Abstract
Cell migration and invasion in glioblastoma (GBM), the most lethal form of primary brain tumors, are critically dependent on Ca2+ signaling. Increases of [Ca2+]i in GBM cells often result from Ca2+ release from the endoplasmic reticulum (ER), promoted by a variety of agents present in the tumor microenvironment and able to activate the phospholipase C/inositol 1,4,5-trisphosphate PLC/IP3 pathway. The Ca2+ signaling is further strengthened by the Ca2+ influx from the extracellular space through Ca2+ release-activated Ca2+ (CRAC) currents sustained by Orai/STIM channels, meant to replenish the partially depleted ER. Notably, the elevated cytosolic [Ca2+]i activates the intermediate conductance Ca2+-activated K (KCa3.1) channels highly expressed in the plasma membrane of GBM cells, and the resulting K+ efflux hyperpolarizes the cell membrane. This translates to an enhancement of Ca2+ entry through Orai/STIM channels as a result of the increased electromotive (driving) force on Ca2+ influx, ending with the establishment of a recurrent cycle reinforcing the Ca2+ signal. Ca2+ signaling in migrating GBM cells often emerges in the form of intracellular Ca2+ oscillations, instrumental to promote key processes in the migratory cycle. This has suggested that KCa3.1 channels may promote GBM cell migration by inducing or modulating the shape of Ca2+ oscillations. In accordance, we recently built a theoretical model of Ca2+ oscillations incorporating the KCa3.1 channel-dependent dynamics of the membrane potential, and found that the KCa3.1 channel activity could significantly affect the IP3 driven Ca2+ oscillations. Here we review our new theoretical model of Ca2+ oscillations in GBM, upgraded in the light of better knowledge of the KCa3.1 channel kinetics and Ca2+ sensitivity, the dynamics of the Orai/STIM channel modulation, the migration and invasion mechanisms of GBM cells, and their regulation by Ca2+ signals.
1. The Glioblastoma
The large majority (more than 90%) of cancer deaths are due not to the primary tumor per se, but to relapses arising from new foci established in distant organs via metastasis [1]. Glioblastoma (GBM), the most common and aggressive form of primary brain tumors, is no exception, though it does not metastasize in the classical way (that is, by colonizing other tissues via the bloodstream), but invades brain parenchyma by detaching from the original tumor mass and infiltrating into the healthy tissue by degrading the extracellular matrix or squeezing through the brain interstitial spaces. The urgency of tackling the migration and invasion issues of GBM tumors is clear.
The 2016 World Health Organization classification of the various types of brain tumors regards the presence of isocitrate dehydrogenase gene (IDH1/2) mutations as one of the most critical biomarkers. Accordingly, IDH1/2 wildtype gliomas are categorized as glioblastoma (formerly primary glioblastoma) and IDH1/2-mutated gliomas (including formerly classified secondary glioblastoma) as astrocytic glioma and oligodendroglioma. GBMs are further subdivided into four groups (Proneural, Neural, Classical, and Mesenchymal), mainly based on the abnormally high levels of mutated genes (i.e., EGFR is highly upregulated in >98% of Classical GBM, whereas TP53 (p53), which is most frequently mutated in Proneural GBM (50–60% of patients) is rarely mutated in Classical GBM). In spite of the intensive basic and clinical studies carried out over the past decades, and modern diagnostics and treatments, the average life expectancy for GBM patients is still only around 15 months. The major obstacle with GBM remains its high migratory and invasive potential into healthy brain parenchyma, which prevents complete surgical removal of tumor cells. Even with full clinical treatment (temozolomide-based chemotherapy and radiation therapy), tumors normally recur at some distance from the site of resection, establishing new tumor lesions that are by far the primary cause of mortality in GBM patients. Arguably, at the time of surgery, large numbers of cells have already detached from the original tumor mass and invaded normal brain tissue. Although GBM cell migration and invasion have been deeply investigated, many aspects of these processes are still poorly understood.
GBM cell migration is a highly regulated multistep process that initiates with GBM cells losing adhesion with surrounding elements, avoiding the cell death often associated with extracellular matrix (ECM) disconnection, and acquiring a highly migratory phenotype, which is a critical feature of the invasive process. The basic mechanisms underlying migration of GBM cells are common to most types of migratory cells. Migration is a property of many non-tumor cells, although it is often restricted to specific developmental stages or environmental conditions; the migration of tumor cells could be viewed as the result of mutation-induced dysregulation of specific biochemical pathways that in healthy tissue keep cell migration dormant.
2. Glioblastoma Cell Migration and Ca2+ Signaling
2.1. Cell Migration
The basic mechanisms of cell locomotion are now fairly well established. Locomotion can be described as the cyclical repeating of two main processes: (i) protrusion of the cell front due to local gain of cell volume mostly generated by active Na+/K+/2Cl− cotransport accompanied by isoosmotically obliged water, and actin polymerization, with formation of pseudopods; (ii) retraction of the rear cell body in the direction of motion, due to forces produced by actomyosin contraction, accompanied by loss of cell volume generated by passive ion (mainly K+ and Cl−) fluxes and osmotic water [2,3]. These two processes involve the coordinated and localized formation of integrin-dependent cell adhesions at the leading edge, and their disassembly at the cell rear [4,5].
Protrusion of the cell front is sustained by localized polymerization of submembrane actin-based cytoskeleton that generates the pushing force and forms flat lamellipodia or needle-like filipodia. A large variety of signaling molecules have been shown to play a leading role in these processes, including the Rho GTPases family (that act as molecular switches to control downstream transduction pathways), and their effector proteins CDC42, RAC1, and RhoA. PI3 kinases have also been deeply implicated in controlling actin polymerization and lamellipodium extension. Activation of PI3 kinase by the pro-invasive signal molecules present in the tumor microenvironment functions as the trigger of the process, in that its activation initiates actin polymerization and generates membrane protrusion [5].
The retraction of the cell rear depends on the contractile forces generated by the activation of the myosin motors along crosslinked actin filaments. The myosin molecule is formed by two heavy chains that make up the two heads and the coiled-coil tail, and four—two essential and two regulatory—myosin light chains (MLCs). The myosin motor is primarily activated by a Ca2+-dependent cascade whereby a cytosolic Ca2+ increase activates a MLC kinase (MLCK) that leads to the regulatory MLC phosphorylation, which allows the myosin motor to crosslink with actin and produce tension to pull the rear end of the cell (however, some studies claim that the motor activity of myosin is not required for cell migration [6,7], playing instead a role in the establishment of cell polarity and in the coordination between different cell domains [7,8]). Clearly, to make the rear cell effectively move, the focal adhesions that anchor the actin cytoskeleton to the extracellular matrix (ECM) must be disassembled, normally through a proteolytic process that involves calpain, but also actin microtubules and focal adhesion kinase (FAK)-recruited dynamin that internalizes the integrins. Increasing actomyosin tension disrupts the possible residual resistances of focal adhesions at the cell rear [2]. Myosin can also be modulated by other pathways, including a Rho-dependent cascade (Rho → ROCK → MLC phosphorylation, or MLC phosphatase activation) that does not depend on cytosolic Ca2+.
