Allogeneic Mesenchymal Stem Cells and Biomaterials: The Perfect Match for Cardiac Repair?
Abstract
1. Cardiac Diseases: Epidemiology and Etiopathology
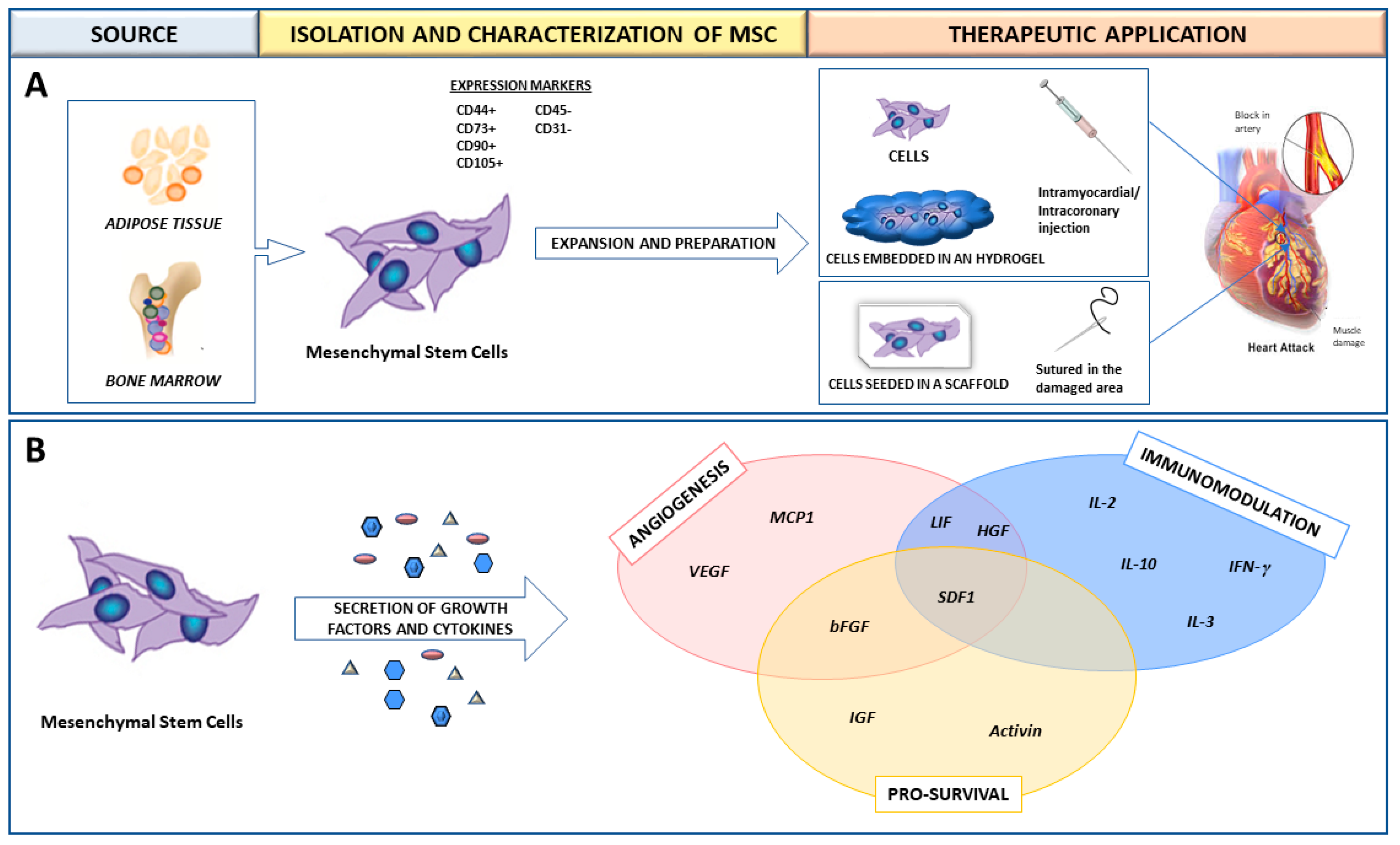
2. Mesenchymal Stem Cells (MSC) for the Treatment of Myocardial Infarction (MI)
3. MSC in Preclinical and Clinical Studies for MI Treatment
4. Allogeneic Transplant Model
5. Allorecognition of MSC by the Host Immune Cells
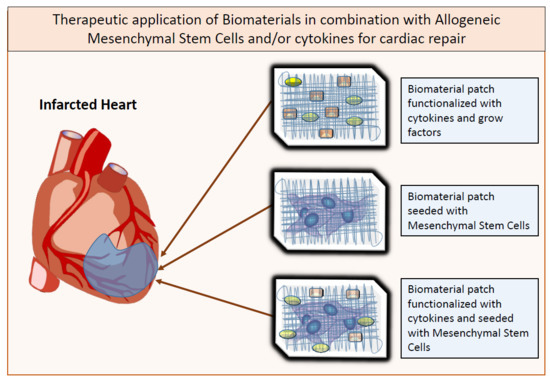
6. Biomaterials as Co-Adjuvants for MSC Therapy in Cardiac Regeneration
7. Use of Hydrogels for Cardiac Application
8. Cardiac Patches and Cellularized Scaffolds
9. Acknowledging the Importance of the Scaffold Substrate in the MSC Cytokine Secretion Profile
10. Biomaterials Functionalization with Growth Factors
11. Future Perspective: Allogeneic MSC-Seeded and Cytokine-Immobilized Patches for Cardiac Regeneration
Funding
Conflicts of Interest
Abbreviations
| ADSC | Adipose Derived Mesenchymal Stem Cells |
| BMMC | Bone Marrow Mononuclear Cell |
| BM-MSC | Bone-Marrow Derived Mesenchymal Stem Cells |
| FGF2 | Basic Fibroblast Growth Factor |
| FS | Fractional Shortening |
| Fstl1 | Follistatin-Like 1 |
| GMP | Good Manufacturing Practices |
| HGF | Hepatocyte Growth Factor |
| IGF | Insulin-Like Growth Factor |
| IL-6 | Interleukin-6 |
| iPSC | Induced Pluripotent Stem Cells |
| LIF | Leukaemia Inhibitory Factor |
| LV | Left Ventricle |
| MHC | Mayor Histocompatibility Complex |
| MI | Myocardial Infarction |
| MSC | Mesenchymal Stem Cells |
| NK | Natural Killer Cell |
| PCL | Polycaprolactone |
| PDGF | Platelet-Derived Growth Factor |
| PGA | Polyglycolic Acid |
| PGCL | Poly-Glycolide-co-Caprolactone |
| PLA | Polylactic Acid |
| SAE | Secondary Adverse Effects |
| SC | Stem Cell |
| VEGF | Vascular Endothelial Growth Factor |
References
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; de Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive summary: Heart disease and stroke statistics—2010 update: A report from the american heart association. Circulation 2010, 121, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Gonzalez, I. The epidemiology of coronary heart disease. Rev. Esp. Cardiol. 2014, 67, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nigro, P.; Bassetti, B.; Cavallotti, L.; Catto, V.; Carbucicchio, C.; Pompilio, G. Cell therapy for heart disease after 15 years: Unmet expectations. Pharmacol. Res. 2018, 127, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Thakker, R.; Yang, P. Mesenchymal stem cell therapy for cardiac repair. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 323. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringden, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Rasmusson, I. Immune modulation by mesenchymal stem cells. Exp. Cell Res. 2006, 312, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Maggini, J.; Mirkin, G.; Bognanni, I.; Holmberg, J.; Piazzon, I.M.; Nepomnaschy, I.; Costa, H.; Canones, C.; Raiden, S.; Vermeulen, M.; et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE 2010, 5, e9252. [Google Scholar] [CrossRef] [PubMed]
