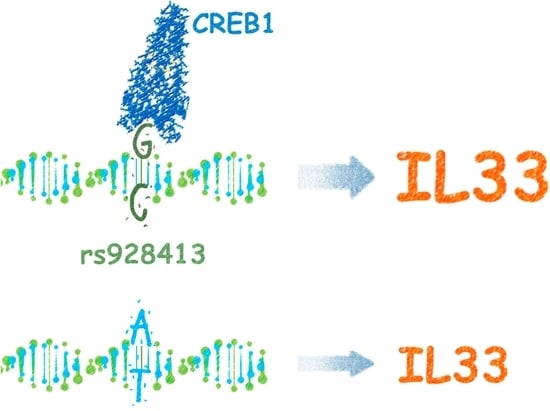
The Risk G Allele of the Single-Nucleotide Polymorphism rs928413 Creates a CREB1-Binding Site That Activates IL33 Promoter in Lung Epithelial Cells
Abstract
1. Introduction
2. Results
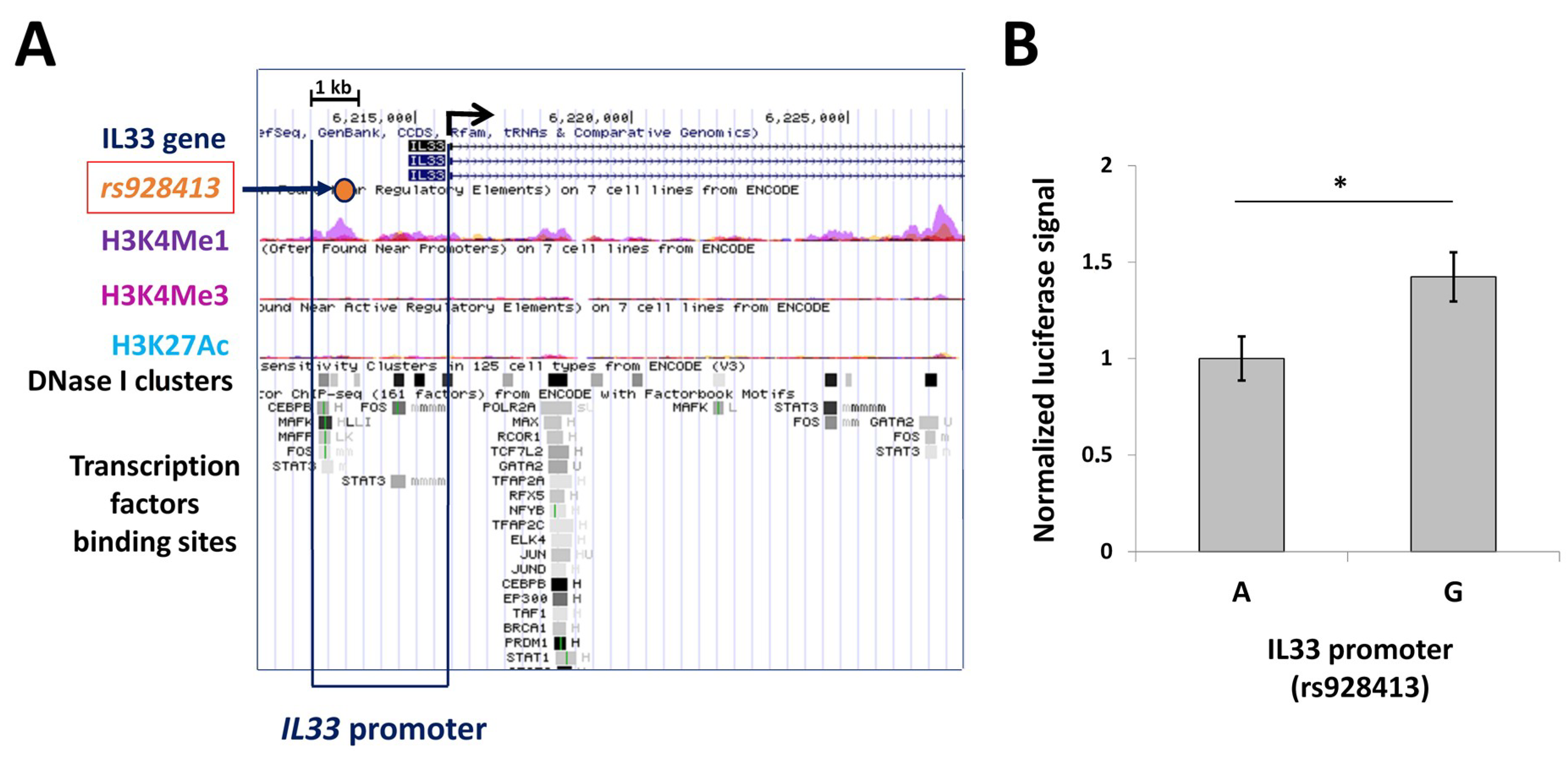
2.1. Presence of “G” Allele of rs928413 Is Associated with Increased IL33 Promoter Activity
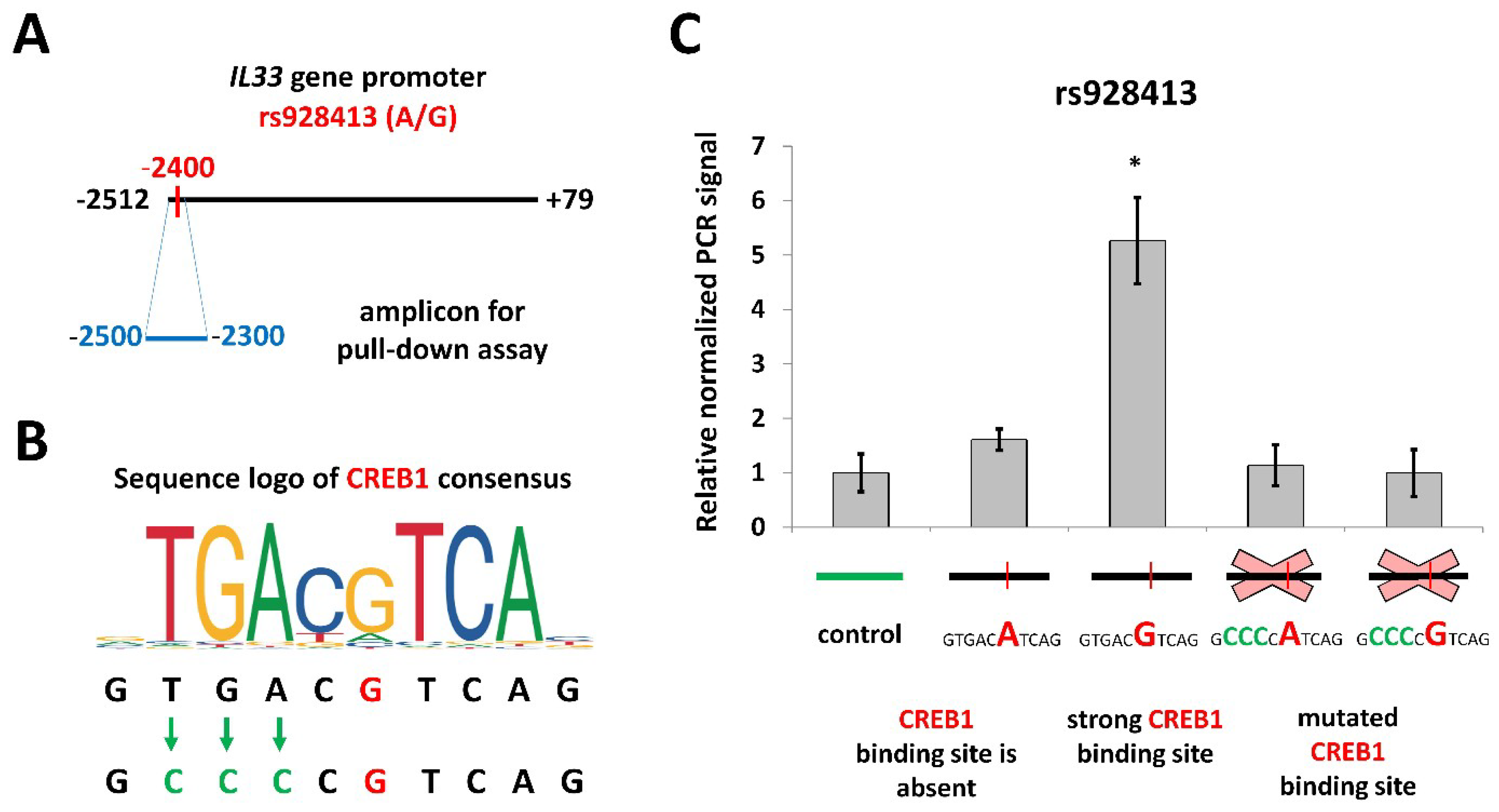
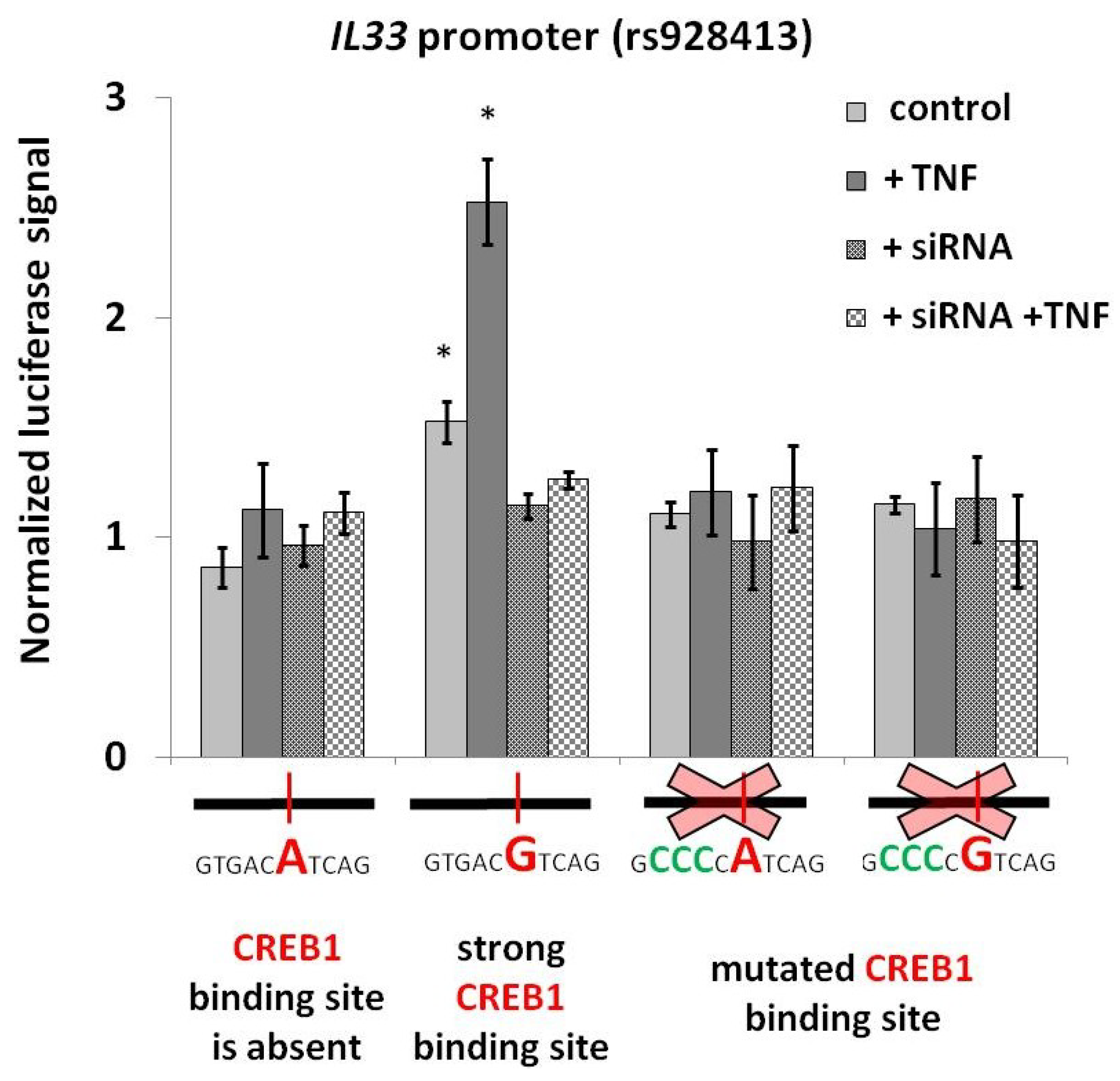
2.2. Risk Allele of rs928413 in IL33 Promoter Creates CREB1-Binding Site
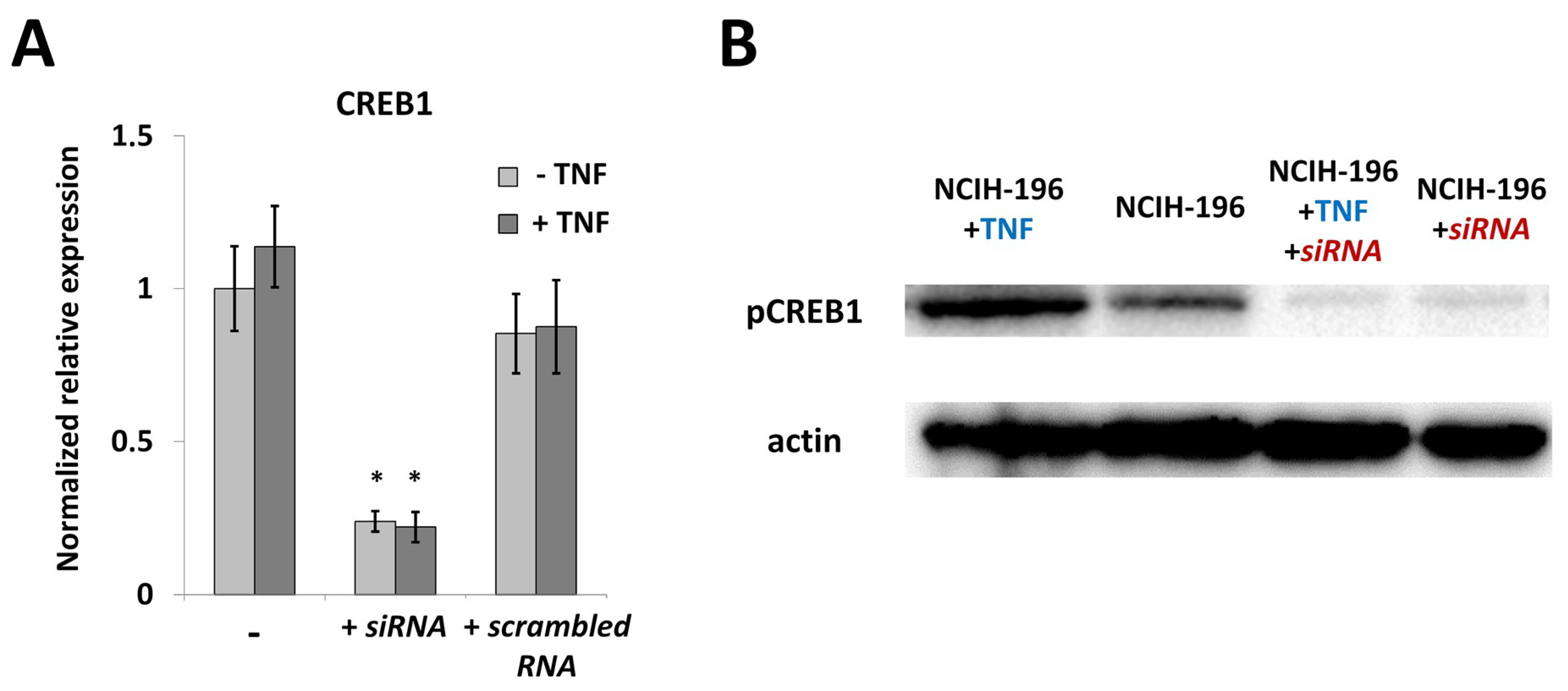
2.3. CREB1 Activation Is Associated with Elevated IL33 Promoter Activity
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Ethical Approval
4.3. Luciferase Reporter Constructss
4.4. NCIH-196 Transfection and Luciferase Reporter Assay
4.5. Pull-Down Assay
4.6. CREB1 Knockdown Using siRNA
4.7. Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.P. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lott, J.M.; Sumpter, T.L.; Turnquist, H.R. New dog and new tricks: Evolving roles for IL-33 in type 2 immunity. J. Leukoc. Biol. 2015, 97, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.E. IL-33: A tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin. Exp. Allergy 2009, 40, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Pitman, N.I.; McInnes, I.B. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat. Rev. Immunol. 2010, 10, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.A.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Préfontaine, D.; Lajoie-Kadoch, S.; Foley, S.; Audusseau, S.; Olivenstein, R.; Halayko, A.J.; Lemière, C.; Martin, J.G.; Hamid, Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J. Immunol. 2009, 183, 5094–5103. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Stolarski, B.; Kewin, P.; Murphy, G.; Corrigan, C.J.; Ying, S.; Pitman, N.; Mirchandani, A.; Rana, B.; van Rooijen, N.; et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 2009, 183, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Préfontaine, D.; Nadigel, J.; Chouiali, F.; Audusseau, S.; Semlali, A.; Chakir, J.; Martin, J.G.; Hamid, Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J. Allergy Clin. Immunol. 2010, 125, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh Jahromi, S.; Mahesh, P.A.; Jayaraj, B.S.; Madhunapantula, S.R.V.; Holla, A.D.; Vishweswaraiah, S.; Ramachandra, N.B. Serum levels of IL-10, IL-17F and IL-33 in patients with asthma: A case-control study. J. Asthma 2014, 51, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Christianson, C.A.; Goplen, N.P.; Zafar, I.; Irvin, C.; Good, J.T.; Rollins, D.R.; Gorentla, B.; Liu, W.; Gorska, M.M.; Chu, H.W.; et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J. Allergy Clin. Immunol. 2015, 136, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Bjornsdottir, U.S.; Halapi, E.; Helgadottir, A.; Sulem, P.; Jonsdottir, G.M.; Thorleifsson, G.; Helgadottir, H.; Steinthorsdottir, V.; Stefansson, H.; et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat. Genet. 2009, 41, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, M.; Gut, I.; Demenais, F.; Strachan, D.; Bouzigon, E.; Heath, S. A Large-Scale, Consortium-Based Genomewide Association Study of Asthma. N. Engl. J. Med. 2010, 363, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, D.G.; Ampleford, E.J.; Chiu, G.Y.; Gauderman, W.J.; Gignoux, C.R.; Graves, P.E.; Himes, B.E.; Levin, A.M.; Mathias, R.A.; Hancock, D.B.; et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat. Genet. 