Abstract
The family of cyclin-dependent kinases (CDKs) has critical functions in cell cycle regulation and controlling of transcriptional elongation. Moreover, dysregulated CDKs have been linked to cancer initiation and progression. Pharmacological CDK inhibition has recently emerged as a novel and promising approach in cancer therapy. This idea is of particular interest to combat pancreatic ductal adenocarcinoma (PDAC), a cancer entity with a dismal prognosis which is owed mainly to PDAC’s resistance to conventional therapies. Here, we review the current knowledge of CDK biology, its role in cancer and the therapeutic potential to target CDKs as a novel treatment strategy for PDAC.
1. Background
Despite advances in diagnosis and therapy, carcinoma of the exocrine pancreas, especially pancreatic ductal adenocarcinoma (PDAC), imposes a grand challenge for oncology. PDAC is one out of only two cancers where there has been virtually no progress in survival rates over the last decades, the second one being lung cancer [1]. Because pancreatic cancer presents with non-specific symptoms, like diffuse abdominal discomfort and loss of appetite [2], it is often only diagnosed at a late stage, when it is more difficult to treat [3].
With an incidence of 4.2 per 100,000, pancreatic cancer is the 12th-most common cancer in humans [4]. Within malignancies of the gastrointestinal tract, PDAC ranks second behind the most commonly occurring colorectal cancer [1,5]. PDAC is one of the deadliest cancer entities, with a cumulative five-year-survival rate of only 8% [1,3]. This meagre survival is partly caused by late diagnosis as suggested by the fact that a solely localised tumour without metastases offers a four-times improved survival rate of 28% [1]. In cancer-associated mortality statistics, pancreatic cancer ranks fourth in both sexes [1]. It is a cancer entity associated with old age, as pancreatic cancer arises most frequently in the seventh and eighth decade, although, less frequently, it is also reported in people under 40 years of age [6].
The only potential cure for PDAC is surgical resection [2,7,8]. However, this possibility is hampered by the aggressiveness of this disease, presenting early spreading, invasion, distant metastases, and a typical resistance towards conventional radio- and chemotherapy [2,3,9,10,11]. At the time of diagnosis, only 15 to 20% of pancreatic cancers are operable (i.e., have not spread to other organs), whereas the rest can only be treated through palliative chemotherapy, often with unfavourable results [12]. Most patients that have been treated with curative intent develop on-site relapse and distant metastases [2,13]. Therefore, clinicians are in need of adequate and effective chemotherapeutic regimens. Adjuvant treatments are mostly based on gemcitabine; palliative disease is mostly treated with gemcitabine or FOLFIRINOX (FOL = Folinic acid = Leucovorin, F = 5-FU = 5-Fluorouracil, IRIN = Irinotecan, OX = Oxaliplatin) or tyrosine-kinase inhibitor erlotinib [14,15].
The prodrug gemcitabine has been the gold standard for adjuvant treatment for a long time. However, the effects on improving median survival are only marginal at best [16,17]. Paclitaxel, nowadays prepared as nab-paclitaxel (nanoparticle albumin-bound), combined with gemcitabine improved therapeutic success, although not to a satisfactory level [18,19,20]. FOLFIRINOX has been a second line regime for advanced and metastatic pancreatic cancer since its introduction as PDAC treatment a few years ago [21,22]. However, new trends [23] indicate FOLFIRINOX as a first-line medicament in curative and adjuvant settings, as it was shown to be superior to gemcitabine, as it had a significantly better overall survival, disease-free survival, and metastasis-free survival during a randomized clinical trial [24,25].
Unfortunately, gemcitabine, nab-paclitaxel, and FOLFIRINOX are highly toxic [13,20,26,27]. In addition, currently approved therapies for PDAC yield unsatisfactory results. Hence, new therapeutic strategies with lowered toxicity and higher on-target efficacy are needed. Cyclin-dependent kinases (CDKs) have been proposed as possible targets of pharmacological inhibition for several cancer entities. Indeed, there are some successful examples of CDK inhibition in clinical settings, particularly against breast cancer, non-small-cell lung cancer, melanoma, and head and neck squamous cell carcinoma (reviewed in [28]). In the case of pancreatic cancer, the available clinical and pre-clinical evidence for a benefit in inhibiting CDKs is still scarce, although CDKs play an important role in the pathobiology of this disease.
2. Family of CDKs
Cyclin-dependent kinases (CDKs) are serine/threonine kinases that control cell cycle progression and other critical functions within the cell. Based on the homology of their catalytic domains, CDKs, along with the Mitogen-activated protein kinases (MAPKs), Glycogen synthase kinase-3 β (Gsk3β), members of the dual-specificity tyrosine-regulated kinase (DYRK) family and CDK-like kinases belong to the CMGC group of kinases (named after the initials of its members) [29,30]. CDKs are activated by cyclins and act as a regulatory subunit. These CDK/cyclin complexes are fundamental to the orderly progression of the cell [31]. Studies examining the functions of CDKs and cyclins have uncovered that in fact, these proteins have other relevant roles besides regulation of the cell cycle [31,32]. These other roles include, for example, regulation of transcription, epigenetic regulation, metabolism, stem cell renewal, and spermatogenesis [33,34,35,36].
According to the classification of Malumbres et al. [37], there are 21 CDKs (see Table 1), sharing a conserved catalytic domain that comprises an ATP-binding pocket, the amino acid sequence PSTAIRE as a cyclin-binding domain and an activating T-loop motif. Active CDK/cyclin complexes depend on the phosphorylation of the T-loop on the respective CDK. Cyclins interact with the PSTAIRE helix, displacing the T-loop and exposing the substrate binding domain of the kinase thereby allowing for its phosphorylation. Unlike CDKs, cyclins are heterogeneous proteins, characterised by the presence of the so-called cyclin-box, which mediates binding to CDKs [38]. Outside this domain, their sequences are quite diverse. Most known cyclins promote CDK activity. There are several regulatory mechanisms for CDKs at the post-transcriptional level, CDK inhibitors exerting a negative regulation, phosphorylation status, protein folding, and subcellular localisation [39,40,41]. Phosphorylation can regulate CDKs in a negative and positive manner [42]. For example, CDK1 has inhibitory (threonine 14, T14; tyrosine 15, Y15 in CDK1) and stimulatory (threonine 161, T161 in CDK1) phosphorylation sites [43,44]. Phosphorylation at T14 and Y15 within the ATP-binding site by inhibitory kinases Wee1 and Myt1 interferes with proper ATP alignment, whereas T-loop phosphorylation at T161 by CDK-activating kinases (CAKs) improves substrate binding and complex stability to enable full CDK activation [42]. In this review, a summarized overview of the role of CDKs during cell cycle and transcription will be provided.

Table 1.
Cyclin-Dependent Kinases with corresponding cyclins and their main function.
3. Cell Cycle Regulation by CDKs
The cell cycle is one of the most essential and evolutionarily conserved cellular processes. CDKs and cyclins are the main proteins responsible for driving it [93,94,95]. Typically, these protein families act as heterodimers regulating different steps of the cell cycle, as CDK/cyclin complexes (Figure 1). CDKs unbound to cyclins are usually inactive, but upon the formation of a complex with a cyclin partner, they become able to phosphorylate different targets, resulting in target inactivation or repression. CDKs are constitutively expressed in the cell while cyclin expression is highly dependent on the stage of the cell cycle, hence their name. This fluctuating expression pattern of cyclins constitutes a mechanism by which the orderly progression of the cell cycle is regulated.
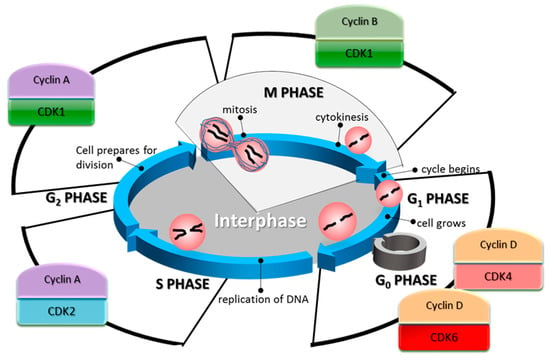
Figure 1.
The cell cycle phases and their associated cyclin-dependent kinases (CDK)/cyclin complexes. In the G1 phase of the cell cycle, the synthesis of cyclin D is increased. This cyclin partners with CDK4/6 to promote cell cycle entry, and its progression through G1, as well as the G1/S transition. During the S phase, CDK2 in complex with cyclin A controls the phosphorylation of targets involved in DNA replication. Cyclin A is found highly expressed in this phase and until the last stages of G2. In the G2 phase, the primary regulator of the cell cycle is CDK1.
In the G1 phase of the cell cycle, the synthesis of cyclin D is stimulated. This cyclin is a partner of CDKs 4 and 6 [96]. Complexes of these CDKs and cyclin D promote cell cycle entry, progression through G1, and G1/S transition [56,97]. Cyclin D regulation is complex, depending mainly on the RAS/RAF/MEK/ERK signalling pathway for its transcription [98,99]. It is also regulated by its constant rate of degradation through the proteasome, mediated by GSK3β [100]. GSK3β phosphorylates cyclin D1, which primes it for its subsequent ubiquitination. The primary cell cycle-related target of the CDK4/6-cyclin D complex is the Retinoblastoma protein (Rb). Rb is one of the main repressors of the G1 to S phase transition [101], through its inhibitory activity on E2F, a transcription factor that promotes the expression of genes required for DNA synthesis and mitosis [102]. In humans, Rb contains 16 known phosphorylation sites that are targeted by CDKs [103,104], and its phosphorylation status varies along the cell cycle [105]. In its hypophosphorylated form, Rb can form a complex with E2F and repress its activity. Besides CDK4/cyclin D and CDK6/cyclin D, two other related complexes are known for their ability to hyperphosphorylate Rb: CDK2/cyclin E during G1, and CDK2/cyclin A during S phase [106].
One of the transcriptional targets of E2F is CCNE1 encoding cyclin E [107], which in turn represses Rb when is in a heterodimer with CDK2 [56]. CDK2/Cyclin E complexes act mainly in the G1 and S phases of the cell cycle [96], however, its levels are reduced after the early stages of the S phase by proteasomal degradation. This particular complex has a broader range of targets related to the cell cycle, such as DNA replication and centrosome duplication [108]. CDK2 in complex with cyclins A1 or A2 is associated with S phase, with A2 being ubiquitously expressed in mice germ cells [109]. During this phase, cyclin A partners with CDK2 to phosphorylate targets involved in DNA replication [110]. Cyclin A is found highly expressed in this phase and until the last stages of G2. At the G1/S checkpoint, the cell halts its progression in the cell cycle if the conditions are not favourable for division. This checkpoint is partly controlled by the inhibition of the CDK4/cyclin D complex by the Inhibitor of CDK4 (INK4) family. These inhibitors competitively bind to CDK4 and CDK6, preventing, in turn, their binding to cyclin D, which is then degraded [100]. Either growth-induced or oncogene-induced overexpression of cyclin D alters this dynamic and pushes the cell towards the S phase [111].
In G2 phase, after the cell has duplicated its DNA during S phase, the primary regulator of the cell cycle is the complex formed between CDK1 and cyclin B. So far, more than 70 proteins have been identified as cellular targets of phosphorylation mediated by this complex [94], influencing many cell cycle-critical events, such as the separation of centrosomes [112], the condensation of chromosomes [113], breakdown of the nuclear lamina [114], and disassembly of the Golgi apparatus [115]. The activation of the CDK1/cyclin B complex is inhibited when DNA damage of genotoxic stress is present [116]. Also, its subcellular localisation is a regulation mechanism. CDK1 can be sequestered in the cytoplasm by the protein 14-3-3σ when it is separated from its partner cyclin B, either by competitive binding with p21Cip1 or directly dissociated by the Growth Arrest and DNA Damage-inducible GADD45 [117].
