Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo
Abstract
1. Introduction
2. Results
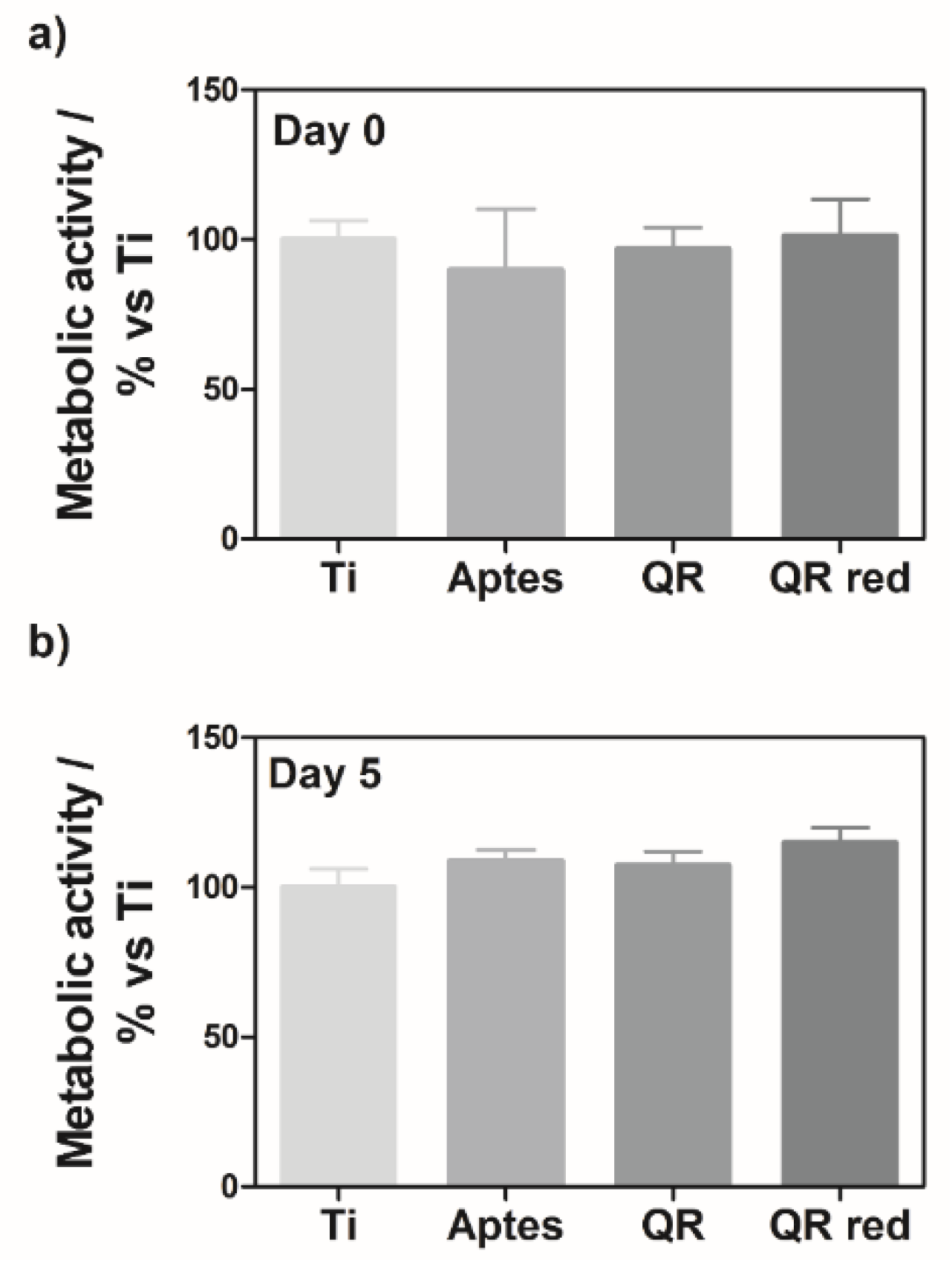
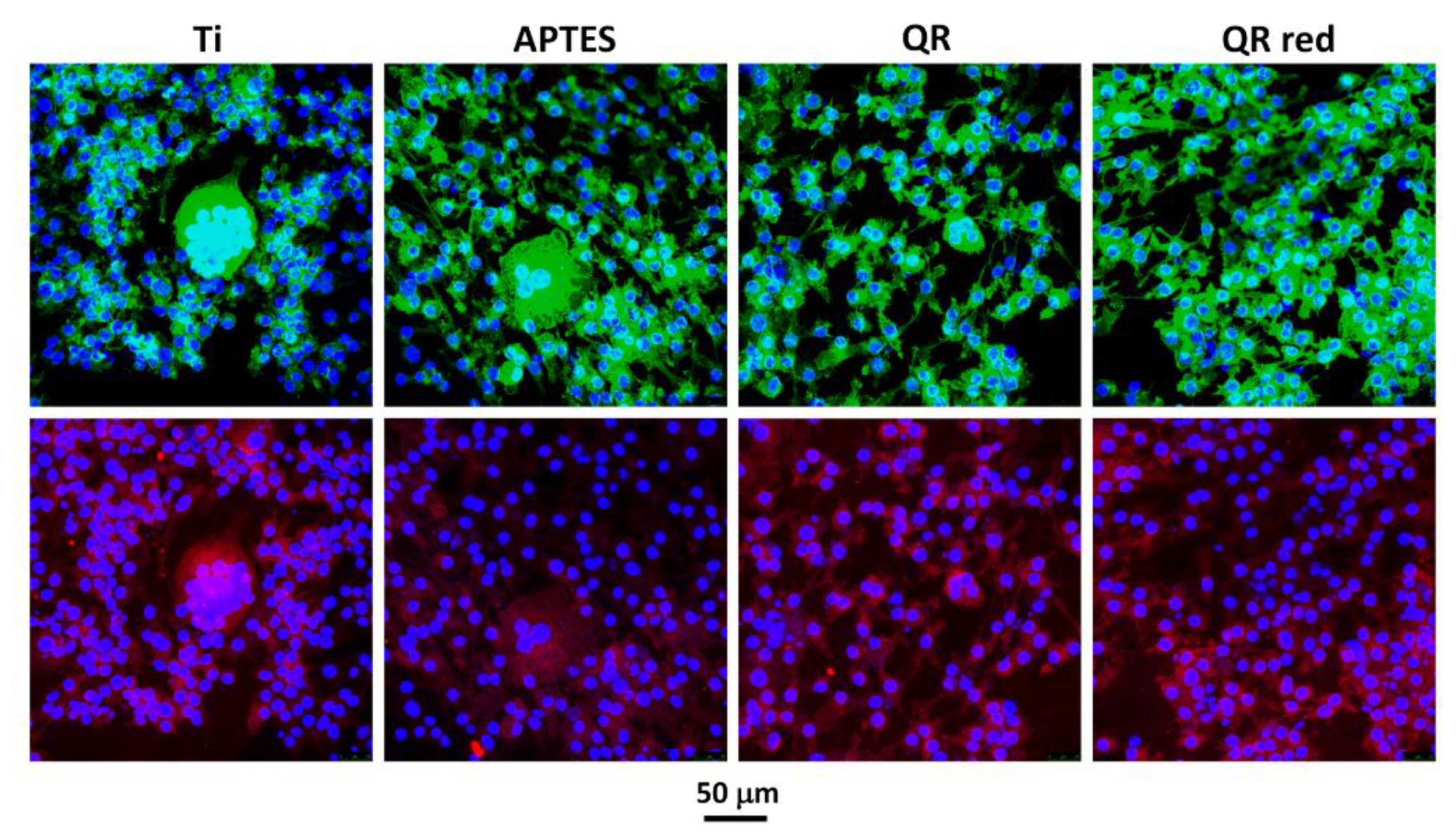
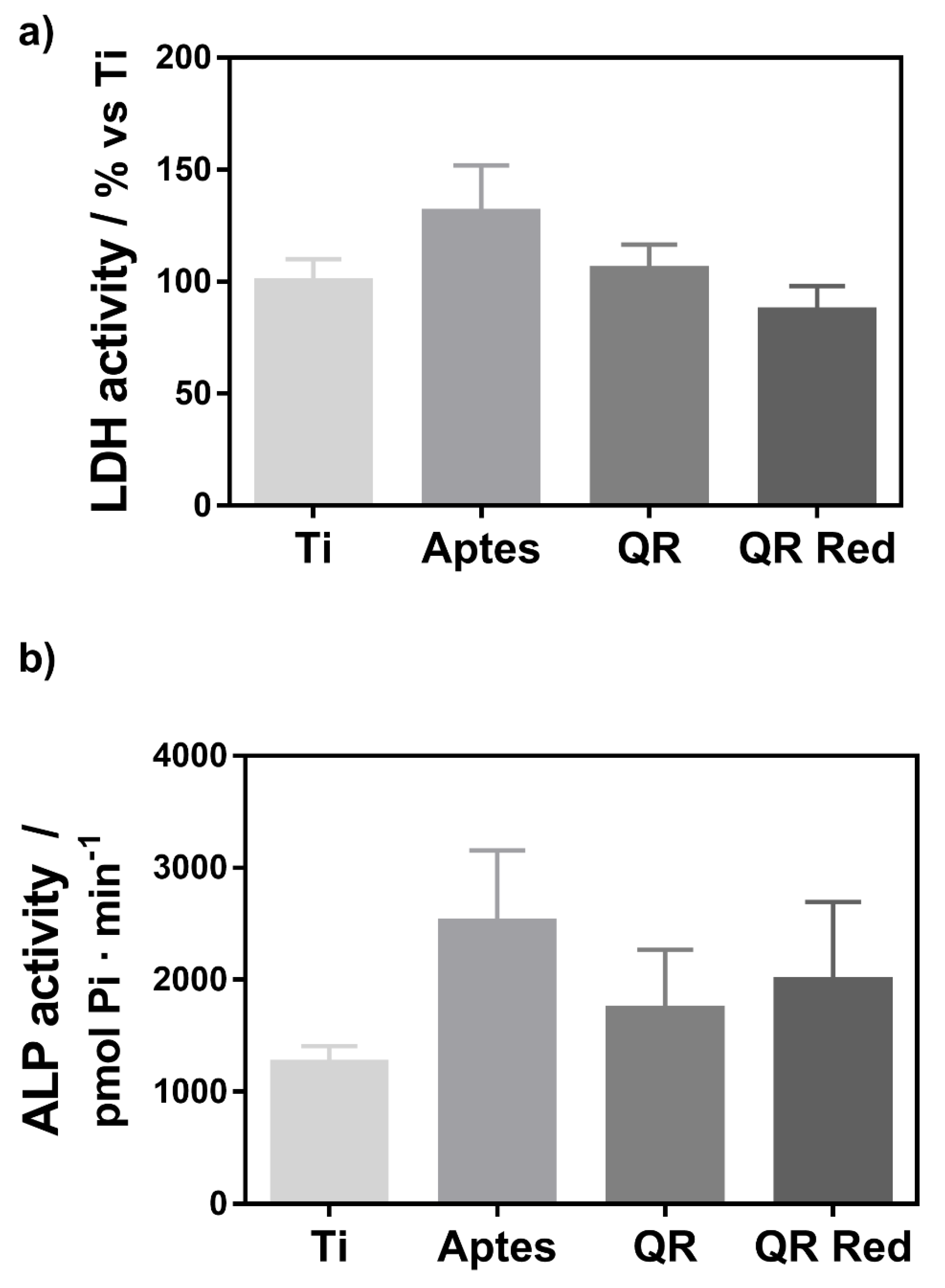
2.1. Effect of the Quercitrin Surfaces on Osteoclastogenesis In Vitro
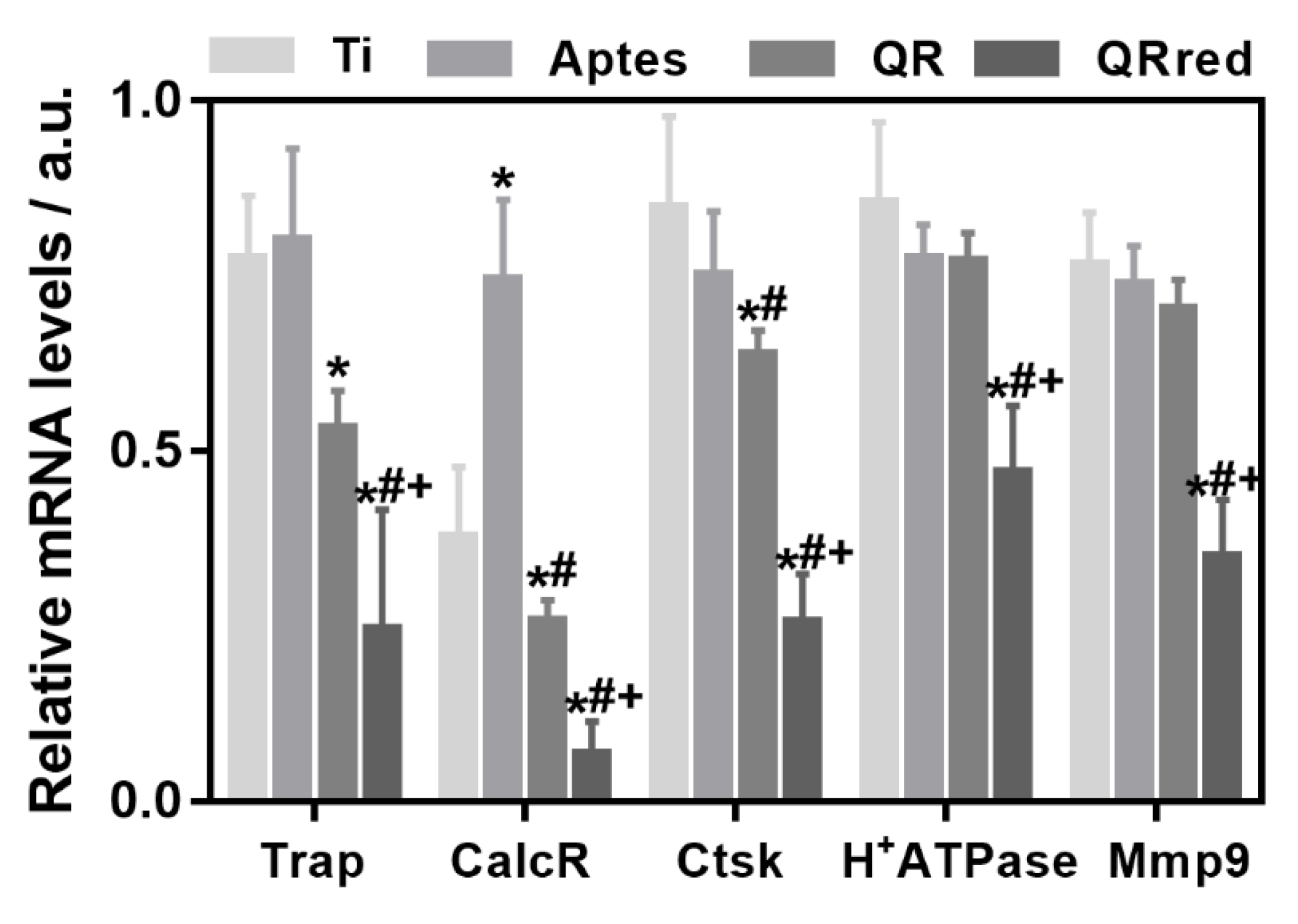
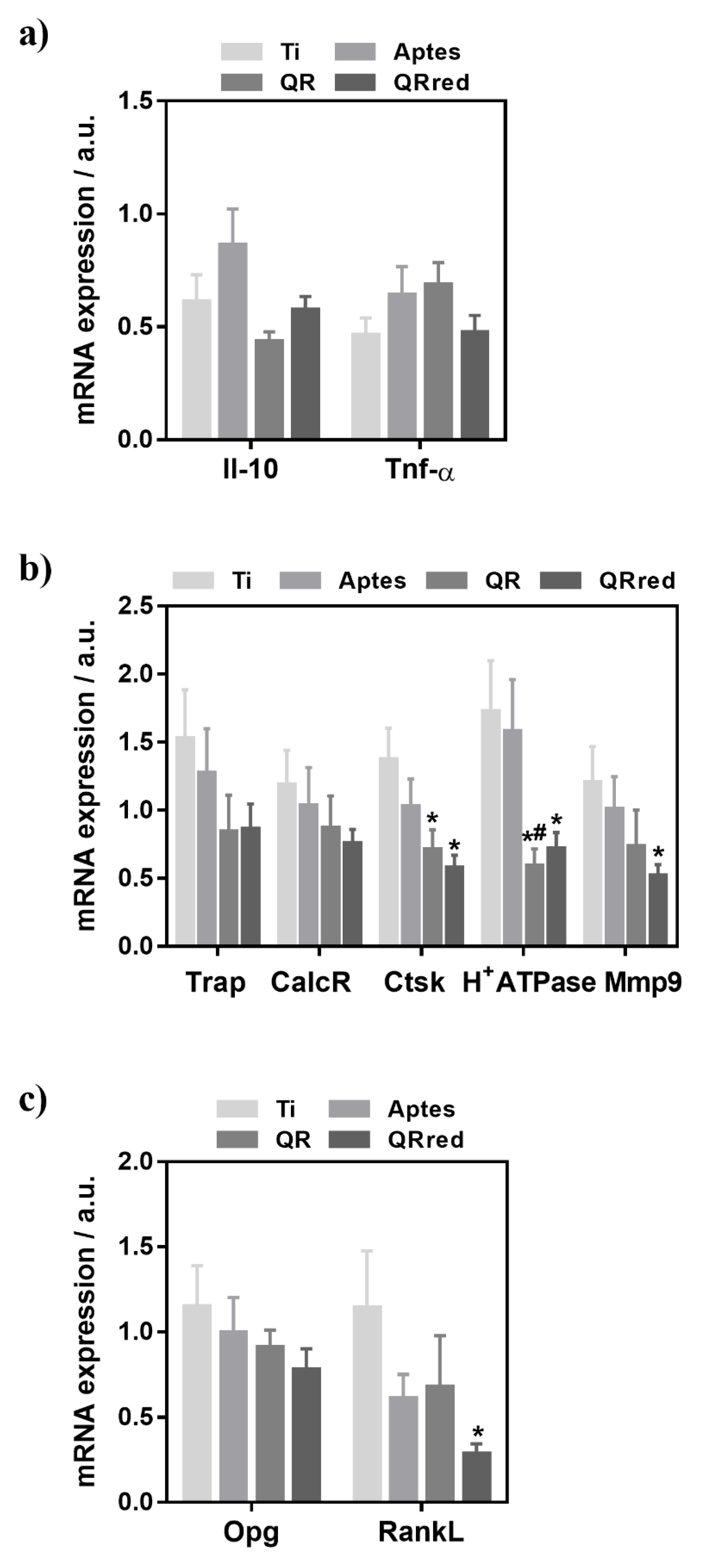
2.2. Effect of the Quercitrin Surfaces on Osseointegration and Osteoclastogenesis In Vivo
3. Discussion
4. Materials and Methods
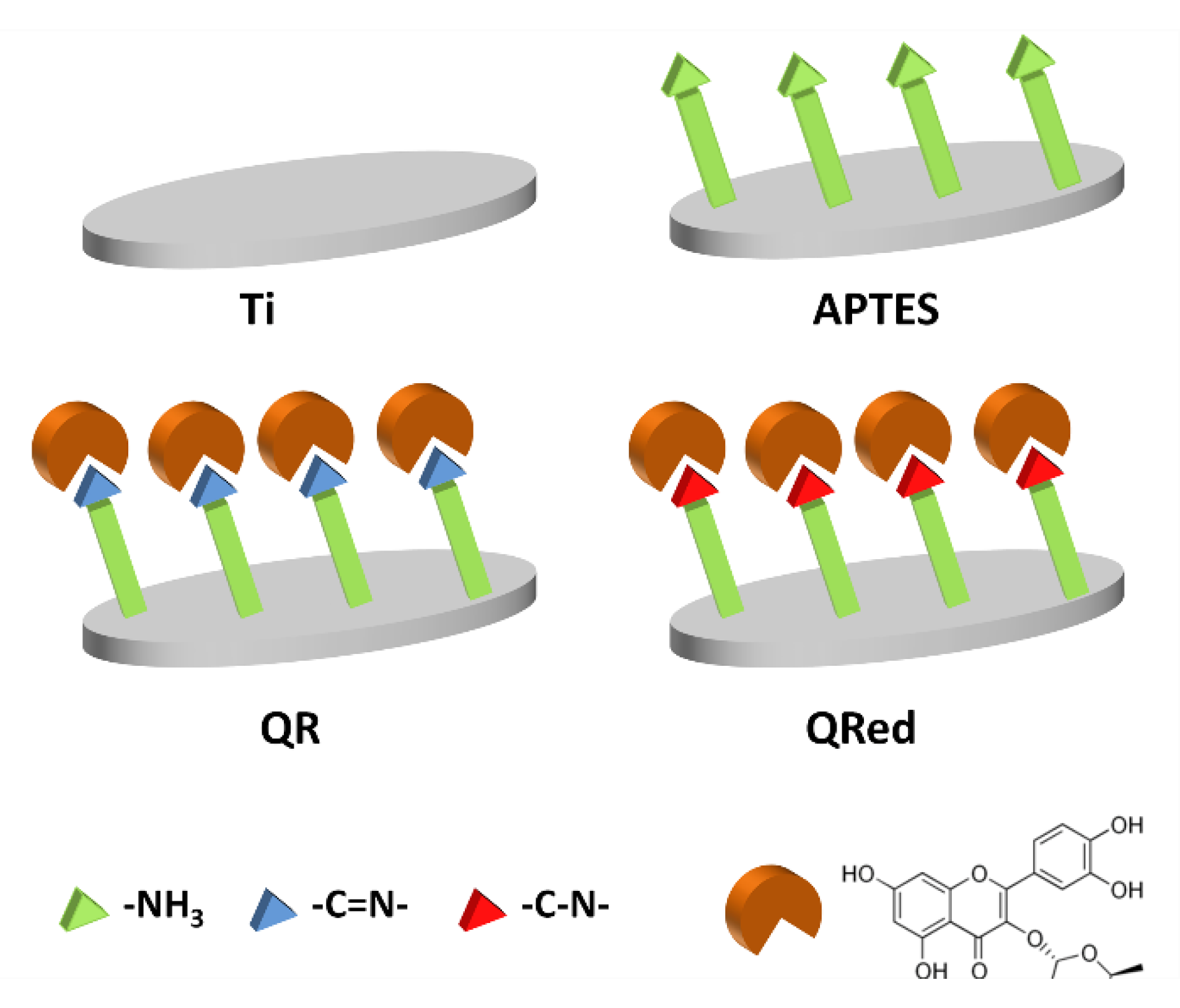
4.1. Modification of the Implant Surfaces with Quercitrin
4.2. Cell Culture and Generation of Osteoclast-Like Cells In Vitro
4.3. Cell Viability
4.4. Cell Staining
4.5. Animal Study
4.6. RNA Isolation and Gene Expression Analysis by Real Time RT-PCR
4.7. Statistics
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moraschini, V.; Poubel, L.A.; Ferreira, D.C.; Barboza, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implants Res. 2008, 19, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implants Res. 2012, 23 (Suppl. 6), 67–76. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20 (Suppl. 4), 185–206. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces. Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interface 2010, 7 (Suppl. 5), 515S–S527. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, J.; Tengvall, P.; Aspenberg, P. A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone 2012, 50, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Madrid, C.; Sanz, M. What impact do systemically administrated bisphosphonates have on oral implant therapy? A systematic review. Clin. Oral Implants Res. 2009, 20, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Amić, D.; Davidović-Amić, D.; Beslo, D.; Rastija, V.; Lucić, B.; Trinajstić, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2014, 19, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Welch, A.A.; Hardcastle, A.C. The effects of flavonoids on bone. Curr. Osteoporos. Rep. 2014, 12, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Fabjan, N.; Rode, J.; Kosir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Satué, M.; Arriero, M.D.M.; Monjo, M.; Ramis, J.M. Quercitrin and Taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem. Pharmacol. 2013, 86, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Identification of Quercitrin as a Potential Therapeutic Agent for Periodontal Applications. J. Periodontol. 2014, 85, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Hierro-Oliva, M.; Petzold, C.; Lyngstadaas, S.P.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Flavonoid-Modified Surfaces: Multifunctional Bioactive Biomaterials with Osteopromotive, Anti-Inflammatory, and Anti-Fibrotic Potential. Adv. Healthc. Mater. 2015, 4, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, A.; Monjo, M.; Hierro-Oliva, M.; González-Martín, M.L.; Ramis, J.M. Bioinspired Quercitrin Nanocoatings: A Fluorescence-Based Method for Their Surface Quantification, and Their Effect on Stem Cell Adhesion and Differentiation to the Osteoblastic Lineage. ACS Appl. Mater. Interfaces 2015, 7, 16857–16864. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pacha-Olivenza, M.A.; Fernández-Calderón, M.C.; Córdoba, A.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Quercitrin-nanocoated titanium surfaces favour gingival cells against oral bacteria. Sci. Rep. 2016, 6, 22444. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.-J.; Kim, S.-Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zhai, Z.; Jiang, C.; Liu, X.; Li, H.; Qu, X.; Ouyang, Z.; Fan, Q.; Tang, T.; Qin, A.; et al. Geraniin suppresses RANKL-induced osteoclastogenesis in vitro and ameliorates wear particle-induced osteolysis in mouse model. Exp. Cell Res. 2015, 330, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.-G.; Chen, K.-M.; Xian, C.J. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J. Cell. Physiol. 2013, 228, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-P.; Sheu, S.-Y.; Sun, J.-S.; Chen, M.