Chlorophyll Composition, Chlorophyll Fluorescence, and Grain Yield Change in esl Mutant Rice
Abstract
1. Introduction
2. Results
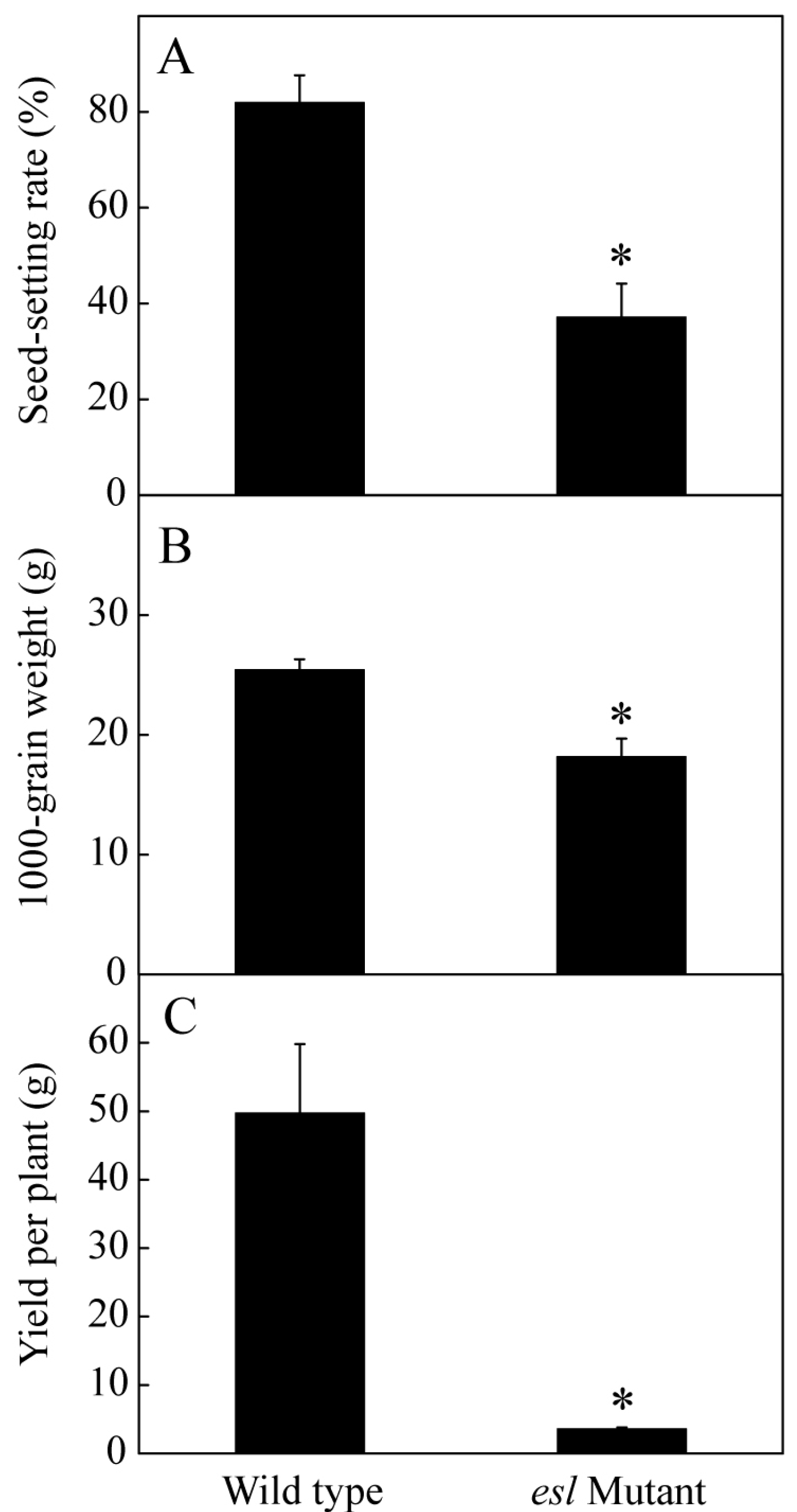
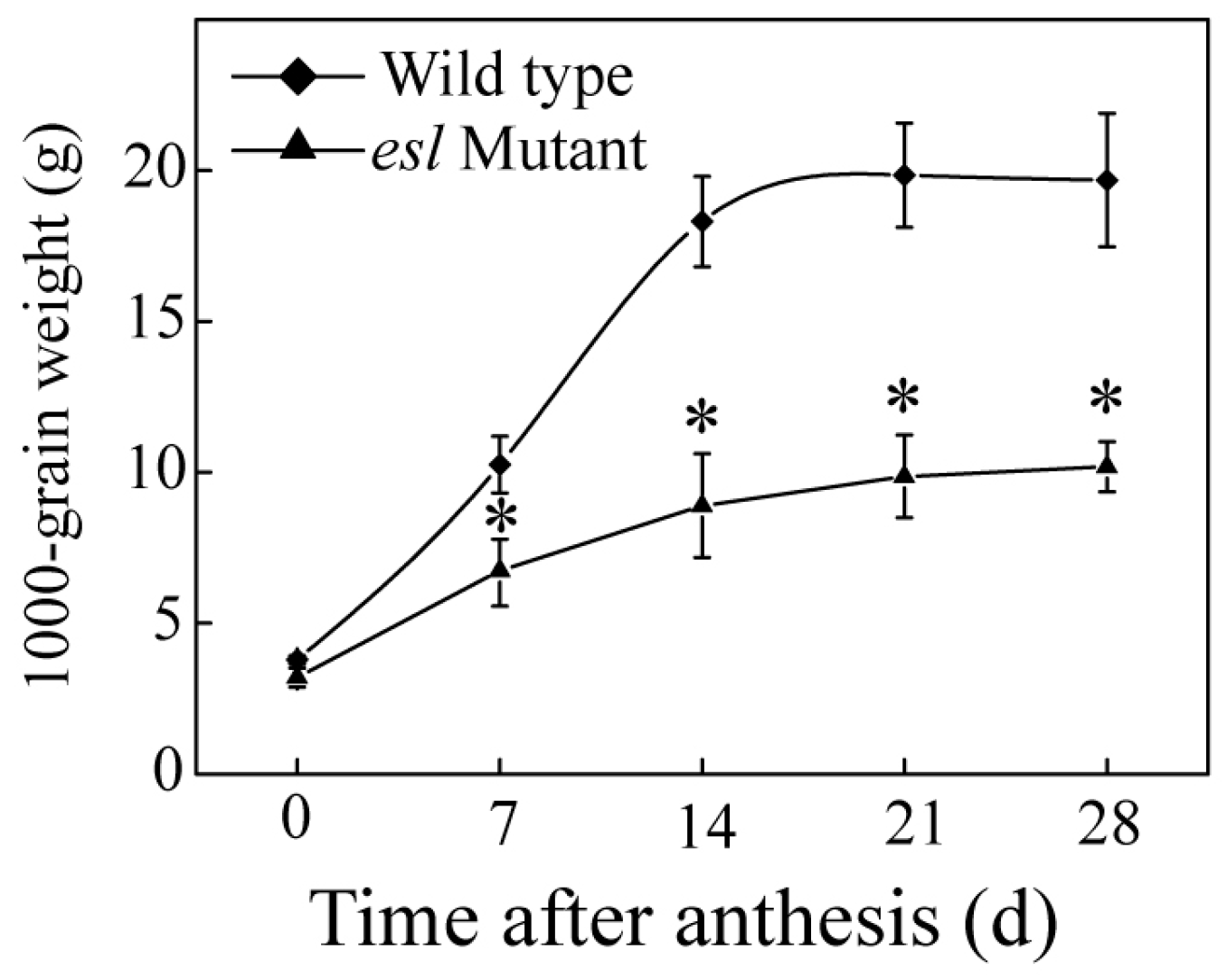
2.1. Genotype-Dependent Differences in the Yield Properties and Grain Filling
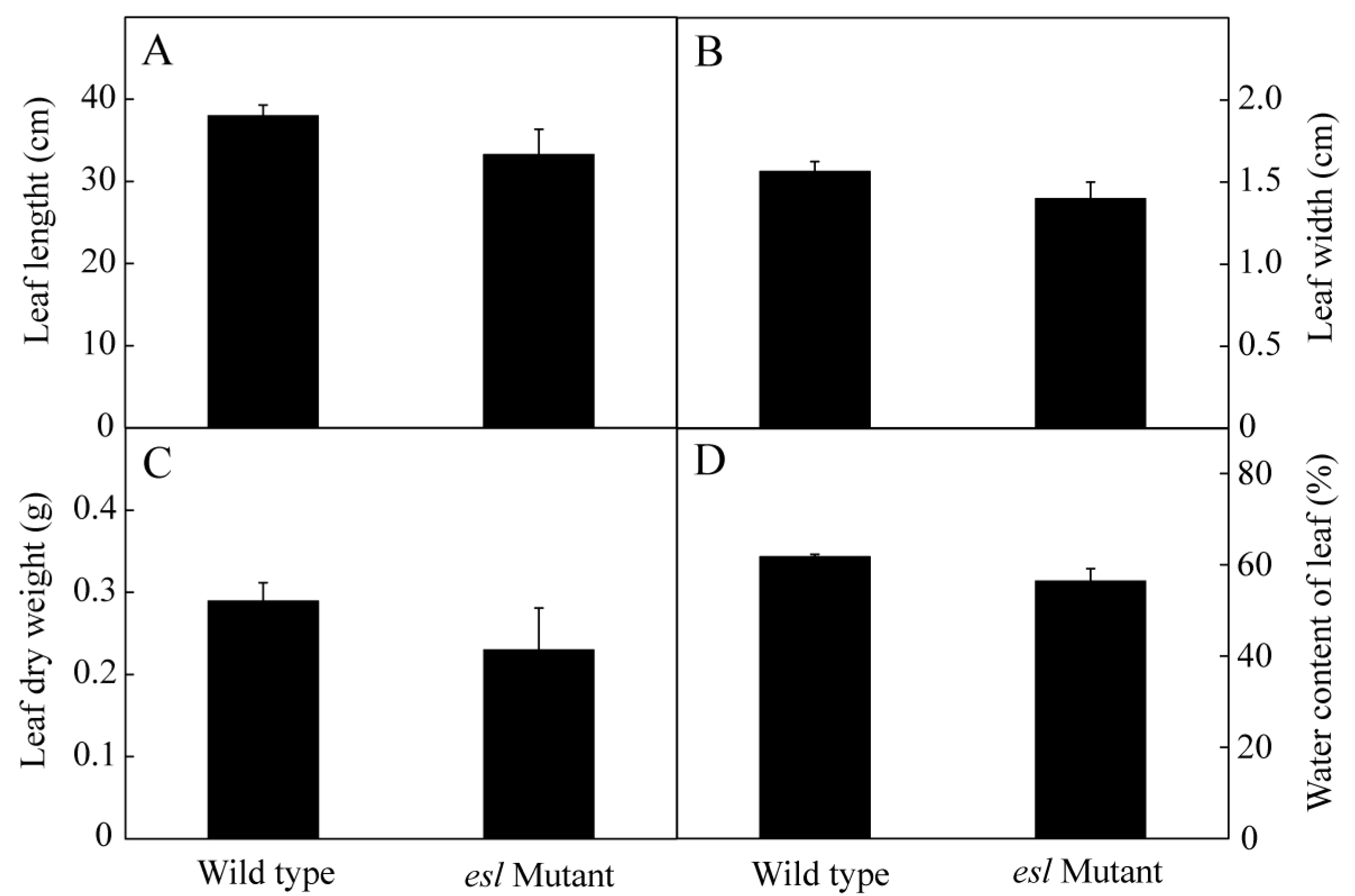
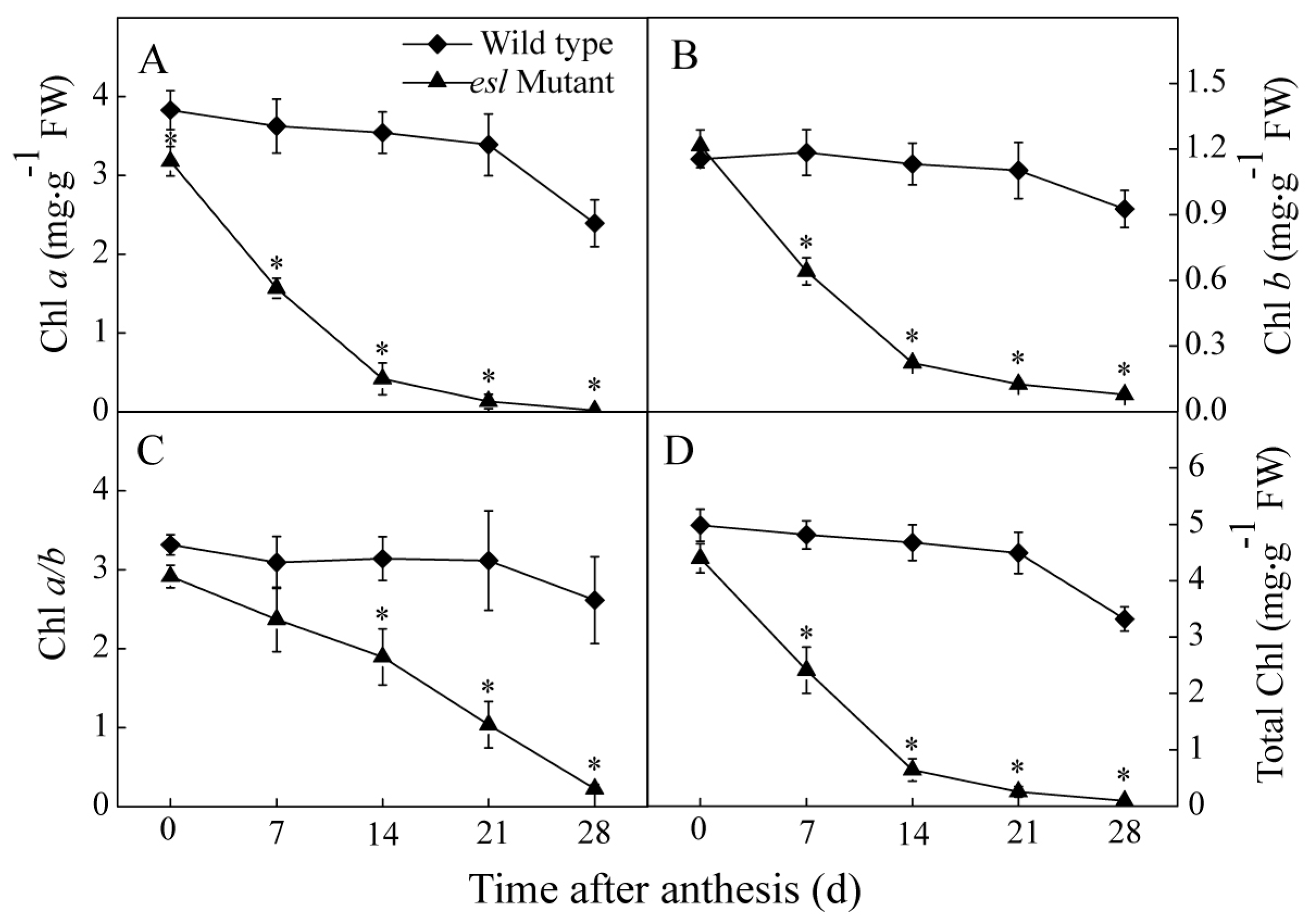
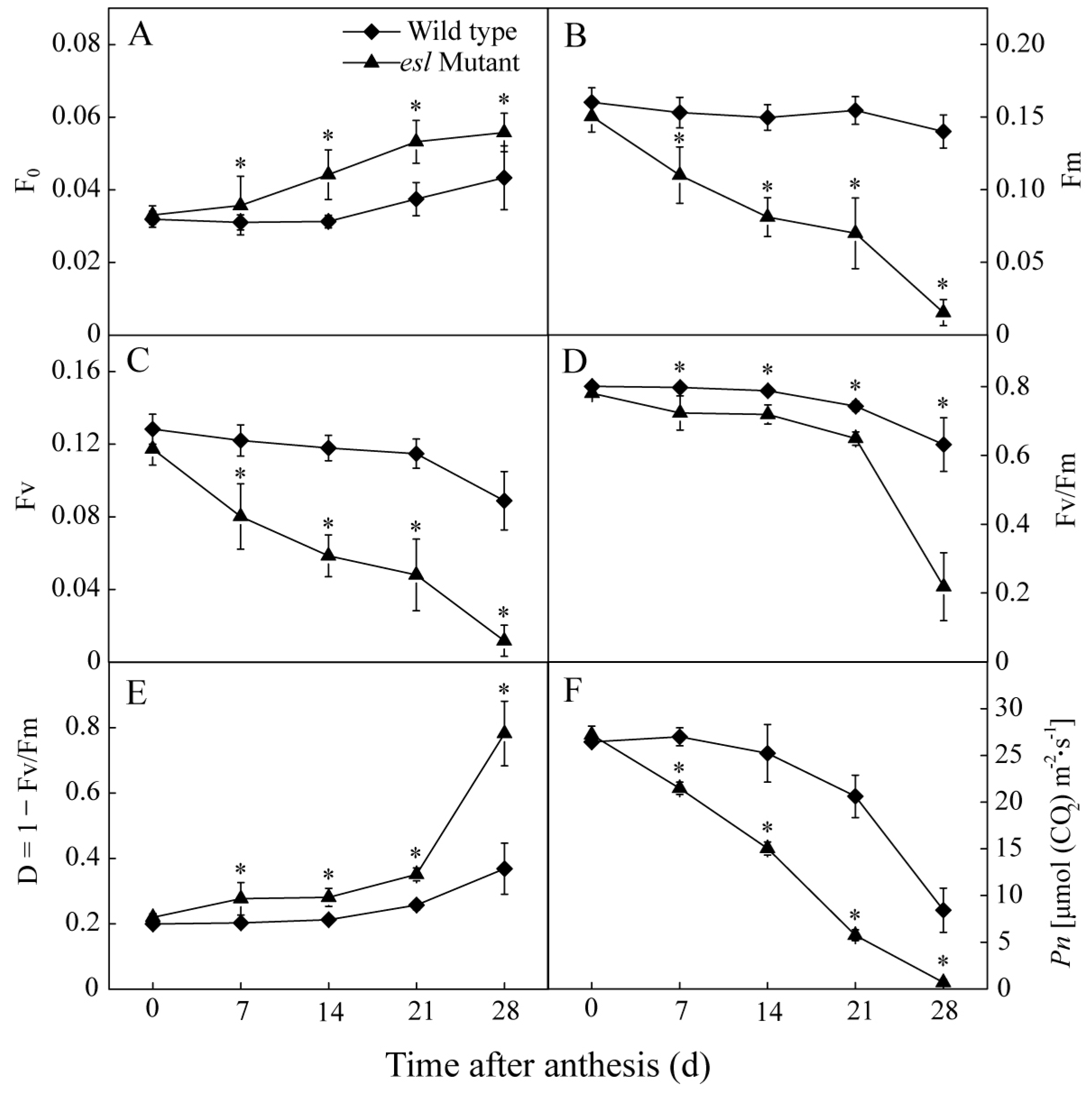
2.2. Genotype-Dependent Differences in Leaf Characteristics and Photosynthetic Performance during the Grain-Filling Stage
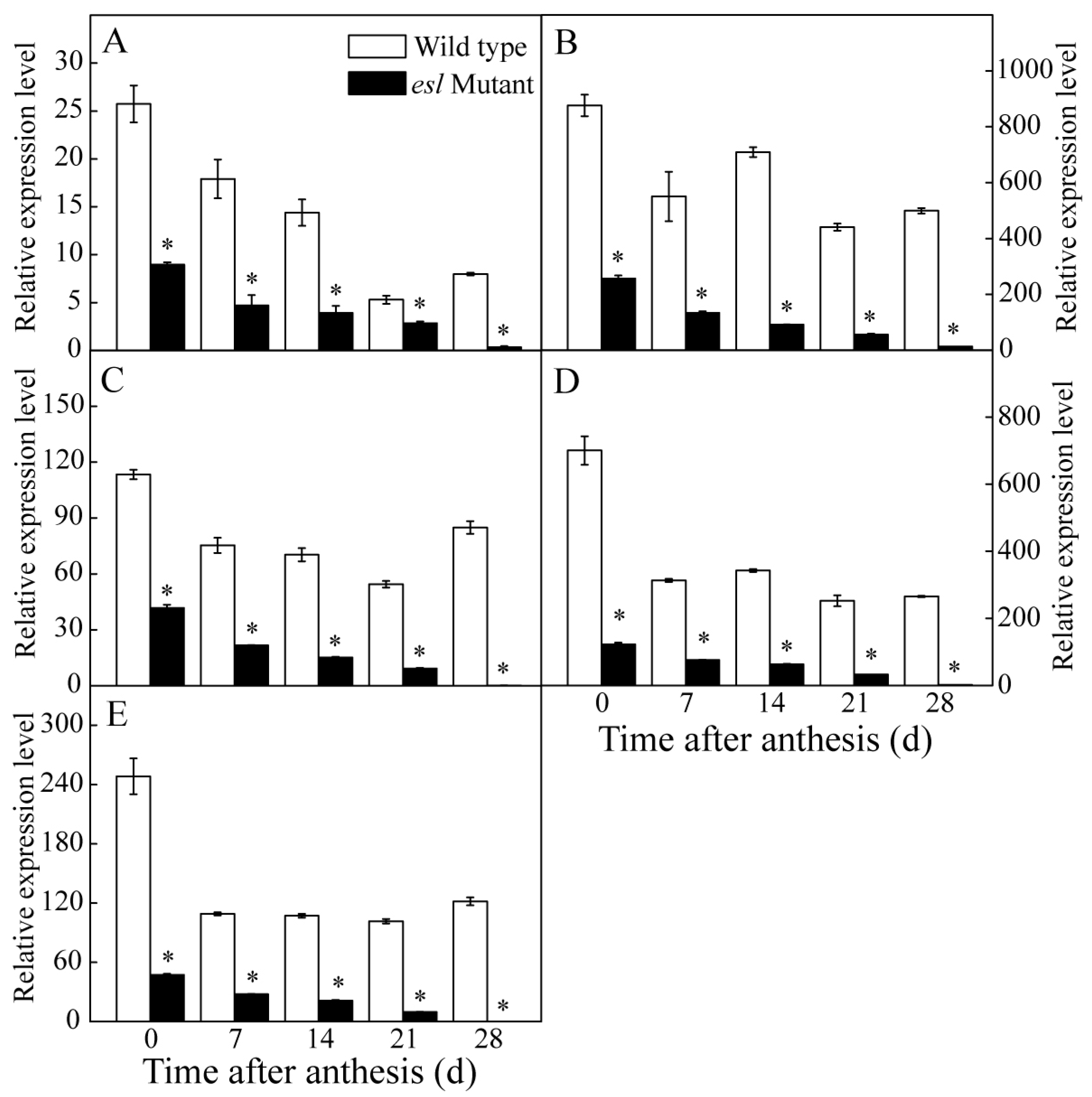
2.3. Genotype-Dependent Differences in the Expression of Cab and Key Genes Encoding for the Reaction Center of the PSII Complex
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Field Study
4.3. Determination of Photosynthetic Pigments
4.4. Measurement of Chl Fluorescence Parameters and Net Photosynthetic Rate (Pn)
4.5. RNA Extraction and cDNA Synthesis
4.6. qRT–PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Cab | Chlorophyll a/b binding protein |
| Chl | Chlorophyll |
| esl | Early senescence leaf |
| Fm | Maximal fluorescence yield of the dark-adapted state |
| F0 | Minimal fluorescence yield of the dark-adapted state |
| Fv | Variable fluorescence |