- Karpov, A.A.; Uspenskaya, Y.K.; Minasian, S.M.; Puzanov, M.V.; Dmitrieva, R.I.; Bilibina, A.A.; Anisimov, S.V.; Galagudza, M.M. The effect of bone marrow- and adipose tissue-derived mesenchymal stem cell transplantation on myocardial remodelling in the rat model of ischaemic heart failure. Int. J. Exp. Pathol. 2013, 94, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mazo, M.; Planat-Benard, V.; Abizanda, G.; Pelacho, B.; Leobon, B.; Gavira, J.J.; Penuelas, I.; Cemborain, A.; Penicaud, L.; Laharrague, P.; et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur. J. Heart Fail. 2008, 10, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zeng, Q.; Wang, H.; Shao, S.; Mao, X.; Zhang, F.; Li, S.; Guo, Z. Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coron. Artery Dis. 2007, 18, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xie, Q.; Pan, G.; Wang, J.; Wang, M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur. J. Cardiothorac. Surg. 2006, 30, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Schuleri, K.H.; Feigenbaum, G.S.; Centola, M.; Weiss, E.S.; Zimmet, J.M.; Turney, J.; Kellner, J.; Zviman, M.M.; Hatzistergos, K.E.; Detrick, B.; et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur. Heart J. 2009, 30, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Amado, L.C.; Saliaris, A.P.; Schuleri, K.H.; St John, M.; Xie, J.S.; Cattaneo, S.; Durand, D.J.; Fitton, T.; Kuang, J.Q.; Stewart, G.; et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci USA 2005, 102, 11474–11479. [Google Scholar] [CrossRef] [PubMed]
- Mazo, M.; Hernandez, S.; Gavira, J.J.; Abizanda, G.; Arana, M.; Lopez-Martinez, T.; Moreno, C.; Merino, J.; Martino-Rodriguez, A.; Uixeira, A.; et al. Treatment of reperfused ischemia with adipose-derived stem cells in a preclinical Swine model of myocardial infarction. Cell Transplant. 2012, 21, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.V.; Litovsky, S.; Assad, J.A.; Sousa, A.L.; Martin, B.J.; Vela, D.; Coulter, S.C.; Lin, J.; Ober, J.; Vaughn, W.K.; et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 2005, 111, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ishida, O.; Hagino, I.; Nagaya, N.; Shimizu, T.; Okano, T.; Sawa, Y.; Mori, H.; Yagihara, T. Adipose-derived stem cell sheet transplantation therapy in a porcine model of chronic heart failure. Transl. Res. 2015, 165, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Valina, C.; Pinkernell, K.; Song, Y.H.; Bai, X.; Sadat, S.; Campeau, R.J.; Le Jemtel, T.H.; Alt, E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 2007, 28, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Houtgraaf, J.H.; den Dekker, W.K.; Van Dalen, B.M.; Springeling, T.; de Jong, R.; Van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W.; et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; DiFede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.P.; Fishman, J.; Pattany, P.; McNiece, I.; Conte, J.; et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ. Res. 2014, 114, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Leri, A. Aging and disease as modifiers of efficacy of cell therapy. Circ. Res. 2008, 102, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Beyth, S.; Borovsky, Z.; Mevorach, D.; Liebergall, M.; Gazit, Z.; Aslan, H.; Galun, E.; Rachmilewitz, J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005, 105, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Zangi, L.; Margalit, R.; Reich-Zeliger, S.; Bachar-Lustig, E.; Beilhack, A.; Negrin, R.; Reisner, Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009, 27, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Sun, Z.; Miyagi, Y.; McDonald Kinkaid, H.; Zhang, L.; Weisel, R.D.; Li, R.K. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 2010, 122, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Tano, N.; Kaneko, M.; Ichihara, Y.; Ikebe, C.; Coppen, S.R.; Shiraishi, M.; Shintani, Y.; Yashiro, K.; Warrens, A.; Suzuki, K. Allogeneic mesenchymal stromal cells transplanted onto the heart surface achieve therapeutic myocardial repair despite immunologic responses in rats. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Ishikane, S.; Hosoda, H.; Yamahara, K.; Akitake, Y.; Kyoungsook, J.; Mishima, K.; Iwasaki, K.; Fujiwara, M.; Miyazato, M.; Kangawa, K.; et al. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation 2013, 96, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.H.; Mo, X.M.; Han, Z.C.; Zhou, B. Stem cell engraftment and survival in the ischemic heart. Ann. Thorac. Surg. 2011, 92, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, J.; Yang, J.; Kong, X.; Zheng, F.; Guo, L.; Zhang, L.; Huang, Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur. J. Cardiothorac. Surg. 2009, 36, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kwon, K.; Lim, S.; Kang, S.M.; Ko, Y.G.; Xu, Z.; Chung, J.H.; Kim, B.S.; Lee, H.; Joung, B.; et al. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol. Cells 2005, 19, 402–407. [Google Scholar] [PubMed]
- Rane, A.A.; Christman, K.L. Biomaterials for the treatment of myocardial infarction: A 5-year update. J. Am. Coll. Cardiol. 2011, 58, 2615–2629. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.J.; Coulombe, K.L. Physiologically inspired cardiac scaffolds for tailored in vivo function and heart regeneration. Biomed. Mater. 2015, 10, 034003. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhou, Y.; Cai, H.; Tan, W. Myocardial regeneration: Roles of stem cells and hydrogels. Adv. Drug Deliv. Rev. 2011, 63, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.D.; Wang, H.J.; Tan, Y.Z.; Wu, J.H. Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng. Part. A 2011, 17, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Danoviz, M.E.; Nakamuta, J.S.; Marques, F.L.; dos Santos, L.; Alvarenga, E.C.; dos Santos, A.A.; Antonio, E.L.; Schettert, I.T.; Tucci, P.J.; Krieger, J.E. Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS ONE 2010, 5, e12077. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Du, K.T.; Fang, Q.; Gu, Y.; Mihardja, S.S.; Sievers, R.E.; Wu, J.C.; Lee, R.J. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 2010, 31, 7012–7020. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.J.; Villafuertes, E.; Haeck, J.C.; Koevoet, W.J.; Fernandez-Gutierrez, B.; Hoogduijn, M.J.; Verhaar, J.A.; Bernsen, M.R.; Van Buul, G.M.; Van Osch, G.J. Encapsulation of allogeneic mesenchymal stem cells in alginate extends local presence and therapeutic function. Eur. Cell Mater. 2017, 33, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Linke, A.; Suselbeck, T.; Muller-Ehmsen, J.; Vermeersch, P.; Schoors, D.; Rosenberg, M.; Bea, F.; Tuvia, S.; Leor, J. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: A first-in-man study. Circ. Cardiovasc. Interv. 