-H. Icariin inhibits osteoclast differentiation and bone resorption by suppression of MAPKs/NF-κB regulated HIF-1α and PGE2 synthesis. Phytomedicine 2011, 18, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, L.; Watts, D.C.; Qiu, H.; You, T.; Deng, F.; Wu, X. Controlled-release naringin nanoscaffold for osteoporotic bone healing. Dent. Mater. 2014, 30, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Monjo, M.; Ramis, J.M.; Rønold, H.J.; Taxt-Lamolle, S.F.; Ellingsen, J.E.; Lyngstadaas, S.P. Correlation between molecular signals and bone bonding to titanium implants. Clin. Oral Implants Res. 2013, 24, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Monjo, M.; Lamolle, S.F.; Lyngstadaas, S.P.; Rønold, H.J.; Ellingsen, J.E. In vivo expression of osteogenic markers and bone mineral density at the surface of fluoride-modified titanium implants. Biomaterials 2008, 29, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Oates, T.W.; Valderrama, P.; Bischof, M.; Nedir, R.; Jones, A.; Simpson, J.; Toutenburg, H.; Cochran, D.L. Enhanced implant stability with a chemically modified SLA surface: A randomized pilot study. Int. J. Oral Maxillofac. Implants 2007, 22, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Ganeles, J.; Zöllner, A.; Jackowski, J.; ten Bruggenkate, C.; Beagle, J.; Guerra, F. Immediate and early loading of Straumann implants with a chemically modified surface (SLActive) in