| Fv/Fm | Maximal quantum yield of PSII photochemistry |
| PSII | Photosystem II |
| Pn | Net photosynthetic rate |
| QA | Primary quinone acceptor |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
References
- International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Pan, Y.; Ma, Y.; Ling, Z.; Bao, W.; Jing, Y.; You, S. Physiological and biochemical studies on leaf senescence at heading and grain formation stage in hybrid rice. Sci. Agric. Sin. 1988, 3, 004. [Google Scholar]
- Duan, J.; Liang, C.; Huang, Y. Studies on leaf senescence of hybrid rice at flowing and grain formation stage. Acta Photophysiol. Sin. 1997, 23, 139–144. [Google Scholar]
- Li, Z.; Wang, F.; Lin, W.; Zhao, Q.; Liu, J.; Cheng, F. Carbon reserve and remobilization in leaf sheaths during the grain-filling stage in response to leaf early senescence. Acta Physiol. Plant. 2017, 39, 10. [Google Scholar] [CrossRef]
- Panda, D.; Sarkar, R.K. Natural leaf senescence: Probed by chlorophyll fluorescence, CO2 photosynthetic rate and antioxidant enzyme activities during grain filling in different rice cultivars. Physiol. Mol. Biol. Plants 2013, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lu, D. Effect of irrigation modes on the senescence and physiological activity in hybrid rice after heading. Chin. J. Rice Sci. 1990, 4, 56–62. [Google Scholar]