2011, 43, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Bonnelykke, K.; Sleiman, P.; Nielsen, K.; Kreiner-møller, E.; Mercader, J.M.; Belgrave, D.; Dekker, H.T.; Den Husby, A.; Sevelsted, A.; Faura-tellez, G.; et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat. Genet. 2013, 46, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Hu, H.; Jin, Y.; Xue, M. Polymorphisms of RAD50, IL33 and IL1RL1 are associated with atopic asthma in Chinese population. Tissue Antigens 2015, 86, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Schroder, P.; Casaca, V.; Illi, S.; Schieck, M.; Michel, S.; Bock, A.; Roduit, C.; Frei, R.; Lluis, A.; Shaub, B. IL-33 polymorphisms are associated with increased risk of hay fever and reduced Regulatory T cells in a birth cohort. Pediatr. Allergy Immunol. 2016, 27, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, S.; Hayakawa, M.; Tsuda, H.; Ohta, S.; Yanagisawa, K. Presence of a novel exon 2E encoding a putative transmembrane protein in human IL-33 gene. Biochem. Biophys. Res. Commun. 2013, 430, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Komine, M.; Tominaga, S.; Ohtsuki, M. Identification of the promoter region of human IL-33 responsive to induction by IFNγ. J. Dermatol. Sci. 2017, 85, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Kryukov, G.V.; Sonkin, D.; Reddy, A.; Liu, M.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 463, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Vorontsov, I.E.; Kulakovskiy, I.V.; Khimulya, G.; Nikolaeva, D.D.; Makeev, V.J. PERFECTOS-APE: Predicting regulatory functional effect of SNPs by approximate P-value estimation. In Proceedings of the Bioinformatics 2015 6th International Conference on Bioinformatics Models, Methods and Algorithms, Lisbon, Portugal, 12–15 January 2015; pp. 102–108. [Google Scholar] [CrossRef]