This complex network of CDK/cyclin interactions is not fully understood, not only because many other functions of these proteins have emerged in recent years, but also because there are many instances of functional redundancy in the cell cycle. For example, in the absence of CDK4/6, CDK2 can take over their functions when in complex with cyclin D [36]. In a similar manner, CDK1 can substitute for CDK2 and 4. In fact, the only essential CDK in the cell cycle is CDK1 which cannot be substituted for by another CDK [48]. In the absence of CDK2, CDK3, CDK4, and CDK6 in mouse embryos, CDK1 was able to bind to all cyclins, leading to the phosphorylation of Rb, an event required for cell cycle progression. However, the embryos were unable to develop past the morula and blastocyst stages in the absence of CDK1, showing that this CDK can drive cell division by itself [48].
4. Transcriptional Regulation by CDKs
Transcription is a process that can be influenced at several levels by CDKs, such as with their influence on E2F, [105,118] and the transcription factor FoxM1 during G2 phase by CDK2/cyclin A and CDK1/cyclin B [119,120,121,122]. Also, CDKs are also able to influence the transcription process more directly through regulation of RNA polymerase II (RNA Pol II)-dependent transcription (Figure 2). CDKs can both negatively and positively influence the functionality of RNA Pol II. CDK8 and CDK19 are components of the Mediator complex as part of a 4-subunit subcomplex with cyclin C, Mediator complex subunits MED12 and MED13. This complex acts as an inhibitor of RNA Pol II by phosphorylating its C-terminal domain (CTD), a process which blocks RNA Pol II participation in the pre-initiation complex that drives transcription in eukaryotes [123,124,125]. In contrast to this, there is also CDK-mediated phosphorylation of the RNA Pol II CTD at distinct sites leading to positive regulation of RNA Pol II activity. The pre-initiation complex includes CDK7, its partner cyclin H, and MAT1 (Menage à Trois 1) as a catalytic subunit (named TFIIH) that phosphorylates the CTD of RNA Pol II. This phosphorylation allows for initiation of transcription and elongation to happen [126]. TFIIH, in turn, can also be negatively regulated by CDK8-mediated phosphorylation of cyclin H preventing TFIIH-mediated activatory phosphorylation of the RNA Pol II CTD [67]. CDK9-mediated phosphorylation of the CTD stimulates RNA polymerisation, exerting a positive regulation.
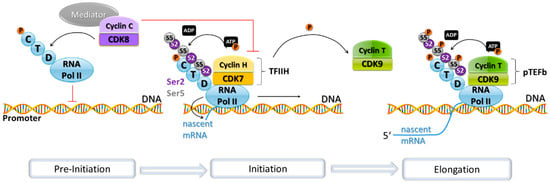
Figure 2.
Transcription and its associated CDK/cyclin complexes. RNA Pol II forms part of the pre-initiation complex that starts gene transcription in eukaryotes. This pre-initiation complex is inhibited by the Mediator complex, of which CDK8 and cyclin C are part. The Mediator complex represses transcription by phosphorylating the C-terminal domain (CTD) of RNA Pol II to prevent its recruitment to the promoter and by phosphorylating cyclin H. Cyclin H in complex with CDK7 forms part of the transcription factor complex TFIIH, which in turn phosphorylates the CTD of RNA Pol II, triggering the transition from transcription initiation to mRNA elongation. TFIIH phosphorylates cyclin T as well. The CDK9/cyclin T complex forms part of the positive transcription elongation factor b (pTEFb), which phosphorylates the RNA Pol II CTD, thus promoting the extension of the pre-mRNA.
CDK9 promotes phosphorylation of RNA Pol II as a subunit of the positive transcription elongation factor b (pTEFb), where it is found bound to cyclin T1. It also phosphorylates the negative elongation factor NELF, allowing for the extension of the nascent pre-mRNA [127,128]. A mouse study indicates that CDK9 is essential for development and embryonic genome activation. Inhibition of the kinase activity of positive transcription elongation factor b (pTEFb) using the CDK9-specific inhibitor, flavopiridol, caused defects in transcription, abnormal localisation of pTEFb, and developmental arrest in mouse two-cell stage embryos [129]. pTEFb is required for transcription of most genes [130], which explains the drastic consequences observed in this study.
Other CDKs involved in the transcription process are CDK11, 12, and 13. CDK11 has been found to co-immunoprecipitate with the elongation factors ELL2, TFIIF, TFIIS, and FACT, as well as with RNA Pol II itself [31]. Moreover, CDK11 is thought to phosphorylate RNA Pol II on its CTD [131]. CDK12 and CDK13 are also able to phosphorylate RNA Pol II, when in complex with cyclin K [132,133].
Another fundamental process via which CDKs influence transcription is the canonical Wnt pathway. CDK8 kinase activity has been found to be necessary for β-catenin-driven transformation and expression of several β-catenin transcriptional targets [69], while CDK14/cyclin Y complexes are responsible for the phosphorylation of Lrp6, a Wnt coreceptor. This phosphorylation primes Lrp6 for its phosphorylation by the membrane-bound Casein Kinase 1 gamma, leading to the binding of Axin, which is then unable to form part of the destruction complex that ubiquitinylates β-catenin. This chain of events leads to increased TCF/LEF mediated transcription [31].
As such, the examples mentioned above illustrate the diverse roles CDKs play in major cellular processes, and it is likely that more roles of this family will be discovered. It is clear that although their role in the cell cycle has been extensively studied, more research is needed to fully understand CDKs’ integral role in the cell and other central processes in cell biology.
5. The Role of CDKs in Pancreatic Cancer
The dysregulation of CDK and cyclin activity in the cell cycle is often found elevated in human tumours and is associated with the unrestrained proliferation of cells, an essential hallmark of cancer [134,135,136]. To maintain these malignant rates at which cells cycle, cancer cells acquire additional mutations which help to circumvent cell cycle checkpoints that usually safeguard genomic integrity and control proliferation [135,136]. A plethora of aberrant CDK behaviour has been described for various tumour entities [44,134,137,138,139]. However, in this review, we will focus on CDK dysregulation described for pancreatic cancer.
Signal transduction cascades activated by proto-oncogene KRAS, CDKN2A (encoding the tumour suppressors p16INK4a and p14ARF) and TP53 intersect on the cell cycle, thereby promoting the transition from G0 or G1 phase into S phase [140]. Since pancreatic cancers carry activating mutations in KRAS, loss-of-function mutations in TP53, and CDKN2A in >90%, 50%, and 80% of tumours respectively, controlling CDKs might significantly impact PDAC [3,141,142,143]. So far, histopathological investigations of pancreatic cancer tissue have identified intraepithelial neoplasia (PanIN) lesions which illustrate the multistep genetic progression leading to invasive PDAC. Early stages include the activation of KRAS with subsequent inactivation of TP53 and CDKN2A/2B in advanced stages [143].
A crucial checkpoint, controlled by the CDK4/6-cyclin D-Rb pathway, is the transition from G1 to S phase [144]. Consequently, dysregulation of the Rb pathway results in enhanced proliferation [145,146,147,148]. Against the background of a high frequency of KRAS mutations, which is the case in PDAC, the CDK4/6 inhibitor encoding function of the CDKN2A locus is principally relevant [26]. Despite its oncogenic function, if mutated KRAS is expressed in cells with intact cell cycle checkpoints, it triggers a senescent-like state referred to oncogene-induced senescence leading to cell cycle arrest in G1 phase thereby preventing further transformation of the tissue [149,150,151,152]. Oncogene-induced senescence is triggered by either p53 or p16INK4a encoded by CDKN2A that inhibits CDK4/CDK6 and prevents the catalytic activity of the CDK/cyclin D complexes [153,154,155,156,157]. CDK4/6-cyclin D-associated hyperphosphorylation of Rb is prevented and consequently S-phase entry through suppression of E2F-mediated transcription [154,158]. In fully developed PDAC, oncogene-induced senescence is bypassed through inactivating of either Rb and/or TP53 or mutation of p16INK4a [158]. In line with this, germ-line mutations of p16INK4a have been associated with an elevated risk for developing PDAC through uncontrolled CDK4/6-mediated proliferation [149,159]. Besides, recent data underpin a concerted action of mutant KRAS with CDK5 and its activators to intensify malignant progression, migration, and invasion of pancreatic cancer cells [160]. CDK5 holds a unique position within the CDK family, as it becomes activated by the non-cyclin proteins p35 and p39. Although CDK5 shares high homology with CDK2, it has a marginal role in cell cycle control. Most substrates of CDK5 are associated with cell morphology and motility as well as cell–cell communication [161]. Different studies support the relationship between KRAS altered status and CDK inhibition as a potential avenue of treatment in cancer. CDK8 expression was found increased in KRAS-mutated pancreatic cancer samples [162], while KRAS-mutated PDAC was shown to be sensitive to CDK9-inhibition in patient-derived xenografts [163], and downstream inhibition of KRAS via AKT-inhibition combined with CDK-inhibition via dinaciclib showed dramatic improvements—even complete responses—in patient-derived xenograft models of pancreatic cancer in mice [164]. Of note, CDK4 inhibition was shown to be synthetically lethal with KRAS mutations in non-small cell lung cancer (NSCLC), an observation yet to be explored for PDAC [165].
Interestingly, pancreatic acinar cells of adult mice have been found to be resistant to transformation by KRAS with or without loss of p16Ink4a/p19Arf or TP53, yet they will show PanIN and even PDAC if mice were experimentally induced to develop pancreatitis. If expression of oncogenic KRAS was induced after pancreatitis induction, this would only induce PDAC in mice if residual inflammation was still present [166]. In this case, it is thought that the inflammatory response reduces the occurrence of senescence observed in benign lesions. Importantly, it has been found that the CDK4/6 inhibitor palbociclib is able to induce senescence in a breast cancer cell line [167], highlighting CDK inhibition as an interesting avenue of research in senescence induction and its role against oncogenic transformation.
DNA lesions caused by destructive reagents such as chemotherapeutics may trigger checkpoint arrest in the cell cycle and block CDK activity [168,169,170,171,172,173]. DNA damage regularly initiates the p53/p21 pathway resulting in G1 arrest to allow the repair machinery to restore genome stability. There is plenty of evidence in mice and human cells showing the p53-induced regulation of CDK2 by the inhibitor protein p21 [168,173]. Interestingly, it was also shown that apart from that checkpoint element, the binding of CDK2/cyclin E complexes, p21 binds CDK4/cyclin D and suppresses their kinase activities in a p53-dependent manner [174]. Since a vast number of pancreatic tumours carry silencing mutations of TP53, this checkpoint is also eliminated.
In case of overexpressed p16, the cellular response is either cell cycle arrest or apoptosis in different pancreatic cancer cell lines [175]. While p16 inhibits CDK4/6 activity leading to growth arrest, p16-induced apoptosis requires activation of p53-dependent cell death pathways [176,177,178]. In line with these findings, earlier results indicated the disruption of CDK4/6 by p16 leading to the rearrangement of the CDK inhibitors p21Cip1 and p27Kip1 [39]. p21/p27 are involved in binding and activity of CDK4/6-cyclin D enzymes, while they are also potent inhibitors of CDK2. p27 loss is frequently associated with tumorigenesis and it has been found that KRAS activation in the pancreas is associated with p27 mislocalization, while the absence of p27 or its ability to bind CDKs triggers the mislocalization of acinar polarity markers related to metaplasia and induces the nuclear expression of transcription factors involved in acinar-to-ductal metaplasia [179]. Also, it was shown that cyclin E overexpression facilitates p16-mediated circumvention of G1 arrest [180,181]. The molecular mechanism of the various fate of pancreatic cancer cells upon CDK4/6 inactivation is associated with the modulation of CDK2 activity [175].
