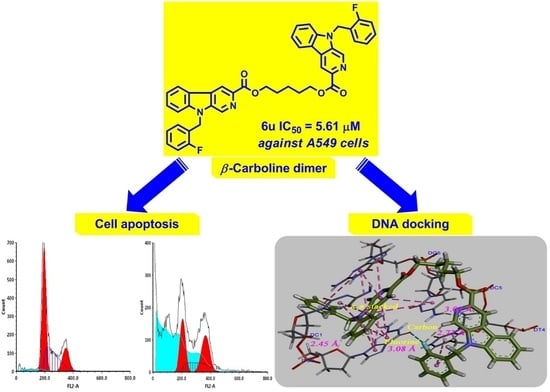
Synthesis and In Vitro Antitumor Activity of Novel Bivalent β-Carboline-3-carboxylic Acid Derivatives with DNA as a Potential Target
Abstract
1. Introduction
2. Results
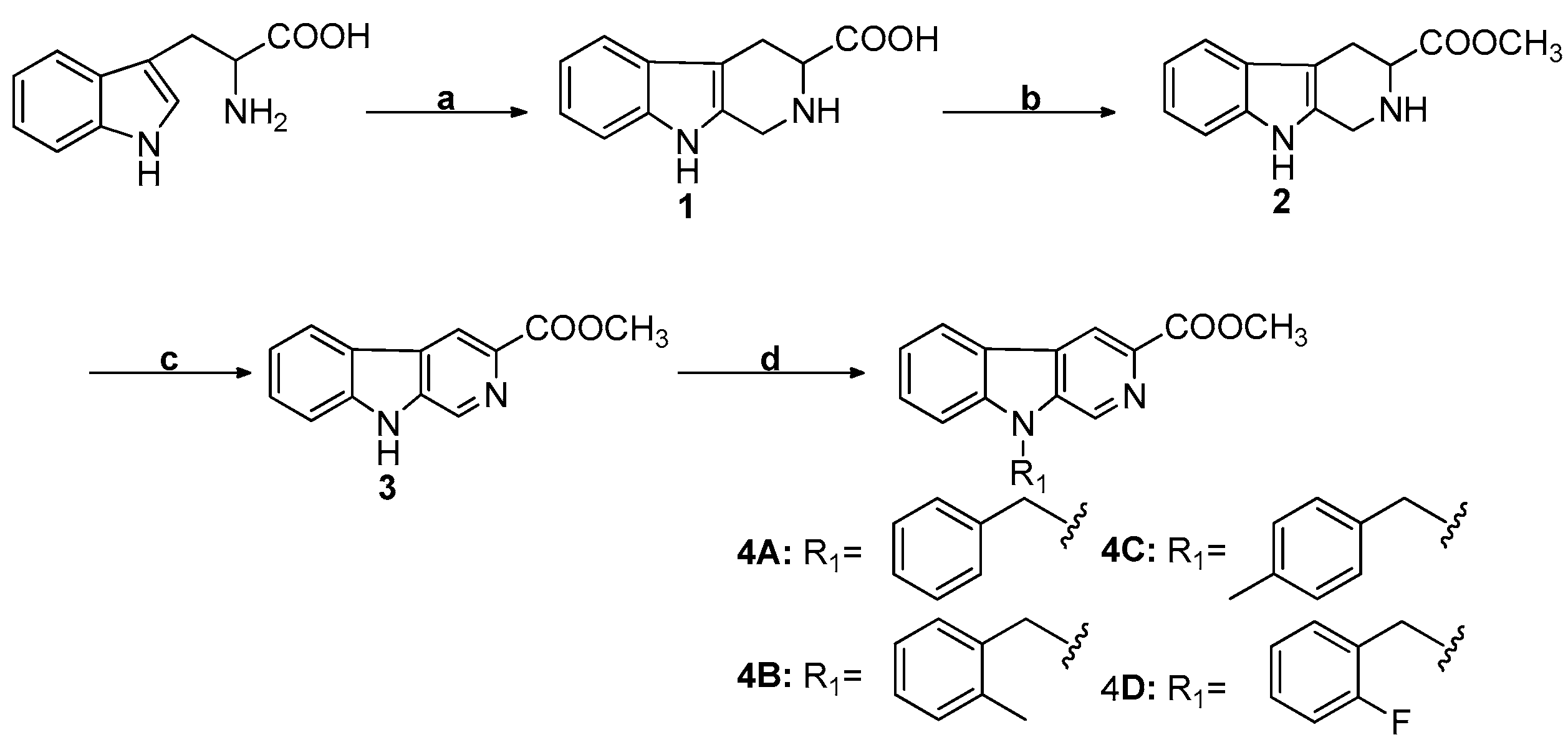
2.1. Chemistry
2.2. In Vitro Cytotoxicity Assay
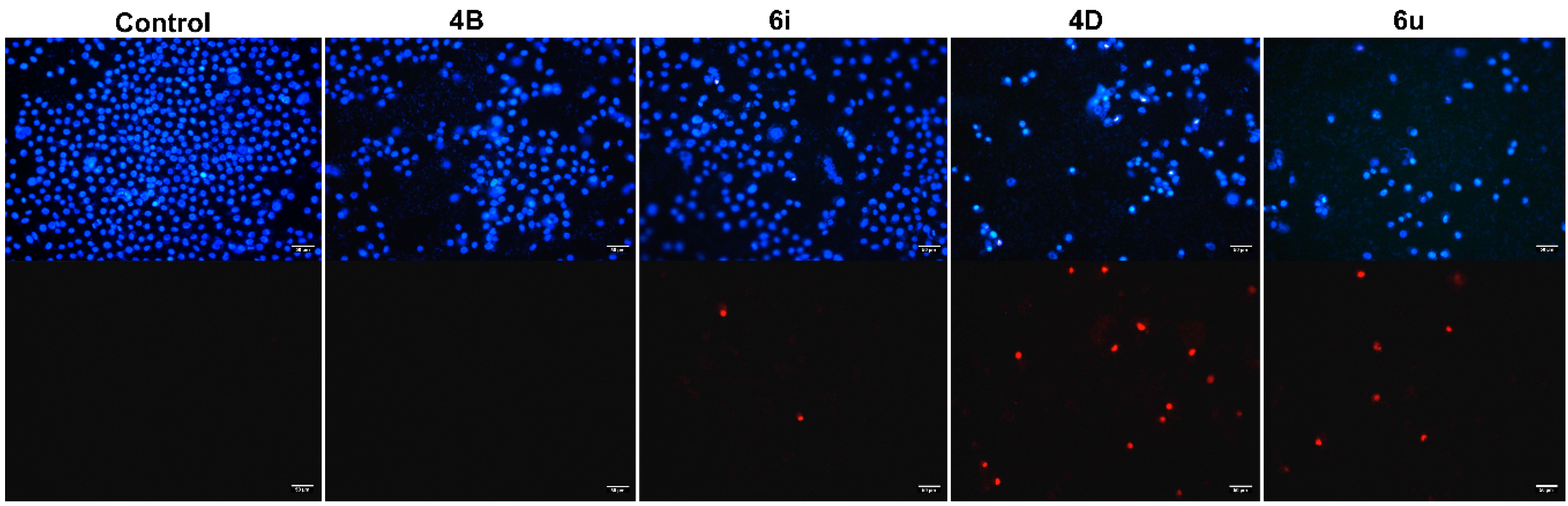
2.3. The Morphological Observation of Cell Apoptosis
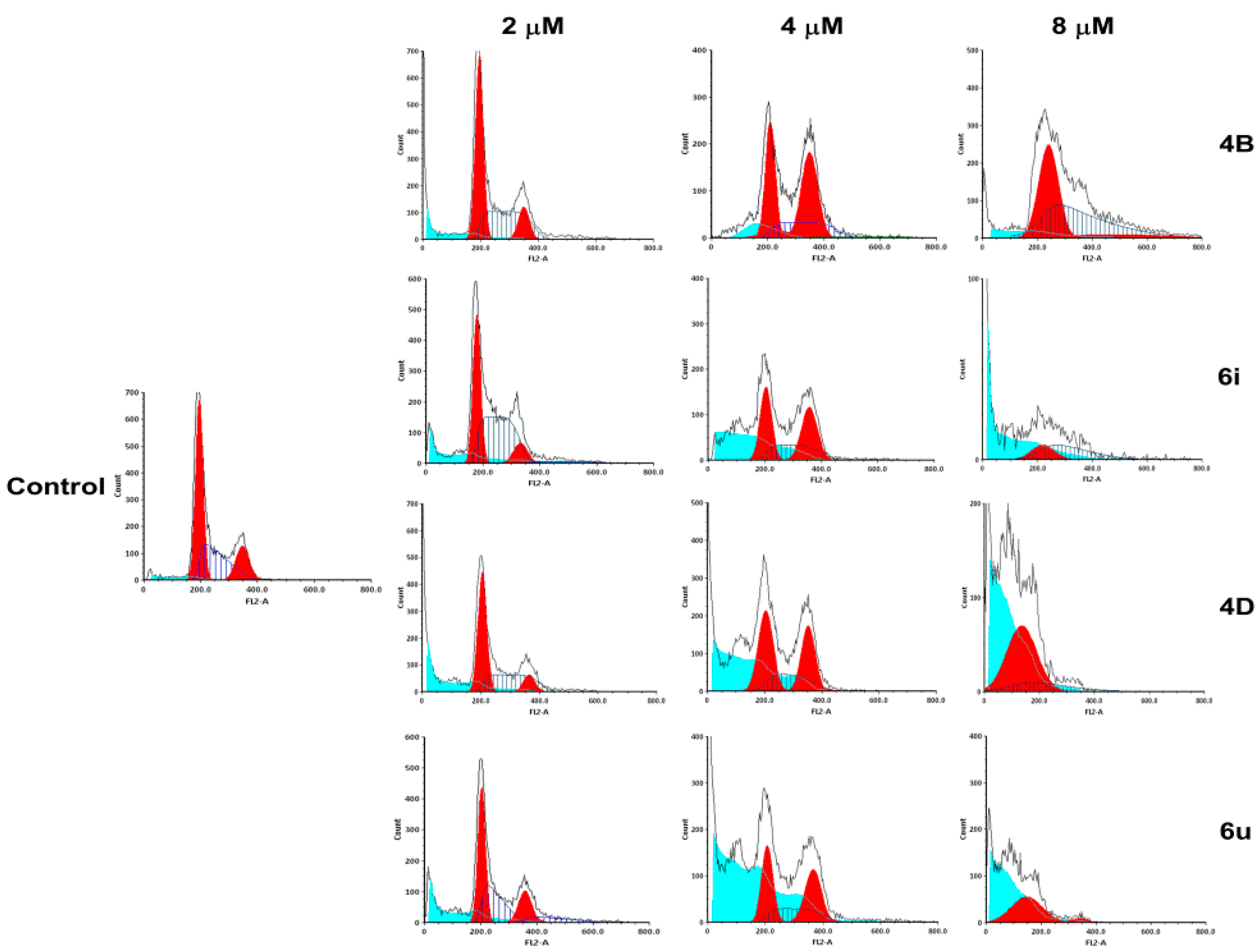
2.4. Cell Cycle Assay
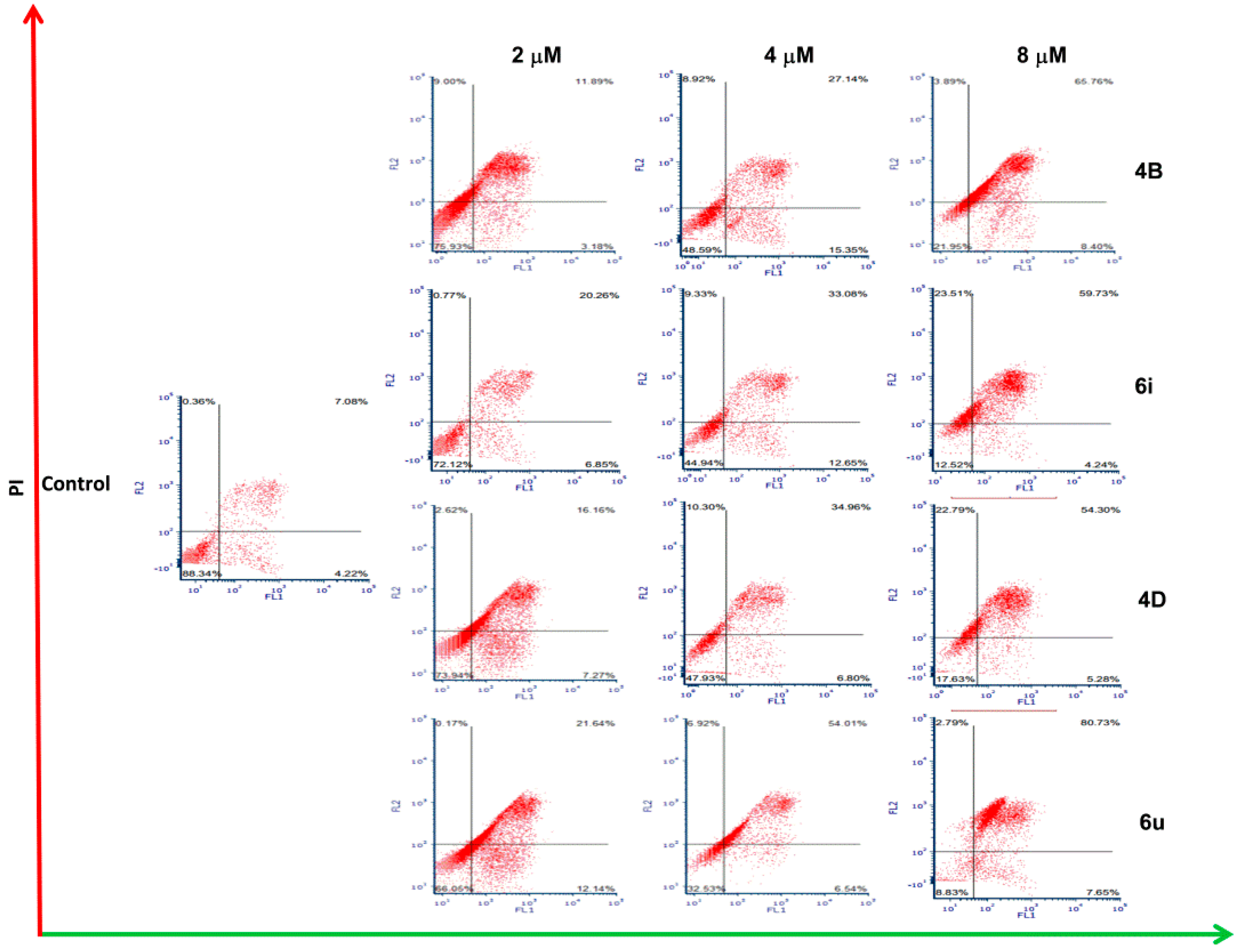
2.5. Cell Apoptosis Assay
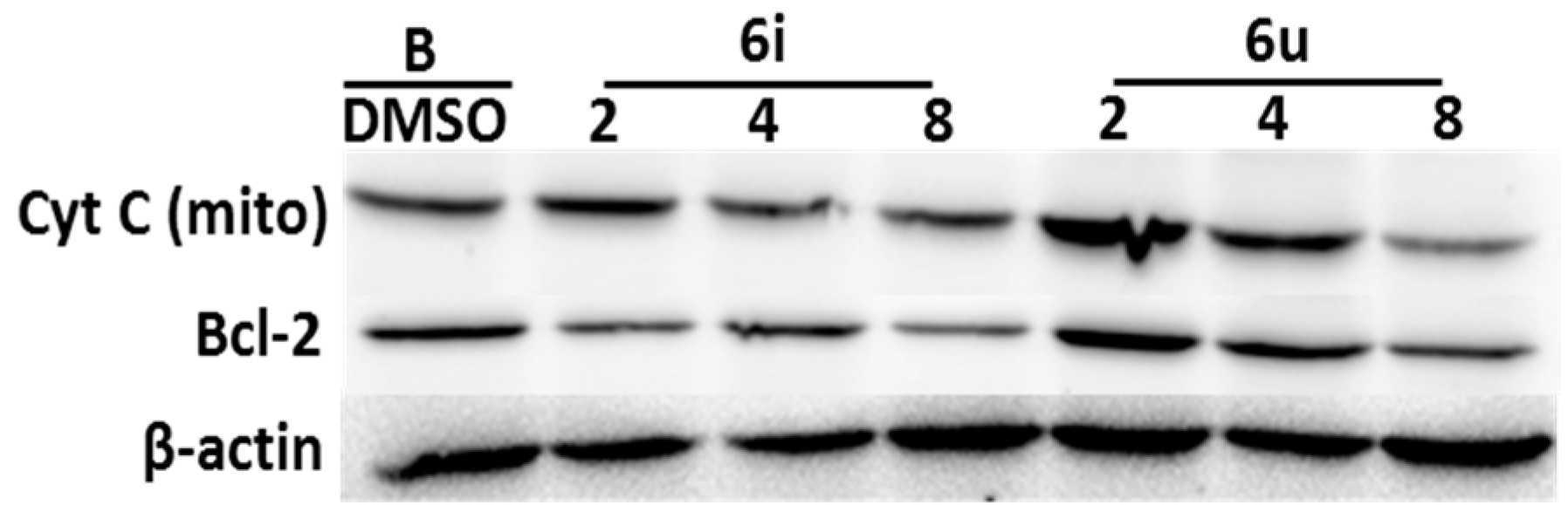
2.6. The Detection of Apoptosis-Related Protein (Cytochrome C, bcl-2)
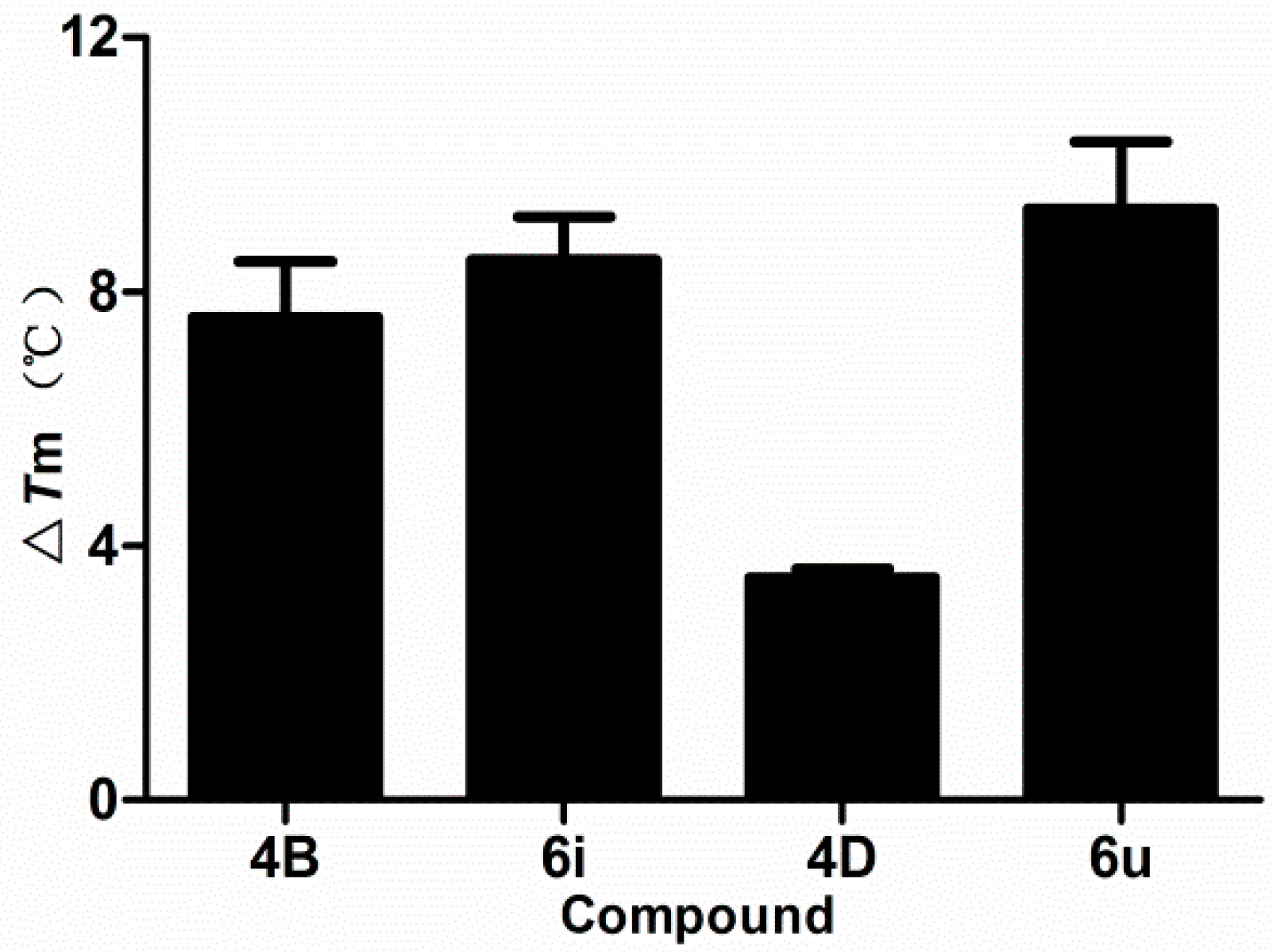
2.7. DNA Binding Studies
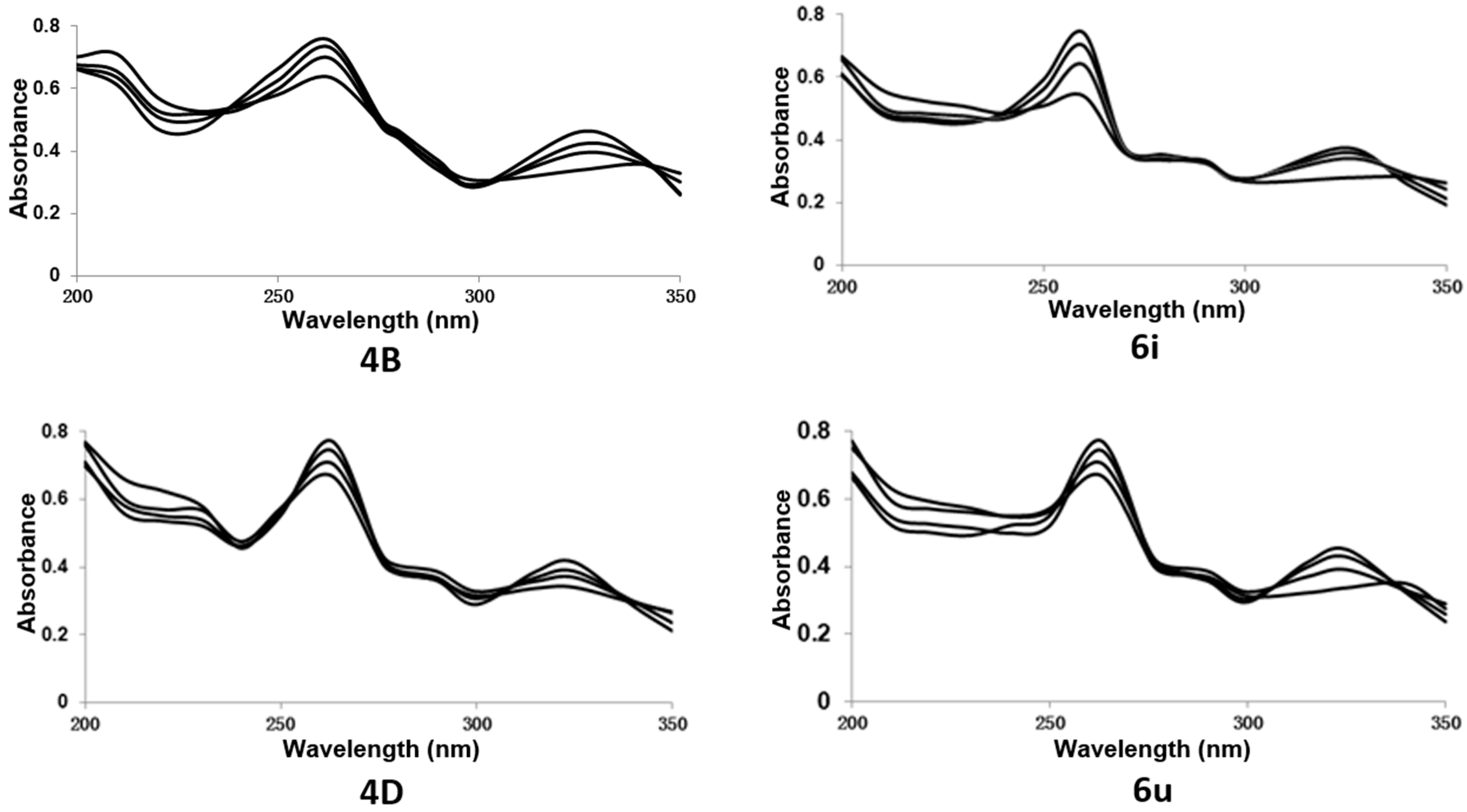
2.7.1. UV-Visible Spectral Study
2.7.2. Thermal Denaturation Study
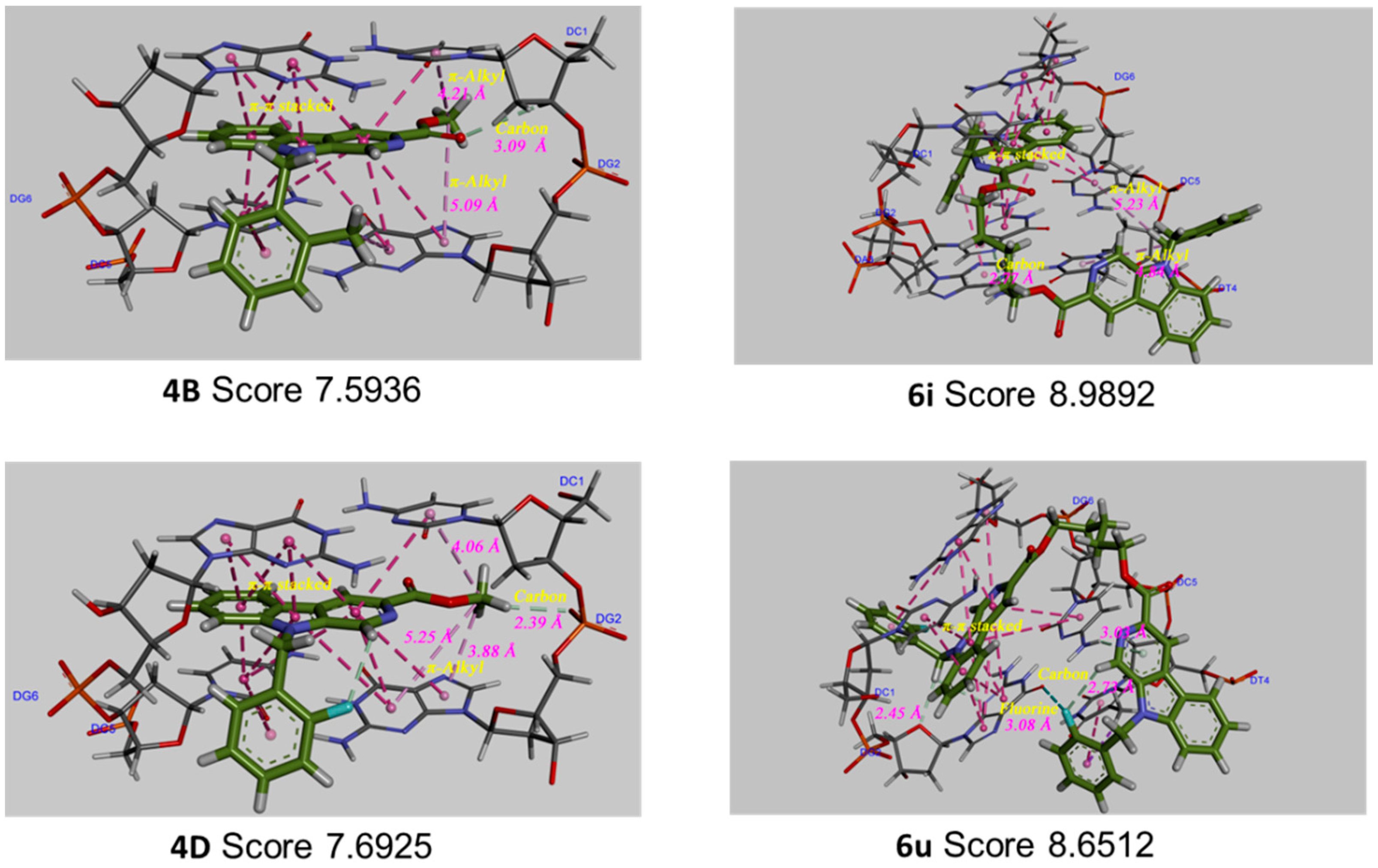
2.7.3. Molecular Docking Study
3. Discussion
4. Experimental Section
4.1. Materials and Methods
4.2. Chemistry
4.2.1. General Synthesis Procedure for N9 Substituted β-Carboline-3-carboxylic Acid Methyl Esters (4A, 4B, 4C, and 4D)
4.2.2. General Reaction of Synthesis of Bivalent β-Carboline Derivatives (6a-6f, 6g-6l, 6m-6r, and 6s-6x)
4.3. MTT Assay
4.4. Hoechst 33342/PI Dual Staining Assay
4.5. Cell Cycle Assay
4.6. Cell Apoptosis Assay
4.7. Western Blot Assay
4.8. UV-Visible Spectral Study
4.9. Thermal Denaturation Study
4.10. Molecular Modeling Study
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bluefarb, S.M. Peganum harmala. Int. J. Dermatol. 1980, 19, 535. [Google Scholar] [CrossRef]
- Allen, J.R.F.; Holmstedt, B.R. The simple β-carboline alkaloids. Phytochemistry 1980, 19, 1573–1582. [Google Scholar] [CrossRef]
- Dai, J.; Dan, W.; Li, N.; Wang, J. Computer-aided drug discovery: Novel 3,9-disubstituted eudistomin U derivatives as potent antibacterial agents. Eur. J. Med. Chem. 2018, 157, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Dan, W.; Schneider, U.; Wang, J. β-Carboline alkaloid monomers and dimers: Occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018, 157, 622–656. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Chen, Q.; Hou, X.; Chen, H.; Guan, H.; Ma, Y.; Peng, W.; Xu, A. Synthesis, acute toxicities, and antitumor effects of novel 9-substituted β-carboline derivatives. Bioorg. Med. Chem. 2004, 12, 4613–4623. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Chen, H.; Peng, W.; Ma, Y.; Hou, X.; Guan, H.; Liu, X.; Xu, A. Design, synthesis and in vitro and in vivo antitumor activities of novel β-carboline derivatives. Eur. J. Med. Chem. 2005, 40, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Chen, H.; Peng, W.; Ma, Y.; Cao, R.; Liu, X.; Xu, A. Design of β-carboline derivatives as DNA-targeting antitumor agents. Eur. J. Med. Chem. 2006, 41, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Chang, Y.