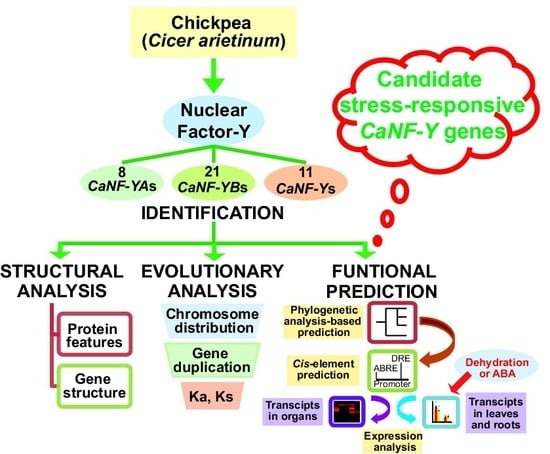
Identification, Structural Characterization and Gene Expression Analysis of Members of the Nuclear Factor-Y Family in Chickpea (Cicer arietinum L.) under Dehydration and Abscisic Acid Treatments
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification and Annotation of the CaNF-Y Genes in Chickpea
2.2. Chromosomal Localization and Prediction of the Duplication Events of CaNF-Y Genes
2.3. Phylogenetic Analysis-Based Prediction of the CaNF-Y Genes with Drought-Related Functions
2.4. Prediction of the Cis-Acting Motifs in the Promoter Region of CaNF-Y Genes
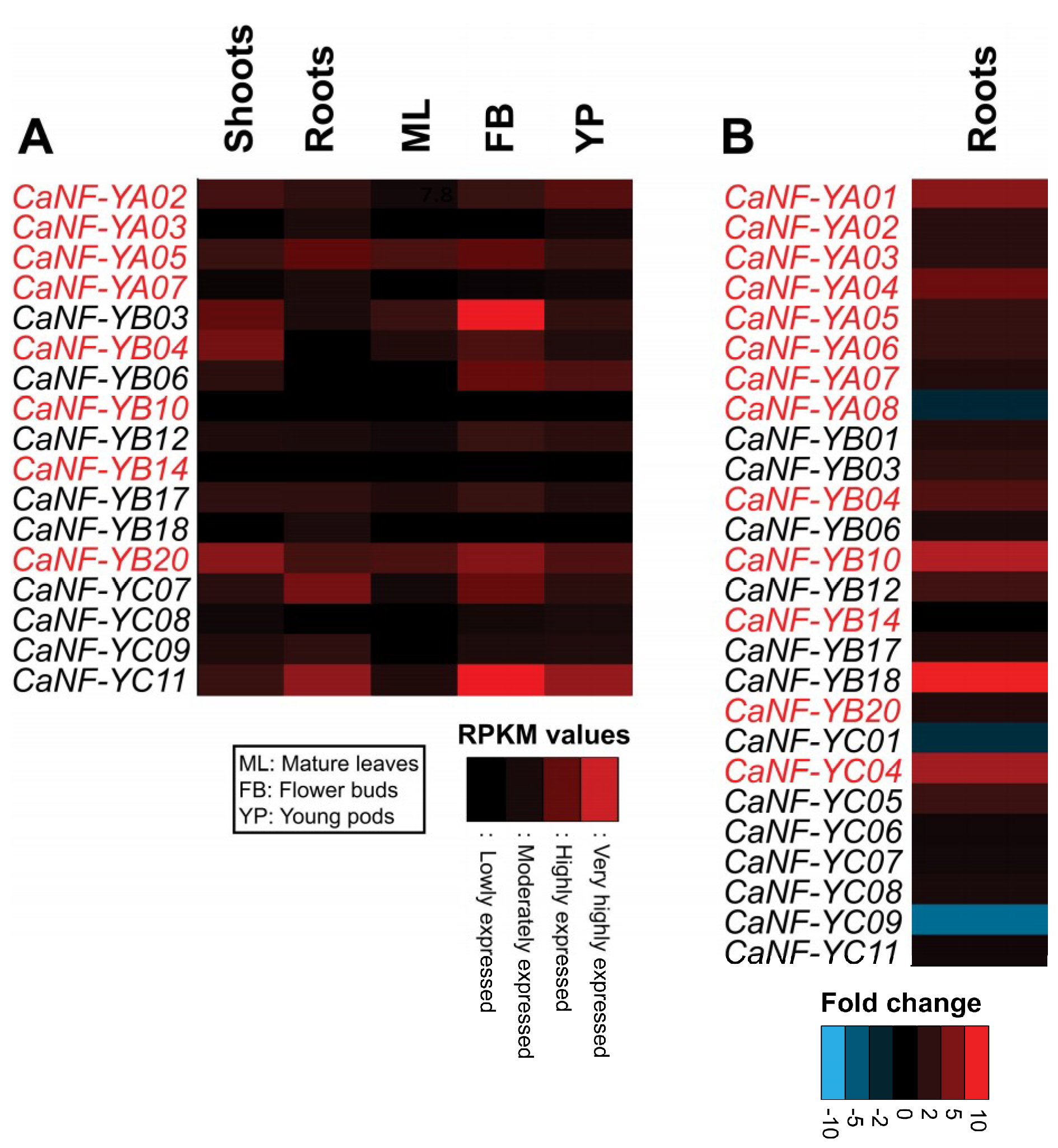
2.5. Transcript Patterns of the CaNF-Y Genes in Major Organs of Chickpea Plants during Growth and Development
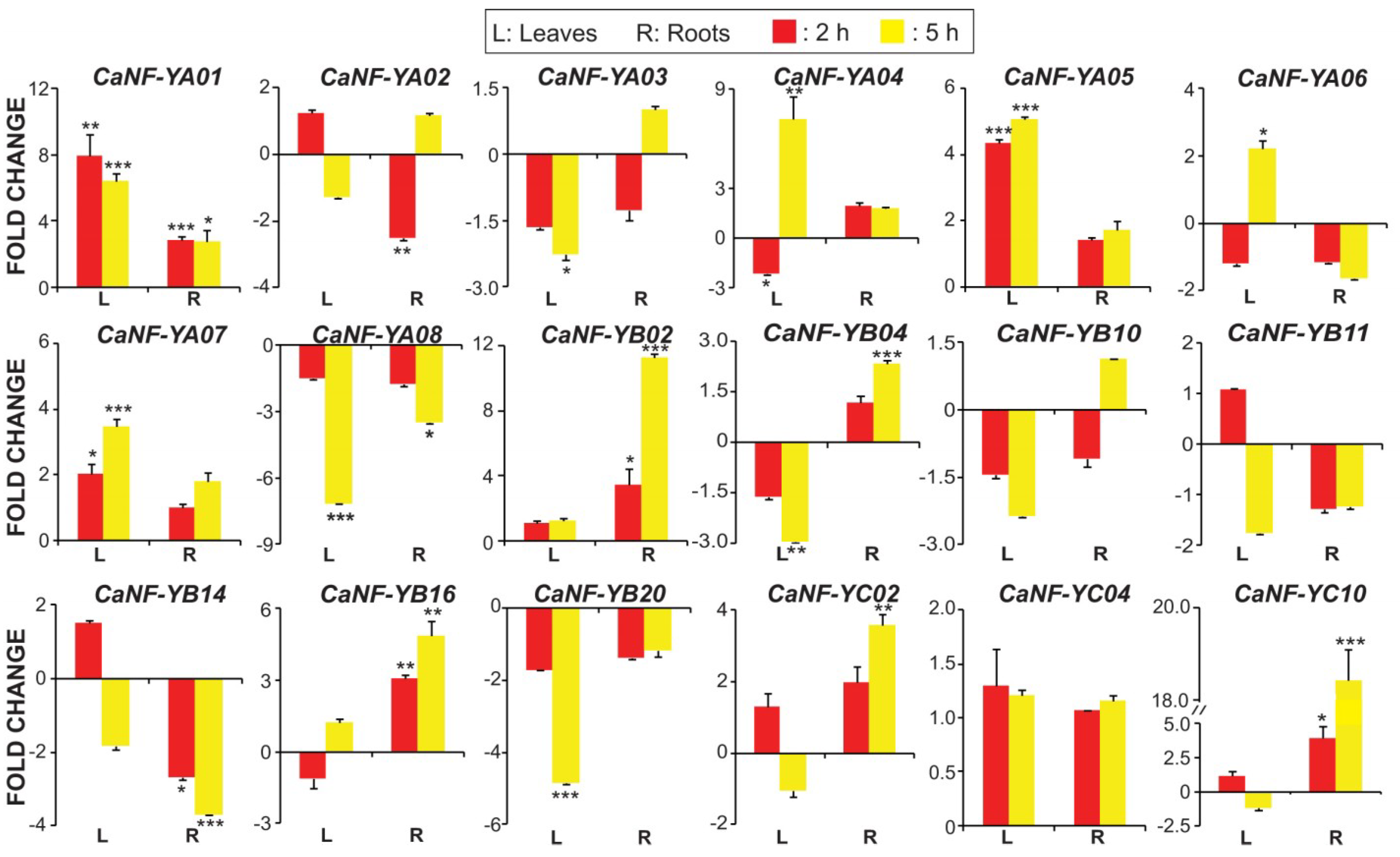
2.6. Quantification of Transcript Levels of the Predicted Stress-Related CaNF-Y Genes in Roots and Leaves of Chickpea Plants Exposed to Dehydration or ABA Treatment
3. Materials and Methods
3.1. Identification and Annotation of the CaNF-Y Genes
3.2. Gene Duplication Analysis of the CaNF-Ys
3.3. Protein Features and Gene Organization of the CaNF-Ys
3.4. Conserved Domains and Phylogenetic Analysis-Based Prediction of the CaNF-Y Proteins
3.5. Prediction of the Stress- and Hormone-Related Cis-Regulatory Elements
3.6. Transcript Patterns of the CaNF-Y Genes in Different Organs
3.7. Growth, and Dehydration and ABA Treatments of Chickpea Plants
3.8. Expression Analysis of CaNF-Y Genes by qPCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ohri, D.; Pal, M. The origin of chickpea (Cicer arietinum L.): Karyotype and nuclear DNA amount. Heredity 1991, 66, 367–372. [Google Scholar] [CrossRef]
- Rachwa-Rosiak, D.; Nebesny, E.; Budryn, G. Chickpeas—Composition, nutritional value, health benefits, application to bread and snacks: A review. Crit. Rev. Food Sci. Nutr. 2013, 55, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, S.; Sarmah, B.K. Biotechnologically generating ‘super chickpea’ for food and nutritional security. Plant Sci. 2013, 207, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Bird, L.G.; Pilkington, C.L.; Saputra, A.; Serventi, L. Products of chickpea processing as texture improvers in gluten-free bread. Food Sci. Technol. Int. 2017, 23, 690–698. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; El-Senousi, N.A.; Ali, Z.A.; Omran, A.A. The impact of using chickpea flour and dried carp fish powder on pizza quality. PLoS ONE 2017, 12, e0183657. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.N.; Sulieman, S.; Schulze, J.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Approaches for enhancement of N2 fixation efficiency of chickpea (Cicer arietinum L.) under limiting nitrogen conditions. Plant Biotechnol. J. 2014, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, M. Effect of drought stress on yield and water relative content in chickpea. Int. J. Agron. Plant Prod. 2013, 4, 1168–1172. [Google Scholar]