CDK1 monitors M phase entry and exit and binds either cyclin A or cyclin B [134]. In addition to G2/M checkpoint maintenance, the activation of CDK1 is essential to governing mitosis, execution of apoptosis, pluripotency, and genomic stability in human pluripotent stem cells, their proliferation comparable to those of cancer cells [182]. Inactive CDK1, induced for example by cyclin damage, is a condition leading to a shift from M phase and the reconstruction of the G1 phase [183,184]. However, CDK1 activity was identified as essential for tumorigenesis as cell proliferation in transformed cells is mediated by CDK1 [184]. Tumour cells have some proliferation characteristics in common with stem cells; nevertheless, they appear more sensitive to loss of CDK activity [185]. The downregulation of CDK1 in human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPSC) leads to cell cycle modifications arresting the cells in G2 phase at the expense of G1 and S phase. In both cell types, the loss of the pluripotent stem cell morphology could be detected upon CDK1 downregulation. Knockdown of CDK1 resulted in a significant rise of double-strand breaks [182]. The growth of pancreatic cancer cell lines was reduced when CDC25B, an activator of CDK1, was inhibited resulting in accumulation of phosphorylated CDK1 and G2/M arrest [186].
In addition to their cell cycle-regulating features, a subset of CDKs, most importantly CDK7 and CDK9, have been identified to regulate RNA Pol II-mediated transcription [187,188]. We could recently demonstrate that the upregulation of CDK9 in human pancreatic cancer tissue negatively correlated with patients’ survival. These results became even more significant when the cohort was subdivided by tumour grades revealing a strong correlation between high CDK9 expression levels and reduced overall survival of PDAC patients with well-differentiated tumours (grade 1, grade 2) [139].
6. CDK Inhibition in PDAC
Due to their central role in cell cycle control, it is hardly surprising that CDKs and their substrates and regulators are identified as targets of genetic manipulations in various human cancers, which has accelerated the development of small molecule inhibitors against CDKs as an anticancer approach [26,189,190]. The “standard of care” for pancreatic cancer, which means the best treatment known so far, is still disappointing regarding the median overall survival of only six months [17,20,191]. Thus, there is a growing demand for new, more effective treatment possibilities, going in the direction of targeted therapies [26,190]. Of note, unfavourable toxicity profiles and severe adverse effects limited clinical success of potential CDK inhibitor candidates which is thought to be caused by inhibitory activity towards CDK1 which is known to be essential for all living cells [190].
Since the loss of p16INK4a is a standard feature in KRAS-driven PDAC [143], specific pharmacological inhibition of CDK4/6 is of substantial interest and presents a possible target for treatment. So far it has not been possible to design an inhibitor that is completely selective for a specific CDK, due to the lack of three-dimensional structure-displaying models of various CDKs and the high homology within the CDK family.
Currently, the oral CDK4/6-specific compounds palbociclib (PD-0332991) [192], ribociclib (LEE-011) [193], and abemaciclib (LY2835219) [194] are approved for the treatment of breast cancer. They inhibit retinoblastoma (Rb) protein phosphorylation in early G1 phase. Inhibition of Rb phosphorylation, in turn, avoids CDK-mediated G1-S phase transition, thereby arresting the cells in the G1 phase, suppressing DNA synthesis and constraining cancer cell growth. Notably, the approach is likely to succeed in cells exhibiting intact Rb, whereas palbociclib has been demonstrated failing in Rb-negative cancers [192]. Loss-of-function mutations in p16(INK4A) (CDKN2A) and therefore in the Rb pathway is a common feature of PDAC, and cause of an early progression in the disease. While this silencing activates CDK4/6, p16(INK4A)-deficient PDAC is nonetheless widely resistant to pharmacological CDK4/6 inhibition [195]. Furthermore, Chou and collaborators showed that inhibition of CDK4 with palbociclib significantly induces apoptosis in pancreatic tumour overexpressing Rb and also enhances the apoptotic effect of chemotherapeutic gemcitabine in patient-derived xenografts in mice. These studies suggest that a combination therapy including CDK4/6 inhibitors could be beneficial in Rb-positive pancreatic tumours and Rb can be used as a tool to select the patients that would benefit the most from this strategy. The approach of CDK4/6 inhibition by palbociclib was also evaluated in combination with IGF1 receptor inhibitors [196] and patient-derived models of pancreatic cancer primary tumour explants. The targeted therapy combinations exhibited robust cytotoxicity towards tumour cells [196]. The CDK4/6 inhibition resulted in potent suppression of tumour growth in the primary tumour explants illustrating the similarities of the biology between primary tumour model and tumour of origin [192]. LY2835219 inhibits CDK4/6 leading to reduced tumour growth in human xenografts, however, not demonstrated up until now for pancreatic cancer. The effect was enhanced in an epithelial tumour xenograft when the inhibitor was administered in combination with gemcitabine [194].
Although the underlying mechanism needs to be clarified, metabolic functions organise cell division [197] as the execution of the cell cycle entry is accompanied by an increase in cellular mass and accretion of energetic metabolites necessary for cell division [198]. However, much of the metabolic network is driven by mutated KRAS in a concerted manner with tumorigenic proliferation [199,200]. As metabolic characteristics of cancer gradually evolve as a therapeutic target, the CDK4/6 inhibitor-mediated metabolic state might be considered as a therapeutic target [201]. During the cell cycle, CDK4/6 inhibition has different effects but leads to the increase of tumour-associated mitochondrial mass via Rb and reactive oxygen species (ROS). Oxidative and glycolytic metabolic pathways are stimulated upon CDK4/6 inhibition. Apart from that, mTOR signalling is activated as a consequence of CDK4/6 blockade in vivo. These results indicate an active, feed-forward loop involving mTOR pathways for metabolic reprogramming. Consequently, cooperating effects could be shown with mTOR inhibitors resulting in apoptosis induction [201]. The fact that treatment alone with CDK4/6 inhibitors seems not very promising for pancreatic cancer [26], the CDK4/6 inhibition-mediated metabolic state might be additionally targeted [202]. Alternatively, the activity of CDK4/6 inhibitors can be exploited by the combination with mTOR pathway-selective compounds [203]. To overcome the robust apoptotic resistance of pancreatic cancer cells [9], the targeting of the tumour necrosis factor-related, apoptosis-inducing ligand receptors (TRAIL-R1/2) by their ligand TRAIL, in combination with CDK4/6 inhibitors could be used as an anticancer strategy. Indeed, blockage of CDK4/6 sensitises PDAC cells towards TRAIL-induced apoptosis [204]. However, there is additional need for optimisation of these treatment strategies since CDK4/6 inhibition antagonises chemotherapy and radiotherapy as their efficacy mostly relies on the fact that cancer cells undergo fast and uninhibited cell divisions [205,206].
Conversely, there is evidence that release from CDK4/6 inhibition may synchronise cells to go through the cell cycle in a concerted manner and eventually sensitise them to subsequent chemotherapy or may avoid current proliferation or repopulation of cells between treatment cycles [97]. P276-00, a CDK1, CDK4, and CDK9 inhibitor, could sensitise pancreatic cancer cells to gemcitabine-induced apoptosis. The data indicate that the combination might simultaneously target CDKs and Akt/mTOR signalling to inhibit both tumour progress and angiogenesis [207]. Angiogenesis is further influenced by CDK8, which has been found that when overexpressed, it promotes angiogenesis in pancreatic cancer via activation of CDK8/β-catenin/KLF2 signalling, while silencing of CDK8 inhibits angiogenesis in pancreatic cancer in vitro and in nude mice xenograft models [208]. However, the transfer of these hopeful preclinical models to the clinic, where vast genetic tumour heterogeneity and, consequently, dynamics of tumour growth are highly inconsistent between patients, is extremely challenging [209,210].
Dinaciclib (SCH727965) is a small molecule inhibitor with on-target activity in the low nanomolar range against CDK1, CDK2, CDK5, and CDK9. Pre-clinical testing of the substance showed acceptable toxicity and promising efficacy in mouse models and was well tolerated and active in phase I clinical trials [211,212]. Therefore, dinaciclib entered phase III clinical trials with refractory chronic lymphocytic leukaemia (CLL) [213]. In preclinical models for PDAC, monotherapy with dinaciclib inhibited growth, migration, and colony formation of pancreatic cancer cells through the blockage of cell cycle progression and reduction in Rb phosphorylation. Furthermore, this inhibition reduces RalA activity [214]. RalA is an effector of RAS signalling and plays a critical role in tumorigenicity of PDAC, observed for both human and mice [215,216,217]. RalA can be blocked by inactivation of CDK5 probably through effector cascades downstream of oncogenic KRAS. In PDAC xenografts the effects could be verified shown by a significant reduction in tumour growth [214].
Indeed, it has been demonstrated that CDK9 facilitates resistance to apoptosis through the transcriptional control of anti-apoptotic proteins such as Mcl-1 [218]. SNS-032 is a CDK9-selective inhibitor already undergoing clinical testing [219,220,221]. CDK9 was shown to be overexpressed in PDAC tissue and the in vitro response of pancreatic cancer cell lines results in markedly reduced cell viability upon CDK9 inhibition [139]. CDK9 inhibition by SNS-032 might, therefore, pose a promising therapeutic approach for pancreatic cancer. Mechanistically, CDK9 inhibition leads to apoptosis induction by shifting the ratio of anti-apoptotic and pro-apoptotic proteins in line with previous reports describing the inhibition of transcriptional elongation as an antagonist of short-lived anti-apoptotic proteins. As additive effects of CDK inhibition could be achieved in combination with chemotherapeutics, it might be promising to explore this approach as an alternative to conventional treatment strategies [188].
Rohitukine, a plant-derived chromone alkaloid, is the precursor of the promising anticancer clinical candidates flavopiridol (also known as alvocidib, HMR 1275, L86-8275) [222,223,224] and P276-00 [223,225]. However, the antitumor activity, pharmacokinetics, and CDK inhibitory potential of this parental product were not studied in detail for a long time. Eventually, in vitro toxicity could be reported in a variety of cancer cell lines including pancreatic cancer by the inhibition of CDK2 and CDK9 [226]. With the knowledge of the physicochemical properties and targets, the scaffold of rohitukine might be used for the design of further derivates.
7. Clinical Trials of CDK-Inhibitors
As discussed above, CDK-inhibition could be a promising strategy for new, advanced therapy protocols to treat pancreatic cancer. There are several ongoing clinical trials with a high number of CDK-inhibitors which we have summarized in Table 2. Unfortunately, pancreatic cancer as an entity is vastly underrepresented in these studies, with only a few results obtained so far.

Table 2.
CDK-specific inhibitors currently studied in clinical trials.
Flavopiridol (Alvocidib) was one of the first CDK inhibitors, with a wave of publications in the 2000s–2010s. Acting as a pan-CDK-inhibitor (targeting CDK1, 2, 4, 6, 7 and 9) [26], its first results in phase I studies in chronic lymphocytic leukemia (CLL) seemed encouraging, as most of the patients had a reduction in tumour mass; some even had complete responses [27]. Another phase I study, this time in solid tumours, could not confirm this enthusiasm: of 42 subjects, 22 had progression of the disease, 13 had a stable disease, and only seven subjects showed an improvement when flavopiridol was combined with conventional chemotherapy (Oxaliplatin, FOLFOX). Intriguingly, there was one complete response measurable in a case of pancreatic cancer [227]. Another study of this type, with different cytostatics (FOLFIRI; FOL = Folinic acid, F = 5-FU, IRI = Irinotecan), showed similar results [228]. A phase I dose-finding study with docetaxel and flavopiridol in advanced solid tumours showed cases of complete and partial responses towards this regime in pancreatic cancer, but a comparable amount of stable disease [229]. Again, Phase II studies could not confirm these first results. Until now, additional studies have been undertaken in metastatic melanoma, endometrial adenocarcinoma and multiple myeloma, without objective signs of antitumoural activity [190]. Moreover, flavopiridol showed severe adverse effects, and consequently, it was discontinued [240]. Interestingly, flavopiridol in combination with several classical chemotherapeutics, like doxorubicin, docetaxel and FOLFOX (FOL = Folinic acid, F = 5-FU, OX = Oxaliplatin), seems to exacerbate their anti-tumourigenic effect, although this is controversially discussed, as there are also contradictory results on this matter [230,232,258]. One phase II study directed solely towards pancreatic cancer (ClinicalTrials.gov Identifier NCT00331682) showed no benefit from treatment of gemcitabine-refractory, metastatic pancreatic cancer with a combination of docetaxel and flavopiridol [232,233]. Another study (ClinicalTrials.gov Identifier NCT00047307) currently investigates the effects of flavopiridol in combination with radiation, followed by gemcitabine on locally advanced, unresectable pancreatic cancer with no results published so far [231].