-T.; Lin, C.-L.; Liaw, C.-C.; Kuo, Y.H.; Tu, L.-C.; Yeh, S.F.; Chern, J.-W. Synthesis of 1-Substituted Carbazolyl-1,2,3,4-tetrahydro-and Carbazolyl-3,4-dihydro-β-carboline Analogs as Potential Antitumor Agents. Mar. Drugs 2011, 9, 256–277. [Google Scholar] [CrossRef] [PubMed]
- Taira, Z.; Kanzawa, S.; Dohara, C.; Ishida, S.; Matsumoto, M.; Sakiya, Y. Intercalation of Six β-Carboline Deriyatives into DNA. Jpn. J. Toxicol. Environ. Health 1997, 42, 83–91. [Google Scholar] [CrossRef]
- Cao, R.H.; Peng, W.L.; Chen, H.S.; Ma, Y.; Liu, X.D.; Hou, X.R.; Guan, H.J.; Xu, A.L. DNA binding properties of 9-substituted harmine derivatives. Biochem. Biophys. Res. Commun. 2005, 338, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Cao, R.H.; Yu, L.A.; Shi, B.X.; Sun, J.; Guo, L.A.; Ma, Q.; Yi, W.; Song, X.A.; Song, H.C. Synthesis, cytotoxic activities and DNA binding properties of beta-carboline derivatives. Eur. J. Med. Chem. 2010, 45, 4740–4745. [Google Scholar] [CrossRef] [PubMed]
- Shankaraiah, N.; Siraj, K.P.; Nekkanti, S.; Srinivasulu, V.; Sharma, P.; Senwar, K.R.; Sathish, M.; Vishnuvardhan, M.V.P.S.; Ramakrishna, S.; Jadala, C.; et al. DNA-binding affinity and anticancer activity of β-carboline–chalcone conjugates as potential DNA intercalators: Molecular modelling and synthesis. Bioorg. Chem. 2015, 59, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Sathish, M.; Nayak, V.L.; Srinivasulu, V.; Kavitha, B.; Tangella, Y.; Thummuri, D.; Bagul, C.; Shankaraiah, N.; Nagesh, N. Design and synthesis of dithiocarbamate linked β-carboline derivatives: DNA topoisomerase II inhibition with DNA binding and apoptosis inducing ability. Bioorg. Med. Chem. 2015, 23, 5511–5526. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.M.; Labroli, M.A.; Dieckhaus, C.M.; Barthen, M.T.; Smith, K.S.; Macdonald, T.L. The Synthesis of Amino-Acid Functionalized β-Carbolines as Topoisomerase II Inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 1251–1255. [Google Scholar] [CrossRef]
