3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells
Abstract
1. Introduction
2. Use of 3D Scaffolds in Basic Studies: Evaluation of MSC Multilineage Potential and Study of Human Hematopoiesis
3. Effective Coupling of Cells and Scaffolds: The Material Choice for Better MSC Performance
4. MSC-Scaffold Interaction: Mechanosensing and Mechanotransduction
5. 3D Bioprinting for MSC Basic and Translational Studies
6. MSC-Seeded Scaffolds as a Source of Soluble Factors
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| AcvR2a | Activin A Receptor Type 2A |
| AEF | aligned electrospun fiber |
| AKT | Protein kinase B |
| bFGF | basic fibroblast growth factor |
| Bioink | biocompatible ink |
| BMP2 | Bone Morphogenetic Protein 2 |
| BMPs | Bone Morphogenetic Proteins |
| CaSO4 | Calcium sulfate |
| COX2 | cyclooxygenase-2 |
| ECM | extracellular matrix |
| FGF | Fibroblast Growth Factor |
| FGF9 | Fibroblast Growth Factor 9 |
| GFP | Green Fluorescent Protein |
| GFs | Growth Factors |
| HA/TCP | HA/tricalcium phosphate |
| HA | Hydroxyapatite |
| HGF | Hepatocyte growth factor |
| hMSCs | human Mesenchymal Stem Cells |
| HSC | Hematopoietic Stem Cell |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| IGF-I | Insulin-like Growth Factor-I |
| IGF-II | Insulin-like Growth Factor-II |
| iNOS | inducible Nitric Oxide Synthase |
| LH | Large Hexagonal |
| LIPUS | Low Intensity Pulsed Ultrasound Stimulation |
| LS | Large Square |
| MAPK | Mitogen-Activated Protein Kinase |
| MEF | Mesh Electrospun Fiber |
| Mg2+ | Magnesium ion |
| MgApRCP | Mineralized, magnesium-doped recombinant type I collagen enriched with the RGD sequence |
| MgCHA | Magnesium and carbonate hydroxyapatite |
| MgHA/ColI | Magnesium-doped hydroxyapatite and type I collagen |
| miRNAs | microRNA |
| Mmp13 | Matrix Metallopeptidase 13 |
| Mmp2 | Matrix Metallopeptidase 2 |
| MPa | Megapascal |
| MSCs | Mesenchymal Stem Cells |
| MT | Masson’s Trichrome |
| NCs | nasal chondrocytes |
| NF-κB | nuclear factor of kappa light polypeptide gene enhancer in B cells |
| NSG | NOD scid gamma |
| PCL | poly(e-caprolactone) |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3 kinase |
| PLGA | poly-lactic-co-glycolic acid |
| PLLA | poly-L-lactic acid |
| PSR | PicroSirius Red |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| RCP | Recombinant Collagen Peptide |
| REF | Random Electrospun Fiber |
| RGD | Arginine-Glycine-Aspartic acid |
| RGDS | Arginine-Glycine-Aspartic acid-Serine |
| Runx2 | Runt-related transcription factor 2 |
| SH | Small Hexagonal |
| Smad5 | SMAD Family Member 5 |
| SS | Small Square |
| TGFβ | Transforming Growth Factor beta |
| TGFβ3 | Transforming Growth Factor beta-3 |
| TSG6 | TNF-Stimulated Gene 6 protein |
| V-Cam1 | Vascular Cell adhesion molecule 1 |
| VEGF | Vascular-Endothelial Growth Factor |
| WT | Wild Type |
| YAP/TAZ | Yes-associated protein/Tafazzin |
| Zo-1 | Zonula occludens-1 |
References
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, A.; Swiech, K. Mesenchymal Stromal Cells: From Discovery to Manufacturing and Commercialization. Stem Cells Int. 2018, 2018, 4083921. [Google Scholar] [CrossRef] [PubMed]
- Ghasroldasht, M.M.; Irfan-Maqsood, M.; Matin, M.M.; Bidkhori, H.R.; Naderi-Meshkin, H.; Moradi, A.; Bahrami, A.R. Mesenchymal stem cell based therapy for osteo-diseases. Cell Boil. Int. 2014, 38, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Burdick, J.A. Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell Stem Cell 2018, 22, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Assis-Ribas, T.; Forni, M.F.; Winnischofer, S.M.B.; Sogayar, M.C.; Trombetta-Lima, M. Extracellular matrix dynamics during mesenchymal stem cells differentiation. Dev. Boil. 2018, 437, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Bayona, S.; Guiza-Arguello, V.; Qu, X.; Munoz-Pinto, D.J.; Hahn, M.S. Influence of select extracellular matrix proteins on mesenchymal stem cell osteogenic commitment in three-dimensional contexts. Acta Biomater. 