Dinaciclib, also acting as a multi-CDK-inhibitor, was first described in 2010 [211]. It showed desirable inhibitory effects towards pancreatic cancer in murine xenograft models [214]. Although clinical studies in humans with pancreatic cancer and dinaciclib are rare, it has been extensively studied in other tumour entities. Dinaciclib is one of the few CDK-inhibitors, next to palbociclib and abemaciclib, which made it past phase II into phase III in CLL [213]. One of the most significant predicted benefits of dinaciclib over flavopiridol is better tolerance over longer timespans, especially in refractory patients [234]. One study showed that with dinaciclib-treatment there is a significant response in CLL patients [234]. Another study demonstrated the efficacy of dinaciclib in relapsed multiple myeloma [237]. In a phase I trial in advanced malignancies dinaciclib was able to stabilise disease while being tolerable over a long time span [212]. In contrast to this, other phase I trials were less favourable. As such, Mitri et al. showed that the combination of dinaciclib with epirubicin could not stop the progression of triple negative breast cancer [235]. Furthermore, this regime came at a price of high toxicity [235]. A phase II study in advanced breast cancer showed capecitabin to be superior over dinaciclib concerning disease-free survival [238]. Dinaciclib showed no antitumoural effects and no superiority over erlotinib in progression-free survival for non-small cell lung cancer in a phase II study [239]. However, most of these studies show good tolerance towards dinaciclib when it is administered as a single drug [212,234,238,239].
Regarding phase III studies, dinaciclib was compared to ofatumumab, an anti-CD-20-antibody [259] in terms of efficacy and safety in relapsed or refractory CLL [213]. This study showed the promising antitumoural activity of dinaciclib in this hematologic neoplasia, with five times better overall response [213]. Currently, there is one already completed phase I study directed towards inoperable pancreatic cancer though there are no results published yet (ClinicalTrials.gov Identifier NCT01783171). In summary, it can be said that while dinaciclib seems like a well tolerable drug, it also seems to show superior anti-tumour activity in non-solid cancers.
SNS-032, a specific inhibitor of CDKs 2, 7, and 9 [219] has only undergone phase I studies until now. It was proven to be tolerable in humans [220]; however, the best clinical response in advanced solid tumours was stable disease in only 15% of cases, whereas the rest of the cases worsened. Again, another phase I study in the hematologic malignancies CLL and multiple myeloma showed better results for SNS-032 treatment [221].
Abemaciclib is a CDK4/6 inhibitor that has made it into phase III clinical studies and is even FDA-approved for certain advanced breast cancers [246]. This drug showed promising results for solid malignancies in several studies, including the extension of progression-free survival [243], even objective response rates in highly advanced breast cancers [242,244]. The JUNIPER study is of particular interest since it investigates the effects of abemaciclib on KRAS-mutated non-small cell lung cancer [245]. However, currently, there are no results posted (ClinicalTrials.gov Identifier: NCT02152631). Furthermore, there is an ongoing study targeting the effects of abemaciclib alone or in combination with other agents on previously treated PDAC (ClinicalTrials.gov Identifier: NCT02981342).
The CDK4/6 inhibitor palbociclib is FDA-approved for advanced breast cancers, and most literature refers to this substance in breast cancer and most studies investigate the effects of this substance in breast cancer [250,251,252,253]. Only a few studies addressing pancreatic cancer exist. These studies investigate the effects of combinatory treatments with palbociclib on pancreatic cancer: ClinicalTrials.gov Identifier: NCT02897375 (Cisplatin, Carboplatin), ClinicalTrials.gov Identifier: NCT03454035 (ERK1/2 Inhibitor Ulixertinib), ClinicalTrials.gov Identifier: NCT03065062 (PI3K/mTOR Inhibitor gedatolisib). As of now, no results have been posted yet.
There are ongoing studies with a number of other CDK-inhibitors that include pancreatic cancer: Ribociclib (ClinicalTrials.gov Identifier: NCT02703571; no results posted), the macrolide lactone molecule bryostatin-1 (ClinicalTrials.gov Identifier: NCT00031694; known for activation of protein kinase C, but also inhibition of CDK2 [190,255]; not effective in combination with paclitaxel in advanced pancreatic carcinoma) [256], as well as milciclib was reportedly able to overcome gemcitabine-resistance in a pancreatic cancer patients [257].
In summary, while mostly well tolerable, CDK-inhibitors seem to induce improved responses in hematologic malignancies more than in solid tumours. Reasons for this remain to be explored. Although there are many trials currently ongoing to address the role of CDKs in PDAC, there are still a lot of studies necessary to further evaluate the promise of CDK-inhibitors. For example, the identification of biomarkers, other than Rb, that can predict the response to CDK inhibitor therapies is needed, both to understand in a deeper manner the mechanisms of action of these inhibitors and to determine which patients benefit the most from combinatorial therapies.
CDK/cyclin complexes have been extensively studied in the past decades, yet their roles in the cell cycle and other related processes are not fully understood. It is likely that new functions of CDK/cyclin complexes will be discovered, adding more layers of complexity to the biological consequences of their inhibition, but also shedding more light into the mechanisms of tumorigenesis involved when these proteins expression and activity are altered. As CDK dysregulation is associated with tumorigenesis, these protein remain as an exciting target for therapeutic inhibition. In pancreatic cancer, there is an urgent need for treatment alternatives, and inhibition of CDKs represent a possible approach against this deadly disease.
Author Contributions
B.G.-R., A.-L.K., J.L. and D.H.-B. designed the study. B.G.-R., A.-L.K., J.-P.R. performed the literature search. A-L.K., B.G.-R. and J.L. prepared the figures. B.G.-R., A.-L.K., A.H., J.-P.R., U.K. and S.v.K. wrote the manuscript. All authors contributed to the preparation of the manuscript. All authors read and approved the manuscript.
Funding
This research was funded by a Deutsche Forschungsgemeinschaft research grant (LE3556/1-1) awarded to J.L. S.v.K. is funded through the Max Eder grant of the Deutsche Krebshilfe.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CAK | CDK-activating kinase |
| CDK | Cyclin-dependent kinase |
| CDKN2A | Cyclin-dependent kinase Inhibitor 2A |
| CLL | Chronic lymphocytic leukemia |
| CMGC | CDKs, MAPKs, Gsk3β, and CDK-like kinases |
| CTD | C-Terminal Domain |
| DYRK | Dual-specificity tyrosine-regulated kinase |
| ELL2 | Elongation Factor for RNA Polymerase II |
| FACT protein | Facilitates chromatin transcription |
| FDA | US Food and Drug Administration |
| FOLFIRINOX | FOL = Folinic acid, L = Leucovorin, F = 5-FU = 5-Fluorouracil, IRIN = Irinotecan, OX = Oxaliplatin |
| FoxM1 | Forkhead box protein M1 |
| GADD45 | Growth Arrest and DNA Damage-inducible protein |
| GSK3β | Glycogen synthase kinase-3 β |
| hESC | Human embryonic stem cells |
| hiPSC | Human induced pluripotent stem cells |
| INK4 | Inhibitor of Cyclin-Dependent Kinase 4 |
| MAPK | Mitogen-activated protein kinase |
| MAT1 | Menage á trois 1 |
| Mcl-1 | Myeloid leukaemia cell differentiation protein 1 |
| MED12 | Mediator complex subunit 12 |
| MED13 | Mediator complex subunit 13 |
| MPK7 | Mitogen-activated protein kinase 7 |
| mTOR | Mechanistic target of rapamycin |
| NSCLC | Non-small cell lung cancer |
| PanIN | Pancreatic intraepithelial neoplasia |
| PDAC | Pancreatic ductal adenocarcinoma |
| pTEFb | Positive transcription elongation factor b |
| RalA | RAS-Like Protein A |
| Rb | Retinoblastoma protein |
| RNA Pol II | RNA polymerase II |
| ROS | Reactive oxygen species |
| TAK | Tat-associated kinase |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| TF | Transcription factor |
| TFIIH | Transcription factor II H |
| TP53 | Tumour protein p53 |
| TPKII | Tau protein kinase II |
| TRAIL-R1/2 | Tumour necrosis factor-related, apoptosis-inducing ligand receptors 1/2 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M. Pancreatic Cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Simoes, P.K.; Olson, S.H.; Saldia, A.; Kurtz, R.C. Epidemiology of pancreatic adenocarcinoma. Chin. Clin. Oncol. 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Kupfer, S.S.; Brim, H.; Carethers, J.M. Accepted Manuscript Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterol. Gastrointest. Cancer Risk Gastroenterol. 2017. [Google Scholar] [CrossRef]
- Sanabria Mateos, R.; Conlon, K.C. Pancreatic cancer. Surgery 2016, 34, 282–291. [Google Scholar] [CrossRef]
- Mancuso, A.; Calabrò, F.; Sternberg, C.N. Current therapies and advances in the treatment of pancreatic cancer. Crit. Rev. Oncol. Hematol. 2006, 58, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; O’Hayer, K.; Blanco, F.F.; Winter, J.M.; Brody, J.R. The landscape of pancreatic cancer therapeutic resistance mechanisms. Int. J. Biol. Sci. 2016, 12, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Westphal, S.; Kalthoff, H. Apoptosis: Targets in Pancreatic Cancer. Mol. Cancer 2003, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Conroy, T.; Bachet, J.-B.; Ayav, A.; Huguet, F.; Lambert, A.; Caramella, C.; Maréchal, R.; Van Laethem, J.-L.; Ducreux, M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur. J. Cancer 2016, 57, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Kretz, A.-L.; von Karstedt, S.; Hillenbrand, A.; Henne-Bruns, D.; Knippschild, U.; Trauzold, A.; Lemke, J. Should We Keep Walking along the Trail for Pancreatic Cancer Treatment? Revisiting TNF-Related Apoptosis-Inducing Ligand for Anticancer Therapy. Cancers 2018, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Unicancer GI PRODIGE 24/CCTG PA.6 Trial: A Multicenter International Randomized Phase III Trial of Adjuvant mFOLFIRINOX versus Gemcitabine (gem) in Patients with Resected Pancreatic Ductal Adenocarcinomas. 2018 ASCO Annual Meeting Abstracts. Available online: http://abstracts.asco.org/214/AbstView_214_218335.html (accessed on 2 August 2018).
