BCPA {N,N′-1,4-Butanediylbis[3-(2-chlorophenyl)acrylamide]} Inhibits Osteoclast Differentiation through Increased Retention of Peptidyl-Prolyl cis-trans Isomerase Never in Mitosis A-Interacting 1
Abstract
1. Introduction
2. Results
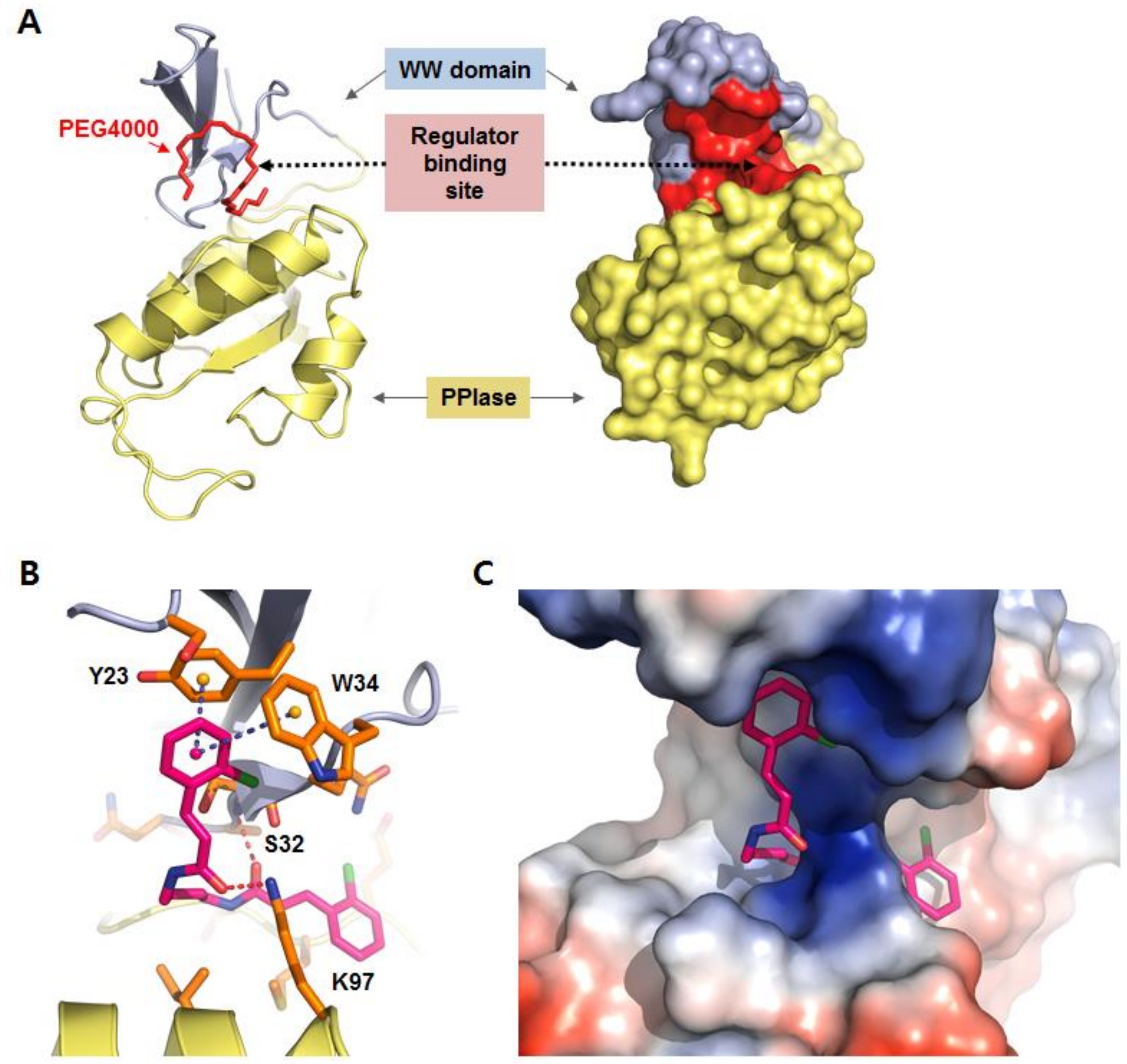
2.1. Identification of BCPA by Receptor-Based in Silico Screening
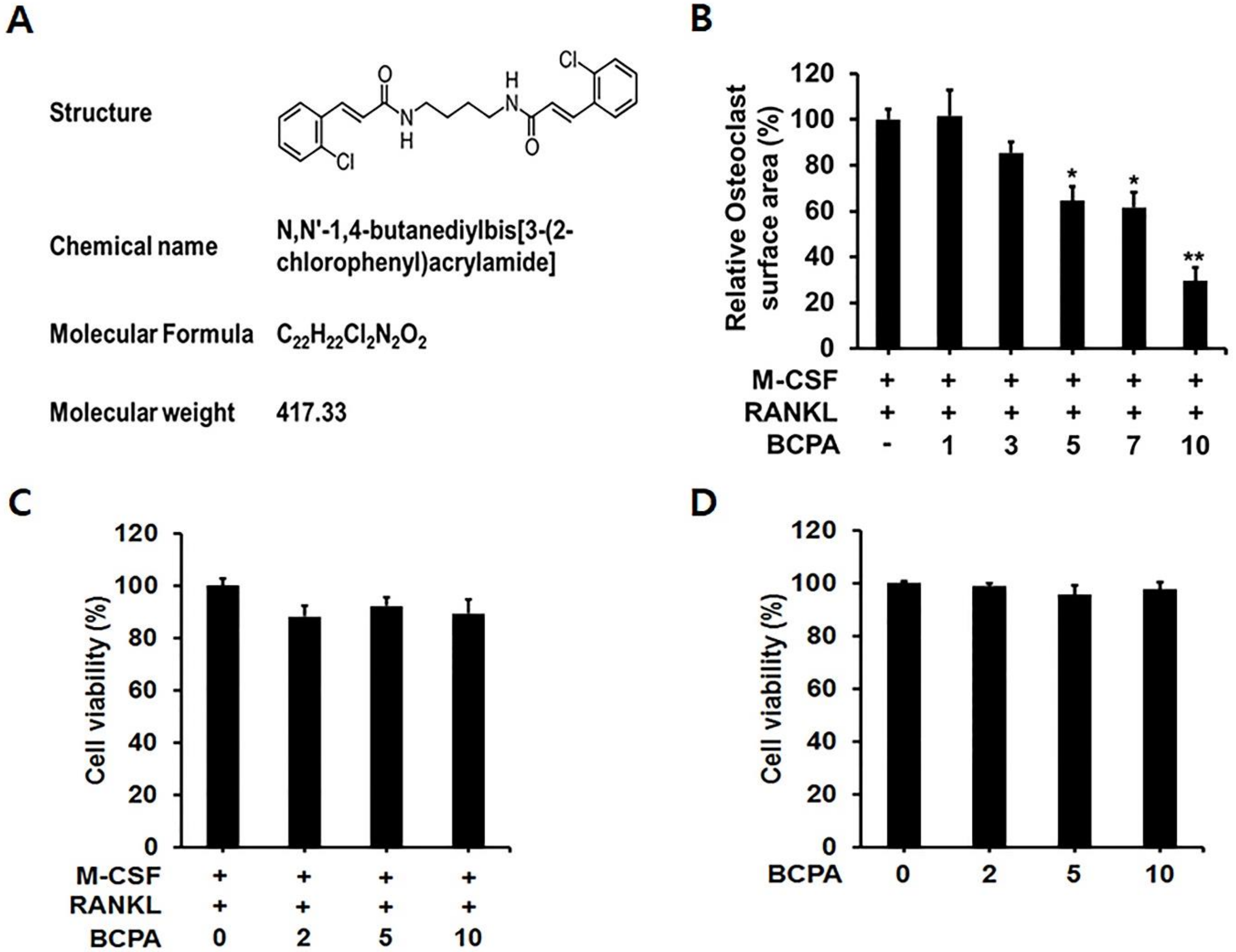
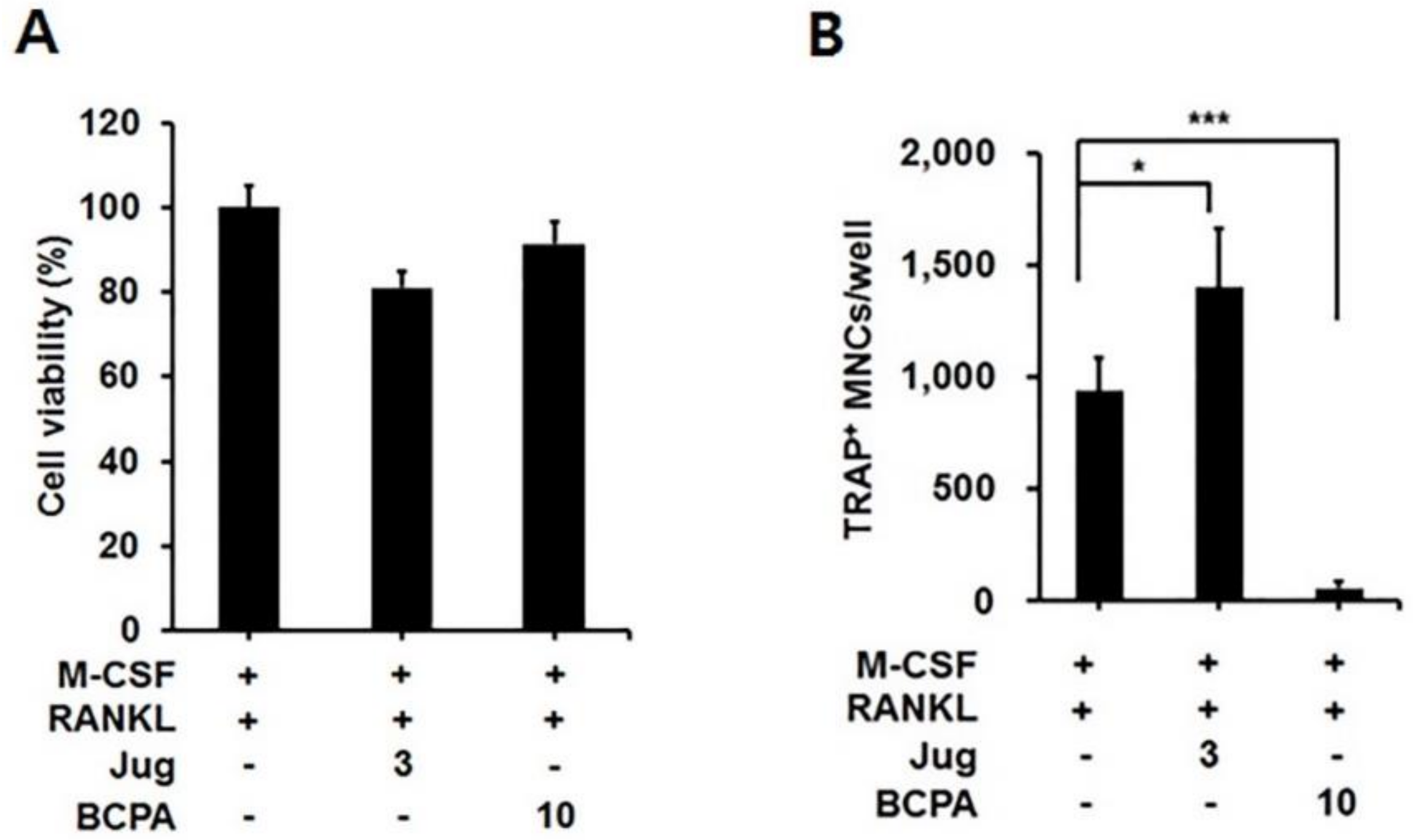
2.2. BCPA Suppresses Osteoclast Differentiation without Cytotoxicity
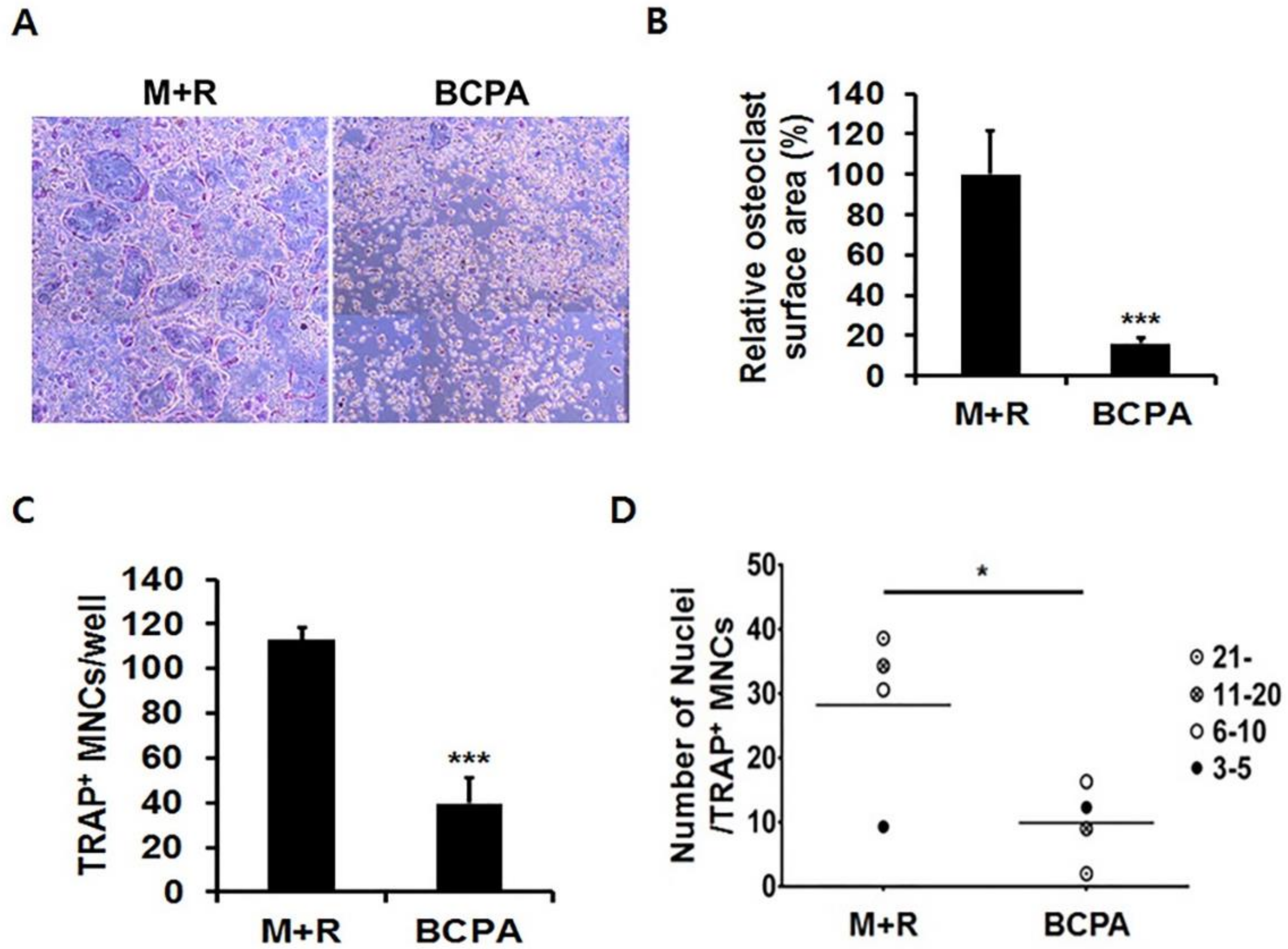
2.3. BCPA Inhibits Osteoclastogenesis
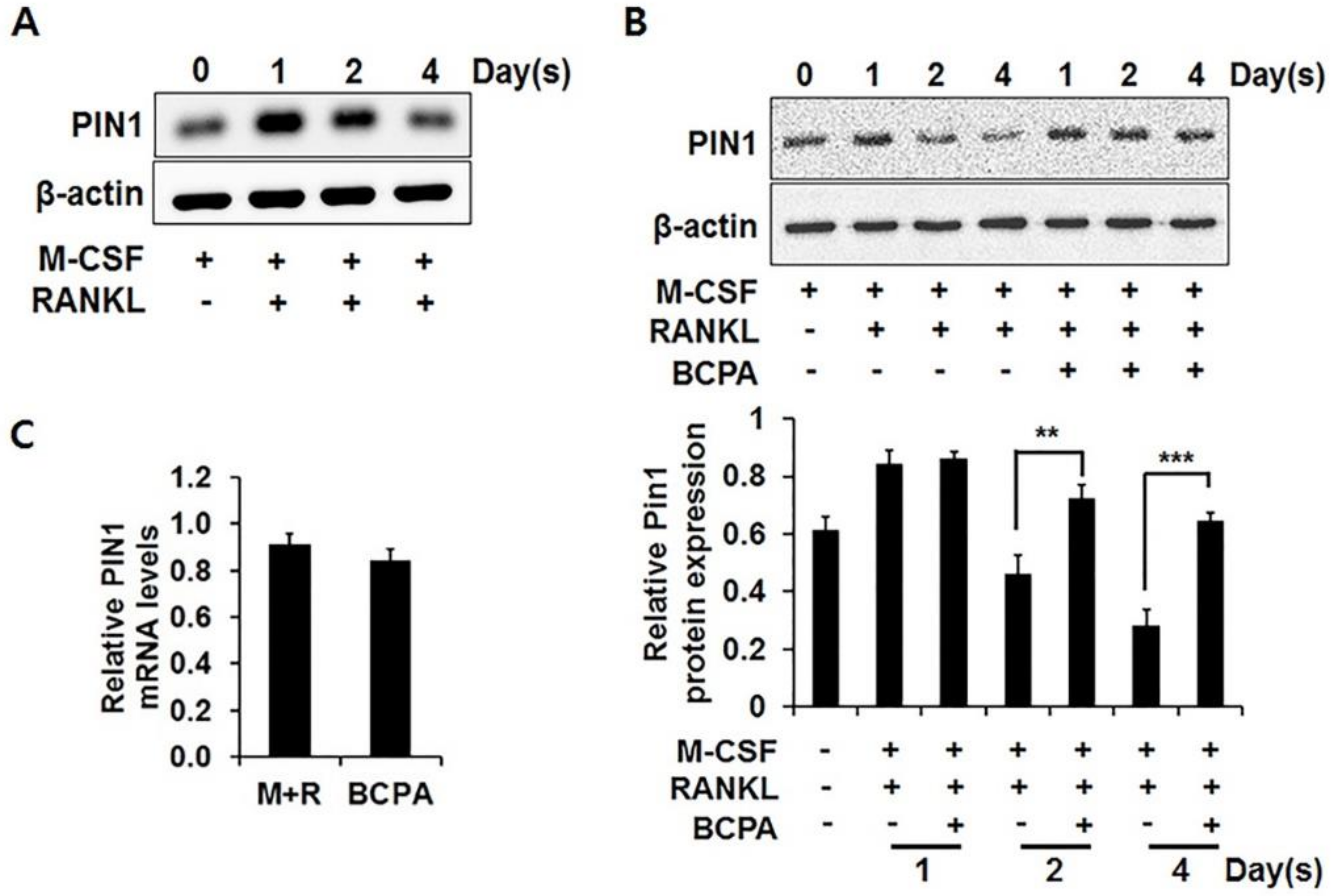
2.4. BCPA Regulates Osteoclastogenesis by Attenuating Pin1 Reduction
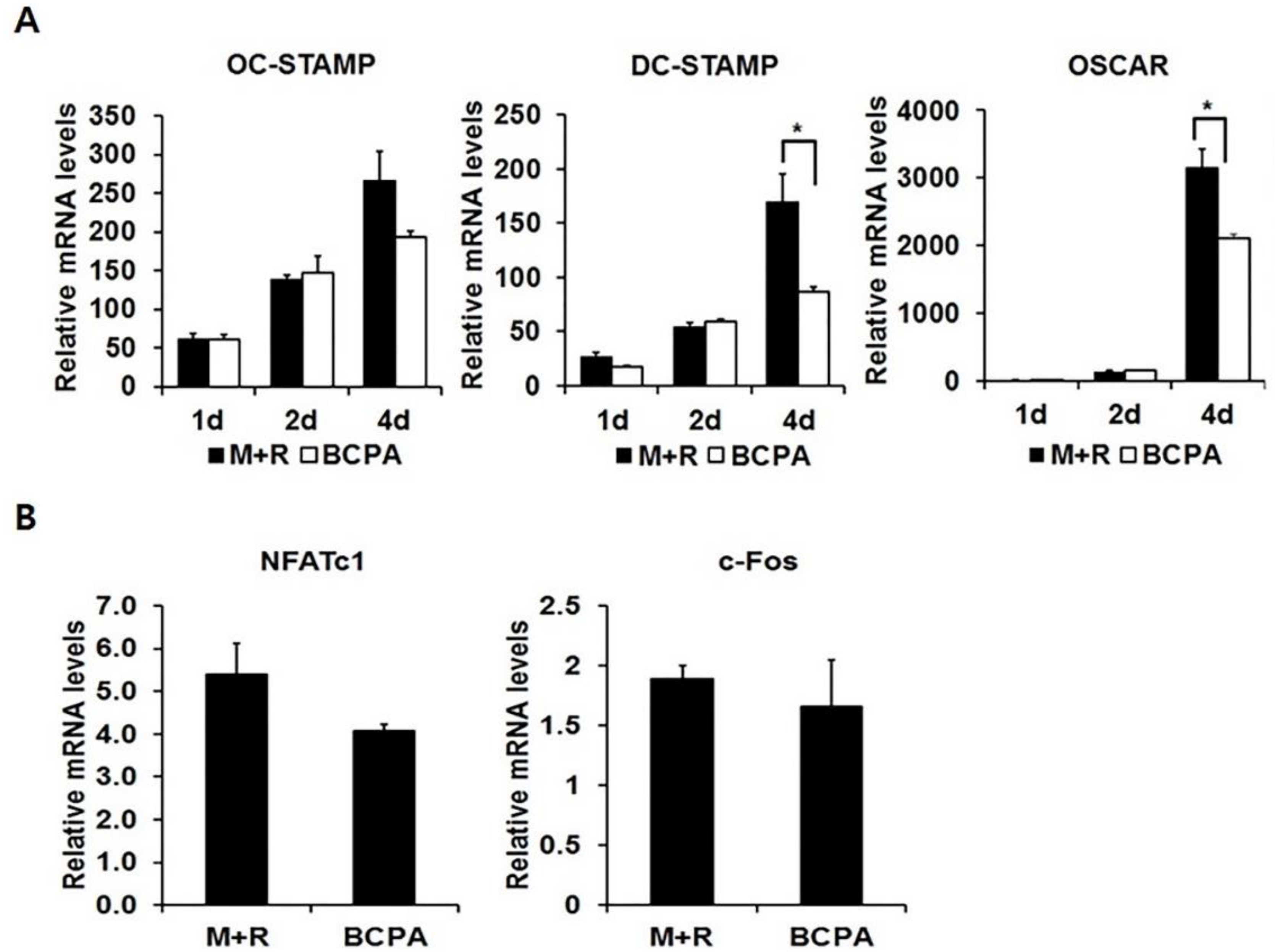
2.5. BCPA Inhibits Fusion of Osteoclasts by Reducing DC-STAMP
2.6. Pin1 Inhibition Enhances Osteoclastogenesis
3. Discussion
4. Materials and Methods
4.1. In Silico Screening of a Compound Library for Pin1 Regulators
4.2. Materials
4.3. Cell Culture
4.4. Isolation and Differentiation of Mouse Bone Marrow-Derived Macrophages (BMMs)
4.5. MTT Assay
4.6. Western Blot Analysis
4.7. Quantitative Real-Time PCR
4.8. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMMs | bone marrow-derived macrophages |
| MNCs | multinucleated giant cells |
| Pin1 | peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
| BCPA | N,N′-1,4-butanediylbis[3-(2-chlorophenyl)acrylamide] |
| mRNA | messenger ribonucleic acid |
| PCR | polymerase chain reaction |