2012, 8, 4397–4404. [Google Scholar] [CrossRef] [PubMed]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Boil. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tsai, T.L.; Li, W.J. Strategies to retain properties of bone marrow-derived mesenchymal stem cells ex vivo. Ann. N. Y. Acad. Sci. 2017, 1409, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Follin, B.; Juhl, M.; Cohen, S.; Perdersen, A.E.; Kastrup, J.; Ekblond, A. Increased Paracrine Immunomodulatory Potential of Mesenchymal Stromal Cells in Three-Dimensional Culture. Tissue Eng. Part B Rev. 2016, 22, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.; Vissers, C.B.; Leach, J.K. Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels. Stem Cell Transl. Med. 2016, 5, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Sittinger, M.; Bujia, J.; Rotter, N.; Reitzel, D.; Minuth, W.W.; Burmester, G.R. Tissue engineering and autologous transplant formation: Practical approaches with resorbable biomaterials and new cell culture techniques. Biomaterials 1996, 17, 237–242. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25 (Suppl. 1), S101–S103. [Google Scholar] [CrossRef] [PubMed]
- Oyen, M.L. The materials science of bone: Lessons from nature for biomimetic materials synthesis. Mrs Bull 2008, 33, 49–55. [Google Scholar] [CrossRef]
- Shpichka, A.; Koroleva, A.; Kuznetsova, D.; Dmitriev, R.I.; Timashev, P. Fabrication and Handling of 3D Scaffolds Based on Polymers and Decellularized Tissues. Adv. Exp. Med. Boil. 2017, 1035, 71–81. [Google Scholar] [CrossRef]
- Cui, Z.; Lin, L.; Si, J.; Luo, Y.; Wang, Q.; Lin, Y.; Wang, X.; Chen, W. Fabrication and characterization of chitosan/OGP coated porous poly(epsilon-caprolactone) scaffold for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 826–845. [Google Scholar] [CrossRef] [PubMed]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Boil. 2009, 3, 189–196. [Google Scholar] [CrossRef]
- Sobacchi, C.; Palagano, E.; Villa, A.; Menale, C. Soluble Factors on Stage to Direct Mesenchymal Stem Cells Fate. Front. Bioeng. Biotechnol. 2017, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef] [PubMed]
- Roothaer, X.; Delille, R.; Morvan, H.; Bennani, B.; Markiewicz, E.; Fontaine, C. A three-dimensional geometric quantification of human cortical canals using an innovative method with micro-computed tomographic data. Micron 2018, 114, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, H.K.; Kim, K.S. Intrinsic and extrinsic mechanical properties related to the differentiation of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2016, 473, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.Y. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Piccinini, E.; Takizawa, H.; Todorov, A.; Bourgine, P.; Papadimitropoulos, A.; Barbero, A.; Manz, M.G.; Martin, I. Engineering of a functional bone organ through endochondral ossification. Proc. Natl. Acad. Sci. USA 2013, 110, 3997–4002. [Google Scholar] [CrossRef] [PubMed]
- Robey, P.G.; Kuznetsov, S.A.; Riminucci, M.; Bianco, P. Bone marrow stromal cell assays: In vitro and in vivo. Methods Mol. Boil. 2014, 1130, 279–293. [Google Scholar] [CrossRef]