This locomotion cycle can be initiated by a variety of external stimuli or directional cues which are sensed and decoded by specialized receptors on the plasma membrane and transferred to the cell interior via distinct signaling pathways to actuate the mechanical response. Most common migration stimuli rely on cells sensing (by specific G protein-coupled receptors) local gradients in the concentration of chemical factors (chemoattractants) [9] present in the tumor microenvironment (for instance chemokines IL-8 or CXCL12), or certain growth factors, including the platelet-derived growth factor (PDGF) that plays an important role in tumor metastasis [10]. Cancer cells can also be guided by gradients of bound ligands. Generally attached to the ECM, these ligands are recognized by specific integrins that form oriented focal adhesion to direct the formation of invadopodia, thus the direction of movement. Common is also the observation of cancer cells being guided by mechanical stimuli (mechanotaxis), namely the stiffness of the extracellular matrix they try to penetrate [11]. It is important to note that in vivo cells can be simultaneously subjected to multiple types of cues that need be evaluated as a whole in order to provide appropriate responses.
2.2. Cell Volume Changes Associated with Migration
It is important to recognize that these two steps—cell front protrusion and cell rear retraction—normally occur in succession, implying that cells are subjected to major changes in cell volume, especially the cell rear retraction. This requires compensatory ion fluxes (and osmotic water flow) across the plasma membrane in order to maintain proper cytosol osmolarity. Along the line proposed by [12] with regard to cell migration in general, during the protrusion of the cell front the maintenance of a stable osmolarity is sustained by cotransporters and ion exchangers, for instance Na+/K+/2Cl− cotransport, followed by osmotically driven water. By contrast, the control of osmolarity during cell rear retraction critically depends on the activation of K channels and ensuing efflux of K+ ions (accompanied by Cl− fluxes, to which the membrane has become highly permeant, and osmotic water). Following the opening of K channels the membrane potential hyperpolarizes to a value between the equilibrium potentials of Cl− and K+ (ECl and EK), a condition that allows a significant efflux of KCl with consequent loss of water and cell volume decrease that facilitates cell body retraction. This view can explain the slowing of cell locomotion following the inhibition of K and Cl channels [13]. A number of papers indicate that the channel types primarily involved in osmolarity control are the intermediate conductance Ca2+-activated K channel (KCa3.1) and the Cl channel ClC3 [13,14].
2.3. Ca2+ Oscillations
Much work shows that cell migration is strictly regulated by Ca2+, which is no surprise given that many proteins involved in migration such as myosin, myosin light chain kinase (MLCK), Ca2+/calmodulin-dependent protein kinase II are Ca2+ sensitive [15]. In the resting cell Ca2+ concentration is kept very low (<100 nM) to prevent activation of Ca2+-sensitive proteins that act as Ca2+ sensor and initiation of unwanted biological processes. Ca2+ concentration can easily increase more than ten-fold (well over 1 µM) following several types of cell stimulation, and this would bring many of the major cytoplasmic Ca2+-sensitive proteins (calmodulin, PKA, Ca2+-activated channels, etc.) to activate. This opens the question of how Ca2+ signaling, under these conditions, can selectively activate a specific Ca2+ sensor protein and the associated biochemical cascade. Several strategies have evolved in this respect, but specificity is mostly attained by confining the signal at sub-cellular level, or by regulating kinetics and magnitude of the Ca2+ signal. Since in the cytoplasm Ca2+ diffusion is extremely low and buffering high, the opening of Ca2+ permeant channels results in a very localized Ca2+ increase (the Ca2+ microdomain), attaining spatial discrimination of the target proteins and linked downstream pathways. Another strategy relies on the temporal features of Ca2+ signals (rising rate, duration, repetition of spikes, etc.) that biological systems are capable to interpret.
In non-excitable cells, including GBM, typical Ca2+ signals induced experimentally by robust chemical (hormone) stimulation are slow, large and generally sustained. Hormones binding to their specific G-protein-coupled receptors (GPCRs) often results in the PLC-dependent synthesis of inositol 1,4,5-trisphosphate (IP3), and consequent Ca2+ release from the endoplasmic reticulum (ER) via IP3 receptors. Eventually, the sustained Ca2+ increase subsides as result of the activation of Ca2+ pumps placed on the sarco/endoplasmic reticulum (SERCA), or the plasma membrane (PMCA) that transfer Ca2+ ions from the cytosol to intracellular stores or the extracellular space.
A physiologically more meaningful type of Ca2+ signal, especially in relation to cell migration, where critical Ca2+-dependent processes are cyclical, comes in the form of Ca2+ oscillations. It has recently become clear that this type of oscillatory Ca2+ increases is what normally occurs when using physiological agonist stimulations. The mechanisms underlying Ca2+ oscillations are not fully understood, and often depend on the cell model and agonist used. In some systems Ca2+ oscillations are secondary to oscillations of the cytoplasmic IP3 level, which may be due to various types of feedback control that for brief intervals uncouple the GTP-coupled receptor from PLC. For instance, the reported negative feedback of the PLC products, IP3 and diacylglycerol (DAG), on PLC itself, or upstream on the GPCR, would result in IP3 oscillations [16], which would in turn generate cyclical release of Ca2+ from intracellular stores, and the Ca2+ oscillations [17].
More commonly, however, Ca2+ oscillations can be observed in the presence of a constant level of IP3. The prevailing view is that, under these conditions, the repetitive Ca2+ oscillations secondary to moderate (physiological) hormone or agonist activation of membrane PLC and production of a constant amount of IP3 arises from the biphasic effects of cytosolic Ca2+ on the IP3 receptor gating, with the first phase being embodied by the establishment of the Ca2+-induced Ca2+ release (CICR) mechanism, whereby a moderate increase of [Ca2+] in the cytosol via IP3 receptor causes a positive feedback activation of the IP3 receptor that results in a higher Ca2+ released from internal stores ([18]; Figure 1A).
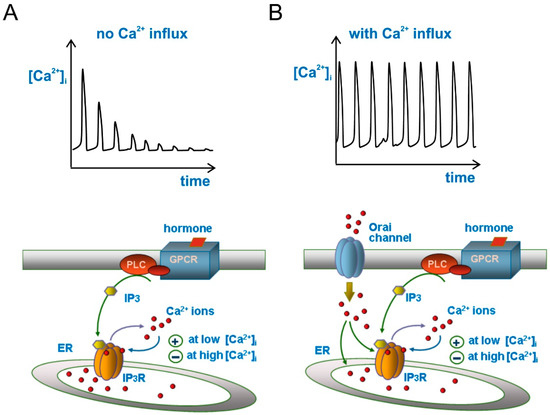
Figure 1.