- Aitola, M.; Carlsson, P.; Mahlapuu, M.; Enerbäck, S.; Pelto-Huikko, M. Forkhead transcription factor FoxF2 is expressed in mesodermal tissues involved in epithelio-mesenchymal. Dev. Dyn. 2000, 218, 136–149. [Google Scholar] [CrossRef]
- Ormestad, M.; Astorga, J.; Landgren, H.; Wang, T.; Carlsson, P. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development 2006, 133, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tamakoshi, T.; Uezato, T.; Shu, F.; Kanzaki-Kato, N.; Fu, Y.; Koseki, H.; Yoshida, N.; Sugiyama, T.; Miura, N. Forkhead transcription factor Foxf2 (LUN)-deficient mice exhibit abnormal development of secondary palate. Dev. Biol. 2003, 259, 83–94. [Google Scholar] [CrossRef]
- Courtois, G.; Morgan, J.; Campbell, L.; Fourel, G.; Crabtree, G. Interaction of a Liver-Specific Nuclear Factor with the Fibrinogen and α1-Antitrypsin Promoters. Science 1987, 238, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Chhabra, K.; Nguen, V.; Lazartigues, E. The transcription factor HNF1α induces expression of angiotensin-converting enzyme 2 (ACE2) in pancreatic islets from evolutionarily conserved promoter motifs. Biochim. Biophys. Acta 2013, 43, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Le Cam, L.; Lacroix, M.; Ciemerych, M.A.; Sardet, C.; Sicinski, P. The E4F Protein Is Required for Mitotic Progression during Embryonic Cell Cycles. Mol. Cell. Biol. 2004, 24, 6467–6475. [Google Scholar] [CrossRef] [PubMed]
- Chagraoui, J.; Niessen, S.; Lessard, J.; Girard, S.; Coulombe, P.; Meloche, S.; Sauvageau, G. p120E4F-1: A novel candidate factor for mediating Bmi-1 function in hematopoietic stem cells. Blood 2004, 104, 307. [Google Scholar]