- Sathish, M.; Kavitha, B.; Nayak, V.L.; Tangella, Y.; Ajitha, A.; Nekkanti, S.; Alarifi, A.; Shankaraiah, N.; Nagesh, N.; Kamal, A. Synthesis of podophyllotoxin linked β-carboline congeners as potential anticancer agents and DNA topoisomerase II inhibitors. Eur. J. Med. Chem. 2018, 144, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, F.S.; Jiang, W.; Yu, F.S.; Cao, R.H.; Ma, Q.H.; Da, X.Y.; Jiang, J.D.; Wang, Y.C.; So, S. DH334, a beta-carboline anti-cancer drug, inhibits the CDK activity of budding yeast. Cancer Biol. Ther. 2007, 6, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liao, S.-Y.; Tan, C.-P.; Ye, R.-R.; Xu, Y.-W.; Zhao, M.; Ji, L.-N.; Mao, Z.-W. Ruthenium–Arene–β-Carboline Complexes as Potent Inhibitors of Cyclin-Dependent Kinase 1: Synthesis, Characterization and Anticancer Mechanism Studies. Chem. Eur. J. 2013, 19, 12152–12160. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.I.; Meyers, M.J.; Anderson, D.R.; Hegde, S.; Mahoney, M.W.; Vernier, W.F.; Buchler, I.P.; Wu, K.K.; Yang, S.; Hartmann, S.J.; et al. Novel tetrahydro-β-carboline-1-carboxylic acids as inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2). Bioorg. Med. Chem. Lett. 2007, 17, 4657–4663. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.C.; Dang, L.C.; Soucy, F.; Grenier, L.; Mazdiyasni, H.; Hottelet, M.; Parent, L.; Pien, C.; Palombella, V.; Adams, J. Novel IKK inhibitors: Beta-carbolines. Bioorg. Med. Chem. Lett. 2003, 13, 2419–2422. [Google Scholar] [CrossRef]
- Hayashi, K.; Nagao, M.; Sugimura, T. Interactions of norharman and harman with DNA. Nucleic Acids Res. 1977, 4, 3679–3685. [Google Scholar] [CrossRef] [PubMed]
- Uezono, T.; Maruyama, W.; Matsubara, K.; Naoi, M.; Shimizu, K.; Saito, O.; Ogawa, K.; Mizukami, H.; Hayase, N.; Shiono, H. Norharman, an indoleamine-derived β-carboline, but not Trp-P-2, a γ-carboline, induces apoptotic cell death in human neuroblastoma SH-SY5Y cells. J. Neural Transm. 2001, 108, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Taira, Z.; Kanzawa, S.; Dohara, C.; Ishida, S.; Matsumoto, M.; Sakiya, Y. Intercalation of Six β-Carboline Derivatives into DNA. Eisei kagaku 1997, 43, 83–91. [Google Scholar] [CrossRef]
- Xiao, S.; Lin, W.; Wang, C.; Yang, M. Synthesis and biological evaluation of DNA targeting flexible side-chain substituted β-carboline derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 437–441. [Google Scholar] [CrossRef]
- Chen, Q.; Chao, R.; Chen, H.; Hou, X.; Yan, H.; Zhou, S.; Peng, W.; Xu, A. Antitumor and neurotoxic effects of novel harmine derivatives and structure-activity relationship analysis. Int. J. Cancer 2005, 114, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Georgi, M.; Adriana, B.; Margarita, K. Novel Approaches Towards Development of Non-Classical Platinum-Based Antineoplastic Agents: Design of Platinum Complexes Characterized by an Alternative DNA-Binding Pattern and/or Tumor-Targeted Cytotoxicity. Curr. Med. Chem. 2005, 12, 2177–2191. [Google Scholar]