Ca2+ oscillations in response to inositol thriphosphate (IP3) increase, with and without Ca2+ influx from extracellular space. (A) Bottom, drawing illustrating the hormone-based production of IP3 that activates the IP3 receptor to release Ca2+ from endoplasmic reticulum (ER). The biphasic effects of cytosolic Ca2+ on IP3 receptor gating (the basic mechanism for Ca2+ oscillations), whereby Ca2+ modulates positively the receptor at low [Ca2+] but negatively at high [Ca2+], is also illustrated. Top, Ca2+ oscillations as produced from the schematics below. Note the decaying trend of Ca2+ spikes due to the absence of Ca2+ influx from extracellular space; (B) Here the drawing has been enriched with a Ca2+ influx apparatus from extracellular space through ER-depletion activated Orai channels on the plasma membrane (bottom), which generates sustained Ca2+ oscillations (top). For clarity, SERCA and PMCA Ca2+ pumps have not been sketched in the drawing, although their activity has always been taken into account. For the same reason, we omitted to draw STIM protein of the ER.
The second phase occurs when the cytosolic [Ca2+] reaches significantly higher levels, shifting the previous positive feedback on IP3 receptors into a delayed negative feedback that results in their closing and [Ca2+]i returning to resting level by the action of both SERCA and PMCA Ca2+ pumps (Figure 1A). This view finds support from the observation that at constant levels of IP3, the activity of the IP3 receptor and the cytosolic Ca2+ concentration display a bell-shaped function, whereby low Ca2+ concentrations activate the receptor, whereas high Ca2+ concentrations inhibit it [19,20,21,22]. Ca2+ oscillations generated according to this scheme (i.e., Figure 1A) would however show a rapid decrease of spike amplitude with time, because part of the Ca2+ released from the ER during each spike will be pumped out of the cell by PMCA, and be no longer available for refilling the ER and contributing to the next Ca2+ spike. This Ca2+ oscillations time course is observed experimentally upon removal of external Ca2+, or blockade of Ca2+ influx from the extracellular space. To have sustained (or slow decaying) Ca2+ oscillations, external Ca2+ pumped out of the cell by PMCAs must be allowed to re-enter the cell. This is most commonly accomplished through the mechanism of store-operated Ca2+ entry (SOCE).
2.4. Store-Operated Ca2+ Entry (SOCE)
When Ca2+ is released from the ER, i.e., following hormone stimulation, the Ca2+ concentration within the organelle decreases. It is this decrease to trigger the process (known as SOCE) whereby Ca2+ channels in the plasma membrane are activated, allowing entry of Ca2+ into the cell. Although the process of SOCE was described some 30 years ago, its mechanisms and molecular counterparts—the STIM and Orai proteins—have been identified much more recently [23,24,25]. The stromal interaction molecules (STIMs) are a family of one-passage integral proteins of the ER that act as sensors of the Ca2+ concentration within the ER. Orai proteins form instead a group of highly selective Ca2+ channels placed in the plasma membrane. Orai channels are neither activated by voltage, nor by agonists (in the classical sense), and are normally silent when the cell is at rest and the ER filled with Ca2+ ions. Orai Ca2+ channels are opened by the interaction with activated STIM proteins, which occurs following depletion of Ca2+ in the ER, and their opening results in Ca2+ ions flowing inside the cell (the mechanism has been recently reviewed in depth on neurons by [26]). This Ca2+ entry into the cytoplasm enables the refilling of Ca2+ depleted ER through the SERCA pumps, and make the Ca2+ oscillations amplitude to be kept stable with time (Figure 1B). In the context of our review topic, this feedback mechanism of store-operated Ca2+ entry gains further relevance for two more reasons: it contributes to further modulating the IP3 receptor (this modulation is also dependent on the cell type—for instance, in U87-MG human GBM cells Ca2+ oscillations are affected only marginally by removal of Ca2+ influx [27], whereas in rat C6 GBM cells this maneuver fully suppresses Ca2+ oscillations [28]), and it represents the target on which the KCa3.1 channel exerts its modulation of Ca2+ oscillations, as we will see later.
It needs to be recalled that the store-operated Ca2+ entry is not the only suggested mechanism—although it is by far the most widely accepted—that links hormone stimulation to increased plasma membrane Ca2+ entry. For instance, heteromers of Orai1 and Orai3 can be activated by arachidonic acid [26], and the Ca2+-permeant TRPC6 by receptor-induced PLC activation via direct action of DAG. Notably, neither pathway involves IP3 receptor or Ca2+ depletion from the ER.
2.5. Ca2+ Oscillations and Cell Migration
Numerous studies have shown that migrating GBM cells display evident Ca2+ oscillations having a period of several tens of seconds, whose presence appears to be essential to the process of migration. For example, in U87-MG cells Ca2+ oscillations may be induced by the pro-migratory fetal calf serum (FCS), and trigger focal adhesion disassembly during cell rear retraction [29,30]. Similarly, in D54-MG cells prolonged exposure to bradykinin induces Ca2+ oscillations, which significantly enhance cell motility [31]. Mechanistically, the Ca2+ signal may translate into actomyosin contraction and rear cell retraction by first binding to calmodulin (CaM). The activated complex, Ca2+/CaM, then activates MLCK, the kinase that phosphorylates the regulatory myosin light chains (MLC), thereby triggering cross-bridge movements and actomyosin contraction. It has been shown that MLCK can be modulated by several other protein kinases including PKA, PKC, and RhoK, indicating that this critical enzyme for cell migration is under the influence and control of many extracellular signals and cytoplasmic pathways. Although the consensus on this mechanism and the role of MLCK is widespread, a little caution is needed as most studies used a pharmacological approach, but really specific MLCK inhibitors were not available [32]. A recent report showed, for instance, that knockout cells for MLCK maintained unaltered (or increased) their migratory ability [33]. This shows that if the critical role of Ca2+ in cell motility is undisputed, uncertainties may remain as to how Ca2+ is linked to the cell migratory machinery. The role of the Ca2+-activated K channels in cell volume changes and the dynamics of Ca2+ signals during migration may be a valid alternative.
3. The Intermediate Conductance Ca2+-Activated K Channel
The intermediate conductance Ca2+-activated K channel, KCa3.1, belongs to the Ca2+-activated K channel (KCa) family, which comprises the large- (KCa1.1), intermediate (KCa3.1), and small conductance (KCa2.1-3) K channels, as originally classified according to their single-channel conductance. Genetic relationship and Ca2+ activation mechanisms have later shown that these channels form two well-defined and distantly related groups. One, including only the large-conductance KCa1.1 channel, is gated by the cooperative action of membrane depolarization and [Ca2+]i, while the other group includes both the intermediate conductance KCa3.1 and small-conductance KCa2.1-3 channels, gated solely by cytosolic Ca2+ increase.