| DMSO | dimethyl sulfoxide |
References
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Leibbrandt, A.; Penninger, J.M. RANK/RANKL: Regulators of immune responses and bone physiology. Ann. N. Y. Acad. Sci. 2008, 1143, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica 2013, 2013, 125705. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M. Aging mechanisms in bone. Bonekey Rep. 2012, 1, 102. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Wu, C.C.; Liao, M.T.; Shyu, J.F.; Hung, C.F.; Yen, T.H.; Lu, C.L.; Lu, K.C. Role of nutritional vitamin D in osteoporosis treatment. Clin. Chim. Acta 2018, 484, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Office of the Surgeon General (US). Bone Health and Osteoporosis: A Report of the Surgeon General. Rockv. (MD) Off. Surg. Gen. 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK45031/ (accessed on 11 September 2018).
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.P.; Zhou, X.Z. The prolyl isomerase PIN1: A pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Islam, R.; Cho, Y.D.; Woo, K.M.; Baek, J.H.; Uchida, T.; Van Komori Wijnen, A.; Stein, J.L.; Lian, J.B.; Stein, G.S.; et al. Pin1-mediated Runx2 modification is critical for skeletal development. J. Cell. Physiol. 2013, 228, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Islam, R.; Cho, Y.D.; Ryu, K.M.; Shin, H.R.; Woo, K.M.; Baek, J.H.; Ryoo, H.M. Pin1 plays a critical role as a molecular switch in canonical BMP signaling. J. Cell. Physiol. 