- Cao, R.H.; Peng, W.L.; Chen, H.S.; Hou, X.R.; Guan, H.J.; Chen, Q.; Ma, Y.; Xu, A.L. Synthesis and in vitro cytotoxic evaluation of 1,3-bisubstituted and 1,3,9-trisubstituted beta-carboline derivatives. Eur. J. Med. Chem. 2005, 40, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Cao, R.H.; Shi, B.X.; Guo, L.; Sun, J.; Ma, Q.; Fan, W.X.; Song, H.C. Synthesis and biological evaluation of 1,9-disubstituted beta-carbolines as potent DNA intercalating and cytotoxic agents. Eur. J. Med. Chem. 2011, 46, 5127–5137. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.S.N.; Tonin, L.T.D.; Foglio, M.A.; Madjarof, C.; de Carvalho, J.E.; da Costa, W.F.; Cardoso, F.P.; Sarragiotto, M.H. Synthesis and antitumoral activity of novel 3-(2-substituted-1,3,4-oxadiazol-5-yl) and 3-(5-substituted-1,2,4-triazol-3-yl) β-carboline derivatives. Bioorg. Med. Chem. 2008, 16, 9660–9667. [Google Scholar] [CrossRef] [PubMed]
- Savariz, F.C.; Foglio, M.A.; de Carvalho, J.E.; Ruiz, A.L.; Duarte, M.C.; da Rosa, M.F.; Meyer, E.; Sarragiotto, M.H. Synthesis and evaluation of new beta-carboline-3-(4-benzylidene)-4H-oxazol-5-one derivatives as antitumor agents. Molecules 2012, 17, 6100–6113. [Google Scholar] [CrossRef] [PubMed]
- Shankaraiah, N.; Nekkanti, S.; Chudasama, K.J.; Senwar, K.R.; Sharma, P.; Jeengar, M.K.; Naidu, V.G.M.; Srinivasulu, V.; Srinivasulu, G.; Kamal, A. Design, synthesis and anticancer evaluation of tetrahydro-β-carboline-hydantoin hybrids. Bioorg. Med. Chem. Lett. 2014, 24, 5413–5417. [Google Scholar] [CrossRef] [PubMed]
- Gaugain, B.; Barbet, J.; Capelle, N.; Roques, B.P.; Le Pecq, J.B.; Le Bret, M. DNA Bifunctional Intercalators. 2. Fluorescence Properties and DNA Binding Interaction of an Ethidium Homodimer and an Acridine Ethidium Heterodimer. Biochemistry 1978, 17, 5078–5088. [Google Scholar] [CrossRef] [PubMed]
- Capelle, N.; Barbet, J.; Dessen, P.; Blanquet, S.; Roques, B.P.; Le Pecq, J.B. Deoxyribonucleic acid bifunctional intercalators: Kinetic investigation of the binding of several acridine dimers to deoxyribonucleic acid. Biochemistry 1979, 18, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Rook, Y.; Schmidtke, K.-U.; Gaube, F.; Schepmann, D.; Wünsch, B.; Heilmann, J.; Lehmann, J.; Winckler, T. Bivalent β-Carbolines as Potential Multitarget Anti-Alzheimer Agents. J. Med. Chem. 2010, 53, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Cao, R.; Fan, W.; Guo, L.; Ma, Q.; Chen, X.; Zhang, G.; Qiu, L.; Song, H. Design, synthesis and in vitro and in vivo antitumor activities of novel bivalent β-carbolines. Eur. J. Med. Chem. 2013, 60, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.F.; Bai, Z.S.; Ma, Q.; Fan, W.X.; Guo, L.; Zhang, G.X.; Qiu, L.Q.; Yu, H.J.; Shao, G.; Cao, R.H. Synthesis and biological evaluation of novel bivalent beta-carbolines as potential antitumor agents. MedChemComm 2014, 5, 953–957. [Google Scholar] [CrossRef]