2014, 7, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Giraud, M.N.; Ayuni, E.; Cook, S.; Siepe, M.; Carrel, T.P.; Tevaearai, H.T. Hydrogel-based engineered skeletal muscle grafts normalize heart function early after myocardial infarction. Artif. Organs 2008, 32, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.; Liu, H.; Fan, T.H.; Nerem, R.; Dudley, S.C., Jr. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells 2007, 25, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Arana, M.; Mazo, M.; Aranda, P.; Pelacho, B.; Prosper, F. Adipose tissue-derived mesenchymal stem cells: Isolation, expansion, and characterization. Methods Mol. Biol. 2013, 1036, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Kameli, M.; Khorramirouz, R.; Eftekharzadeh, S.; Fendereski, K.; Daryabari, S.S.; Tavangar, S.M.; Kajbafzadeh, A.M. Application of tissue-engineered pericardial patch in rat models of myocardial infarction. J. Biomed. Mater. Res. A 2018. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Hill, K.L.; Li, Q.; Suntharalingam, P.; Mansoor, A.; Wang, X.; Jameel, M.N.; Zhang, P.; Swingen, C.; Kaufman, D.S.; et al. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells 2011, 29, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Kwon, J.S.; Piao, S.; Sohn, J.H.; Lee, Y.S.; Bae, J.W.; Hwang, K.K.; Kim, D.W.; Jeon, O.; Kim, B.S.; et al. Effects of cardiac patches engineered with bone marrow-derived mononuclear cells and PGCL scaffolds in a rat myocardial infarction model. Biomaterials 2007, 28, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Mickle, D.A.; Weisel, R.D.; Koyama, N.; Ozawa, S.; Li, R.K. Optimal biomaterial for creation of autologous cardiac grafts. Circulation 2002, 106, I176–I182. [Google Scholar] [PubMed]
- Chen, J.; Zhan, Y.; Wang, Y.; Han, D.; Tao, B.; Luo, Z.; Ma, S.; Wang, Q.; Li, X.; Fan, L.; et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Osama, I.; Gorenkova, N.; McKittrick, C.M.; Wongpinyochit, T.; Goudie, A.; Seib, F.P.; Carswell, H.V.O. In vitro studies on space-conforming self-assembling silk hydrogels as a mesenchymal stem cell-support matrix suitable for minimally invasive brain application. Sci. Rep. 2018, 8, 13655. [Google Scholar] [CrossRef] [PubMed]
- Chachques, J.C.; Trainini, J.C.; Lago, N.; Cortes-Morichetti, M.; Schussler, O.; Carpentier, A. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM trial): Clinical feasibility study. Ann. Thorac. Surg. 2008, 85, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Leuning, D.G.; Beijer, N.R.M.; du Fosse, N.A.; Vermeulen, S.; Lievers, E.; Van Kooten, C.; Rabelink, T.J.; Boer, J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials 2017, 140, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Darnell, M.; O’Neil, A.; Mao, A.; Gu, L.; Rubin, L.L.; Mooney, D.J. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8368–E8377. [Google Scholar] [CrossRef] [PubMed]
