ijms19113558
Vilchinskaya NA, Krivoi II, Shenkman BS. AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling. International Journal of Molecular Sciences. 2018; 19(11):3558. https://doi.org/10.3390/ijms19113558
Chicago/Turabian StyleVilchinskaya, Natalia A., Igor I. Krivoi, and Boris S. Shenkman. 2018. "AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling" International Journal of Molecular Sciences 19, no. 11: 3558. https://doi.org/10.3390/ijms19113558
APA StyleVilchinskaya, N. A., Krivoi, I. I., & Shenkman, B. S. (2018). AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling. International Journal of Molecular Sciences, 19(11), 3558. https://doi.org/10.3390/ijms19113558