- Du, H.; Tian, S.; Chen, J.; Gu, H.; Li, N.; Wang, J. Synthesis and biological evaluation of N9-substituted harmine derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4015–4019. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Gu, H.; Li, N.; Wang, J. Synthesis and biological evaluation of bivalent β-carbolines as potential anticancer agents. MedChemComm 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Kerr, J.F.R. Morphological criteria for identifying apoptosis. Cell Biol. Lab. Handb. 1994, 1, 319–329. [Google Scholar]
- Escrich, L.; Grau, N.; Meseguer, M.; Pellicer, A.; Escribá, M.-J. Morphologic indicators predict the stage of chromatin condensation of human germinal vesicle oocytes recovered from stimulated cycles. Fertil. Steril. 2010, 93, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Weir, N.M.; Selvendiran, K.; Kutala, V.K.; Tong, L.; Vishwanath, S.; Rajaram, M.; Tridandapani, S.; Anant, S.; Kuppusamy, P. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating akt and p38 mAPK. Cancer Biol. Ther. 2007, 6, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Wlodkowic, D.; Skommer, J.; Darzynkiewicz, Z. Cytometry in cell necrobiology revisited. Recent advances and new vistas. Cytom. Part A 2010, 77, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Fidan, M.; Nowak, M.W.; Wilford, G.G.; Hogan, E.L.; Banik, N.L. Oxidative stress and Ca2+ influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res. 2000, 852, 326–334. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Chang, H.-A.; Lin, Y.-H.; Lin, Y.-S.; Chang, H.-T.; Lin, H.-H.; Huang, S.-C.; Tang, M.-J.; Shen, M.-R. Bcl-2 regulates store-operated Ca2+ entry to modulate ER stress-induced apoptosis. Cell Death Discov. 2018, 4, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Savariz, F.C.; Silva-Júnior, E.F.; de Aquino, T.M.; Sarragiotto, M.H.; Santos, J.C.C.; Figueiredo, I.M. Interaction of β-Carbolines with DNA: Spectroscopic Studies, Correlation with Biological Activity and Molecular Docking. J. Braz. Chem. Soc. 2016, 27, 1558–1568. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Shu, B.; Cui, G.; Zhong, G. Cytotoxic and Apoptotic Activity of the Novel Harmine Derivative ZC-14 in Sf9 Cells. Int. J. Mol. Sci. 2018, 19, 811. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.H.; Peng, W.L.; Wang, Z.H.; Xu, A.L. beta-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Sun, R.-Q.; Jia, Y.-F.; Chen, Q.; Tu, R.-F.; Li, K.-K.; Zhang, X.-D.; Du, R.-L.; Cao, R.-H. Synthesis and mechanisms of action of novel harmine derivatives as potential antitumor agents. Sci. Rep. 2016, 6, 33204–33219. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, G.; Guo, L.; Fan, W.; Ma, Q.; Zhang, X.; Du, R.; Cao, R. Synthesis and biological evaluation of novel alkyl diamine linked bivalent β-carbolines as angiogenesis inhibitors. Eur. J. Med. Chem. 2016, 124, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture: Methods and Protocols; Cree, I.A., Ed.; Series 731; Humana Press: New York, NY, USA, 2011; pp. 237–245. [Google Scholar]
- Chen, Q.; Shao, X.; Ling, P.; Liu, F.; Shao, H.; Ma, A.; Wu, J.; Zhang, W.; Liu, F.; Han, G.; et al. Low molecular weight xanthan gum suppresses oxidative stress-induced apoptosis in rabbit chondrocytes. Carbohydr. Polym. 2017, 169, 255–263. [Google Scholar] [CrossRef] [PubMed]
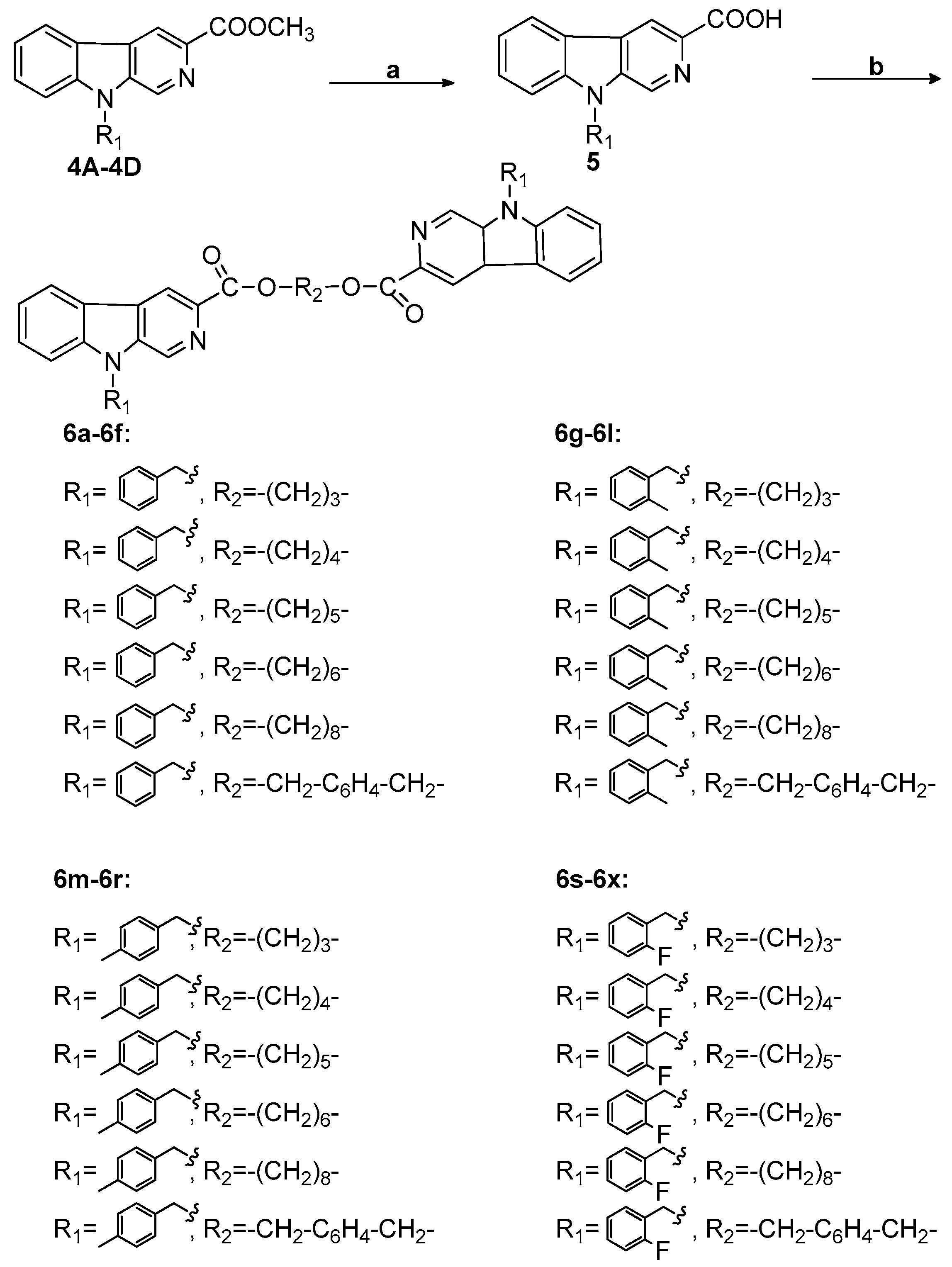
| Compounds | Substituents | IC50 (μM) Mean ± SD a | ||||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | A549 b | SGC-7901 b | Hela b | SMMC-7721 b | MCF-7 b | MRC5 | |
| 4A |  | - | >80 | >80 | >80 | >80 | >80 | 35.60 ± 0.30 |
| 4B | 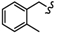 | - | 39.20 ± 1.18 | 42.31 ± 2.13 | 31.28 ± 2.33 | >80 | >80 | 51.78 ± 0.10 |
| 4C | 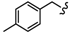 | - | 28.06 ± 0.13 | 47.98 ± 0.09 | 42.55 ± 0.98 | >80 | >80 | 35.35 ± 0.30 |
| 4D | 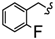 | - | 13.94 ± 0.07 | 17.84 ± 0.66 | 26.36 ± 1.33 | 35.61 ± 1.33 | 53.92 ± 2.02 | 35.38 ± 0.01 |
| 6a | 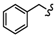 | -(CH2)3- | 24.27 ± 0.12 | 17.76 ± 0.17 | 17.89 ± 0.03 | 25.73 ± 0.32 | 73.45 ± 0.88 | 32.57 ± 0.10 |
| 6b | 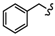 | -(CH2)4- | 12.26 ± 0.04 | 15.48 ± 0.32 | 18.22 ± 0.52 | 66.53 ± 0.31 | 70.88 ± 2.33 | 64.44 ± 0.09 |
| 6c | 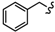 | -(CH2)5- | 17.65 ± 0.09 | 18.72 ± 0.07 | 18.15 ± 0.03 | 55.15 ± 0.03 | 76.85 ± 3.01 | 39.34 ± 0.11 |
| 6d | 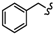 | -(CH2)6- | 40.03 ± 0.15 | 58.41 ± 0.72 | 32.36 ± 0.02 | 33.49 ± 0.01 | 75.47 ± 2.32 | 66.99 ± 0.10 |
| 6e | 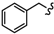 | -(CH2)8- | 50.32 ± 3.15 | 69.97 ± 0.13 | 53.97 ± 0.56 | 75.36 ± 0.89 | >80 | >100 |