- Wu, X.Y.; Kuai, B.K.; Jia, J.Z.; Jing, H.C. Regulation of leaf senescence and crop genetic improvement. J. Integr. Plant Biol. 2012, 54, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Distelfeld, A.; Avni, R.; Fischer, A.M. Senescence, nutrient remobilization, and yield in wheat and barley. J. Exp. Bot. 2014, 65, 3783–3798. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.L.; Holm, P.B.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Krupinska, K.; Humbeck, K. Photosynthesis and chloroplast breakdown. In Plant Cell Death Processes, 1st ed.; Elsevier: San Diego, CA, USA, 2003; pp. 169–187. [Google Scholar]
- Shu, S.; Guo, S.R.; Sun, J.; Yuan, L.Y. Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumissativus and its protection by exogenous putrescine. Physiol. Plant. 2012, 146, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Wen, X.G.; Lu, C.M. Differential changes in degradation of chlorophyll-protein complexes of photosystem I and photosystem II during flag leaf senescence of rice. Plant Physiol. Biochem. 2005, 43, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Falqueto, A.R.; Silva, F.S.P.; Cassol, D.; MagalhãesJúnior, A.M.; Oliveira, A.C.; Bacarin, M.A. Chlorophyll fluorescence in rice: Probing of senescence driven changes of PSII activity on rice varieties differing in grain yield capacity. Braz. J. Plant Physiol. 2010, 22, 35–41. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.; Hannaway, D.; Lu, H. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigmahemsleyanum Diels et Gilg. Environ. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.J.; Anjum, S.A.; Wang, L.; Song, J.X.; Zong, X.F.; Lv, J.; Zohaib, A.; Ali, I.; Yan, R.; Zhang, Y.; et al. Effect of foliar application of brassinolide on photosynthesis and chlorophyll fluorescence traits of Leymuschinensis under varying levels of shade. Photosynthetica 2018, 56, 873–883. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Hossain, Z. Chlorophyll a fluorescence—A useful tool for the early detection of temperature stress in spring barley (Hordeumvulgare L.). Omics 2011, 15, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, M.; Yotsova, E.; Markovska, Y.; Apostolova, E.L. Effect of high light intensity on the photosynthetic apparatus of two hybrid lines of Paulownia grown on soils with different salinity. Photosynthetica 2018, 56, 832–840. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, C.; Zhang, J.; Kuang, T. Photosynthesis and chlorophyll a fluorescence during flag leaf senescence of field-grown wheat plants. J. Plant Physiol. 2002, 159, 1173–1178. [Google Scholar] [CrossRef]
- Wingler, A.; Marès, M.; Pourtau, N. Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence. New Phytol. 2004, 161, 781–789. [Google Scholar] [CrossRef]
- Tollenaar, M.; Wu, J. Yield improvement in temperate maize is attributable to greater stress tolerance. Crop. Sci. 1999, 39, 1597–1604. [Google Scholar] [CrossRef]
- Thomas, H.; Ougham, H. The stay-green trait. J. Exp. Bot. 2014, 65, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Mondal, W.A.; Choudhuri, M.A. Regulation of leaf senescence, grain-filling and yield of rice by kinetin and abscisic acid. Physiol. Plant. 1983, 59, 343–346. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Egli, D.B. Seed-fill duration and yield of grain crops. Adv. Agron. 2004, 83, 243–279. [Google Scholar]
- Gan, S.S. Leaf senescence as an important target for improving crop production. Adv. Crop. Sci. Technol. 2014, 2, e116. [Google Scholar] [CrossRef]
- Sheela, G.; Shai, V.N.; Saran, S. Role of flag leaf on grain yield and spikelet sterility in rice cultivars. Oryza 1990, 27, 87–88. [Google Scholar]