- Ha, C.V.; Esfahani, M.N.; Watanabe, Y.; Tran, U.T.; Sulieman, S.; Mochida, K.; Nguyen, D.V.; Tran, L.S. Genome-wide identification and expression analysis of the CaNAC family members in chickpea during development, dehydration and ABA treatments. PLoS ONE 2014, 9, e114107. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Shankar, R.; Thakkar, B.; Kudapa, H.; Krishnamurthy, L.; Mantri, N.; Varshney, R.K.; Bhatia, S.; Jain, M. Transcriptome analyses reveal genotype- and developmental stage-specific molecular responses to drought and salinity stresses in chickpea. Sci. Rep. 2016, 6, 19228. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi Mashaki, K.; Garg, V.; Nasrollahnezhad Ghomi, A.A.; Kudapa, H.; Chitikineni, A.; Zaynali Nezhad, K.; Yamchi, A.; Soltanloo, H.; Varshney, R.K.; Thudi, M. RNA-Seq analysis revealed genes associated with drought stress response in kabuli chickpea (Cicer arietinum L.). PLoS ONE 2018, 13, e0199774. [Google Scholar] [CrossRef] [PubMed]
- Myers, Z.A.; Holt, B.F., 3rd. NUCLEAR FACTOR-Y: Still complex after all these years? Curr. Opin. Plant Biol. 2018, 45, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.E.; Ripodas, C.; Niebel, A. Plant NF-Y transcription factors: Key players in plant-microbe interactions, root development and adaptation to stress. Biochim. Biophys. Acta 2017, 1860, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.F.A.; Sajad, M.; Nazaruddin, N.; Fauzi, I.A.; Murad, A.M.A.; Zainal, Z.; Ismail, I. Microrna and transcription factor: Key players in plant regulatory network. Front. Plant Sci. 2017, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Petroni, K.; Kumimoto, R.W.; Gnesutta, N.; Calvenzani, V.; Fornari, M.; Tonelli, C.; Holt, B.F., 3rd; Mantovani, R. The promiscuous life of plant NUCLEAR FACTOR Y transcription factors. Plant Cell 2012, 24, 4777–4792. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.J.; McIntyre, C.L.; Collet, C.; Xue, G.P. Genome-wide identification and expression analysis of the NF-Y family of transcription factors in Triticum aestivum. Plant Mol. Biol. 2007, 65, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, T.; Ito, Y.; Kubo, T.; Serizawa, A.; Kurata, N. Identification, characterization and interaction of HAP family genes in rice. Mol. Genet. Genom. 2008, 279, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lu, Z.; Xiong, Y.; Yao, J. Genome-wide identification and co-expression network analysis of the OsNF-Y gene family in rice. Crop J. 2017, 5, 21–31. [Google Scholar] [CrossRef]
- Cao, S.; Kumimoto, R.W.; Siriwardana, C.L.; Risinger, J.R.; Holt, B.F., 3rd. Identification and characterization of NF-Y transcription factor families in the monocot model plant Brachypodium distachyon. PLoS ONE 2011, 6, e21805. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Hole, D.; Wu, J.; Blake, T.; Wu, Y. Expression and functional analysis of NUCLEAR FACTOR-Y, subunit B genes in barley. Planta 2012, 235, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-J.; He, G.-H.; Zheng, W.-J.; Lu, P.-P.; Chen, M.; Gong, Y.; Ma, Y.-Z.; Xu, Z.