- Hatchi, E.; Rodier, G.; Lacroix, M.; Caramel, J.; Kirsh, O.; Jacquet, C.; Schrepfer, E.; Lagarrigue, S.; Linares, L.K.; Lledo, G.; et al. E4F1 deficiency results in oxidative stress-mediated cell death of leukemic cells. J. Exp. Med. 2011, 208, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, A.J.; Greenberg, M.E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 1999, 68, 821–861. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I.M. Transcription factors and asthma. Eur. Respir. J. 1998, 12, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Bartel, S.; Schulz, N.; Alessandrini, F.; Schamberger, A.C.; Pagel, P.; Theis, F.J.; Milger, K.; Noessner, E.; Stick, S.M.; Kicic, A.; et al. Pulmonary microRNA profiles identify involvement of Creb1 and Sec14l3 in bronchial epithelial changes in allergic asthma. Sci. Rep. 2017, 7, 46026. [Google Scholar] [CrossRef] [PubMed]
- Chiappara, G.; Chanez, P.; Bruno, A.; Pace, E.; Pompeo, F.; Bousquet, J.; Bonsignore, G.; Gjomarkaj, M. Variable p-CREB expression depicts different asthma phenotypes. Allergy Eur. J. Allergy Clin. Immunol. 2007, 62, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Hisada, T.; Ishizuka, T.; Utsugi, M.; Ono, A.; Yatomi, M.; Kamide, Y.; Aoki-Saito, H.; Tsurumaki, H.; Dobashi, K.; et al. CREB regulates TNF-α-induced GM-CSF secretion via p38 MAPK in human lung fibroblasts. Allergol. Int. 2016, 65, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liang, Q.; Balzar, S.; Wenzel, S.; Gorska, M.; Alam, R. Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. J. Allergy Clin. Immunol. 2008, 121, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. New therapies for asthma: Is there any progress? Trends Pharmacol. Sci. 2010, 31, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Robinson, D.S.; Varney, V.; Meng, Q.; Tsicopoulos, A.; Moqbel, R.; Durham, S.R.; Kay, A.B.; Hamid, Q. TNF alpha mRNA expression in allergic