- Rahman, M.A.; Haque, M.E.; Sikdar, B.; Islam, M.A.; Matin, M.N. Correlation analysis of flag leaf with yield in several rice cultivars. J. Life Earth Sci. 2013, 8, 49–54. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Islam, M.R.; Ismail, M.R.; Shahidullah, S.M.; Hanafi, M.M. Evaluation of six aromatic rice varieties for yield and yield contributing characters. Int. J. Agric. Biol. 2009, 11, 616–620. [Google Scholar]
- Faisal, M.M.A.T. Flag leaf characteristics and relationship with grain yield and grain protein percentage for three cereals. J. Med. Plants Stud. 2014, 2, 1–7. [Google Scholar]
- Hisir, Y.; Kara, R.; Dokuyucu, T. Evaluation of oat (Avena sativa L.) genotypes for grain yield and physiological traits. Zemdirb. Agric. 2012, 99, 55–60. [Google Scholar]
- He, Y.; Li, L.; Zhang, Z.; Wu, J.L. Identification and comparative analysis of premature senescence leaf mutants in rice (Oryza sativa L.). Int. J. Mol. Sci. 2018, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, E.; Sikora, A.; Charzyniska, M.; Mostowska, A. Aspects of programmed cell death during leaf senescence of mono- and dicotyledonous plants. Protoplasma 2000, 214, 93–101. [Google Scholar] [CrossRef]