2015, 230, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, H.M.; Han, Y.; Cheong, H.; Kang, B.Y.; Lee, K.Y. Prolyl isomerase Pin1 regulates the osteogenic activity of Osterix. Mol. Cell. Endocrinol. 2015, 15, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Islam, R.; Yoon, W.J.; Lee, T.; Cho, Y.D.; Bae, H.S.; Kim, B.S.; Woo, K.M.; Baek, J.H.; Ryoo, H.M. Pin1-mediated Modification Prolongs the Nuclear Retention of β-Catenin in Wnt3a-induced Osteoblast Differentiation. J. Biol. Chem. 2016, 291, 5555–5565. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 1, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Ritchlin, C.T. DC-STAMP: A Key Regulator in Osteoclast Differentiation. J. Cell. Physiol. 2016, 231, 2402–2407. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Uematsu, S.; Kondo, T.; Takeuchi, O.; Martino, M.M.; Kawasaki, T.; Akira, S. Strawberry notch homologue 2 regulates osteoclast fusion by enhancing the expression of DC-STAMP. J. Exp. Med. 2013, 23, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Iwasaki, M.; Ochi, H.; Fukuda, T.; Ma, C.; Miyamoto, T.; Takitani, K.; Negishi-Koga, T.; Sunamura, S.; Kodama, T.; et al. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat. Med. 2012, 18, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Bae, H.S.; Yoon, W.J.; Woo, K.M.; Baek, J.H.; Kim, H.H.; Uchida, T.; Ryoo, H.M. Pin1 regulates osteoclast fusion through suppression of the master regulator of cell fusion DC-STAMP. J. Cell. Physiol. 