- Scott, M.A.; Levi, B.; Askarinam, A.; Nguyen, A.; Rackohn, T.; Ting, K.; Soo, C.; James, A.W. Brief review of models of ectopic bone formation. Stem Cells Dev. 2012, 21, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Riminucci, M.; Majolagbe, A.; Kuznetsov, S.A.; Collins, M.T.; Mankani, M.H.; Corsi, A.; Bone, H.G.; Wientroub, S.; Spiegel, A.M.; et al. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2000, 15, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Miura, M.; Gronthos, S.; Allen, M.R.; Cao, C.; Uveges, T.E.; Bi, Y.; Ehirchiou, D.; Kortesidis, A.; Shi, S.; et al. Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proc. Natl. Acad. Sci. USA 2005, 102, 14022–14027. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Sonoyama, W.; Wang, Z.; Jin, Q.; Zhang, C.; Krebsbach, P.H.; Giannobile, W.; Shi, S.; Wang, C.Y. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J. Boil. Chem. 2007, 282, 30938–30948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zheng, C.; Shou, P.; Li, N.; Cao, G.; Chen, Q.; Xu, C.; Du, L.; Yang, Q.; Cao, J.; et al. SHP1 Regulates Bone Mass by Directing Mesenchymal Stem Cell Differentiation. Cell Rep. 2016, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Scaglione, S.; Sandri, M.; Alvarez-Perez, M.A.; Tampieri, A.; Quarto, R.; Ambrosio, L. MgCHA particles dispersion in porous PCL scaffolds: In vitro mineralization and in vivo bone formation. J. Tissue Eng. Regen. Med. 2014, 8, 291–303. [Google Scholar] [CrossRef] [PubMed]
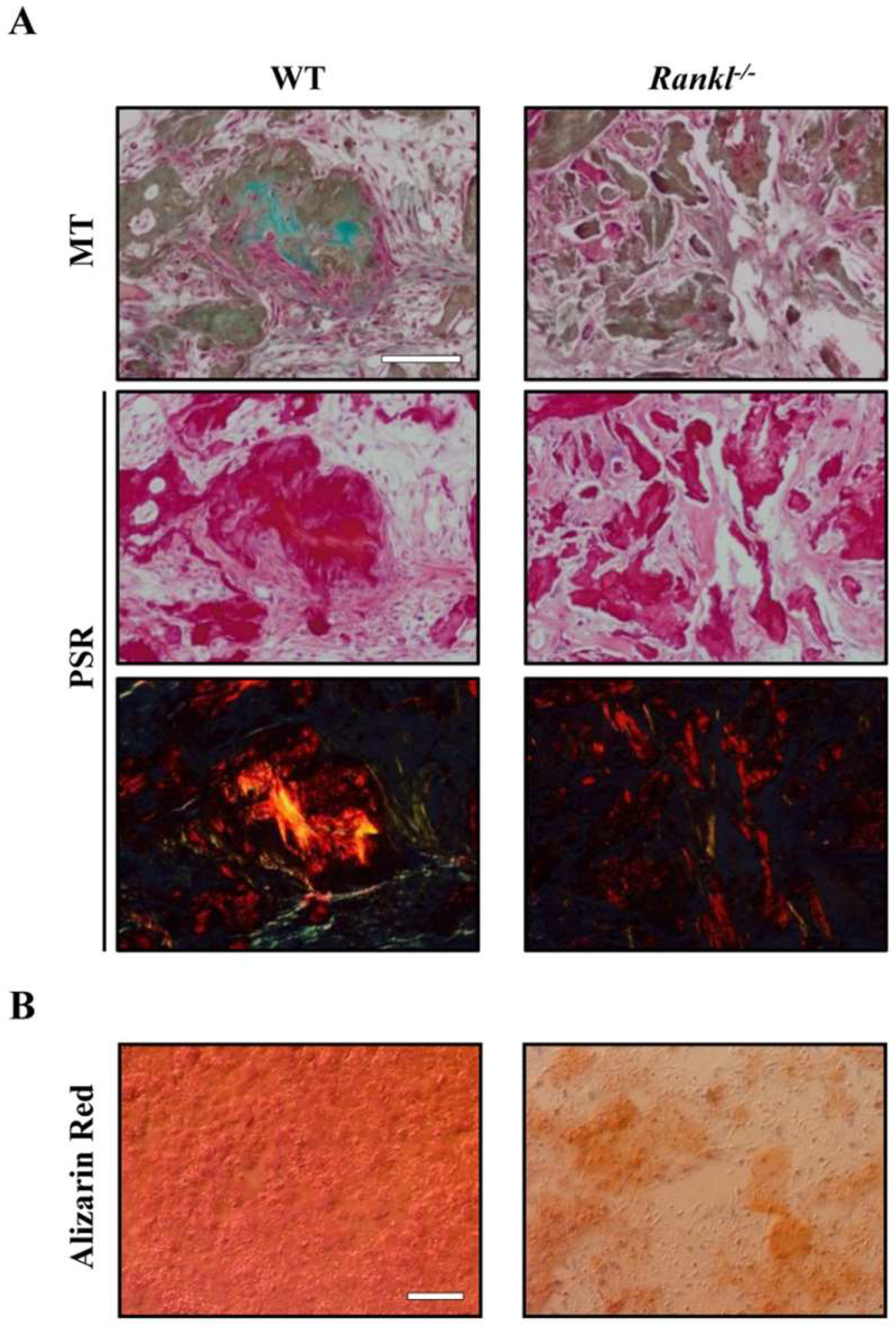
- Schena, F.; Menale, C.; Caci, E.; Diomede, L.; Palagano, E.; Recordati, C.; Sandri, M.; Tampieri, A.; Bortolomai, I.; Capo, V.; et al. Murine Rankl(-/-) Mesenchymal Stromal Cells Display an Osteogenic Differentiation Defect Improved by a RANKL-Expressing Lentiviral Vector. Stem Cells 2017, 35, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, P.E.; Klein, T.; Paczulla, A.M.; Shimizu, T.; Kunz, L.; Kokkaliaris, K.D.; Coutu, D.L.; Lengerke, C.; Skoda, R.; Schroeder, T.; et al. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc. Natl. Acad. Sci. USA 2018, 115, E5688–E5695. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Choy, T.H.; Ho, F.C.; Chan, P.B. Scaffold composition affects cytoskeleton organization, cell-matrix interaction and the cellular fate of human mesenchymal stem cells upon chondrogenic differentiation. Biomaterials 2015, 52, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mezour, M.A.; Badran, Z.; Tamimi, F. Extracellular Matrices for Bone Regeneration: A Literature Review. Tissue Engineering. Part A 2017, 23, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Matsiko, A.; Kelly, D.J.; Gleeson, J.P.; O’Brien, F.J. An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo. Tissue Eng. Part A 2016, 22, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Rubert Perez, C.M.; Stephanopoulos, N.; Sur, S.; Lee, S.S.; Newcomb, C.; Stupp, S.I. The powerful functions of peptide-based bioactive matrices for regenerative medicine. Ann. Biomed. Eng. 2015, 43, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.B.; Montesi, M.; Panseri, S.; Sprio, S.; Tampieri, A.; Sandri, M. Biomineralized Recombinant Collagen-Based Scaffold Mimicking Native Bone Enhances Mesenchymal Stem Cell Interaction and Differentiation. Tissue Eng. Part A 2017, 23, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Dankova, J.; Buzgo, M.; Vejpravova, J.; Kubickova, S.; Sovkova, V.; Vyslouzilova, L.; Mantlikova, A.; Necas, A.; Amler, E. Highly efficient mesenchymal stem cell proliferation on poly-epsilon-caprolactone nanofibers with embedded magnetic nanoparticles. Int. J. Nanomed. 2015, 10, 7307–7317. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Iafisco, M.; Sandri, M.; Panseri, S.; Cunha, C.; Sprio, S.; Savini, E.; Uhlarz, M.; Herrmannsdorfer, T. Magnetic bioinspired hybrid nanostructured collagen-hydroxyapatite scaffolds supporting cell proliferation and tuning regenerative process. ACS Appl. Mater. Interfaces 2014, 6, 15697–15707. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Lee, E.S.; Kim, M.J.; Kim, J.J.; Lee, J.H.; Lee, H.H.; Park, K.R.; Yi, J.K.; Kim, H.W.; Kim, E.C. Magnetic Nanocomposite Scaffold-Induced Stimulation of Migration and Odontogenesis of Human Dental Pulp Cells through Integrin Signaling Pathways. PLoS ONE 2015, 10, e0138614. [Google Scholar] [CrossRef] [PubMed]