-S. Foxtail millet NF-Y families: Genome-wide survey and evolution analyses identified two functional genes important in abiotic stresses. Front. Plant Sci. 2015, 6, 1142. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Liu, C.; Yu, T.; Liu, X.; Xu, D.; Wang, J.; Wang, G.; Cai, Y. Identification and characterization of paternal-preferentially expressed gene NF-YC8 in maize endosperm. Mol. Genet. Genom. 2015, 290, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Zhang, C.; Zou, H.; Wu, Z. Isolation, structural analysis, and expression characteristics of the maize nuclear factor Y gene families. Biochem. Biophys. Res. Commun. 2016, 478, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Malviya, N.; Jaiswal, P.; Yadav, D. Genome-wide characterization of Nuclear Factor Y (NF-Y) gene family of sorghum [Sorghum bicolor (L.) Moench]: A bioinformatics approach. Physiol. Mol. Biol. Plants 2016, 22, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Gusmaroli, G.; Tonelli, C.; Mantovani, R. Regulation of the CCAAT-binding NF-Y subunits in Arabidopsis thaliana. Gene 2001, 264, 173–185. [Google Scholar] [CrossRef]
- Siefers, N.; Dang, K.K.; Kumimoto, R.W.; Bynum, W.E.T.; Tayrose, G.; Holt, B.F., 3rd. Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 2009, 149, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Ripodas, C.; Castaingts, M.; Clua, J.; Blanco, F.; Zanetti, M.E. Annotation, phylogeny and expression analysis of the nuclear factor Y gene families in common bean (Phaseolus vulgaris). Front. Plant Sci. 2014, 5, 761. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yin, X.; Lin, Z.; Zheng, Q.; Liu, G.; Zhao, G. Identification and characterization of NF-Y transcription factor families in canola (Brassica napus L.). Planta 2014, 239, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lin, Z.; Tao, Q.; Liang, M.; Zhao, G.; Yin, X.; Fu, R. Multiple NUCLEAR FACTOR Y transcription factors respond to abiotic stress in Brassica napus L. PLoS ONE 2014, 9, e111354. [Google Scholar] [CrossRef] [PubMed]
- Quach, T.N.; Nguyen, H.T.; Valliyodan, B.; Joshi, T.; Xu, D.; Nguyen, H.T. Genome-wide expression analysis of soybean NF-Y genes reveals potential function in development and drought response. Mol. Genet. Genom. 2015, 290, 1095–1115. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Z.; Wang, Y.; Li, S.; Liang, Z. Genome-wide identification and characterization of the NF-Y gene family in grape (Vitis vinifera L.). BMC Genom. 2016, 17, 605. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, K.; Ju, Z.; Cao, D.; Fu, D.; Zhu, H.; Zhu, B.; Luo, Y. Genome-wide analysis of tomato NF-Y factors and their role in fruit ripening. BMC Genom. 2016, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Z.; Xiao, Y.; Wang, P.; Wang, Y.; Ge, X.; Zhang, C.; Zhang, X.; Li, F. Genome-wide analysis of the NF-YB gene family in Gossypium hirsutum L. and characterization of the role of GhDNF-YB22 in embryogenesis. Int. J. Mol. Sci. 2018, 19, 483. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L.S.; Martins, C.P.S.; Sousa, A.O.; Camillo, L.R.; Araujo, C.P.; Alcantara, G.M.; Camargo, D.S.; Cidade, L.C.; de Almeida, A.F.; Costa, M.G.C. Genome-wide characterization and expression analysis of citrus NUCLEAR FACTOR-Y (NF-Y) transcription factors identified a novel NF-YA gene involved in drought-stress response and tolerance. PLoS ONE 2018, 13, e0199187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.; Chen, Z.; Han, B.; Haque, M.E.; Liu, A. Gene structure, expression pattern and interaction of Nuclear Factor-Y family in castor bean (Ricinus communis). Planta 2018, 247, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Song, C.; Saxena, R.K.; Azam, S.; Yu, S.; Sharpe, A.G.; Cannon, S.; Baek, J.; Rosen, B.D.; Tar’an, B.; et al. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotechnol. 2013, 31, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Misra, G.; Patel, R.K.; Priya, P.; Jhanwar, S.; Khan, A.W.; Shah, N.; Singh, V.K.; Garg, R.; Jeena, G.; et al. A draft genome sequence of the pulse crop chickpea (Cicer arietinum L.). Plant J. 2013, 74, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, C.; Sun, H.; Rosli, H.G.; Pombo, M.A.; Zhang, P.; Banf, M.; Dai, X.; Martin, G.B.; Giovannoni, J.J.; et al. iTAK: A program for genome-wide prediction and classification of plant transcription factors, transcriptional regulators, and protein kinases. Mol. Plant 2016, 9, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, L.; Fedorov, A. Introns in gene evolution. Genetica 2003, 118, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Le Hir, H.; Nott, A.; Moore, M.J. How introns influence and enhance eukaryotic gene expression. Trends Biochem. Sci. 2003, 28, 215–220. [Google Scholar] [CrossRef]
- Roy, S.W.; Gilbert, W. The evolution of spliceosomal introns: Patterns, puzzles and progress. Nat. Rev. Genet. 2006, 7, 211–221. [Google Scholar] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Guruprasad, K.; Reddy, B.V.; Pandit, M.W. Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. 1990, 4, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Mantovani, R. The molecular biology of the CCAAT-binding factor NF-Y. Gene 1999, 239, 15–27. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Cannon, S.; Mitra, A.; Baumgarten, A.; Young, N.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; De la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Vang, S.; Yu, J.; Wong, G.K.-S.; Wang, J. Correlation between Ka/Ks and Ks is related to substitution model and evolutionary lineage. J. Mol. Evol. 2009, 68, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiu, L.-J. Genome-wide analysis of the Dof transcription factor gene family reveals soybean-specific duplicable and functional characteristics. PLoS ONE 2013, 8, e76809. [Google Scholar] [CrossRef] [PubMed]
- Gabaldón, T. Evolution of proteins and proteomes: A phylogenetics approach. Evol. Bioinform. 2005, 1, 51–61. [Google Scholar] [CrossRef]