| 6f | 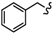 | -CH2-C6H4-CH2- | 29.04 ± 0.06 | 51.24 ± 3.22 | 66.3 ± 1.02 | 57.35 ± 1.13 | >80 | >100 |
| 6g | 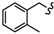 | -(CH2)3- | 18.33 ± 0.28 | 29.42 ± 1.03 | 17.99 ± 0.15 | 73.49 ± 8.06 | 53.14 ± 2.28 | 32.61 ± 0.10 |
| 6h | 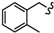 | -(CH2)4- | 8.09 ± 0.25 | 17.75 ± 0.93 | 45.75 ± 0.55 | 24.71 ± 0.5 | 76.73 ± 5.0 | 50.78 ± 0.09 |
| 6i | 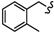 | -(CH2)5- | 6.93 ± 0.33 | 7.18 ± 0.01 | 9.88 ± 0.04 | 14.22 ± 0.28 | 59.29 ± 5.86 | 37.99 ± 0.10 |
| 6j | 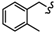 | -(CH2)6- | 12.18 ± 0.06 | 26.26 ± 1.03 | 18.05 ± 1.03 | 16.68 ± 0.03 | 64.49 ± 3.31 | 41.0 ± 0.07 |
| 6k | 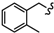 | -(CH2)8- | 37.36 ± 2.08 | 35.00 ± 2.09 | 36.69 ± 2.02 | 33.93 ± 0.52 | 44.93 ± 2.99 | 65.85 ± 0.07 |
| 6l | 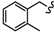 | -CH2-C6H4-CH2- | 30.13 ± 0.37 | 39.26 ± 1.22 | 59.68 ± 1.52 | 69.66 ± 0.30 | 70.76 ± 2.02 | 37.66 ± 0.13 |
| 6m |  | -(CH2)3- | 19.84 ± 0.04 | 18.41 ± 0.31 | 17.95 ± 0.02 | 33.32 ± 0.03 | 28.43 ± 0.28 | 49.05 ± 0.12 |
| 6n | 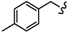 | -(CH2)4- | 13.36 ± 0.35 | 37.79 ± 1.02 | 27.96 ± 0.99 | 42.72 ± 2.18 | 49.06 ± 2.23 | 26.39 ± 0.01 |
| 6o | 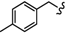 | -(CH2)5- | 11.23 ± 0.61 | 17.18 ± 0.03 | 18.76 ± 0.46 | 36.62 ± 0.12 | 49.37 ± 1.65 | 31.28±0.07 |
| 6p | 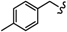 | -(CH2)6- | 19.28 ± 0.02 | 17.84 ± 0.22 | 15.66 ± 0.33 | 26.75 ± 0.51 | 55.04 ± 2.22 | 47.60 ± 0.02 |
| 6q | 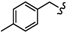 | -(CH2)8- | 23.65 ± 0.05 | 35.78 ± 0.82 | 45.21 ± 1.66 | 29.82 ± 0.93 | 43.05 ± 2.01 | 42.06 ± 0.09 |
| 6r | 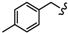 | -CH2-C6H4-CH2- | 46.61 ± 0.03 | 65.93 ± 1.18 | 58.25 ± 2.98 | >80 | 68.26 ± 1.25 | 56.11 ± 0.01 |
| 6s | 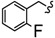 | -(CH2)3- | 6.12 ± 0.03 | 8.06 ± 0.08 | 13.77 ± 0.11 | 14.71 ± 0.08 | 25.20 ± 1.12 | 22.82 ± 0.02 |
| 6t |  | -(CH2)4- | 12.39 ± 0.59 | 9.01 ± 0.19 | 15.20 ± 0.32 | 18.26 ± 0.18 | 25.69 ± 1.21 | >100 |
| 6u |  | -(CH2)5- | 5.61 ± 0.04 | 5.86 ± 0.08 | 14.63 ± 0.36 | 16.68 ± 0.11 | 36.03 ± 2.99 | 39.89 ± 0.05 |
| 6v | 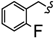 | -(CH2)6- | 7.95 ± 0.06 | 8.60 ± 0.13 | 22.85 ± 1.36 | 24.65 ± 0.06 | 42.41 ± 2.08 | 67.75 ± 0.11 |
| 6w |  | -(CH2)8- | 16.54 ± 0.33 | 29.98 ± 0.07 | 31.34 ± 1.88 | 44.57 ± 0.02 | 76.32 ± 3.36 | 60.78 ± 0.16 |
| 6x | 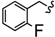 | -CH2-C6H4-CH2- | 34.27 ± 0.05 | 45.00 ± 0.16 | 39.61 ± 2.59 | >80 | >80 | 63.28 ± 0.10 |