- Rossi, M.L.; Rehman, A.A.; Gondi, C.S. Therapeutic options for the management of pancreatic cancer. World J. Gastroenterol. 2014, 20, 11142–11159. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Van Buren, G.; Fisher, W.E. Pancreatic cancer: Advances in treatment. World J. Gastroenterol. 2014, 20, 9354–9360. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, W.; Huang, P.; Xu, Y.Z.; Heinemann, V.; Grunewald, R.; Gandhi, V. Gemcitabine: Metabolism, mechanisms of action, and self-potentiation. Semin. Oncol. 1995, 22, 3–10. [Google Scholar] [PubMed]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, S.W. Systemic Chemotherapy in Advanced Pancreatic Cancer. Gut Liver 2016, 10, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Saung, M.T.; Zheng, L. Current Standards of Chemotherapy for Pancreatic Cancer. Clin. Ther. 2017, 39, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Assaf, E.; Verlinde-Carvalho, M.; Delbaldo, C.; Grenier, J.; Sellam, Z.; Pouessel, D.; Bouaita, L.; Baumgaertner, I.; Sobhani, I.; Tayar, C.; et al. 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology 2011, 80, 301–306. [Google Scholar] [CrossRef] [PubMed]
- de W Marsh, R.; Talamonti, M.S.; Baker, M.S.; Posner, M.; Roggin, K.; Matthews, J.; Catenacci, D.; Kozloff, M.; Polite, B.; Britto, M.; et al. Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: A pilot study. J. Surg. Oncol. 2018, 117, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Gunturu, K.S.; Yao, X.; Cong, X.; Thumar, J.R.; Hochster, H.S.; Stein, S.M.; Lacy, J. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: Single institution retrospective review of efficacy and toxicity. Med. Oncol. 2013, 30, 361. [Google Scholar] [CrossRef] [PubMed]
- Faris, J.E.; Blaszkowsky, L.S.; McDermott, S.; Guimaraes, A.R.; Szymonifka, J.; Huynh, M.A.; Ferrone, C.R.; Wargo, J.A.; Allen, J.N.; Dias, L.E.; et al. FOLFIRINOX in locally advanced pancreatic cancer: The Massachusetts General Hospital Cancer Center experience. Oncologist 2013, 18, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.T.; Jones, J.A.; Maddocks, K.; Poi, M.; Grever, M.R.; Johnson, A.; Byrd, J.C.; Andritsos, L.A. A phase 1 clinical trial of flavopiridol consolidation in chronic lymphocytic leukemia patients following chemoimmunotherapy. Ann. Hematol. 2016, 95, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Hanks, S.K.; Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995, 9, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Mikolcevic, P.; Rainer, J.; Geley, S. Orphan kinases turn eccentric: A new class of cyclin Y-activated, membrane-targeted CDKs. Cell Cycle 2012, 11, 3758–3768. [Google Scholar] [CrossRef] [PubMed]
- Rane, S.G.; Dubus, P.; Mettus, R.V.; Galbreath, E.J.; Boden, G.; Reddy, E.P.; Barbacid, M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat. Genet. 1999, 22, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Berthet, C.; Aleem, E.; Coppola, V.; Tessarollo, L.; Kaldis, P. Cdk2 knockout mice are viable. Curr. Biol. 2003, 13, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.; Prieto, I.; Odajima, J.; Martin, A.; Dubus, P.; Sotillo, R.; Barbero, J.L.; Malumbres, M.; Barbacid, M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 2003, 35, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Sotillo, R.; Santamaria, D.; Galan, J.; Cerezo, A.; Ortega, S.; Dubus, P.; Barbacid, M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 2004, 118, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Harlow, E.; Hunt, T.; Hunter, T.; Lahti, J.M.; Manning, G.; Morgan, D.O.; Tsai, L.H.; Wolgemuth, D.J. Cyclin-dependent kinases: A family portrait. Nat. Cell Biol. 2009, 11, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, L.; Ratnacaram, C.K.; Kaldis, P. Established and novel Cdk/cyclin complexes regulating the cell cycle and development. Results Probl. Cell Differ. 2011, 53, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. To cycle or not to cycle: A critical decision in cancer. Nat. Rev. Cancer 2001, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bendris, N.; Lemmers, B.; Blanchard, J.M. Cell cycle, cytoskeleton dynamics and beyond: The many functions of cyclins and CDK inhibitors. Cell Cycle 2015, 14, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Pavletich, N.P. Mechanisms of cyclin-dependent kinase regulation: Structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 1999, 287, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.O. Principles of CDK regulation. Nature 1995, 374, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Norbury, C.J.; Spurr, N.K.; Nurse, P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature 1988, 333, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Jing, T.; Liu, B.; Yao, J.; Tan, M.; McDonnell, T.J.; Hung, M.C. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol. Cell 1998, 2, 581–591. [Google Scholar] [CrossRef]
- Moore, J.D.; Kirk, J.A.; Hunt, T. Unmasking the S-phase-promoting potential of cyclin B1. Science 2003, 300, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, D.; Barriere, C.; Cerqueira, A.; Hunt, S.; Tardy, C.; Newton, K.; Caceres, J.F.; Dubus, P.; Malumbres, M.; Barbacid, M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 2007, 448, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.H.; Harlow, E.; Meyerson, M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature 1991, 353, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Sheaff, R.J.; Groudine, M.; Gordon, M.; Roberts, J.M.; Clurman, B.E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997, 11, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliffe, E.H.; Li, C.; Thompson, E.A.; Maller, J.L.; Sluder, G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science 1999, 283, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Maller, J.L. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science 2004, 306, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Zariwala, M.; Liu, J.; Xiong, Y. Cyclin E2, a novel human G1 cyclin and activating partner of CDK2 and CDK3, is induced by viral oncoproteins. Oncogene 1998, 17, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhu, C.; Harper, J.W. A premature-termination mutation in the Mus musculus cyclin-dependent kinase 3 gene. Proc. Natl. Acad. Sci. USA 2001, 98, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Rollins, B.J. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 2004, 117, 239–251. [Google Scholar] [CrossRef]
- Harbour, J.W.; Luo, R.X.; Dei Santi, A.; Postigo, A.A.; Dean, D.C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 1999, 98, 859–869. [Google Scholar] [CrossRef]
- Lazarov, M.; Kubo, Y.; Cai, T.; Dajee, M.; Tarutani, M.; Lin, Q.; Fang, M.; Tao, S.; Green, C.L.; Khavari, P.A. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat. Med. 2002, 8, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, I.; Denissova, N.G.; Wang, G.; He, D.; Long, J.; Liu, F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 2004, 430, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, M.; Harlow, E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell Biol. 1994, 14, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Fernandes, H.; Rocha, B. High expression of active CDK6 in the cytoplasm of CD8 memory cells favors rapid division. Nat. Immunol. 2004, 5, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.N.; Zukerberg, L.; Nikolic, M.; de la Monte, S.; Dikkes, P.; Tsai, L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 1999, 402, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Dhavan, R.; Tsai, L.H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001, 2, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Brinkkoetter, P.T.; Olivier, P.; Wu, J.S.; Henderson, S.; Krofft, R.D.; Pippin, J.W.; Hockenbery, D.; Roberts, J.M.; Shankland, S.J. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Investig. 2009, 119, 3089–3101. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Hashimoto, T.; Nakashima, A.; Hisanaga, S.; Kikkawa, U.; Kamada, S. Cyclin I is involved in the regulation of cell cycle progression. Cell Cycle 2013, 12, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.P.; Morgan, D.O. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 1994, 78, 713–724. [Google Scholar] [CrossRef]
- Shiekhattar, R.; Mermelstein, F.; Fisher, R.P.; Drapkin, R.; Dynlacht, B.; Wessling, H.C.; Morgan, D.O.; Reinberg, D. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature 1995, 374, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Akoulitchev, S.; Chuikov, S.; Reinberg, D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 2000, 407, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Tassan, J.P.; Jaquenoud, M.; Leopold, P.; Schultz, S.J.; Nigg, E.A. Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc. Natl. Acad. Sci. USA 1995, 92, 8871–8875. [Google Scholar] [CrossRef] [PubMed]
- Firestein, R.; Bass, A.J.; Kim, S.Y.; Dunn, I.F.; Silver, S.J.; Guney, I.; Freed, E.; Ligon, A.H.; Vena, N.; Ogino, S.; et al. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature 2008, 455, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.; Fukasawa, R.; Tanaka, A.; Hirose, Y.; Ohkuma, Y. Identification of target genes for the CDK subunits of the Mediator complex. Genes Cells 2011, 16, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.M.; Byers, S.A.; Maury, W.; Price, D.H. Identification of a novel isoform of Cdk9. Gene 2003, 307, 175–182. [Google Scholar] [CrossRef]
- Garriga, J.; Grana, X. Cellular control of gene expression by T-type cyclin/CDK9 complexes. Gene 2004, 337, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Guen, V.J.; Gamble, C.; Flajolet, M.; Unger, S.; Thollet, A.; Ferandin, Y.; Superti-Furga, A.; Cohen, P.A.; Meijer, L.; Colas, P. CDK10/cyclin M is a protein kinase that controls ETS2 degradation and is deficient in STAR syndrome. Proc. Natl. Acad. Sci. USA 2013, 110, 19525–19530. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Wang, Y.C.; Fann, M.J. Identification and characterization of the CDK12/cyclin L1 complex involved in alternative splicing regulation. Mol. Cell. Biol. 2006, 26, 2736–2745. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yang, W.; Ni, T.; Tang, Z.; Nakadai, T.; Zhu, J.; Roeder, R.G. RNA polymerase II-associated factor 1 regulates the release and phosphorylation of paused RNA polymerase II. Science 2015, 350, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Kohoutek, J.; Blazek, D. Cyclin K goes with Cdk12 and Cdk13. Cell Div. 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Mayeda, A.; Trembley, J.H.; Lahti, J.M.; Kidd, V.J. CDK11 complexes promote pre-mRNA splicing. J. Biol. Chem. 2003, 278, 8623–8629. [Google Scholar] [CrossRef] [PubMed]
- Loyer, P.; Trembley, J.H.; Grenet, J.A.; Busson, A.; Corlu, A.; Zhao, W.; Kocak, M.; Kidd, V.J.; Lahti, J.M. Characterization of cyclin L1 and L2 interactions with CDK11 and splicing factors: Influence of cyclin L isoforms on splice site selection. J. Biol. Chem. 2008, 283, 7721–7732. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.; Shen, J.; Huang, Y.L.; Su, Y.; Karaulanov, E.; Bartscherer, K.; Hassler, C.; Stannek, P.; Boutros, M.; Niehrs, C. Cell cycle control of wnt receptor activation. Dev. Cell 2009, 17, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Romano, G. Deregulations in the cyclin-dependent kinase-9-related pathway in cancer: Implications for drug discovery and development. ISRN Oncol. 2013, 2013, 305371. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, S.Y.; Kim, Y.J.; Chung, Y.H. ALS2CR7 (CDK15) attenuates TRAIL induced apoptosis by inducing phosphorylation of survivin Thr34. Biochem. Biophys. Res. Commun. 2014, 450, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Dixon-Clarke, S.E.; Shehata, S.N.; Krojer, T.; Sharpe, T.D.; von Delft, F.; Sakamoto, K.; Bullock, A.N. Structure and inhibitor specificity of the PCTAIRE-family kinase CDK16. Biochem. J. 2017, 474, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Tamaru, T.; Okumura, N.; Nagai, K.; Okada, M. PCTAIRE 2, a Cdc2-related serine/threonine kinase, is predominantly expressed in terminally differentiated neurons. Eur. J. Biochem. 1997, 249, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Besset, V.; Rhee, K.; Wolgemuth, D.J. The cellular distribution and kinase activity of the Cdk family member Pctaire1 in the adult mouse brain and testis suggest functions in differentiation. Cell Growth Differ. 1999, 10, 173–181. [Google Scholar] [PubMed]