- Sulieman, S.; Van Ha, C.; Nasr Esfahani, M.; Watanabe, Y.; Nishiyama, R.; Pham, C.T.; Van Nguyen, D.; Tran, L.S. DT2008: A promising new genetic resource for improved drought tolerance in soybean when solely dependent on symbiotic N2 fixation. Biomed. Res. Int. 2015, 2015, 687213. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mallano, A.I.; Bo, L.; Wang, T.; Nisa, Z.; Li, Y. Soybean transcription factor GmNF-YB1 confers abiotic stress tolerance to transgenic Arabidopsis plants. Can. J. Plant Sci. 2016, 97, 501–515. [Google Scholar]
- Esfahani, M.N.; Sulieman, S.; Schulze, J.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Mechanisms of physiological adjustment of N2 fixation in Cicer arietinum L. (chickpea) during early stages of water deficit: single or multi-factor controls. Plant J. 2014, 79, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Mochida, K.; Yoshida, T.; Sakurai, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. In silico analysis of transcription factor repertoires and prediction of stress-responsive transcription factors from six major gramineae plants. DNA Res. 2011, 18, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ruan, J.; Ho, T.H.; You, Y.; Yu, T.; Quatrano, R.S. Cis-regulatory element based targeted gene finding: Genome-wide identification of abscisic acid- and abiotic stress-responsive genes in Arabidopsis thaliana. Bioinformatics 2005, 21, 3074–3081. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell. Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, R.; Muthurajan, R.; Subramaniyam, S.; Jegadeesan, R. In silico analysis of drought tolerant genes in rice. Int. J. Biol. Med. Res. 2010, 1, 36–40. [Google Scholar]
- Maruyama, K.; Todaka, D.; Mizoi, J.; Yoshida, T.; Kidokoro, S.; Matsukura, S.; Takasaki, H.; Sakurai, T.; Yamamoto, Y.Y.; Yoshiwara, K.; et al. Identification of cis-acting promoter elements in cold- and dehydration-induced transcriptional pathways in Arabidopsis, rice, and soybean. DNA Res. 2012, 19, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.S.; Nakashima, K.; Sakuma, Y.; Simpson, S.D.; Fujita, Y.; Maruyama, K.; Fujita, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 2004, 16, 2481–2498. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Tran, L.S.; Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Mostofa, M.G.; Li, W.; Van Ha, C.; Watanabe, Y.; Le, D.T.; Thao, N.P.; Tran, L.-S.P. The soybean transcription factor GmNAC085 enhances drought tolerance in Arabidopsis. Environ. Exp. Bot. 2018, 151, 12–20. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Q.Y.; Cheng, X.G.; Xu, Z.S.; Li, L.C.; Ye, X.G.; Xia, L.Q.; Ma, Y.Z. GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 2007, 353, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Ali, M.; Feng, X.; Li, X. The essence of NAC gene family to the cultivation of drought-resistant soybean (Glycine max L. Merr.) cultivars. BMC Plant Biol. 