the posterior mandible and maxilla: 1-year results from a prospective multicenter study. Clin. Oral Implants Res. 2008, 19, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.M.; Moon, H.J.; Kwon, Y.D.; Yoo, J.Y.; Pae, A.; Kwon, I.K. Osteoblastic and osteoclastic differentiation on SLA and hydrophilic modified SLA titanium surfaces. Clin. Oral Implants Res. 2014, 25, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Lotz, E.M.; Berger, M.B.; Schwartz, Z.; Boyan, B.D. Regulation of osteoclasts by osteoblast lineage cells depends on titanium implant surface properties. Acta Biomater. 2018, 68, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ge, X.-Y.; Hao, K.-Y.; Zhang, B.-R.; Jiang, X.; Lin, Y.; Zhang, Y. Simple 3,4-Dihydroxy-l-Phenylalanine Surface Modification Enhances Titanium Implant Osseointegration in Ovariectomized Rats. Sci. Rep. 2017, 7, 17849. [Google Scholar] [CrossRef] [PubMed]
- Lamolle, S.F.; Monjo, M.; Lyngstadaas, S.P.; Ellingsen, J.E.; Haugen, H.J. Titanium implant surface modification by cathodic reduction in hydrofluoric acid: Surface characterization and in vivo performance. J. Biomed. Mater. Res. Part A 2009, 88A, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Advances in bone biology: The osteoclast. Endocr. Rev. 1996, 17, 308–332. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Drake, F.H.; Dodds, R.A.; James, I.E.; Connor, J.R.; Debouck, C.; Richardson, S.; Lee-Rykaczewski, E.; Coleman, L.; Rieman, D.; Barthlow, R.; et al. But not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J. Biol. Chem. 1996, 271, 12511–12516. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, H.K.; Karhukorpi, E.K.; Sundquist, K.; Wallmark, B.; Roininen, I.; Hentunen, T.; Tuukkanen, J.; Lakkakorpi, P. Evidence for the presence of a proton pump of the vacuolar H+-ATPase type in the ruffled borders of osteoclasts. J. Cell Biol. 1990, 111, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Engsig, M.T.; Chen, Q.J.; Vu, T.H.; Pedersen, A.C.; Therkidsen, B.; Lund, L.R.; Henriksen, K.; Lenhard, T.; Foged, N.T.; Werb, Z.; et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J. Cell Biol. 2000, 151, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence | Product Size (bp) | GeneBank Accession Nr. |
|---|---|---|---|
| Gapdh | S 5′-ACC CAG AAG ACT GTG GAT GG-3′ A 5′-CAC ATT GGG GGT AGG AAC AC-3′ | 171 | XM_132897 |
| 18s rRNA | S 5′-GTA ACC CGT TGA ACC CCA TT-3′ A 5′-CCA TCC AAT CGG TAG TAG CG-3′ | 151 | X00686 |
| Trap | S 5′-GCG ACC ATT GTT AGC CAC ATA CG-3′ A 5′-CGT TGA TGT CGC ACA GAG GGA T-3′ | 144 | NM_007388.2 |
| CalcR | S 5′-TGG TGC GGC GGG ATC CTA TAA GT-3′ A 5′-AGC GTA GGC GTT GCT CGT CG-3′ | 150 | NM_001042725 |