- Okuda, T.; Cleveland, J.L.; Downing, J.R. PCTAIRE-1 and PCTAIRE-3, two members of a novel cdc2/CDC28-related protein kinase gene family. Oncogene 1992, 7, 2249–2258. [Google Scholar] [PubMed]
- Barone, G.; Staples, C.J.; Ganesh, A.; Patterson, K.W.; Bryne, D.P.; Myers, K.N.; Patil, A.A.; Eyers, C.E.; Maslen, S.; Skehel, J.M.; et al. Human CDK18 promotes replication stress signaling and genome stability. Nucleic Acids Res. 2016, 44, 8772–8785. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kawamoto, K.; Miyamoto, K.; Tsuji, A.; Yuasa, K. PCTK3/CDK18 regulates cell migration and adhesion by negatively modulating FAK activity. Sci. Rep. 2017, 7, 45545. [Google Scholar] [CrossRef] [PubMed]
- Wohlbold, L.; Larochelle, S.; Liao, J.C.; Livshits, G.; Singer, J.; Shokat, K.M.; Fisher, R.P. The cyclin-dependent kinase (CDK) family member PNQALRE/CCRK supports cell proliferation but has no intrinsic CDK-activating kinase (CAK) activity. Cell Cycle 2006, 5, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, J.; Lu, Y.; Zhang, S.; Huang, J.; Chen, J.; Bei, J.X.; Yang, K.; Wu, G.; Huang, K.; et al. CDK20 interacts with KEAP1 to activate NRF2 and promotes radiochemoresistance in lung cancer cells. Oncogene 2017, 36, 5321–5330. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Fabbro, D.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The Concise Guide to Pharmacology 2017/18: Enzymes. Br. J. Pharmacol. 2017, 174 (Suppl. 1), S272–S359. [Google Scholar] [CrossRef]
- Tang, D.; Chun, A.C.; Zhang, M.; Wang, J.H. Cyclin-dependent kinase 5 (Cdk5) activation domain of neuronal Cdk5 activator. Evidence of the existence of cyclin fold in neuronal Cdk5a activator. J. Biol. Chem. 1997, 272, 12318–12327. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.C.; Watenpaugh, K.D.; Heinrikson, R.L. A model of the complex between cyclin-dependent kinase 5 and the activation domain of neuronal Cdk5 activator. Biochem. Biophys. Res. Commun. 1999, 259, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Woo, R.A.; Poon, R.Y. Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle 2003, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Sanso, M.; Fisher, R.P. Modelling the CDK-dependent transcription cycle in fission yeast. Biochem. Soc. Trans. 2013, 41, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004, 18, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Di Liberto, M.; Jayabalan, D.; Liang, J.; Ely, S.; Bretz, J.; Shaffer, A.L., 3rd; Louie, T.; Chen, I.; Randolph, S.; et al. Prolonged early G(1) arrest by selective CDK4/CDK6 inhibition sensitizes myeloma cells to cytotoxic killing through cell cycle-coupled loss of IRF4. Blood 2012, 120, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.; Dong, F.; Pledger, W.J. Differential modulation of G1 cyclins and the Cdk inhibitor p27kip1 by platelet-derived growth factor and plasma factors in density-arrested fibroblasts. J. Biol. Chem. 1996, 271, 11253–11260. [Google Scholar] [CrossRef] [PubMed]
- Aktas, H.; Cai, H.; Cooper, G.M. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol. Cell. Biol. 1997, 17, 3850–3857. [Google Scholar] [CrossRef] [PubMed]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Cobrinik, D. Pocket proteins and cell cycle control. Oncogene 2005, 24, 2796–2809. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998, 12, 2245–2262. [Google Scholar] [CrossRef] [PubMed]
- Friend, S.H.; Bernards, R.; Rogelj, S.; Weinberg, R.A.; Rapaport, J.M.; Albert, D.M.; Dryja, T.P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 1986, 323, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.A.; Buchkovich, K.J.; Marshak, D.R.; Anderson, C.W.; Harlow, E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991, 10, 4279–4290. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Dean, D.C. Rb function in cell-cycle regulation and apoptosis. Nat. Cell Biol. 2000, 2, E65–E67. [Google Scholar] [CrossRef] [PubMed]
- Narasimha, A.M.; Kaulich, M.; Shapiro, G.S.; Choi, Y.J.; Sicinski, P.; Dowdy, S.F. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. eLife 2014, 3, e02872. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, C.; Klier, S.; McGowan, C.; Wittenberg, C.; de Bruin, R.A. Chk1 inhibits E2F6 repressor function in response to replication stress to maintain cell-cycle transcription. Curr. Biol. 2013, 23, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.K.; Wang, X.; Li, Y.; Ekholm-Reed, S.; Wu, X.; Wang, P.; Reed, S.I. Cyclin E deregulation promotes loss of specific genomic regions. Curr. Biol. 2015, 25, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.; Murphy, M.; Kubelka, M.; Ravnik, S.E.; Hawkins, C.F.; Wolgemuth, D.J.; Carrington, M. A distinct cyclin A is expressed in germ cells in the mouse. Development 1996, 122, 53–64. [Google Scholar] [PubMed]
- Coverley, D.; Laman, H.; Laskey, R.A. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat. Cell Biol. 2002, 4, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Wang, C.; Li, Z.; Sakamaki, T.; Pestell, R.G. Minireview: Cyclin D1: Normal and abnormal functions. Endocrinology 2004, 145, 5439–5447. [Google Scholar] [CrossRef] [PubMed]
- Crasta, K.; Lim, H.H.; Zhang, T.; Nirantar, S.; Surana, U. Consorting kinases, end of destruction and birth of a spindle. Cell Cycle 2008, 7, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Drozdowska, A.; Dobrynin, G. A role for Cdc48/p97 and Aurora B in controlling chromatin condensation during exit from mitosis. Biochem. Cell Biol. 2010, 88, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Gavet, O.; Pines, J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J. Cell Biol. 2010, 189, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Draviam, V.M.; Orrechia, S.; Lowe, M.; Pardi, R.; Pines, J. The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. J. Cell Biol. 2001, 152, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Mailand, N.; Podtelejnikov, A.V.; Groth, A.; Mann, M.; Bartek, J.; Lukas, J. Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 2002, 21, 5911–5920. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, K.A.; Michelson, R.J.; Putnam, C.W.; Weinert, T.A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 2002, 36, 617–656. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, J.M.; Lees, J.A. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 2002, 3, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Dominguez-Brauer, C.; Wang, Z.; Asara, J.M.; Costa, R.H.; Tyner, A.L.; Lau, L.F.; Raychaudhuri, P. A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1. J. Biol. Chem. 2009, 284, 30695–30707. [Google Scholar] [CrossRef] [PubMed]
- Major, M.L.; Lepe, R.; Costa, R.H. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol. Cell. Biol. 2004, 24, 2649–2661. [Google Scholar] [CrossRef] [PubMed]
- Laoukili, J.; Alvarez, M.; Meijer, L.A.; Stahl, M.; Mohammed, S.; Kleij, L.; Heck, A.J.; Medema, R.H. Activation of FoxM1 during G2 requires cyclin A/Cdk-dependent relief of autorepression by the FoxM1 N-terminal domain. Mol. Cell. Biol. 2008, 28, 3076–3087. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Wang, Z.; Costa, R.H.; Tyner, A.; Lau, L.F.; Raychaudhuri, P. An N-terminal inhibitory domain modulates activity of FoxM1 during cell cycle. Oncogene 2008, 27, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Rickert, P.; Corden, J.L.; Lees, E. Cyclin C/CDK8 and cyclin H/CDK7/p36 are biochemically distinct CTD kinases. Oncogene 1999, 18, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, J.O.; Zhu, X.; Gustafsson, C.M. The multitalented Mediator complex. Trends Biochem. Sci. 2013, 38, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Nemet, J.; Jelicic, B.; Rubelj, I.; Sopta, M. The two faces of Cdk8, a positive/negative regulator of transcription. Biochimie 2014, 97, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, H.; Makela, T.P.; Conaway, J.W.; Conaway, R.C.; Weinberg, R.A.; Young, R.A. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 1995, 374, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, M.; Marion, J.; Zhu, Y.; Price, D.H. RNA polymerase II elongation control. Cold Spring Harb. Symp. Quant. Biol. 1998, 63, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.J.; Peng, J.; Lee, G.; Price, D.H.; Flores, O. Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J. Biol. Chem. 1999, 274, 34527–34530. [Google Scholar] [CrossRef] [PubMed]
- Oqani, R.K.; Kim, H.R.; Diao, Y.F.; Park, C.S.; Jin, D.I. The CDK9/cyclin T1 subunits of P-TEFb in mouse oocytes and preimplantation embryos: A possible role in embryonic genome activation. BMC Dev. Biol. 2011, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yik, J.H. The Yin and Yang of P-TEFb regulation: Implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol. Mol. Biol. Rev. 2006, 70, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.A.; Edgar, A.J.; Ehley, J.; Gottesfeld, J.M. Cyclin L is an RS domain protein involved in pre-mRNA splicing. J. Biol. Chem. 2002, 277, 25465–25473. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, B.; Liu, P.; Phatnani, H.P.; Fuda, N.J.; Cooper, J.J.; Price, D.H.; Adelman, K.; Lis, J.T.; Greenleaf, A.L. CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev. 2010, 24, 2303–2316. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.W.; Kuzyk, M.A.; Moradian, A.; Ichu, T.A.; Chang, V.C.; Tien, J.F.; Vollett, S.E.; Griffith, M.; Marra, M.A.; Morin, G.B. Interaction of cyclin-dependent kinase 12/CrkRS with cyclin K1 is required for the phosphorylation of the C-terminal domain of RNA polymerase II. Mol. Cell. Biol. 2012, 32, 4691–4704. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Bukholm, I.R.; Bukholm, G.; Nesland, J.M. Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int. J. Cancer 2001, 93, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.H.; Fung, T.K.; Poon, R.Y. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 2002, 59, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Kretz, A.L.; Schaum, M.; Richter, J.; Kitzig, E.F.; Engler, C.C.; Leithauser, F.; Henne-Bruns, D.; Knippschild, U.; Lemke, J. CDK9 is a prognostic marker and therapeutic target in pancreatic cancer. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Ying, H.; Tian, T.; Ling, J.; Fu, J.; Lu, Y.; Wu, M.; Yang, L.; Achreja, A.; Chen, G.; et al. Mutant Kras- and p16-regulated NOX4 activation overcomes metabolic checkpoints in development of pancreatic ductal adenocarcinoma. Nat. Commun. 2017, 8, 14437. [Google Scholar] [CrossRef] [PubMed]
- Caldas, C.; Hahn, S.A.; da Costa, L.T.; Redston, M.S.; Schutte, M.; Seymour, A.B.; Weinstein, C.L.; Hruban, R.H.; Yeo, C.J.; Kern, S.E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat. Genet. 1994, 8, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.K.; Grimmond, S.M.; Biankin, A.V. Pancreatic cancer genomics. Curr. Opin. Genet. Dev. 2014, 24, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Wilentz, R.E.; Kern, S.E. Genetic progression in the pancreatic ducts. Am. J. Pathol. 2000, 156, 1821–1825. [Google Scholar] [CrossRef]