3.1. Biophysics, Pharmacology, and Gating of the KCa3.1 Channel
The biophysical properties of the KCa3.1 channel have been investigated using various experimental approaches and cell models. Work on cultured human glioblastoma cells shows high K+ selectivity, moderate inward rectification, and single-channel conductance of 20–60 pS in symmetrical 150 mM K+. The KCa3.1 channel is voltage-insensitive, but highly sensitive to [Ca2+]i showing an EC50 of <200 nM. Ca2+ ions activate the KCa3.1 channel via the Ca2+-binding protein CaM, which is constitutively bound to the membrane-proximal region of the intracellular C terminus of the channel. Binding of Ca2+ to CaM results in conformational changes of the channel, and its opening. In addition to serving as a Ca2+ sensor for channel opening, CaM also regulates the assembly and trafficking of the channel protein to the plasma membrane [34,35].
The KCa3.1 channel can be blocked by peptidic toxins isolated from various scorpions, such as charybdotoxin (ChTx) and maurotoxin (MTx), which display high affinity (IC50 in the nM range). Small synthetic molecules have also been developed, many derived from the clotrimazole template, the classical KCa3.1 channel blocker. The most widely used TRAM-34, developed by Wulff’s group, inhibits KCa3.1 channels with an IC50 < 20 nM and displays high selectivity over the other KCa channels. As for KCa3.1 channel activators, we recall the benzimidazolone 1-EBIO that activates the channels with an EC50 < 30 μM and DC-EBIO that exhibits 10-fold higher potency. Two structurally similar and still more potent molecules are the oxime NS309 and the benzothiazole SKA-31, both showing an EC50 for KCa3.1 channels < 20 nM. We finally mention riluzole, another potent activator of KCa3.1 channels, which is the only FDA-approved drug for treatment of amyotrophic lateral sclerosis (ALS).
3.2. KCa3.1 Channel Expression and Impact on GBM Migration and Invasion
Initial biochemical and electrophysiological studies showed that KCa3.1 channels were diffusely expressed in virtually all cell types investigated. Notably, the KCa3.1 channel was not found in the brain (later the KCa3.1 channel was described in microglia [7], several types of brain neurons [8,36], and the nodes of Ranvier of cerebellar Purkinje neurons [37]), although it was highly expressed in established cell lines from brain tumors, as well as in brain tumors in situ. Moreover, KCa3.1 channel expression was reported in many other tumor types, and implicated in malignancy (increased cell growth, migration, invasion, apoptosis evasion). The KCa3.1 mRNA and protein expression were also found to be significantly enhanced in cancer stem cells derived from both the established cell line U87-MG and primary cell line FCN9 [38]. The REMBRANDT database shows that the gene KCNN4, which encodes the KCa3.1 channel, is overexpressed in more than 30% of gliomas, and its expression is associated with a poor prognosis.
To test the role of the KCa3.1 channel in GBM cells’ infiltration into brain parenchyma, human GL-15 GBM cells were xenografted into the brains of SCID mice and later treated with the specific KCa3.1 blocker TRAM-34. Immunofluorescence analyses of cerebral slices after a five-week treatment revealed a significant reduction of tumor infiltration, compared with TRAM-34 untreated mice [39]. Reduction of tumor infiltration was also observed in the brain of mice transplanted with KCa3.1-silenced GL-15 cells, indicating a direct role of KCa3.1 channels in GBM tumor infiltration [40]. Similarly, KCa3.1 channel block with TRAM-34 or silencing by short hairpin RNA (shRNA) completely abolished CXCL12-induced GL-15 cell migration [40]. A strong correlation of KCa3.1 channel expression with migration was reported by Calogero’s group in GBM-derived cancer stem cells (CSC) [38]. Blockage of the KCa3.1 channel with TRAM-34 was found to have a much greater impact on the motility of CSCs (75% reduction) that express a high level of KCa3.1 channel than on the FCN9 parental population (32% reduction), where the KCa3.1 channel is expressed at much lower levels. Similar results were also observed with the CSCs derived from U87-MG [38] and with mesenchymal glioblastoma stem cells [41]. On the same line stand the results later obtained by Sontheimer’s group showing that pharmacological inhibition of the KCa3.1 channel (with TRAM-34) in U251 glioma cells, or silencing it with inducible siRNA, resulted in a significant reduction of tumor cells’ migration in vitro and invasion into surrounding brain parenchyma of SCID mice [42]. On a retrospective study of a patient genomic database, they further showed that KCa3.1 channel expression was inversely correlated with patient survival.
3.3. Basic Functions of KCa3.1 Channel: Regulation of Cell Ca2+ Signaling
KCa3.1 channels control a number of basic cellular processes involved in the modulation of several higher-order biological functions critical to brain tumors’ malignancy, including migration and invasion. The most relevant and common basic cellular processes controlled by KCa3.1 channels are the modulation of Ca2+ signaling and the control of cell volume. Here we will concentrate on the KCa3.1 channel modulation of Ca2+ signaling, the focus of this review.
In virtually all cells a robust stimulation of PLC-coupled membrane receptors triggers an initial IP3-mediated release of Ca2+ from intracellular stores, followed by Ca2+ influx through store-operated Orai Ca2+ channels, which are activated in response to Ca2+ depletion of the ER. One consequence of this Ca2+ influx, besides the activation of Ca2+-dependent target proteins (ion channels included), is membrane depolarization, which, if left unchecked, would more and more strongly inhibit further Ca2+ influx due to the reduced electrochemical driving force for Ca2+ ions. It has been shown that the efflux of K+ ions following KCa3.1 channels’ activation by incoming Ca2+ hyperpolarizes the membrane towards EK, increasing the electromotive force on incoming Ca2+ ions [43,44]. This represents an indirect modulatory mechanism of Ca2+ entry and, as a result, of cell Ca2+ signaling. The Ca2+-dependent and voltage-independent gating of KCa3.1 channels appears to be particularly well suited for this function, since the coupling of Ca2+ influx and activation of K+ channels will create a positive feedback loop whereby more Ca2+ influx will increase the K+ efflux, hyperpolarize the plasma membrane, and further stimulate Ca2+ entry, thus amplifying the signal transduction.