2014, 229, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.J.; Hu, J.; Ali, A.; Pastor, J.; Shiizaki, K.; Blank, R.D.; Kuro-o, M.; Malter, J.S. Pin1 null mice exhibit low bone mass and attenuation of BMP signaling. PLoS ONE 2013, 8, e63565. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Yoon, W.J.; Ryoo, H.M. Pin1, the Master Orchestrator of Bone Cell Differentiation. J. Cell. Physiol. 2017, 232, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Ranger, A.M.; Gerstenfeld, L.C.; Wang, J.; Kon, T.; Bae, H.; Gravallese, E.M.; Glimcher, M.J.; Glimcher, L.H. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J. Exp. Med. 2000, 191, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Gohda, J.; Akiyama, T.; Koga, T.; Takayanagi, H.; Tanaka, S.; Inoue, J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005, 24, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Ninomiya, K.; Fujita, N.; Suzuki, T.; Iwasaki, R.; Morita, K.; Hosogane, N.; Matsuo, K.; Toyama, Y.; Suda, T.; et al. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J. Bone Miner. Res. 2007, 22, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.J.; Zhou, X.Z.; Liou, Y.C.; Noel, J.P.; Lu, K.P. Critical Role of WW Domain Phosphorylation in Regulating Phosphoserine Binding Activity and Pin1 Function. J. Biol. Chem. 2002, 277, 2381–2384. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Mathonet, P.; Jaulent, A.M.; Ullman, C.G. Selection of a high-affinity WW domain against the extracellular region of VEGF receptor isoform-2 from a combinatorial library using CIS display. Protein Eng. Des. Sel. 2013, 26, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Barman, A.; Hamelberg, D. Coupled Dynamics and Entropic Contribution to the Allosteric Mechanism of Pin1. J. Phys. Chem. B 2016, 120, 8405–8415. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.M.; Lee, S.H.; Fisher, D.E.; Kook, H.; Kim, K.K.; Choi, Y.; Kim, N. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem. 2005, 280, 35209–35216. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Saminathan, H.; Kanthasamy, A.; Anantharam, V.; Jin, H.; Sondarva, G.; Harischandra, D.S.; Qian, Z.; Rana, A.; Kanthasamy, A.G. The peptidyl-prolyl isomerase Pin1 up-regulation and proapoptotic function in dopaminergic neurons: Relevance to the pathogenesis of Parkinson disease. J. Biol. Chem. 2013, 288, 21955–21971. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M. Bisphosphonates for the treatment of osteoporosis: Insights for clinicians. Ther. Adv. Chronic Dis. 2010, 1, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Milat, F.; Ebeling, P.R. Osteoporosis treatment: A missed opportunity. Med. J. Aust. 2016, 205, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Pfeifer, G.P. Genotoxicity of acrylamide and glycidamide. J. Natl. Cancer