- LaPak, K.M.; Burd, C.E. The molecular balancing act of p16(INK4a) in cancer and aging. Mol. Cancer Res. 2014, 12, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, D.L.; Sage, J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer 2008, 8, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Infante, J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016, 45, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Zinczuk, J.; Zareba, K.; Guzinska-Ustymowicz, K.; Kedra, B.; Kemona, A.; Pryczynicz, A. p16, p21, and p53 proteins play an important role in development of pancreatic intraepithelial neoplastic. Ir. J. Med. Sci. 2018, 187, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.M.; Fraser, M.C.; Struewing, J.P.; Hussussian, C.J.; Ranade, K.; Zametkin, D.P.; Fontaine, L.S.; Organic, S.M.; Dracopoli, N.C.; Clark, W.H., Jr.; et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N. Engl. J. Med. 1995, 333, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Bartkova, J.; Rezaei, N.; Liontos, M.; Karakaidos, P.; Kletsas, D.; Issaeva, N.; Vassiliou, L.V.; Kolettas, E.; Niforou, K.; Zoumpourlis, V.C.; et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006, 444, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, H.; Beach, D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 1992, 71, 505–514. [Google Scholar] [CrossRef]
- Serrano, M.; Hannon, G.J.; Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Mammalian G1 cyclins. Cell 1993, 73, 1059–1065. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, H.; Beach, D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993, 7, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; Borja, N.A.; Franco, J.; Brody, J.R.; Yeo, C.J.; Mansour, J.; Choti, M.A.; McCue, P.; Knudsen, E.S. Selective impact of CDK4/6 suppression on patient-derived models of pancreatic cancer. Oncotarget 2015, 6, 15788–15801. [Google Scholar] [CrossRef] [PubMed]
- Chicas, A.; Wang, X.; Zhang, C.; McCurrach, M.; Zhao, Z.; Mert, O.; Dickins, R.A.; Narita, M.; Zhang, M.; Lowe, S.W. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 2010, 17, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Whelan, A.J.; Bartsch, D.; Goodfellow, P.J. Brief report: A familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene. N. Engl. J. Med. 1995, 333, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Eggers, J.P.; Grandgenett, P.M.; Collisson, E.C.; Lewallen, M.E.; Tremayne, J.; Singh, P.K.; Swanson, B.J.; Andersen, J.M.; Caffrey, T.C.; High, R.R.; et al. Cyclin-dependent kinase 5 is amplified and overexpressed in pancreatic cancer and activated by mutant K-Ras. Clin. Cancer Res. 2011, 17, 6140–6150. [Google Scholar] [CrossRef] [PubMed]
- Dhariwala, F.A.; Rajadhyaksha, M.S. An unusual member of the Cdk family: Cdk5. Cell. Mol. Neurobiol. 2008, 28, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Z.; Zhang, W.; Qian, K.; Li, H.; Kong, D.; Li, Y.; Tang, Y. Mutated K-ras activates CDK8 to stimulate the epithelial-to-mesenchymal transition in pancreatic cancer in part via the Wnt/β-catenin signaling pathway. Cancer Lett. 2015, 356, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Allaway, R.J.; Fischer, D.A.; de Abreu, F.B.; Gardner, T.B.; Gordon, S.R.; Barth, R.J.; Colacchio, T.A.; Wood, M.; Kacsoh, B.Z.; Bouley, S.J.; et al. Genomic characterization of patient-derived xenograft models established from fine needle aspirate biopsies of a primary pancreatic ductal adenocarcinoma and from patient-matched metastatic sites. Oncotarget 2016, 7, 17087–17102. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dadon, T.; Chenna, V.; Yabuuchi, S.; Bannerji, R.; Booher, R.; Strack, P.; Azad, N.; Nelkin, B.D.; Maitra, A. Combined Inhibition of Cyclin-Dependent Kinases (Dinaciclib) and AKT (MK-2206) Blocks Pancreatic Tumor Growth and Metastases in Patient-Derived Xenograft Models. Mol. Cancer Ther. 2015, 14, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Puyol, M.; Martin, A.; Dubus, P.; Mulero, F.; Pizcueta, P.; Khan, G.; Guerra, C.; Santamaria, D.; Barbacid, M. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 2010, 18, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.; Collado, M.; Navas, C.; Schuhmacher, A.J.; Hernandez-Porras, I.; Canamero, M.; Rodriguez-Justo, M.; Serrano, M.; Barbacid, M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 2011, 19, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.P.; Peltier, J.; Hartlova, A.; Gierlinski, M.; Jansen, V.M.; Trost, M.; Bjorklund, M. Thermal proteome profiling of breast cancer cells reveals proteasomal activation by CDK4/6 inhibitor palbociclib. EMBO J. 2018, 37. [Google Scholar] [CrossRef] [PubMed]
- Dulic, V.; Kaufmann, W.K.; Wilson, S.J.; Tlsty, T.D.; Lees, E.; Harper, J.W.; Elledge, S.J.; Reed, S.I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 1994, 76, 1013–1023. [Google Scholar] [CrossRef]
- Elledge, S.J. Cell cycle checkpoints: Preventing an identity crisis. Science 1996, 274, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M. Mammalian G1 and G2 phase checkpoints. Cancer Surv. 1997, 29, 151–182. [Google Scholar] [PubMed]
- Bartek, J.; Lukas, J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 2001, 13, 738–747. [Google Scholar] [CrossRef]
- Attardi, L.D.; de Vries, A.; Jacks, T. Activation of the p53-dependent G1 checkpoint response in mouse embryo fibroblasts depends on the specific DNA damage inducer. Oncogene 2004, 23, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Wierod, L.; Rosseland, C.M.; Lindeman, B.; Oksvold, M.P.; Grosvik, H.; Skarpen, E.; Huitfeldt, H.S. Activation of the p53-p21(Cip1) pathway is required for CDK2 activation and S-phase entry in primary rat hepatocytes. Oncogene 2008, 27, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Siddik, Z.H.; Huang, Z.; Wang, R.; Koomen, J.; Kobayashi, R.; Khokhar, A.R.; Kuang, J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 2005, 24, 2929–2943. [Google Scholar] [CrossRef] [PubMed]
- Calbo, J.; Serna, C.; Garriga, J.; Grana, X.; Mazo, A. The fate of pancreatic tumor cell lines following p16 overexpression depends on the modulation of CDK2 activity. Cell Death Differ. 2004, 11, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, S.; Harlow, E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 1993, 262, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, K.; Kataoka, M.; Uno, F.; Murakami, T.; Kondo, T.; Roth, J.A.; Tanaka, N.; Fujiwara, T. Activation of caspase-3 and cleavage of Rb are associated with p16-mediated apoptosis in human non-small cell lung cancer cells. Oncogene 2002, 21, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Katayose, Y.; Rojanala, L.; Shah, S.; Sgagias, M.; Jang, L.; Jung, Y.J.; Lee, S.H.; Hwang, S.G.; Cowan, K.H. Induction of apoptosis in p16INK4A mutant cell lines by adenovirus-mediated overexpression of p16INK4A protein. Cell Death Differ. 2000, 7, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, P.; Callot, C.; Baer, R.; Duquesnes, N.; Guerra, C.; Guillermet-Guibert, J.; Bachs, O.; Besson, A. Loss of p27Kip(1) promotes metaplasia in the pancreas via the regulation of Sox9 expression. Oncotarget 2015, 6, 35880–35892. [Google Scholar] [CrossRef] [PubMed]
- Lukas, J.; Herzinger, T.; Hansen, K.; Moroni, M.C.; Resnitzky, D.; Helin, K.; Reed, S.I.; Bartek, J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997, 11, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Alevizopoulos, K.; Vlach, J.; Hennecke, S.; Amati, B. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 1997, 16, 5322–5333. [Google Scholar] [CrossRef] [PubMed]
- Neganova, I.; Tilgner, K.; Buskin, A.; Paraskevopoulou, I.; Atkinson, S.P.; Peberdy, D.; Passos, J.F.; Lako, M. CDK1 plays an important role in the maintenance of pluripotency and genomic stability in human pluripotent stem cells. Cell Death Dis. 2014, 5, e1508. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, L.T.; Tovar, C.; Chen, S.; Knezevic, D.; Zhao, X.; Sun, H.; Heimbrook, D.C.; Chen, L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA 2006, 103, 10660–10665. [Google Scholar] [CrossRef] [PubMed]
- Diril, M.K.; Ratnacaram, C.K.; Padmakumar, V.C.; Du, T.; Wasser, M.; Coppola, V.; Tessarollo, L.; Kaldis, P. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Berthet, C.; Kaldis, P. Cell-specific responses to loss of cyclin-dependent kinases. Oncogene 2007, 26, 4469–4477. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kleeff, J.; Li, J.; Ding, J.; Hammer, J.; Zhao, Y.; Giese, T.; Korc, M.; Buchler, M.W.; Friess, H. Expression and functional significance of CDC25B in human pancreatic ductal adenocarcinoma. Oncogene 2004, 23, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.P. Secrets of a double agent: CDK7 in cell-cycle control and transcription. J. Cell Sci. 2005, 118, 5171–5180. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fischer, P.M. Cyclin-dependent kinase 9: A key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends Pharmacol. Sci. 2008, 29, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Pevarello, P.; Barbacid, M.; Bischoff, J.R. CDK inhibitors in cancer therapy: What is next? Trends Pharmacol. Sci. 2008, 29, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Schwartz, G.K. Development of cell-cycle inhibitors for cancer therapy. Curr. Oncol. 2009, 16, 36–43. [Google Scholar] [PubMed]
- Lemke, J.; Schäfer, D.; Sander, S.; Henne-Bruns, D.; Kornmann, M. Survival and prognostic factors in pancreatic and ampullary cancer. Anticancer Res. 2014, 34, 3011–3020. [Google Scholar] [PubMed]
- Fry, D.W.; Harvey, P.J.; Keller, P.R.; Elliott, W.L.; Meade, M.; Trachet, E.; Albassam, M.; Zheng, X.; Leopold, W.R.; Pryer, N.K.; et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 2004, 3, 1427–1438. [Google Scholar] [PubMed]
- Rader, J.; Russell, M.R.; Hart, L.S.; Nakazawa, M.S.; Belcastro, L.T.; Martinez, D.; Li, Y.; Carpenter, E.L.; Attiyeh, E.F.; Diskin, S.J.; et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin. Cancer Res. 2013, 19, 6173–6182. [Google Scholar] [CrossRef] [PubMed]
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; Del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig. New Drugs 2014, 32, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, A.M.; Perera, R.M.; Ecker, V.; Nicolay, B.N.; Bardeesy, N.; Benes, C.H.; Dyson, N.J. CDK4/6 and IGF1 receptor inhibitors synergize to suppress the growth of p16INK4A-deficient pancreatic cancers. Cancer Res. 2014, 74, 3947–3958. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.; Froio, D.; Nagrial, A.M.; Parkin, A.; Murphy, K.J.; Chin, V.T.; Wohl, D.; Steinmann, A.; Stark, R.; Drury, A.; et al. Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut 2017. [Google Scholar] [CrossRef] [PubMed]
- Fajas, L. Re-thinking cell cycle regulators: The cross-talk with metabolism. Front. Oncol. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Tu, B.P. Driving the cell cycle through metabolism. Annu. Rev. Cell Dev. Biol. 2012, 28, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Bryant, K.L.; Mancias, J.D.; Kimmelman, A.C.; Der, C.J. KRAS: Feeding pancreatic cancer proliferation. Trends Biochem. Sci. 2014, 39, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.M.; Kimmelman, A.C. The complex landscape of pancreatic cancer metabolism. Carcinogenesis 2014, 35, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.; Balaji, U.; Freinkman, E.; Witkiewicz, A.K.; Knudsen, E.S. Metabolic re-programming of pancreatic cancer mediated by CDK4/6 inhibition elicits unique vulnerabilities. Cell Rep. 2016, 14, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.; Witkiewicz, A.K.; Knudsen, E.S. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget 2014, 5, 6512–6525. [Google Scholar] [CrossRef] [PubMed]