This paradigm was first demonstrated in human macrophages, where KCa3.1 channels have been shown to hyperpolarize the membrane and increase the driving force for Ca2+ ions during store-operated Ca2+ entry [43], and in T cells, where KCa3.1 channels are rapidly upregulated following cell activation, and supposedly used to maximize Ca2+ influx during the reactivation of memory T cells [45,46]. Activated T cells isolated from KCa3.1−/− mice show a defective Ca2+ response to T cell receptor activation [47]. Also, mast cells appear to use KCa3.1 channels to hyperpolarize the membrane and increase the Ca2+ influx following antigen-mediated stimulation. In this case KCa3.1 channels have been found to physically interact with the Orai1 subunit, suggesting that they may be activated by the Ca2+ microdomain that forms close to the store-operated Ca2+ channel [28,48]. A similar conclusion has been reached for rat microglial cells, where the Orai1-KCa3.1 functional coupling could be interrupted by BAPTA but not by EGTA [49]. Since the two Ca2+ chelators have similar Ca2+ affinity but different Ca2+ binding rates (10 times higher for BAPTA), only a physical coupling between Orai1 and KCa3.1, with a separating distance of few nanometers, would explain these results.
Evidence of cross-talk between the KCa3.1 channel and store depletion-activated Ca2+ influx has also been reported for GBM cells. An increase of [Ca2+]i, consisting of a fast peak due to IP3-dependent Ca2+ release from intracellular stores followed by a sustained phase of Ca2+ influx across the plasma membrane, supposedly activated by intracellular stores’ depletion, was found upon prolonged application of histamine on GL-15 GBM cells [44]. The enhancing role of the KCa3.1 channel in sustaining the protracted influx of external Ca2+ was shown by the marked reduction of the sustained histamine-induced [Ca2+]i increase following application of TRAM-34. This observation could be significant with regard to the KCa3.1 channels’ contribution to GBM cell migration exerted through modulation of Ca2+ signals.
In another GBM cell line—U87-MG—we also observed that the promigratory FCS is able to promote Ca2+ oscillations, and these oscillations cyclically activate the KCa3.1 channels during cell migration [13]. Using a modeling approach, we also found that a channel activity with the properties of KCa3.1 channels could modulate these Ca2+ oscillations (it increased the amplitude, duration, and frequency of each Ca2+ spike [50]). The results we obtained, which are illustrated in the following section, were unexpectedly interesting, and also able to explain old experiments showing that the KCa3.1 channel inhibition by ChTx abolishes the bradykinin-induced Ca2+ oscillations in C6 glioma cells [51].
3.4. Modulation of Ca2+ Oscillations by KCa3.1 Channel Activity
We saw earlier that the mechanism underlying Ca2+ oscillations is essentially based on the biphasic effects of Ca2+ on IP3 receptor gating—activatory at low and inhibitory at high concentrations. We also discussed the modulation that the KCa3.1 channel could exert on Ca2+ oscillations. This modulation relies on the high Ca2+ sensitivity and voltage independence of the KCa3.1 channel, and on the output of its activity, that is, the hyperpolarization of the cell membrane potential (Vm) as result of the K+ efflux. As KCa3.1 channels are activated by Ca2+ concentrations well within the range observed during hormone-induced Ca2+ oscillations (150–600 nM; cf. above), they are expected to cyclically open (during the Ca2+ spikes) and cause cyclic membrane hyperpolarizations in phase with the Ca2+ oscillations.
We have observed experimentally such KCa3.1 channel oscillatory activity and associated membrane potential oscillations in U87-MG cells in response to FCS [13], an agent known to induce Ca2+ oscillations in these cells [29,30]. Notably, because of their voltage dependence and much lower Ca2+ sensitivity, the KCa1.1 channels, also highly expressed in GBM cells, could not be activated under these conditions, even at the peak Ca2+ concentration of the oscillations ([13], but see [52] for a case in which the KCa1.1 channel activity may control Ca2+ influx). In conclusion, since Vm controls the driving force for external Ca2+ entry, KCa3.1-dependent Vm oscillations are expected to cause oscillations in the amplitude of Ca2+ influx through the hormone-activated plasma membrane Ca2+ channels, which will in turn feedback onto, and modulate the Ca2+ oscillations themselves.
We recently implemented basic theoretical models of Ca2+ oscillations with the oscillating membrane Vm, as produced by the cyclic activation of KCa3.1 channels. The resulting model was used to predict how the hormone-induced Ca2+ oscillations would be influenced by the KCa3.1 channels-induced Vm oscillations in phase with Ca2+ oscillations. We found that the cyclic activation of KCa3.1 channels by Ca2+ oscillations induces Vm fluctuations that in turn determine oscillations in the Ca2+ influx from the extracellular medium, as a result of the oscillating Vm-dependent changes in the driving force for Ca2+ ions influx. Since Ca2+ influx peaks are in phase with the Ca2+ oscillations, the Ca2+ spikes will also be increased by the in-phase Ca2+ influx. Our model calculations in fact show that KCa3.1-induced Vm oscillations strengthen the hormone-induced [Ca2+]i signals by increasing the amplitude, duration, and oscillatory frequency of Ca2+ spikes (Figure 2; see also [50]).
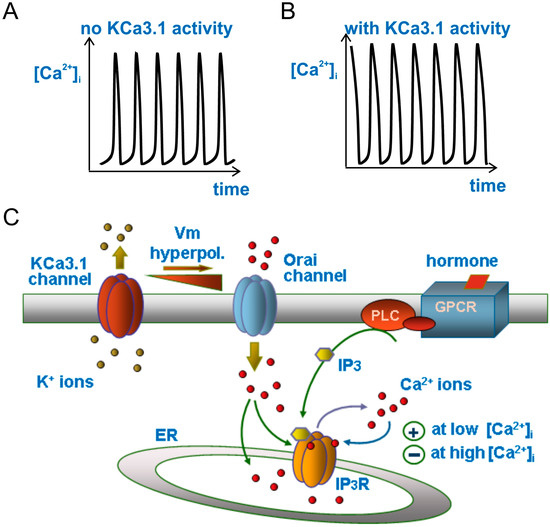
Figure 2.
Modulation of Ca2+ oscillations by the KCa3.1 channel. Drawing (C) illustrating the main ion fluxes generating or modulating the Ca2+ oscillations in our model when the KCa3.1 channel is cut off (A) or introduced into the system (B). Please notice the much longer duration (width) and slightly higher amplitude of the Ca2+ oscillations in the presence of KCa3.1 channels.
3.5. KCa3.1 Channels Switch Ca2+ Oscillations On and Off
Arguably, the KCa3.1 channels can do more than just modulate the amplitude and frequency of Ca2+ oscillations: they can trigger or suppress them. This has been an unexpected prediction of our model that we later found to have been experimentally observed. As experimentally found, our model predicts that Ca2+ oscillations can be generated only within a specific range of IP3 concentrations, while a stable, non-oscillating Ca2+ level is present for both lower and higher IP3 concentrations (Figure 3A). Surprisingly, it was found that when the KCa3.1 conductance is changed in the system, the resulting alteration of the KCa3.1 channels-induced Vm oscillations significantly change the IP3 concentrations needed to produce Ca2+ oscillations. In particular, increasing the activity of KCa3.1 channels shifts leftward the IP3 range within which Ca2+ oscillations are produced. This implies that there are ranges of IP3 levels where the presence or absence of Ca2+ oscillations are only dictated by the activity (conductance) of KCa3.1 channels (Figure 3B bottom, shaded areas).