- Menale, C.; Campodoni, E.; Palagano, E.; Mantero, S.; Erreni, M.; Inforzato, A.; Fontana, E.; Schena, F.; van’t Hof, R.; Sandri, M.; et al. MSC-seeded biomimetic scaffolds as a factory of soluble RANKL in Rankl-deficient osteopetrosis. Stem Cells Transl. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Minardi, S.; Corradetti, B.; Taraballi, F.; Sandri, M.; Van Eps, J.; Cabrera, F.J.; Weiner, B.K.; Tampieri, A.; Tasciotti, E. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 2015, 62, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, G.S.; Gostynska, N.; Dapporto, M.; Campodoni, E.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Evaluation of different crosslinking agents on hybrid biomimetic collagen-hydroxyapatite composites for regenerative medicine. Int. J. Boil. Macromol. 2018, 106, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Zofkova, I.; Davis, M.; Blahos, J. Trace elements have beneficial, as well as detrimental effects on bone homeostasis. Physiol. Res. 2017, 66, 391–402. [Google Scholar] [PubMed]
- Almalki, S.G.; Agrawal, D.K. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Tonti, G.A.; Bagnara, G.P.; Papa, S. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem Cells 2006, 24, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Arealis, G.; Nikolaou, V.S. Bone printing: New frontiers in the treatment of bone defects. Injury 2015, 46 (Suppl. 8), S20–S22. [Google Scholar] [CrossRef]
- Schofer, M.D.; Roessler, P.P.; Schaefer, J.; Theisen, C.; Schlimme, S.; Heverhagen, J.T.; Voelker, M.; Dersch, R.; Agarwal, S.; Fuchs-Winkelmann, S.; et al. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS ONE 2011, 6, e25462. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Martinez, R.; Angelo, A.P.; Pujol, F.V. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem Cell Res. Ther. 2017, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lopez, J.M.; Iafisco, M.; Rodriguez, I.; Tampieri, A.; Prat, M.; Gomez-Morales, J. Crystallization of bioinspired citrate-functionalized nanoapatite with tailored carbonate content. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.B.; Delgado-Lopez, J.M.; Iafisco, M.; Montesi, M.; Sandri, M.; Sprio, S.; Tampieri, A. Biomimetic mineralization of recombinant collagen type I derived protein to obtain hybrid matrices for bone regeneration. J. Struct. Boil. 2016, 196, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, P.; Capitani, D.; Sprio, S.; Sandri, M.; Tampieri, A.; Canella, V.; Nataloni, A.; Schiro, G.R. A new bioinspired collagen-hydroxyapatite bone graft substitute in adult scoliosis surgery: Results at 3-year follow-up. J. Appl. Biomater. Funct. Mater. 2017, 15, E262–E270. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Diachina, S.; Lee, Y.T.; Zhao, L.; Zou, R.; Tang, N.; Han, H.; Chen, X.; Ko, C.C. Decellularized bone matrix grafts for calvaria regeneration. J. Tissue Eng. 2016, 7, 2041731416680306. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Funamoto, S.; Kimura, T.; Nam, K.; Fujisato, T.; Kishida, A. The effect of decellularized bone/bone marrow produced by high-hydrostatic pressurization on the osteogenic differentiation of mesenchymal stem cells. Biomaterials 2011, 32, 7060–7067. [Google Scholar] [CrossRef] [PubMed]
- Papadimitropoulos, A.; Scotti, C.; Bourgine, P.; Scherberich, A.; Martin, I. Engineered decellularized matrices to instruct bone regeneration processes. Bone 2015, 70, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zheng, X.; Zhang, W.; Chen, M.; Wang, Z.; Hao, C.; Huang, J.; Yuan, Z.; Zhang, Y.; Wang, M.; et al. Mesenchymal Stem Cells in Oriented PLGA/ACECM Composite Scaffolds Enhance Structure-Specific Regeneration of Hyaline Cartilage in a Rabbit Model. Stem Cells Int. 2018, 2018, 6542198. [Google Scholar] [CrossRef] [PubMed]