| Ctsk | S 5′-AGC AGA ACG GAG GCA TTG ACT C-3′ A 5′-TTT AGC TGC CTT TGC CGT GGC-3′ | 92 | NM_007802.3 |
| H+ATPase | S 5′-ACG GTG ATG TCA CAG CAG ACG T-3′ A 5′-CCT CTG GAT AGA GCC TGC CGC A-3′ | 153 | NM_175406.3 |
| Mmp9 | S 5′-GCT GAC TAC GAT AAG GAC GGC A-3′ A 5′-GCG GCC CTC AAA GAT GAA CGG-3′ | 114 | NM_013599.2 |
| Gene | Primer Sequence | Primer Source | Product Size (bp) | GeneBank Accession Nr. |
|---|---|---|---|---|
| GAPDH | S 5′- TGC ACC ACC AAC TGC TTA GC-3′ A 5′- GGC ATG GAC TGT GGT CAT GAG-3′ | Human | 87 | M33197 |
| 18s rRNA | S 5’-GTA ACC CGT TGA ACC CCA TT-3’ A 5’-CCA TCC AAT CGG TAG TAG CG-3’ | Mouse | 151 | X00686 |
| IL-10 | S 5′- CCT TTG GCA GGG TGA AGA CT-3′ A 5′- ATG GCT GGA CTC TGG TTC TC-3 | Rabbit | 175 | NM_001082045 |
| Tnf-α | S 5′- TCC GTG AAA ACA GAG CAG AA-3′ A 5′- GAG CAG AGG TTC GGT GAT GT-3′ | Rabbit | 160 | NM_001082263 |
| Opg | S 5′-TCA TCC AAG ATA TTG ACC TCT GTG A-′3 A 5′-GGGGAGCTGCTCACTTGATT-3′ | Rabbit | 170 | XM_002710603.2 |
| RANKL | S 5’- CAG AGC GCA GAT GGA TCC TAA-3′ A 5′- TCC TTT TGC ACA GCT CCT TGA-3′ | Human | 180 | NM_003701.3 |
| Trap | S 5′- CCT GGG CGA CAA CTT TTA CT-3′ A 5′- TTG GAG ACC TTG GAA TAG GC-3′ | Rabbit | 180 | NM_001081988 |
| Calc-R | S 5′- CAA ATG ACA CCC ATC CAA CA-3′ A 5′- ACA TCC ATC CAT CCC AGG TC-3′ | Rabbit | 162 | NM_001082375.2 |
| Ctsk | S 5′-GAC ACC CAG TGG GAG CTA TG-3′ A 5′-TCT TCA CTG GTC ATG TCC CC-3′ | Rabbit | 188 | NM_001082641.1 |