- Wang, D.Y.; Zhang, X.F.; Shao, G.S.; Qian, Q.; Xu, C.M. Leaf senescence of difference leaf color rice and its response to light intensity. Chin. J. Rice Sci. 2008, 22, 77–81. [Google Scholar]
- Scheumann, V.; Ito, H.; Tanaka, A.; Schoch, S.; Rüdiger, W. Substrate specificity of chlorophyll(ide) b reductase in etioplasts of barley (Hordeumvulgare L.). Eur. J. Biochem. 1996, 242, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, D.G.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–355. [Google Scholar] [CrossRef]
- Morita, R.; Sato, Y.; Masuda, Y.; Nishimura, M.; Kusaba, M. Defect in non-yellow coloring 3, an alpha/beta hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J. 2009, 59, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.B.; Huang, F.D.; Cheng, F.M.; Li, Z.W.; Hu, D.W.; Pan, G.; Mao, Y.C. Photosynthesis and chloroplast ultra-structure characteristics of flag leaves for a premature senescence rice mutant. Acta Agron. Sin. 2012, 38, 871–879. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Pairs | Products (Base Pair) |
|---|---|---|
| Actin | 5′-CAGCACATTCCAGCAGATGT-3′ 5′-TAGGCCGGTTGAAAACTTTG-3′ | 198 |
| Cab | 5′-TGGCAGGACATCAAGAACCC-3′ 5′-GCTCCTTCTCCTTGGCCTC-3′ | 145 |