- Matuska, A.M.; Dolwick, M.F.; McFetridge, P.S. Approaches to improve integration and regeneration of an ex vivo derived temporomandibular joint disc scaffold with variable matrix composition. J. Mater. Sci. Mater. Med. 2018, 29, 152. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, P.E.; Scotti, C.; Pigeot, S.; Tchang, L.A.; Todorov, A.; Martin, I. Osteoinductivity of engineered cartilaginous templates devitalized by inducible apoptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 17426–17431. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.H.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Hribar, K.C.; Taylor-Weiner, H.; Chen, S.; Engler, A.J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014, 13, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.R.; Cho, S.; Discher, D.E. Stem Cell Differentiation is Regulated by Extracellular Matrix Mechanics. Physiology 2018, 33, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Holle, A.W.; Young, J.L.; Van Vliet, K.J.; Kamm, R.D.; Discher, D.; Janmey, P.; Spatz, J.P.; Saif, T. Cell-Extracellular Matrix Mechanobiology: Forceful Tools and Emerging Needs for Basic and Translational Research. Nano Lett. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; DelRio, F.W.; Ma, H.; Killaars, A.R.; Basta, L.P.; Kyburz, K.A.; Anseth, K.S. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl. Acad. Sci. USA 2016, 113, E4439–E4445. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bao, M.; Bruekers, S.M.C.; Huck, W.T.S. Collagen Gels with Different Fibrillar Microarchitectures Elicit Different Cellular Responses. ACS Appl. Mater. Interfaces 2017, 9, 19630–19637. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, J.M.; Guillem-Marti, J.; Espanol, M.; Stahli, C.; Dobelin, N.; Ginebra, M.P. In vitro response of mesenchymal stem cells to biomimetic hydroxyapatite substrates: A new strategy to assess the effect of ion exchange. Acta Biomater. 2018, 76, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Gardel, L.S.; Serra, L.A.; Reis, R.L.; Gomes, M.E. Use of perfusion bioreactors and large animal models for long bone tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 126–146. [Google Scholar] [CrossRef] [PubMed]
- Papadimitropoulos, A.; Piccinini, E.; Brachat, S.; Braccini, A.; Wendt, D.; Barbero, A.; Jacobi, C.; Martin, I. Expansion of human mesenchymal stromal cells from fresh bone marrow in a 3D scaffold-based system under direct perfusion. PLoS ONE 2014, 9, e102359. [Google Scholar] [CrossRef] [PubMed]
- Bersini, S.; Arrigoni, C.; Lopa, S.; Bongio, M.; Martin, I.; Moretti, M. Engineered miniaturized models of musculoskeletal diseases. Drug Discov. Today 2016, 21, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Gu, Y.; Wang, H.; Lee, W.Y. Microfluidic 3D bone tissue model for high-throughput evaluation of wound-healing and infection-preventing biomaterials. Biomaterials 2012, 33, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Kim, H.; Lee, E.; Park, M.H.; Chung, S.; Jeon, H.; Ahn, C.H.; Lee, K. Study on chemotaxis and chemokinesis of bone marrow-derived mesenchymal stem cells in hydrogel-based 3D microfluidic devices. Biomater. Res. 2016, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Movilla, N.; Borau, C.; Valero, C.; Garcia-Aznar, J.M. Degradation of extracellular matrix regulates osteoblast migration: A microfluidic-based study. Bone 2018, 107, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Mimicking the 3D biology of osteochondral tissue with microfluidic-based solutions: Breakthroughs towards boosting drug testing and discovery. Drug Discov. Today 2018, 23, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Urrios, A.; Kanga, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, K.M.; Prendergast, M.E.; Miller, M.L.; Kaplan, D.S.; Fisher, J.P. Influence of 3D printed porous architecture on mesenchymal stem cell enrichment and differentiation. Acta Biomater. 