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Pontes, B.; Giráldez, R.; Aguilar-Ruiz, J.S. Configurable pattern-based evolutionary biclustering of gene expression data. Algorithms Mol. Biol. 2013, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Kumar, V.; Patel, R.K.; Garg, R.; Jain, M. CTDB: An integrated chickpea transcriptome database for functional and applied genomics. PLoS ONE 2015, 10, e0136880. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Patel, R.K.; Jhanwar, S.; Priya, P.; Bhattacharjee, A.; Yadav, G.; Bhatia, S.; Chattopadhyay, D.; Tyagi, A.K.; Jain, M. Gene discovery and tissue-specific transcriptome analysis in chickpea with massively parallel pyrosequencing and web resource development. Plant Physiol. 2011, 156, 1661–1678. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Sahoo, A.; Tyagi, A.K.; Jain, M. Validation of internal control genes for quantitative gene expression studies in chickpea (Cicer arietinum L.). Biochem. Biophys. Res. Commun. 2010, 396, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Kumimoto, R.W.; Zhang, Y.; Siefers, N.; Holt, B.F., 3rd. NF-YC3, NF-YC4 and NF-YC9 are required for CONSTANS-mediated, photoperiod-dependent flowering in Arabidopsis thaliana. Plant J. 2010, 63, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kim, S.K.; Lee, K.C.; Chung, Y.S.; Lee, J.H.; Kim, J.K. Functional conservation of rice OsNF-YB/YC and Arabidopsis AtNF-YB/YC proteins in the regulation of flowering time. Plant Cell Rep. 2016, 35, 857–865. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Gilmour, J.; Bonifer, C. The role of the ubiquitously expressed transcription factor Sp1 in tissue-specific transcriptional regulation and in disease. Yale J. Biol. Med. 2016, 89, 513–525. [Google Scholar] [PubMed]
- Nelson, D.E.; Repetti, P.P.; Adams, T.R.; Creelman, R.A.; Wu, J.; Warner, D.C.; Anstrom, D.C.; Bensen, R.J.; Castiglioni, P.P.; Donnarummo, M.G.; et al. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. USA 2007, 104, 16450–16455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, D.; Liu, Y.; Luo, C.; Zhou, Y.; Zhang, L. Overexpression of a NF-YB3 transcription factor from Picea wilsonii confers tolerance to salinity and drought stress in transformed Arabidopsis thaliana. Plant Physiol. Biochem. 2015, 94, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tang, S.; An, Y.; Zheng, D.C.; Xia, X.L.; Yin, W.L. Overexpression of the poplar NF-YB7 transcription factor confers drought tolerance and improves water-use efficiency in Arabidopsis. J. Exp. Bot. 2013, 64, 4589–4601. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Y.; Zhuo, C.; Lu, S.; Guo, Z. Overexpression of a NF-YC transcription factor from bermudagrass confers tolerance to drought and salinity in transgenic rice. Plant Biotechnol. J. 2015, 13, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Mochida, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res. 2011, 18, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [PubMed]



| Gene Name | Protein ID 1 | Locus ID 1,2 | ‘Ca’ Code 3 | Protein Size 4 | pI 4 | mW 4 | II 4 | GRAVY 4 | Number of Exons 5 |
|---|---|---|---|---|---|---|---|---|---|