| PsbA | 5′-ATCTGTAGTTGATAGCCAAGGTCG-3′ 5′-TAGGTCTAGAGGGAAGTTGTGAGC-3′ | 118 |
| PsbB | 5′-ACGGTGGAGTTCTATGGTGG-3′ 5′-CCCTTGGACTGCTGCGAAA-3′ | 154 |
| PsbC | 5′-GGAGCAATGAACCTATTTGAAGTGG-3′ 5′-GCCTAAGACTGCGGAGGAAAT-3′ | 186 |
| PsbD | 5′-AACCGCAGCAGTTTCCACC-3′ 5′-CACCAACGAGTAAAATCCCCTT-3′ | 93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Guo, X.; Pan, X.; Li, Z. Chlorophyll Composition, Chlorophyll Fluorescence, and Grain Yield Change in esl Mutant Rice. Int. J. Mol. Sci. 2018, 19, 2945. https://doi.org/10.3390/ijms19102945
Lin W, Guo X, Pan X, Li Z. Chlorophyll Composition, Chlorophyll Fluorescence, and Grain Yield Change in esl Mutant Rice. International Journal of Molecular Sciences. 2018; 19(10):2945. https://doi.org/10.3390/ijms19102945
Chicago/Turabian StyleLin, Weiwei, Xiaodong Guo, Xinfeng Pan, and Zhaowei Li. 2018. "Chlorophyll Composition, Chlorophyll Fluorescence, and Grain Yield Change in esl Mutant Rice" International Journal of Molecular Sciences 19, no. 10: 2945. https://doi.org/10.3390/ijms19102945
APA StyleLin, W., Guo, X., Pan, X., & Li, Z. (2018). Chlorophyll Composition, Chlorophyll Fluorescence, and Grain Yield Change in esl Mutant Rice. International Journal of Molecular Sciences, 19(10), 2945. https://doi.org/10.3390/ijms19102945