2016, 32, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Savage, N. Technology: The promise of printing. Nature 2016, 540, S56–S57. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.; Willem Visser, C.; Henke, S.; Leijten, J.; Saris, D.B.; Sun, C.; Lohse, D.; Karperien, M. Optimizing cell viability in droplet-based cell deposition. Sci. Rep. 2015, 5, 11304. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Blaeser, A.; Buellesbach, K.; Sen, K.S.; Xun, W.; Tillmann, W.; Fischer, H. Bioprinting Organotypic Hydrogels with Improved Mesenchymal Stem Cell Remodeling and Mineralization Properties for Bone Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Huri, P.Y.; Ozilgen, B.A.; Hutton, D.L.; Grayson, W.L. Scaffold pore size modulates in vitro osteogenesis of human adipose-derived stem/stromal cells. Biomed. Mater. 2014, 9, 045003. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Vorwald, C.E.; Dreher, M.L.; Mott, E.J.; Cheng, M.H.; Cinar, A.; Mehdizadeh, H.; Somo, S.; Dean, D.; Brey, E.M.; et al. Evaluating 3D-printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv. Mater. 2015, 27, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Fosang, A.; Donati, D.M.; Wallace, G.G.; Choong, P.F. 3D Bioprinting of Cartilage for Orthopedic Surgeons: Reading between the Lines. Front. Surg. 2015, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pei, P.; Zhu, M.; Du, X.; Xin, C.; Zhao, S.; Li, X.; Zhu, Y. Three dimensional printing of calcium sulfate and mesoporous bioactive glass scaffolds for improving bone regeneration in vitro and in vivo. Sci. Rep. 2017, 7, 42556. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.J.; Richards, R.G.; Alini, M.; Stoddart, M.J. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: Implications for basic research and the clinic. Stem Cells 2014, 32, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.; Zhu, W.; Li, J.; Lee, J.D.; Zhang, L.G. Development of novel three-dimensional printed scaffolds for osteochondral regeneration. Tissue Eng. Part A 2015, 21, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Castro, N.J.; Zhu, W.; Cui, H.; Aliabouzar, M.; Sarkar, K.; Zhang, L.G. Improved Human Bone Marrow Mesenchymal Stem Cell Osteogenesis in 3D Bioprinted Tissue Scaffolds with Low Intensity Pulsed Ultrasound Stimulation. Sci. Rep. 2016, 6, 32876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chow, S.K.; Leung, K.S.; Cheung, W.H. Ultrasound as a stimulus for musculoskeletal disorders. J. Orthop. Transl. 2017, 9, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, G.M.; Gonzalez-Fernandez, T.; Daly, A.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. Three-Dimensional Bioprinting of Polycaprolactone Reinforced Gene Activated Bioinks for Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Mencia-Castano, I.; Sperger, S.; Chen, G.; Cavanagh, B.; Feichtinger, G.A.; Redl, H.; Hacobian, A.; O’Brien, F.J. Delivery of the improved BMP-2-Advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair. J. Control. Release Off. J. Control. Release Soc. 2018, 283, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Arlov, O.; Aachmann, F.L.; Feyzi, E.; Sundan, A.; Skjak-Braek, G. The Impact of Chain Length and Flexibility in the Interaction between Sulfated Alginates and HGF and FGF-2. Biomacromolecules 2015, 16, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.J.; Lee, H.; Park, S.A.; Lee, J.Y. Three dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering. Carbohydr. Polym. 2018, 196, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Ozturk, E.; Arlov, O.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate-Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Rodeo, S.A.; Fortier, L.A.; Lu, C.; Erisken, C.; Mao, J.J. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 2014, 6, 266ra171. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.; Kalyon, D.M.; Yu, X.; Valdevit, A. Segmental bone replacement via patient-specific, three-dimensional printed bioresorbable graft substitutes and their use as templates for the culture of mesenchymal stem cells under mechanical stimulation at various frequencies. Biotechnol. Bioeng. 2018, 115, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.A.; Elisseeff, J.H.; Dorafshar, A.H.; Grayson, W.L. Three-Dimensional Printing of Bone Extracellular Matrix for Craniofacial Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.H.; Grayson, W.L. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann. Biomed. Eng. 2017, 45, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Boil. 2016, 1416, 123–146. [Google Scholar] [CrossRef]
- Volarevic, V.; Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Djonov, V.; Arsenijevic, N. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. BioFactors 2017, 43, 633–644. [Google Scholar] [CrossRef] [PubMed]
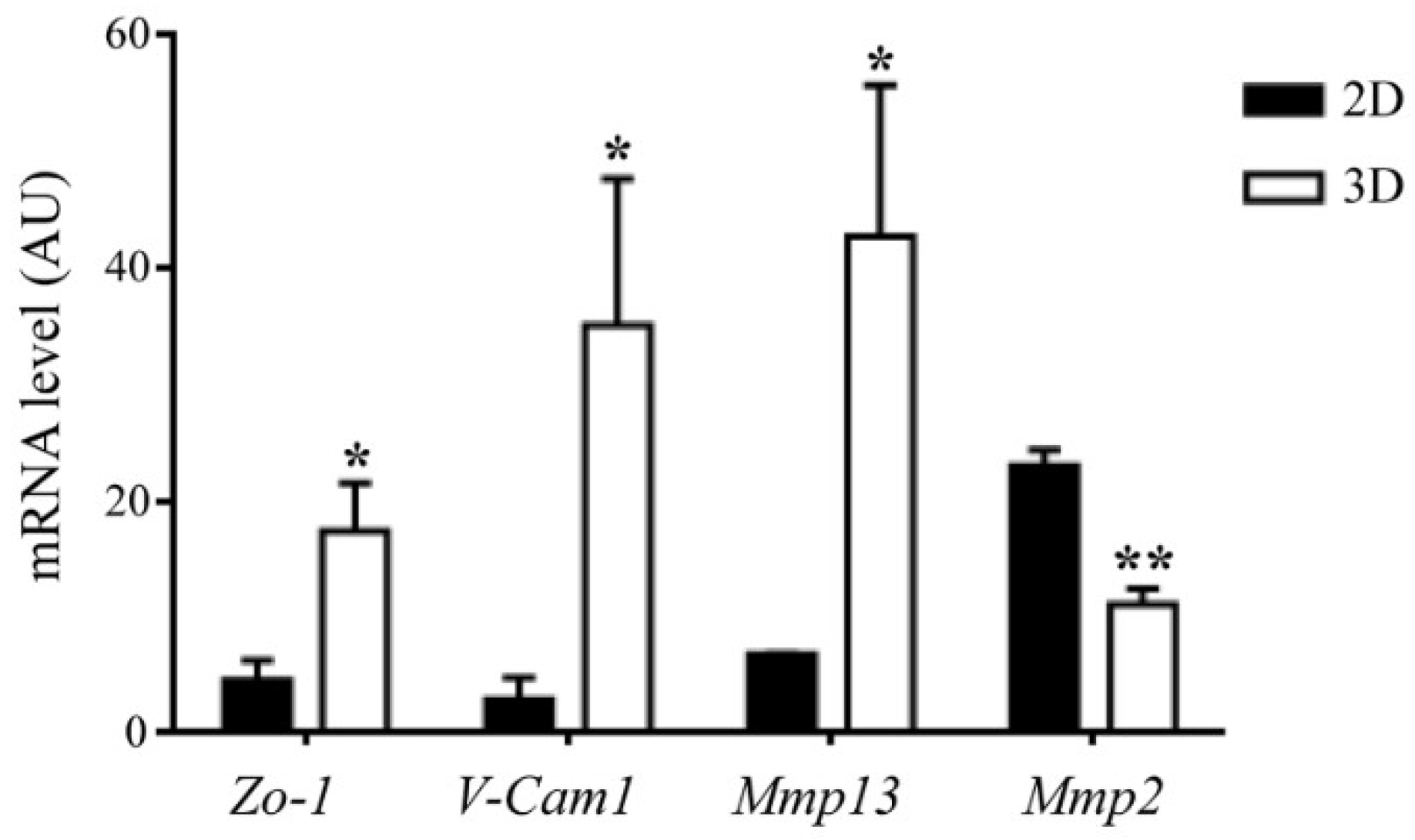
- Su, N.; Gao, P.L.; Wang, K.; Wang, J.Y.; Zhong, Y.; Luo, Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 2017, 141, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, R.; Wang, P.P.; Jian, J.; Jiang, X.L.; Yan, C.; Lin, X.; Wu, L.; Chen, G.Q.; Wu, Q. The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARgamma signaling. Biomaterials 2012, 33, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Feng, B.; Wang, W.; Jiang, Y.; Zhang, W.; Zhou, G.; Jiang, T.; Cao, Y.; Liu, W. Nanoscaled and microscaled parallel topography promotes tenogenic differentiation of ASC and neotendon formation in vitro. Int. J. Nanomed. 2018, 13, 3867–3881. [Google Scholar] [CrossRef] [PubMed]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug Deliv. Rev. 2011, 63, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, I.S.; Cho, T.H.; Lee, K.B.; Hwang, S.J.; Tae, G.; Noh, I.; Lee, S.H.; Park, Y.; Sun, K. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials 2007, 28, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dziak, R.; Yuan, X.; Mao, K.; Genco, R.; Swihart, M.; Sarkar, D.; Li, C.; Wang, C.; Lu, L.; et al. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PLoS ONE 2013, 8, e60473. [Google Scholar] [CrossRef] [PubMed]
- Studle, C.; Vallmajo-Martin, Q.; Haumer, A.; Guerrero, J.; Centola, M.; Mehrkens, A.; Schaefer, D.J.; Ehrbar, M.; Barbero, A.; Martin, I. Spatially confined induction of endochondral ossification by functionalized hydrogels for ectopic engineering of osteochondral tissues. Biomaterials 2018, 171, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Oreffo, R.O. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Yuan, X.; Smith, R.J., Jr.; Guan, H.; Ionita, C.N.; Khobragade, P.; Dziak, R.; Liu, Z.; Pang, M.; Wang, C.; Guan, G.; et al. Hybrid Biomaterial with Conjugated Growth Factors and Mesenchymal Stem Cells for Ectopic Bone Formation. Tissue Eng. Part A 2016, 22, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Taipaleenmaki, H. Regulation of Bone Metabolism by microRNAs. Curr. Osteoporos. Rep. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zheng, S.; Zheng, J. The emerging role of microRNAs in bone remodeling and its therapeutic implications for osteoporosis. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, M.; Browne, S.; Schenke-Layland, K.; Pandit, A. A collagen-based scaffold delivering exogenous microrna-29B to modulate extracellular matrix remodeling. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Chen, Y.; Leong, K.W. MicroRNA delivery for regenerative medicine. Adv. Drug Deliv. Rev. 2015, 88, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Pinese, C.; Lin, J.; Milbreta, U.; Li, M.; Wang, Y.; Leong, K.W.; Chew, S.Y. Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing. Acta Biomater. 2018, 76, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Castano, I.M.; Curtin, C.M.; Duffy, G.P.; O’Brien, F.J. Harnessing a Novel Inhibitory Role of miR-16 in Osteogenesis by Human Mesenchymal Stem Cells for Advanced Scaffold-Based Bone Tissue Engineering. Tissue Eng A 2018. [Google Scholar] [CrossRef]