| CaNF-YA01 | XP_012571402 | LOC101492587 | Ca_02713 | 339 | 8.45 | 37.64 | 54.73 | −0.69 | 5 |
| CaNF-YA02 | XP_004486451 | LOC101496019 | Ca_07995 | 206 | 6.79 | 22.76 | 61.06 | −0.96 | 5 |
| CaNF-YA03 | XP_004494259 | LOC101506076 | Ca_00866 | 333 | 9.43 | 36.16 | 43.44 | −0.50 | 5 |
| CaNF-YA04 | XP_004497643 | LOC101500532 | Ca_14455 | 335 | 9.17 | 36.84 | 60.91 | −0.56 | 5 |
| CaNF-YA05 | XP_004500196 | LOC101504175 | Ca_15574 | 337 | 6.22 | 36.54 | 59.60 | −1.08 | 5 |
| CaNF-YA06 | XP_004510428 | LOC101496153 | Ca_10039 | 313 | 8.70 | 34.32 | 56.63 | −0.60 | 6 |
| CaNF-YA07 | XP_004510989 | LOC101500168 | Ca_20250 | 332 | 9.41 | 37.40 | 59.43 | −0.84 | 6 |
| CaNF-YA08 | XP_012574919 | LOC101488265 | Ca_11593 | 244 | 9.86 | 26.93 | 56.04 | −0.65 | 5 |
| CaNF-YB01 | XP_004486346 | LOC101490518 | Ca_07906 | 157 | 6.43 | 17.42 | 38.92 | −0.59 | 1 |
| CaNF-YB02 | XP_004488253 | LOC101497461 | Ca_20300 | 130 | 5.93 | 14.77 | 40.39 | −0.75 | 1 |
| CaNF-YB03 | XP_004488351 | LOC101507640 | Ca_22468 | 156 | 4.63 | 17.40 | 49.85 | −0.48 | 5 |
| CaNF-YB04 | XP_004491514 | LOC101513559 | Ca_09721 | 194 | 6.21 | 20.99 | 45.91 | −0.67 | 1 |
| CaNF-YB05 | XP_004491660 | LOC101504444 | Ca_09724 | 201 | 4.79 | 22.74 | 52.76 | −0.52 | 2 |
| CaNF-YB06 | XP_004493942 | LOC101512494 | Ca_12013 | 171 | 6.10 | 18.76 | 42.49 | −0.82 | 5 |
| CaNF-YB07 | XP_004498435 | LOC101491567 | Ca_13093 | 147 | 6.51 | 16.06 | 34.12 | −0.57 | 2 |
| CaNF-YB08 | XP_004498102 | LOC101490166 | Ca_20009 | 181 | 4.94 | 20.48 | 50.79 | −0.64 | 1 |
| CaNF-YB09 | XP_004498386 | LOC101500751 | Ca_13089 | 137 | 6.96 | 15.24 | 29.99 | −0.56 | 2 |
| CaNF-YB10 | XP_004495979 | LOC101499868 | Ca_03440 | 142 | 5.39 | 16.49 | 58.00 | −1.07 | 1 |
| CaNF-YB11 | XP_004496024 | LOC101514675 | - | 104 | 7.64 | 11.71 | 20.95 | −0.69 | 3 |
| CaNF-YB12 | XP_004495242 | LOC101489398 | Ca_07860 | 174 | 6.09 | 19.22 | 55.44 | −0.82 | 5 |
| CaNF-YB13 | XP_004503068 | LOC101504826 | - | 184 | 5.04 | 21.01 | 51.90 | −0.84 | 1 |
| CaNF-YB14 | XP_004504028 | LOC101492000 | Ca_09605 | 190 | 5.80 | 20.96 | 46.23 | −0.66 | 1 |
| CaNF-YB15 | XP_004503919 | LOC101512746 | - | 223 | 6.21 | 25.87 | 45.74 | −1.12 | 1 |
| CaNF-YB16 | XP_004508609 | LOC101491613 | Ca_06841 | 146 | 5.31 | 16.73 | 49.21 | −0.91 | 1 |
| CaNF-YB17 | XP_004508593 | LOC101512449 | Ca_06596 | 219 | 5.39 | 24.33 | 46.12 | −0.32 | 6 |
| CaNF-YB18 | XP_004507593 | LOC101497005 | Ca_20238 | 136 | 6.17 | 15.31 | 30.16 | −0.62 | 1 |
| CaNF-YB19 | XP_004507590 | LOC101496026 | - | 244 | 6.49 | 27.34 | 34.09 | −0.89 | 2 |
| CaNF-YB20 | XP_004516103 | LOC101498601 | Ca_20798 | 191 | 6.21 | 20.47 | 37.65 | −0.72 | 1 |
| CaNF-YB21 | XP_004516113 | LOC101501902 | Ca_20790 | 227 | 4.42 | 24.83 | 49.49 | −0.53 | 2 |
| CaNF-YC01 | XP_004488773 | LOC101507861 | Ca_13620 | 357 | 7.31 | 40.52 | 60.84 | −0.71 | 2 |
| CaNF-YC02 | XP_004487742 | LOC101503561 | Ca_06933 | 114 | 8.64 | 12.60 | 57.68 | −0.12 | 1 |
| CaNF-YC03 | XP_004486310 | LOC101502053 | Ca_00637 | 223 | 5.24 | 25.76 | 62.49 | −0.74 | 1 |