| H+ATPase | S 5′ -CCG AAA CCT CCT GAA GAA AA-3′ A 5′- ATA GCC GTG GTG CTG AAG TC-3′ | Rabbit | 165 | NM_000600.3 |
| Mmp9 | S 5′-CAA GGA TGG GAG GTA CTG GC-3′ A 5′-TGT GTA CAC CCA CAC TTG GC-3′ | Rabbit | 172 | NM_001082203.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdoba, A.; Manzanaro-Moreno, N.; Colom, C.; Rønold, H.J.; Lyngstadaas, S.P.; Monjo, M.; Ramis, J.M. Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2018, 19, 3319. https://doi.org/10.3390/ijms19113319
Córdoba A, Manzanaro-Moreno N, Colom C, Rønold HJ, Lyngstadaas SP, Monjo M, Ramis JM. Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo. International Journal of Molecular Sciences. 2018; 19(11):3319. https://doi.org/10.3390/ijms19113319
Chicago/Turabian StyleCórdoba, Alba, Nahuel Manzanaro-Moreno, Carme Colom, Hans J. Rønold, Staale P. Lyngstadaas, Marta Monjo, and Joana M. Ramis. 2018. "Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo" International Journal of Molecular Sciences 19, no. 11: 3319. https://doi.org/10.3390/ijms19113319
APA StyleCórdoba, A., Manzanaro-Moreno, N., Colom, C., Rønold, H. J., Lyngstadaas, S. P., Monjo, M., & Ramis, J. M. (2018). Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo. International Journal of Molecular Sciences, 19(11), 3319. https://doi.org/10.3390/ijms19113319