inflammation. Clin. Exp. Allergy 1991, 21, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Lee, W.; Chung, C.; Koo, J.S.; Yang, E.J.; Choi, Y.; Yoon, J.; Song, K.S.; Lee, W.; Chung, K.C.; et al. Mechanisms of Signal Transduction: Interleukin-1β and Tumor Necrosis Factor-α Induce MUC5AC Overexpression through a Mechanism Involving ERK/p38 Mitogen-activated Protein Kinases-MSK1-CREB Activation in Human Airway Epithelial Cells. J. Biol. Chem. 2003, 278, 23243–23250. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Uehara, A.; Fujimoto, Y.; Fukase, K.; Takada, H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol. Immunol. 2007, 44, 3100–3111. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.A.; Standiford, T.J. Toll like receptors in diseases of the lung. Int. Immunopharmacol. 2011, 11, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Juárez, M.M.; Kolls, J.K.; Evans, S.E. Lung epithelial cells: Therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 2018, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Asokananthan, N.; Graham, P.T.; Fink, J.; Knight, D.A.; Bakker, A.J.; McWilliam, A.S.; Thompson, P.J.; Stewart, G.A. Activation of Protease-Activated Receptor (PAR)-1, PAR-2, and PAR-4 Stimulates IL-6, IL-8, and Prostaglandin E2 Release from Human Respiratory Epithelial Cells. J. Immunol. 2002, 168, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Chieppa, M.; Perros, F.; Willart, M.A.; Germain, R.N.; Lambrecht, B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009, 15, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Nathan, A.T.; Peterson, E.A.; Chakir, J.; Wills-Karp, M. Innate immune responses of airway epithelium to house dust mite are mediated through β-glucan-dependent pathways. J. Allergy Clin. Immunol. 