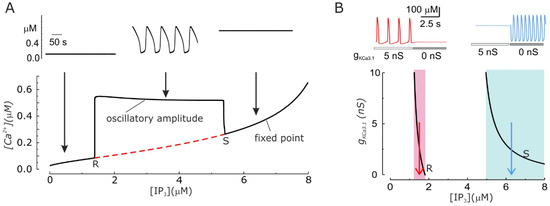
Figure 3.
Ca2+ oscillation development depends on the IP3 concentration and the activity of KCa3.1 channels. (A) Bottom. Bifurcation diagram showing that IP3 concentration determines the establishment of the Ca2+ oscillations. The red dashed line represents the unstable equilibrium [Ca2+]i. The computation was performed with gKCa3.1 = 5 nS. R and S indicate the Hopf bifurcations where Ca2+ oscillations appear and disappear, respectively, upon increasing the IP3 levels. Top. The traces show the temporal changes of the [Ca2+]i for three different concentrations of IP3 (indicated by arrows in the bottom part). (B) Bottom. Plot of the two Hopf bifurcations (R and S) as a function of the IP3 concentration and gKCa3.1. Three different ranges of IP3 concentrations, where the KCa3.1-induced Vm oscillations appear to have different effects in the modulation of Ca2+ oscillations are evidenced (shaded areas; see text). More specifically in the pink region the presence of KCa3.1 channels is necessary for the existence of Ca2+ oscillations, in the light blue region KCa3.1 channels prevents Ca2+ oscillations, and finally in the white region in between KCa3.1 channels modulate the shape and duration of oscillation. Top. Simulated Ca2+ oscillations obtained with two IP3 concentrations (1.5 left and 6.2 μM right, as indicated by the red and blue arrows below) within the regions where removing KCa3.1 channels make Ca2+ oscillations disappear or appear. (Modified from [48].)
These observations could explain a number of experimental results where KCa3.1 channel modulation was shown to have an effect on Ca2+ oscillations. In both activated T lymphocytes and C6 glioma cells, inhibition of Vm oscillations by blocking KCa3.1 channels is able to suppress Ca2+ oscillations [51,53]. Moreover ras-induced transformation of fibroblasts, which has been reported to upregulate KCa3.1 channels, has also been shown to trigger Ca2+ oscillations [54,55]. Finally, KCa3.1 inhibition leads to induction of Ca2+ oscillations in about half of the tested glioblastoma cells [52].
3.6. Glioblastoma KCa3.1 Channels Are Activated by Serum-Induced Ca2+ Oscillations and Participate to Cell Migration
Glioblastoma cells in vivo are exposed to a variety of tumor microenvironment components and soluble factors that can markedly affect their migratory ability. Among them there are unknown serum components that infiltrate into the tumor area of high-grade gliomas as result of the blood brain barrier breakdown [6,56]. As already shown, FCS enhances migration of U87-MG glioblastoma cells, and does so by inducing Ca2+ oscillations that are critically involved in the detachment of focal adhesions (by activating the focal adhesion kinase), and subsequent retraction of the cell rear [29,30]. On the same cell model we further reported that FCS, besides Ca2+ oscillations, induces an oscillatory activity of KCa3.1 channels, and this KCa3.1 channel activity is a necessary step to promote U87-MG cell migration by FCS [13]. In the same study, we also found a stable activation of Cl− currents upon FCS stimulation. Altogether these observations sketch a coherent picture of the cell migratory process, in that, the retraction of the cell rear after the detachment of focal adhesions involves a major cell shape rearrangement and volume reduction. In these instances, the (oscillatory) KCa3.1 channel activity and the consequent cyclical K+ efflux, in combination with Cl− efflux (and essential osmotic water), form part of the whole machinery for achieving the cyclical volume reduction needed.
Cell locomotion is thought to be promoted by the cycling replication of four main steps: (i) protrusion of the cell front, associated to asymmetric polymerization of the actin-based cytoskeleton; (ii) adhesion of the protruded cell front to the substratum, mediated by the binding of focal adhesions to the extracellular polymers in contact with the cell; (iii) unbinding of the focal adhesions at the cell rear; (iv) retraction of the rear cell body in the direction of motion (cf. Figure 4). Two of these steps, namely cell front protrusion and cell rear retraction, involve major changes in cell volume and thus require compensatory ion fluxes (and osmotic water flow) across the membrane. Along the lines proposed by [12], during the protrusion of the cell front the maintenance of a stable osmolarity is sustained by cotransporters and ion exchangers—for instance, Na+/K+/2Cl− cotransport—followed by osmotically driven water. During this phase [Ca2+]i is relatively low (red segment in the Ca2+ oscillation, Figure 4a), a condition in which KCa3.1 channels are closed and the resting membrane potential is near ECl. This condition prevents or strongly limits the loss of KCl via K and Cl channels that would nullify the osmotic work of the Na+/K+/2Cl− cotransport.
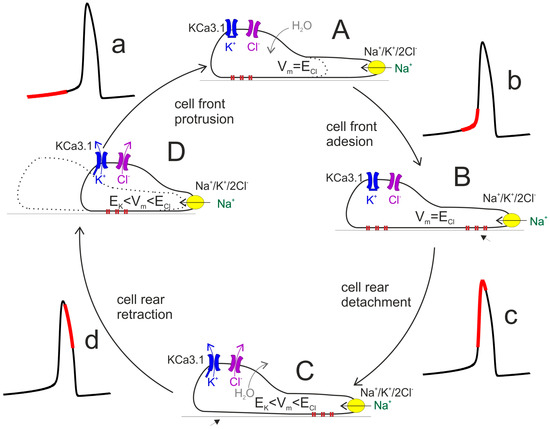
Figure 4.
Schematic diagram of cell migratory cycle. The classic view of the cell migration process can be split down into four main cyclical steps. The cycle begins with the cell front protrusion, due to the activity of Na+/K+/2Cl− cotransport (yellow) (A) and establishment of adhesion structures (B). The elongated cell then removes/weakens the rear adhesions (C) so that ensuing contraction can pull the rear cell portion forward (D). The concomitant values of [Ca2+]i is indicated by the red portion on the associated Ca2+ oscillation. Vm, ion and water fluxes are also illustrated (see text for details).
By contrast, the control of osmolarity during cell rear retraction critically depends on the activation of KCa3.1 channels and ensuing efflux of K+ ions (accompanied by Cl− fluxes, to which membrane has become highly permeant, and osmotic water). Following the increase in [Ca2+]i towards the peak of the Ca2+ oscillation (Figure 4c,d), KCa3.1 channels open and the membrane potential hyperpolarizes to a value between ECl and EK, a condition that would allow a significant efflux of KCl with consequent loss of water and cell volume decrease (Figure 4C,D). This would facilitate the process of cell body retraction. This view can explain the slowing of cell locomotion following the inhibition of K and Cl channels [13].