- Ruvinov, E.; Leor, J.; Cohen, S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 2011, 32, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Silva, E.A.; Mansson-Broberg, A.; Grinnemo, K.H.; Siddiqui, A.J.; Dellgren, G.; Wardell, E.; Brodin, L.A.; Mooney, D.J.; Sylven, C. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc. Res. 2007, 75, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, J.; Gao, Y.; Wang, C.; Zhao, Y.; Chen, B.; Xiao, Z.; Miao, Q.; Dai, J. A myocardial patch made of collagen membranes loaded with collagen-binding human vascular endothelial growth factor accelerates healing of the injured rabbit heart. Tissue Eng. Part. A 2011, 17, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Serpooshan, V.; Hurtado, C.; Diez-Cunado, M.; Zhao, M.; Maruyama, S.; Zhu, W.; Fajardo, G.; Noseda, M.; Nakamura, K.; et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015, 525, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, Y.; Zeng, F.; Huang, X.P.; Foltz, W.D.; Wu, J.; Mihic, A.; Yau, T.M.; Weisel, R.D.; Li, R.K. Surgical ventricular restoration with a cell- and cytokine-seeded biodegradable scaffold. Biomaterials 2010, 31, 7684–7694. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Xie, B.D.; Wu, J.; Lv, B.; Chuai, J.B.; Li, J.Z.; Cai, J.; Wu, H.; Jiang, S.L.; Leng, X.P.; et al. Improved left ventricular aneurysm repair with cell- and cytokine-seeded collagen patches. Stem Cells Int. 2018, 2018, 4717802. [Google Scholar] [CrossRef] [PubMed]

| ALLOGENEIC MSC | AUTOLOGOUS MSC | |
|---|---|---|
| PROS | Cell availability (“ready-to-use” format) Right trophic properties Lower costs in logistics | Perfect immune match Same MHC haplotype |
| CONS | Low/mild immune response Shorter survival time in the implant area | Cells available at medium term Dysfunctional cells in sick/old patients Higher costs in logistics |
| Formula | Biodegradability | Stiffness | E [kPa] | Cell Adhesion | Other Properties | ||
|---|---|---|---|---|---|---|---|
| NATURAL POLYMERS | Gelatin |  | +++ | − | 0.1−30 | +++ |
|
| Collagen | 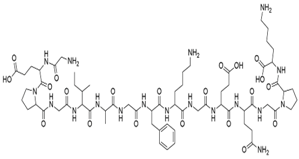 | +++ | + | 0.1−50 | +++ |
| |
| Chitosan | 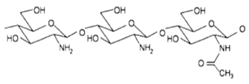 | ++ | + | 0.1−50 | + |
| |
| Fibrin | 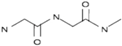 | +++ | + | 0.1−20 | ++ |
| |
| Alginate | 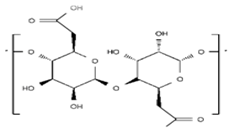 | +++ | − | 0.1−50 | ++ |
| |
| SYNTHETIC POLYMERS | PCL | 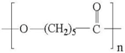 | − | +++ | >100 | − |
|
| PGA | 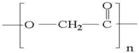 | ++ | ++ | Depends on composition | + |
| |
| PLA | 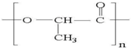 | + | ++ | Depends on composition | + |
| |
| PLGA | 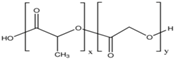 | ++ | + | Depends on composition | + |
| |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Estenaga, I.; Prosper, F.; Pelacho, B. Allogeneic Mesenchymal Stem Cells and Biomaterials: The Perfect Match for Cardiac Repair? Int. J. Mol. Sci. 2018, 19, 3236. https://doi.org/10.3390/ijms19103236
Perez-Estenaga I, Prosper F, Pelacho B. Allogeneic Mesenchymal Stem Cells and Biomaterials: The Perfect Match for Cardiac Repair? International Journal of Molecular Sciences. 2018; 19(10):3236. https://doi.org/10.3390/ijms19103236
Chicago/Turabian StylePerez-Estenaga, Inigo, Felipe Prosper, and Beatriz Pelacho. 2018. "Allogeneic Mesenchymal Stem Cells and Biomaterials: The Perfect Match for Cardiac Repair?" International Journal of Molecular Sciences 19, no. 10: 3236. https://doi.org/10.3390/ijms19103236
APA StylePerez-Estenaga, I., Prosper, F., & Pelacho, B. (2018). Allogeneic Mesenchymal Stem Cells and Biomaterials: The Perfect Match for Cardiac Repair? International Journal of Molecular Sciences, 19(10), 3236. https://doi.org/10.3390/ijms19103236