- Mencia Castano, I.; Curtin, C.M.; Shaw, G.; Murphy, J.M.; Duffy, G.P.; O’Brien, F.J. A novel collagen-nanohydroxyapatite microRNA-activated scaffold for tissue engineering applications capable of efficient delivery of both miR-mimics and antagomiRs to human mesenchymal stem cells. J. Control. Release Off. J. Control. Release Soc. 2015, 200, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B.; Hafizi, M.; Ghaemi, N.; Soleimani, M. Down-regulation of miRNA-221 triggers osteogenic differentiation in human stem cells. Biotechnol. Lett. 2012, 34, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Bakhshandeh, B.; Dehghan, M.M.; Mehrnia, M.R.; Khojasteh, A. Functional synergy of anti-mir221 and nanohydroxyapatite scaffold in bone tissue engineering of rat skull. J. Mater. Sci. Mater. Med. 2016, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, X.; She, F.; Gao, Y.; Tang, P. miR-628-3p regulates osteoblast differentiation by targeting RUNX2: Possible role in atrophic non-union. Int. J. Mol. Med. 2017, 39, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Farina, N.H.; Matzelle, M.M.; Fanning, P.J.; Lian, J.B.; Gravallese, E.M. Synovium-Derived MicroRNAs Regulate Bone Pathways in Rheumatoid Arthritis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Oteri, G.; Allegra, A.; Mania, M.; D’Ascola, A.; Avenoso, A.; Innao, V.; Allegra, A.G.; Campo, S. Altered microRNA expression profile in the peripheral lymphoid compartment of multiple myeloma patients with bisphosphonate-induced osteonecrosis of the jaw. Ann. Hematol. 2018, 97, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Centola, M.; Abbruzzese, F.; Scotti, C.; Barbero, A.; Vadala, G.; Denaro, V.; Martin, I.; Trombetta, M.; Rainer, A.; Marsano, A. Scaffold-based delivery of a clinically relevant anti-angiogenic drug promotes the formation of in vivo stable cartilage. Tissue Eng. Part A 2013, 19, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, H.; Zhang, F.; Wang, L.; Chen, B.; Reynolds, M.A.; Ma, J.; Schneider, A.; Gu, N.; Xu, H.H.K. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, H.; Zhang, F.; Bao, C.; Weir, M.D.; Reynolds, M.A.; Ma, J.; Gu, N.; Xu, H.H.K. Gold nanoparticles in injectable calcium phosphate cement enhance osteogenic differentiation of human dental pulp stem cells. Nanomed. Nanotechnol. Boil. Med. 2018, 14, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Marolt, D.; Knezevic, M.; Novakovic, G.V. Bone tissue engineering with human stem cells. Stem Cell Res. Ther. 2010, 1, 10. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobacchi, C.; Erreni, M.; Strina, D.; Palagano, E.; Villa, A.; Menale, C. 3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 3150. https://doi.org/10.3390/ijms19103150
Sobacchi C, Erreni M, Strina D, Palagano E, Villa A, Menale C. 3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2018; 19(10):3150. https://doi.org/10.3390/ijms19103150
Chicago/Turabian StyleSobacchi, Cristina, Marco Erreni, Dario Strina, Eleonora Palagano, Anna Villa, and Ciro Menale. 2018. "3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells" International Journal of Molecular Sciences 19, no. 10: 3150. https://doi.org/10.3390/ijms19103150
APA StyleSobacchi, C., Erreni, M., Strina, D., Palagano, E., Villa, A., & Menale, C. (2018). 3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells. International Journal of Molecular Sciences, 19(10), 3150. https://doi.org/10.3390/ijms19103150