4. Conclusions
Substantial evidence suggests that the oscillatory activity of the KCa3.1 channel during Ca2+ oscillations may have both direct and indirect modulatory effects on GBM cell migration. As has long been recognized, the K+ efflux through KCa3.1 channels directly participates in the cell volume changes during cell rear retraction. The same oscillatory K+ efflux, however, will also determine the membrane potential oscillations that alter the driving force for Ca2+ influx and indirectly modulate the properties and even the presence of Ca2+ oscillations, and thus the timing of the migratory machinery. Both modulatory roles make KCa3.1 channels pivotal in GBM cell migration, and thus potential pharmacological targets for this deadly tumor. Although this review concentrates on the role of KCa3.1 and STIM/Orai channels in modulating Ca2+ oscillations and cell migration, it needs to be said that other channels including TRPM8 and KCa1.1, also abundantly expressed in glioblastoma cells, may also be important in these processes. Accordingly, TRPM8 [41] and KCa1.1 channel signaling [57] have been demonstrated to be required for basal and radiation-induced migration, respectively, and certainly contribute to Ca2+ oscillations.
Author Contributions
Both L.C. and F.F. performed literature search and wrote the paper.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J. Life at the leading edge. Cell 2011, 145, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Carragher, N.O.; Frame, M.C. Focal adhesion and actin dynamics: A place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004, 14, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Lund, C.V.; Nguyen, M.T.; Owens, G.C.; Pakchoian, A.J.; Shaterian, A.; Kruse, C.A.; Eliceiri, B.P. Reduced glioma infiltration in Src-deficient mice. J. Neurooncol. 2006, 78, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wessels, D.; Soll, D.R.; Knecht, D.; Loomis, W.F.; De Lozanne, A.; Spudich, J. Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev. Biol. 1988, 128, 164–177. [Google Scholar] [CrossRef]
- Lombardi, M.L.; Knecht, D.A.; Dembo, M.; Lee, J. Traction force microscopy in Dictyostelium reveals distinct roles for myosin II motor and actin-crosslinking activity in polarized cell movement. J. Cell Sci. 2007, 120 Pt 9, 1624–1634. [Google Scholar] [CrossRef]
- Roca-Cusachs, P.; Sunyer, R.; Trepat, X. Mechanical guidance of cell migration: Lessons from chemotaxis. Curr. Opin. Cell Biol. 2013, 25, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Ostman, A.; Heldin, C.H. PDGF receptors as targets in tumor treatment. Adv. Cancer Res. 2007, 97, 247–274. [Google Scholar] [PubMed]
- Lo, C.M.; Wang, H.B.; Dembo, M.; Wang, Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of ion channels and transporters in cell migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Aiello, F.; Fioretti, B.; Sforna, L.; Castigli, E.; Ruggieri, P.; Tata, A.M.; Calogero, A.; Franciolini, F. Serum-activated K and Cl currents underlay U87-MG glioblastoma cell migration. J. Cell Physiol. 2011, 226, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Cuddapah, V.A.; Habela, C.W.; Watkins, S.; Moore, L.S.; Barclay, T.T.; Sontheimer, H. Kinase activation of ClC-3 accelerates cytoplasmic condensation during mitotic cell rounding. Am. J. Physiol. Cell Physiol. 2012, 302, C527–C538. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, X.; Zheng, M.; Cheng, H. Calcium gradients underlying cell migration. Curr. Opin. Cell Biol. 2012, 24, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, K.S.; Chay, T.R. Modelling receptor-controlled intracellular calcium oscillators. Cell Calcium 1991, 12, 97–109. [Google Scholar] [CrossRef]
- Gaspers, L.D.; Bartlett, P.J.; Politi, A.; Burnett, P.; Metzger, W.; Johnston, J.; Joseph, S.K.; Höfer, T.; Thomas, A.P. Hormone-induced calcium oscillations depend on cross-coupling with inositol 1,4,5-trisphosphate oscillations. Cell Rep. 2014, 9, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Dupont, G.; Combettes, L.; Bird, G.S.; Putney, J.W. Calcium oscillations. Cold Spring Harb. Perspect. Biol. 2011, 3, a004226. [Google Scholar] [CrossRef] [PubMed]
- Iino, M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J. Gen. Physiol. 1990, 95, 1103–1122. [Google Scholar] [CrossRef] [PubMed]
- Bezprozvanny, I.; Watras, J.; Ehrlich, B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 1991, 351, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Finch, E.A.; Turner, T.J.; Goldin, S.M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science 1991, 252, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Kaznacheyeva, E.; Lupu, V.D.; Bezprozvanny, I. Single-channel properties of inositol (1,4,5)-trisphosphate receptor heterologously expressed in HEK-293 cells. J. Gen. Physiol. 1998, 111, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Peinelt, C.; Vig, M.; Koomoa, D.L.; Beck, A.; Nadler, M.J.; Koblan-Huberson, M.; Lis, A.; Fleig, A.; Penner, R.; Kinet, J.P. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat. Cell Biol. 2006, 8, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Ruffinatti, F.A.; Zuccolo, E. Intracellular Ca2+ Signals to Reconstruct a Broken Heart: Still a Theoretical Approach? Curr. Drug Targets 2015, 6, 793–815. [Google Scholar] [CrossRef]
- Dubois, C.; Vanden Abeele, F.; Lehen’kyi, V.; Gkika, D.; Guarmit, B.; Lepage, G.; Slomianny, C.; Borowiec, A.S.; Bidaux, G.; Benahmed, M.; et al. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell 2014, 26, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.M.; Ashmole, I.; Smallwood, D.T.; Leyland, M.L.; Bradding, P. Orai/CRACM1 and KCa3.1 ion channels interact in the human lung mast cell plasma membrane. Cell Commun. Signal. 2015, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Rondé, P.; Giannone, G.; Gerasymova, I.; Stoeckel, H.; Takeda, K.; Haiech, J. Mechanism of calcium oscillations in migrating human astrocytoma cells. Biochim. Biophys. Acta 2000, 1498, 273–280. [Google Scholar] [CrossRef]
- Giannone, G.; Rondé, P.; Gaire, M.; Haiech, J.; Takeda, K. Calcium oscillations trigger focal adhesion disassembly in human U87 astrocytoma cells. J. Biol. Chem. 2002, 277, 26364–26371. [Google Scholar] [CrossRef] [PubMed]