Inst. 2004, 96, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Koyama, N.; Yasui, M.; Oda, Y.; Suzuki, S.; Satoh, T.; Suzuki, T.; Matsuda, T.; Masuda, S.; Kinae, N.; Honma, M. Genotoxicity of acrylamide in vitro: Acrylamide is not metabolically activated in standard in vitro systems. Environ. Mol. Mutagen. 2011, 52, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, Y.H.; Kim, Y.J.; Choi, H.S.; Yeo, C.Y.; Lee, K.Y. Prolyl isomerase Pin1 enhances osteoblast differentiation through Runx2 regulation. FEBS Lett. 2013, 587, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, S.; Kobayashi, Y.; Yamashita, T.; Miyamoto, T.; Cui, M.; Asada, R.; Cui, X.; Hino, K.; Kaneko, M.; Takai, T.; et al. Luman is involved in osteoclastogenesis through the regulation of DC-STAMP expression, stability and localization. J. Cell Sci. 2015, 128, 4353–4365. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.J.; Park, J.S.; Kang, S.K.; Kwon, I.K.; Kim, E.C. Effects of Melatonin and Its Underlying Mechanism on Ethanol-Stimulated Senescence and Osteoclastic Differentiation in Human Periodontal Ligament Cells and Cementoblasts. Int. J. Mol. Sci. 2018, 19, 1742. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.G.; Zhang, Y.; Mercedes-Camacho, A.Y.; Etzkorn, F.A. A reduced-amide inhibitor of Pin1 binds in a conformation resembling a twisted-amide transition state. Biochemistry 2011, 50, 