- Retzer-Lidl, M.; Schmid, R.M.; Schneider, G. Inhibition of CDK4 impairs proliferation of pancreatic cancer cells and sensitizes towards TRAIL-induced apoptosis via downregulation of survivin. Int. J. Cancer 2007, 121, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.J.; Bisi, J.E.; Strum, J.C.; Combest, A.J.; Darr, D.B.; Usary, J.E.; Zamboni, W.C.; Wong, K.K.; Perou, C.M.; Sharpless, N.E. Multiple Roles of Cyclin-Dependent Kinase 4/6 Inhibitors in Cancer Therapy. J. Natl. Cancer Inst. 2012, 104, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.L.; McClendon, A.K.; Knudsen, E.S. Modification of the DNA damage response by therapeutic CDK4/6 inhibition. J. Biol. Chem. 2012, 287, 29075–29087. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; Periyasamy, G.; Ponnurangam, S.; Chakrabarti, D.; Sugumar, A.; Padigaru, M.; Weir, S.J.; Balakrishnan, A.; Sharma, S.; Anant, S. CDK-4 inhibitor P276 sensitizes pancreatic cancer cells to gemcitabine-induced apoptosis. Mol. Cancer Ther. 2012, 11, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Kong, L.; Xiao, Y.; Yuan, H.; Song, Y.; Wang, J.; Yu, H.; Mao, S.; Xu, W. CDK8 regulates the angiogenesis of pancreatic cancer cells in part via the CDK8-β-catenin-KLF2 signal axis. Exp. Cell Res. 2018, 369, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Raffenne, J.; Couvelard, A.; Pote, N. Tumor Heterogeneity in Pancreatic Adenocarcinoma. Pathobiology 2018, 85, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, V.L.; Garcia-Garijo, A.; van Laarhoven, H.W.; Bijlsma, M.F. Extracellular Influences: Molecular Subclasses and the Microenvironment in Pancreatic Cancer. Cancers 2018, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.; Guzi, T.; Shanahan, F.; Davis, N.; Prabhavalkar, D.; Wiswell, D.; Seghezzi, W.; Paruch, K.; Dwyer, M.P.; Doll, R.; et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol. Cancer Ther. 2010, 9, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.J.; Small, K.A.; Kirschmeier, P.; Zhang, D.; Zhu, Y.; Jou, Y.M.; Statkevich, P.; Yao, S.L.; Bannerji, R. A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J. Transl. Med. 2013, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Scarfò, L.; Perez, S.; Pathiraja, K.; Derosier, M.; Small, K.; McCrary Sisk, C.; Patton, N. Efficacy and safety of dinaciclib vs ofatumumab in patients with relapsed/refractory chronic lymphocytic leukemia. Blood 2017, 129, 1876–1878. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, G.; Mishra, A.; Bisht, S.; Karikari, C.; Garrido-Laguna, I.; Rasheed, Z.; Ottenhof, N.A.; Dadon, T.; Alvarez, H.; Fendrich, V.; et al. Cyclin-dependent kinase inhibitor Dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. Cancer Biol. Ther. 2011, 12, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Hamad, N.M.; Elconin, J.H.; Karnoub, A.E.; Bai, W.; Rich, J.N.; Abraham, R.T.; Der, C.J.; Counter, C.M. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002, 16, 2045–2057. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Baines, A.T.; Fiordalisi, J.J.; Shipitsin, M.; Feig, L.A.; Cox, A.D.; Der, C.J.; Counter, C.M. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 2005, 7, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; O’Hayer, K.; Adam, S.J.; Kendall, S.D.; Campbell, P.M.; Der, C.J.; Counter, C.M. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr. Biol. 2006, 16, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wierda, W.G.; Chubb, S.; Hawtin, R.E.; Fox, J.A.; Keating, M.J.; Gandhi, V.; Plunkett, W. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood 2009, 113, 4637–4645. [Google Scholar] [CrossRef] [PubMed]
- Conroy, A.; Stockett, D.E.; Walker, D.; Arkin, M.R.; Hoch, U.; Fox, J.A.; Hawtin, R.E. SNS-032 is a potent and selective CDK 2, 7 and 9 inhibitor that drives target modulation in patient samples. Cancer Chemother. Pharmacol. 2009, 64, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I.; Bible, K.; Martell, R.E.; Adelman, D.C.; Lorusso, P.M. A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Investig. New Drugs 2008, 26, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.G.; Chen, R.; Plunkett, W.; Siegel, D.; Sinha, R.; Harvey, R.D.; Badros, A.Z.; Popplewell, L.; Coutre, S.; Fox, J.A.; et al. Phase I and Pharmacologic Study of SNS-032, a Potent and Selective Cdk2, 7, and 9 Inhibitor, in Patients with Advanced Chronic Lymphocytic Leukemia and Multiple Myeloma. J. Clin. Oncol. 2010, 28, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Senderowicz, A.M. Flavopiridol: The first cyclin-dependent kinase inhibitor in human clinical trials. Investig. New Drugs 1999, 17, 313–320. [Google Scholar] [CrossRef]
- Cassaday, R.D.; Goy, A.; Advani, S.; Chawla, P.; Nachankar, R.; Gandhi, M.; Gopal, A.K. A phase II, single-arm, open-label, multicenter study to evaluate the efficacy and safety of P276-00, a cyclin-dependent kinase inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Clin. Lymphoma Myeloma Leuk. 2015, 15, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Stetler-Stevenson, M.; Sebers, S.; Worland, P.; Sedlacek, H.; Myers, C.; Czech, J.; Naik, R.; Sausville, E. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J. Natl. Cancer Inst. 1992, 84, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.S.; Rathos, M.J.; Mahajan, P.; Wagh, V.; Shenoy, S.; Bhatia, D.; Chile, S.; Sivakumar, M.; Maier, A.; Fiebig, H.H.; et al. P276-00, a novel cyclin-dependent inhibitor induces G1-G2 arrest, shows antitumor activity on cisplatin-resistant cells and significant in vivo efficacy in tumor models. Mol. Cancer Ther. 2007, 6, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Guru, S.K.; Jain, S.K.; Joshi, P.; Gandhi, S.G.; Bharate, S.B.; Bhushan, S.; Bharate, S.S.; Vishwakarma, R.A. A chromatography-free isolation of rohitukine from leaves of Dysoxylum binectariferum: Evaluation for in vitro cytotoxicity, CDK inhibition and physicochemical properties. Bioorg. Med. Chem. Lett. 2016, 26, 3457–3463. [Google Scholar] [CrossRef] [PubMed]
- Rathkopf, D.; Dickson, M.A.; Feldman, D.R.; Carvajal, R.D.; Shah, M.A.; Wu, N.; Lefkowitz, R.; Gonen, M.; Cane, L.M.; Dials, H.J.; et al. Phase I study of flavopiridol with oxaliplatin and fluorouracil/leucovorin in advanced solid tumors. Clin. Cancer Res. 2009, 15, 7405–7411. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Shah, M.A.; Rathkopf, D.; Tse, A.; Carvajal, R.D.; Wu, N.; Lefkowitz, R.A.; Gonen, M.; Cane, L.M.; Dials, H.J.; et al. A phase I clinical trial of FOLFIRI in combination with the pan-cyclin-dependent kinase (CDK) inhibitor flavopiridol. Cancer Chemother. Pharmacol. 2010, 66, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Fornier, M.N.; Rathkopf, D.; Shah, M.; Patil, S.; O’Reilly, E.; Tse, A.N.; Hudis, C.; Lefkowitz, R.; Kelsen, D.P.; Schwartz, G.K. Phase I Dose-Finding Study of Weekly Docetaxel Followed by Flavopiridol for Patients with Advanced Solid Tumors. Clin. Cancer Res. 2007, 13. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; D’Adamo, D.R.; Dickson, M.A.; Keohan, M.L.; Carvajal, R.D.; Maki, R.G.; de Stanchina, E.; Musi, E.; Singer, S.; Schwartz, G.K. The cyclin-dependent kinase inhibitor flavopiridol potentiates doxorubicin efficacy in advanced sarcomas: Preclinical investigations and results of a phase I dose-escalation clinical trial. Clin. Cancer Res. 2012, 18, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT00047307. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Carvajal, R.D.; Tse, A.; Shah, M.A.; Lefkowitz, R.A.; Gonen, M.; Gilman-Rosen, L.; Kortmansky, J.; Kelsen, D.P.; Schwartz, G.K.; O’Reilly, E.M. A phase II study of flavopiridol (Alvocidib) in combination with docetaxel in refractory, metastatic pancreatic cancer. Pancreatology 2009, 9, 404–409. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT00331682. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Flynn, J.; Jones, J.; Johnson, A.J.; Andritsos, L.; Maddocks, K.; Jaglowski, S.; Hessler, J.; Grever, M.R.; Im, E.; Zhou, H.; et al. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia 2015, 29, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Mitri, Z.; Karakas, C.; Wei, C.; Briones, B.; Simmons, H.; Ibrahim, N.; Alvarez, R.; Murray, J.L.; Keyomarsi, K.; Moulder, S. A phase 1 study with dose expansion of the CDK inhibitor dinaciclib (SCH 727965) in combination with epirubicin in patients with metastatic triple negative breast cancer. Investig. New Drugs 2015, 33, 890–894. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT01783171. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Kumar, S.K.; LaPlant, B.; Chng, W.J.; Zonder, J.; Callander, N.; Fonseca, R.; Fruth, B.; Roy, V.; Erlichman, C.; Stewart, A.K.; et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015, 125, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.M.; Joy, A.A.; Mita, A.; Sankhala, K.; Jou, Y.-M.; Zhang, D.; Statkevich, P.; Zhu, Y.; Yao, S.-L.; Small, K.; et al. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin. Breast Cancer 2014, 14, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.J.; Nemunaitis, J.; Joy, A.A.; Martin, J.C.; Jou, Y.-M.; Zhang, D.; Statkevich, P.; Yao, S.-L.; Zhu, Y.; Zhou, H.; et al. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer 2014, 83, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, S.; Liu, Q.; Yuan, X.; Mani, S.; Pestell, R.G.; Wu, K. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J. Hematol. Oncol. 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT02981342. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2−Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Shi, P.; Reck, M.; Paz-Ares, L.; Koustenis, A.; Hurt, K.C. Treatment Rationale and Study Design for the JUNIPER Study: A Randomized Phase III Study of Abemaciclib With Best Supportive Care Versus Erlotinib With Best Supportive Care in Patients with Stage IV Non-Small-Cell Lung Cancer with a Detectable KRAS Mutati. Clin. Lung Cancer 2016, 17, 80–84. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Press Announcements—FDA Approves New Treatment for Certain Advanced or Metastatic Breast Cancers. Available online: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm578071.htm (accessed on 31 July 2018).
- U.S. National Library of Medicine. Identifier: NCT02897375. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- U.S. National Library of Medicine. Identifier: NCT03454035. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- U.S. National Library of Medicine. Identifier: NCT03065062. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Ma, C.X.; Gao, F.; Luo, J.; Northfelt, D.W.; Goetz, M.; Forero, A.; Hoog, J.; Naughton, M.; Ademuyiwa, F.; Suresh, R.; et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin. Cancer Res. 2017, 23, 4055–4065. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Lu, J. Palbociclib: A first-in-class CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive advanced breast cancer. J. Hematol. Oncol. 2015, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT02703571. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Asiedu, C.; Biggs, J.; Lilly, M.; Kraft, A.S. Inhibition of leukemic cell growth by the protein kinase C activator bryostatin 1 correlates with the dephosphorylation of cyclin-dependent kinase 2. Cancer Res. 1995, 55, 3716–3720. [Google Scholar] [PubMed]
- Lam, A.P.; Sparano, J.A.; Vinciguerra, V.; Ocean, A.J.; Christos, P.; Hochster, H.; Camacho, F.; Goel, S.; Mani, S.; Kaubisch, A. Phase II study of paclitaxel plus the protein kinase C inhibitor bryostatin-1 in advanced pancreatic carcinoma. Am. J. Clin. Oncol. 2010, 33, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Aspeslagh, S.; Shailubhai, K.; Bahleda, R.; Gazzah, A.; Varga, A.; Hollebecque, A.; Massard, C.; Spreafico, A.; Reni, M.; Soria, J.-C. Phase I dose-escalation study of milciclib in combination with gemcitabine in patients with refractory solid tumors. Cancer Chemother. Pharmacol. 2017, 79, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Delohery, T.M.; Schwartz, G.K.; Patil, S.; O’Reilly, E.; Tse, A.N.; Hudis, C.; Lefkowitz, R.; Kelsen, D.P.; Schwartz, G.K. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin. Cancer Res. 1999, 5, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Ofatumumab. mAbs 2009, 1, 326–331. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).