| 5-FU c | C4H3FN2O2 | 15.39 ± 0.09 | >80 | >80 | 52.32 ± 2.06 | 57.69 ± 1.39 | 16.33 ± 0.30 | |
| Compounds | Concentration (μM) | Percentage of Cell Cycles (%) | |||
|---|---|---|---|---|---|
| SubG1 | G1 | S | G2/M | ||
| 4B | 2 | 6.56 | 59.38 | 25.16 | 15.46 |
| 4 | 10.49 | 43.06 | 9.98 | 46.96 | |
| 8 | 4.15 | 52.09 | 45.81 | 2.1 | |
| 6i | 2 | 6.86 | 57.38 | 32.08 | 10.54 |
| 4 | 26.33 | 45.19 | 12.41 | 42.40 | |
| 8 | 21.32 | 46.64 | 53.30 | 0.06 | |
| 4D | 2 | 6.41 | 56.89 | 24.17 | 18.94 |
| 4 | 39.03 | 47.21 | 17.33 | 35.46 | |
| 8 | 46.29 | 88.01 | 11.69 | 0.3 | |
| 6u | 2 | 6.84 | 56.24 | 23.79 | 19.97 |
| 4 | 46.25 | 47.06 | 12.60 | 40.34 | |
| 8 | 44.92 | 81.62 | 0.08 | 18.30 | |
| Control | 1% DMSO | 1.78 | 60.15 | 27.6 | 12.25 |
| Comp. | Concentration (μM) | Percentage of Cell Apoptosis (%) | ||
|---|---|---|---|---|
| Normal Living Cell | Early Apoptosis | Late Apoptosis and Necrosis | ||
| 4B | 2 | 75.93 | 3.18 | 20.89 |
| 4 | 48.59 | 15.35 | 36.06 | |
| 8 | 21.95 | 8.40 | 69.65 | |
| 6i | 2 | 72.12 | 6.85 | 21.03 |
| 4 | 44.94 | 12.65 | 42.41 | |
| 8 | 12.52 | 4.24 | 83.24 | |
| 4D | 2 | 73.94 | 7.27 | 18.78 |
| 4 | 47.93 | 6.80 | 45.26 | |
| 8 | 17.63 | 5.28 | 77.09 | |
| 6u | 2 | 66.05 | 12.14 | 21.81 |
| 4 | 32.53 | 6.54 | 60.93 | |
| 8 | 8.83 | 7.65 | 83.52 | |
| Control | 1% DMSO | 88.34 | 4.22 | 7.44 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Li, N.; Dai, J.; Xi, Y.; Wang, S.; Wang, J. Synthesis and In Vitro Antitumor Activity of Novel Bivalent β-Carboline-3-carboxylic Acid Derivatives with DNA as a Potential Target. Int. J. Mol. Sci. 2018, 19, 3179. https://doi.org/10.3390/ijms19103179
Gu H, Li N, Dai J, Xi Y, Wang S, Wang J. Synthesis and In Vitro Antitumor Activity of Novel Bivalent β-Carboline-3-carboxylic Acid Derivatives with DNA as a Potential Target. International Journal of Molecular Sciences. 2018; 19(10):3179. https://doi.org/10.3390/ijms19103179
Chicago/Turabian StyleGu, Hongling, Na Li, Jiangkun Dai, Yaxi Xi, Shijun Wang, and Junru Wang. 2018. "Synthesis and In Vitro Antitumor Activity of Novel Bivalent β-Carboline-3-carboxylic Acid Derivatives with DNA as a Potential Target" International Journal of Molecular Sciences 19, no. 10: 3179. https://doi.org/10.3390/ijms19103179
APA StyleGu, H., Li, N., Dai, J., Xi, Y., Wang, S., & Wang, J. (2018). Synthesis and In Vitro Antitumor Activity of Novel Bivalent β-Carboline-3-carboxylic Acid Derivatives with DNA as a Potential Target. International Journal of Molecular Sciences, 19(10), 3179. https://doi.org/10.3390/ijms19103179