9545–9550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.J.; Chen, X.; Bowman, M.E.; Luo, Y.; Noel, J.P.; Ellington, A.D.; Etzkorn, F.A.; Zhang, Y. Structural and kinetic analysis of prolyl-isomerization/phosphorylation cross-talk in the CTD code. ACS Chem. Biol. 2012, 7, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Elokely, K.M.; Doerksen, R.J. Docking challenge: Protein sampling and molecular docking performance. J. Chem. Inf. Model. 2013, 53, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sense Primer Antisense Primer | Tm (°C) | Accession Number |
|---|---|---|---|
| mPin1 | 5′-TTAATGGAAGGTGCGTAGGGT-3′ | 60 | NM_023371 |
| 5′-TTAATGGAAGGTGCGTAGGGT-3′ | |||
| mc-Fos | 5′-CGAAGGGAACGGAATAAGATG-3′ | 55 | NM_010234 |
| 5′-GCTGCCAAAATAAACTCCAG-3′ | |||
| mNFATc1 | 5′-ACCACCTTTCCGCAACCA-3′ | 56 | NM_016791 |
| 5′-GGTACTGGCTTCTCTTCCGT-3′ | |||
| mOC-STAMP | 5′-CAGAGTGACCACCTGAACAA-3′ | 56 | NM_029021 |
| 5′-TGCCTGAGGTCCCTGTGACT-3′ | |||
| mDC-STAMP | 5′-GGGAGTCCTGCACCATATGG-3′ | 56 | NM_029442 |
| 5′-AGGCCAGTGCTGACTAGGAT-3′ | |||
| mOSCAR | 5′-TCTGCCCCCTATGTGCTATC-3′ | 58 | NM_175632 |
| 5′-CAGCCCCAAACGGATGAG-3′ | |||
| mGAPDH | 5′-TGTGTCCGTCGTGGATCTGA-3′ | 56 | NM_001289726 |
| 5′-GATGCCTGCTTCACCACCTT-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Lee, J.-K.; Lee, J.-Y.; Chen, Z.; Ahn, S.-H.; Kim, N.D.; Kook, M.-S.; Min, S.H.; Park, B.-J.; Lee, T.-H. BCPA {N,N′-1,4-Butanediylbis[3-(2-chlorophenyl)acrylamide]} Inhibits Osteoclast Differentiation through Increased Retention of Peptidyl-Prolyl cis-trans Isomerase Never in Mitosis A-Interacting 1. Int. J. Mol. Sci. 2018, 19, 3436. https://doi.org/10.3390/ijms19113436