| CaNF-YC04 | XP_004494629 | LOC101508013 | Ca_01175 | 260 | 6.03 | 28.95 | 59.81 | −0.61 | 1 |
| CaNF-YC05 | XP_004495741 | LOC101507067 | Ca_03698 | 256 | 5.78 | 28.52 | 67.12 | −0.65 | 1 |
| CaNF-YC06 | XP_004501704 | LOC101502982 | Ca_01433 | 213 | 5.03 | 23.84 | 56.43 | −0.52 | 1 |
| CaNF-YC07 | XP_004499778 | LOC101492987 | Ca_17100 | 295 | 5.11 | 32.73 | 50.38 | −0.88 | 6 |
| CaNF-YC08 | XP_012571846 | LOC101509194 | Ca_11344 | 150 | 5.88 | 17.20 | 75.58 | −0.61 | 1 |
| CaNF-YC09 | XP_004505981 | LOC101509858 | Ca_14578 | 219 | 9.63 | 25.06 | 46.15 | −1.11 | 4 |
| CaNF-YC10 | XP_004507577 | LOC101491701 | - | 124 | 9.15 | 13.57 | 57.66 | 0.08 | 1 |
| CaNF-YC11 | XP_004511091 | LOC101513228 | Ca_20191 | 309 | 5.21 | 33.96 | 46.02 | −0.88 | 6 |
| Gene Pairs | Chromosome Localization | Duplication Event | Identity Level (%) | Ka | Ks | Ka/Ks | T (Mya) |
|---|---|---|---|---|---|---|---|
| CaNF-YA03/CaNF-YA04 | Chr III/Chr IV | Segmental | 76.8 | 0.26 | 0.21 | 1.24 | 16.15 |
| CaNF-YB04/CaNF-YB20 | Chr II/US | Unknown | 72.3 | 0.16 | 0.68 | 0.23 | 52.31 |
| CaNF-YB02/CaNF-YB16 | Chr I/Chr VII | Segmental | 72.3 | 0.13 | 0.73 | 0.18 | 56.15 |
| CaNF-YC02/CaNF-YC10 | Chr I/ Chr VI | Segmental | 76.8 | 0.19 | 0.13 | 1.46 | 10.00 |
| CaNF-YC04/CaNF-YC05 | Chr III/Chr IV | Segmental | 79.6 | 0.20 | 0.31 | 0.64 | 23.85 |
| CaNF-YC07/CaNF-YC11 | Chr V/Chr VII | Segmental | 70.3 | 0.29 | 0.33 | 0.88 | 25.38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.D.; Nguyen, K.H.; Watanabe, Y.; Le, D.T.; Pham, T.L.T.; Mochida, K.; Tran, L.-S.P. Identification, Structural Characterization and Gene Expression Analysis of Members of the Nuclear Factor-Y Family in Chickpea (Cicer arietinum L.) under Dehydration and Abscisic Acid Treatments. Int. J. Mol. Sci. 2018, 19, 3290. https://doi.org/10.3390/ijms19113290
Chu HD, Nguyen KH, Watanabe Y, Le DT, Pham TLT, Mochida K, Tran L-SP. Identification, Structural Characterization and Gene Expression Analysis of Members of the Nuclear Factor-Y Family in Chickpea (Cicer arietinum L.) under Dehydration and Abscisic Acid Treatments. International Journal of Molecular Sciences. 2018; 19(11):3290. https://doi.org/10.3390/ijms19113290
Chicago/Turabian StyleChu, Ha Duc, Kien Huu Nguyen, Yasuko Watanabe, Dung Tien Le, Thu Ly Thi Pham, Keiichi Mochida, and Lam-Son Phan Tran. 2018. "Identification, Structural Characterization and Gene Expression Analysis of Members of the Nuclear Factor-Y Family in Chickpea (Cicer arietinum L.) under Dehydration and Abscisic Acid Treatments" International Journal of Molecular Sciences 19, no. 11: 3290. https://doi.org/10.3390/ijms19113290
APA StyleChu, H. D., Nguyen, K. H., Watanabe, Y., Le, D. T., Pham, T. L. T., Mochida, K., & Tran, L.-S. P. (2018). Identification, Structural Characterization and Gene Expression Analysis of Members of the Nuclear Factor-Y Family in Chickpea (Cicer arietinum L.) under Dehydration and Abscisic Acid Treatments. International Journal of Molecular Sciences, 19(11), 3290. https://doi.org/10.3390/ijms19113290