- Montana, V.; Sontheimer, H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J. Neurosci. 2011, 31, 4858–4867. [Google Scholar] [CrossRef] [PubMed]
- Totsukawa, G.; Wu, Y.; Sasaki, Y.; Hartshorne, D.J.; Yamakita, Y.; Yamashiro, S.; Matsumura, F. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J. Cell Biol. 2004, 164, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tao, T.; Wen, C.; He, W.Q.; Qiao, Y.N.; Gao, Y.Q.; Chen, X.; Wang, P.; Chen, C.P.; Zhao, W.; et al. Myosin light chain kinase (MLCK) regulates cell migration in a myosin regulatory light chain phosphorylation-independent mechanism. J. Biol. Chem. 2014, 289, 28478–28488. [Google Scholar] [CrossRef] [PubMed]
- Sforna, L.; Megaro, A.; Pessia, M.; Franciolini, F.; Catacuzzeno, L. Structure, Gating and Basic Functions of the Ca2+-activated K Channel of Intermediate Conductance. Curr. Neuropharmacol. 2018, 16, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Franciolini, F. Editorial: The Role of Ca2+-activated K+ Channels of Intermediate Conductance in Glioblastoma Malignancy. Curr. Neuropharmacol. 2018, 16, 607. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Koach, M.A.; Whitmore, L.; Lamers, M.L.; Horwitz, A.F. Segregation and activation of myosin IIB creates a rear in migrating cells. J. Cell Biol. 2008, 183, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, V.; Koeberle, P.D.; Wang, Y.; Schlichter, L.C. The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J. Neurosci. 2007, 27, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, P.; Mangino, G.; Fioretti, B.; Catacuzzeno, L.; Puca, R.; Ponti, D.; Miscusi, M.; Franciolini, F.; Ragona, G.; Calogero, A. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PLoS ONE 2012, 7, e47825. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, G.; Catalano, M.; Sciaccaluga, M.; Chece, G.; Cipriani, R.; Rosito, M.; Grimaldi, A.; Lauro, C.; Cantore, G.; Santoro, A.; et al. KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo. Cell Death Dis. 2013, 4, e773. [Google Scholar] [CrossRef] [PubMed]
- Sciaccaluga, M.; Fioretti, B.; Catacuzzeno, L.; Pagani, F.; Bertollini, C.; Rosito, M.; Catalano, M.; D’Alessandro, G.; Santoro, A.; Cantore, G.; et al. CXCL12-induced glioblastoma cell migration requires intermediate conductance Ca2+-activated K+ channel activity. Am. J. Physiol. Cell Physiol. 2010, 299, C175–C184. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, L.; Sezgin, E.C.; Skardelly, M.; Eckert, F.; Huber, S.M. KCa3.1 Channels and Glioblastoma: In Vitro Studies. Curr. Neuropharmacol. 2018, 16, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.L.; Honasoge, A.; Robert, S.M.; McFerrin, M.M.; Sontheimer, H. A proinvasive role for the Ca(2+)-activated K(+) channel KCa3.1 in malignant glioma. Glia 2014, 62, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Hanley, P.J.; Rinné, S.; Zuzarte, M.; Daut, J. Calcium-activated K+ channel (KCa3.1) activity during Ca2+ store depletion and store-operated Ca2+ entry in human macrophages. Cell Calcium 2010, 48, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, B.; Catacuzzeno, L.; Sforna, L.; Aiello, F.; Pagani, F.; Ragozzino, D.; Castigli, E.; Franciolini, F. Histamine hyperpolarizes human glioblastoma cells by activating the intermediate-conductance Ca2+-activated K+ channel. Am. J. Physiol. Cell Physiol. 2009, 297, C102–C110. [Google Scholar] [CrossRef] [PubMed]
- Ghanshani, S.; Wulff, H.; Miller, M.J.; Rohm, H.; Neben, A.; Gutman, G.A.; Cahalan, M.D.; Chandy, K.G. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000, 275, 37137–37149. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Beeton, C.; Chandy, K.G. Potassium channels as therapeutic targets for autoimmune disorders. Curr. Opin. Drug Discov. Dev. 2003, 6, 640–647. [Google Scholar]
- Di, L.; Srivastava, S.; Zhdanova, O.; Ding, Y.; Li, Z.; Wulff, H.; Lafaille, M.; Skolnik, E.Y. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc. Natl. Acad. Sci. USA 2010, 107, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.S.; Berger, P.; Cruse, G.; Yang, W.; Bolton, S.J.; Bradding, P. The K+ channel iKCA1 potentiates Ca2+ influx and degranulation in human lung mast cells. J. Allergy Clin. Immunol. 2004, 114, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Schlichter, L.C. Selective activation of KCa3.1 and CRAC channels by P2Y2 receptors promotes Ca(2+) signaling, store refilling and migration of rat microglial cells. PLoS ONE 2013, 8, e62345. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Fioretti, B.; Franciolini, F. A theoretical study on the role of Ca2+-activated K+ channels in the regulation of hormone-induced Ca2+ oscillations and their synchronization in adjacent cells. J. Theor. Biol. 2012, 309, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Reetz, G.; Reiser, G. [Ca2+]i oscillations induced by bradykinin in rat glioma cells associated with Ca2+ store-dependent Ca2+ influx are controlled by cell volume and by membrane potential. Cell Calcium 1996, 19, 143–156. [Google Scholar] [CrossRef]
- Stegen, B.; Klumpp, L.; Misovic, M.; Edalat, L.; Eckert, M.; Klumpp, D.; Ruth, P.; Huber, S.M. K+ channel signaling in irradiated tumor cells. Eur. Biophys. J. 2016, 45, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Verheugen, J.A.; Vijverberg, H.P. Intracellular Ca2+ oscillations and membrane potential fluctuations in intact human T lymphocytes: Role of K+ channels in Ca2+ signaling. Cell Calcium 1995, 17, 287–300. [Google Scholar] [CrossRef]
- Hashii, M.; Nozawa, Y.; Higashida, H. Bradykinin-induced cytosolic Ca2+ oscillations and inositol tetrakisphosphate-induced Ca2+ influx in voltage-clamped ras-transformed NIH/3T3 fibroblasts. J. Biol. Chem. 1993, 268, 19403–19410. [Google Scholar] [PubMed]
- Rane, S.G. A Ca2(+)-activated K+ current in ras-transformed fibroblasts is absent from nontransformed cells. Am. J. Physiol. 1991, 260, C104–C112. [Google Scholar] [CrossRef] [PubMed]
- Seitz, R.J.; Wechsler, W. Immunohistochemical demonstration of serum proteins in human cerebral gliomas. Acta Neuropathol. 1987, 73, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Steinle, M.; Palme, D.; Misovic, M.; Rudner, J.; Dittmann, K.; Lukowski, R.; Ruth, P.; Huber, S.M. Ionizing radiation induces migration of glioblastoma cells by activating BK K+ channels. Radiother. Oncol. 2011, 101, 122–126. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).