Cho E, Lee J-K, Lee J-Y, Chen Z, Ahn S-H, Kim ND, Kook M-S, Min SH, Park B-J, Lee T-H. BCPA {N,N′-1,4-Butanediylbis[3-(2-chlorophenyl)acrylamide]} Inhibits Osteoclast Differentiation through Increased Retention of Peptidyl-Prolyl cis-trans Isomerase Never in Mitosis A-Interacting 1. International Journal of Molecular Sciences. 2018; 19(11):3436. https://doi.org/10.3390/ijms19113436
Chicago/Turabian StyleCho, Eugene, Jin-Kyung Lee, Jee-Young Lee, Zhihao Chen, Sun-Hee Ahn, Nam Doo Kim, Min-Suk Kook, Sang Hyun Min, Byung-Ju Park, and Tae-Hoon Lee. 2018. "BCPA {N,N′-1,4-Butanediylbis[3-(2-chlorophenyl)acrylamide]} Inhibits Osteoclast Differentiation through Increased Retention of Peptidyl-Prolyl cis-trans Isomerase Never in Mitosis A-Interacting 1" International Journal of Molecular Sciences 19, no. 11: 3436. https://doi.org/10.3390/ijms19113436
APA StyleCho, E., Lee, J.-K., Lee, J.-Y., Chen, Z., Ahn, S.-H., Kim, N. D., Kook, M.-S., Min, S. H., Park, B.-J., & Lee, T.-H. (2018). BCPA {N,N′-1,4-Butanediylbis[3-(2-chlorophenyl)acrylamide]} Inhibits Osteoclast Differentiation through Increased Retention of Peptidyl-Prolyl cis-trans Isomerase Never in Mitosis A-Interacting 1. International Journal of Molecular Sciences, 19(11), 3436. https://doi.org/10.3390/ijms19113436