2009, 123, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Hammad, H.; Van Nimwegen, M.; Kool, M.; Willart, M.A.M.; Muskens, F.; Hoogsteden, H.C.; Luttmann, W.; Ferrari, D.; Di Virgilio, F.; et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 2007, 13, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Willart, M.A.M.; van Nimwegen, M.; Bergen, I.; Pouliot, P.; Virchow, J.C.; Rogers, N.; Osorio, F.; Reis e Sousa, C.; Hammad, H.; et al. An Unexpected Role for Uric Acid as an Inducer of T Helper 2 Cell Immunity to Inhaled Antigens and Inflammatory Mediator of Allergic Asthma. Immunity 2011, 34, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Herre, J.; Gronlund, H.; Brooks, H.; Hopkins, L.; Waggoner, L.; Murton, B.; Gangloff, M.; Opaleye, O.; Chilvers, E.R.; Fitzgerald, K.; et al. Allergens as Immunomodulatory Proteins: The Cat Dander Protein Fel d 1 Enhances TLR Activation by Lipid Ligands. J. Immunol. 2013, 191, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Millien, V.O.; Lu, W.; Shaw, J.; Yuan, X.; Mak, G.; Roberts, L.; Song, L.Z.; Knight, J.M.; Creighton, C.J.; Luong, A.; et al. Cleavage of Fibrinogen by Proteinases Elicits Allergic Responses Through Toll-Like Receptor 4. Science 2013, 341, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. Biology of Lung Dendritic Cells at the Origin of Asthma. Immunity 2009, 31, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Petit-Bertron, A.F.; Bricard, R.; Levasseur, M.; Ramadan, A.; Girard, J.P.; Herbelin, A.; Dy, M. IL-33 Activates Unprimed Murine Basophils Directly In Vitro and Induces Their In Vivo Expansion Indirectly by Promoting Hematopoietic Growth Factor Production. J. Immunol. 2009, 183, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Gomez, E.; Doe, C.; Berair, R.; Woodman, L.; Saunders, R.; Hollins, F.; Rose, F.R.; Amrani, Y.; May, R.; et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: Airway smooth muscle crosstalk. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Kewin, P.; Murphy, G.; Russo, R.C.; Stolarski, B.; Garcia, C.C.; Komai-Koma, M.; Pitman, N.; Li, Y.; McKenzie, A.N.J.; et al. IL-33 Induces Antigen-Specific IL-5+ T Cells and Promotes Allergic-Induced Airway Inflammation Independent of IL-4. J. Immunol. 2008, 181, 4780–4790. [Google Scholar] [CrossRef] [PubMed]
- Bartemes, K.R.; Iijima, K.; Kobayashi, T.; Kephart, G.M.; McKenzie, A.N.; Kita, H. IL-33-Responsive Lineage-CD25+ CD44hi Lymphoid Cells Mediate Innate Type 2 Immunity and Allergic Inflammation in the Lungs. J. Immunol. 2012, 188, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Stolarski, B.; Kurowska-Stolarska, M.; Kewin, P.; Xu, D.; Liew, F.Y. IL-33 Exacerbates Eosinophil-Mediated Airway Inflammation. J. Immunol. 2010, 185, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Lunderius-Andersson, C.; Enoksson, M.; Nilsson, G. Mast cells respond to cell injury through the recognition of IL-33. Front. Immunol. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Suzukawa, M.; Iikura, M.; Koketsu, R.; Nagase, H.; Tamura, C.; Komiya, A.; Nakae, S.; Matsushima, K.; Ohta, K.; Yamamoto, K.; et al. An IL-1 Cytokine Member, IL-33, Induces Human Basophil Activation via Its ST2 Receptor. J. Immunol. 2008, 181, 5981–5989. [Google Scholar] [CrossRef] [PubMed]
- Komai-Koma, M.; Brombacher, F.; Pushparaj, P.N.; Arendse, B.; McSharry, C.; Alexander, J.; Chaudhuri, R.; Thomson, N.C.; McKenzie, A.N.J.; McInnes, I.; et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naive mice. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yang, T.Y.; Park, C.S.; Ahn, S.H.; Son, B.K.; Kim, J.H.; Lim, D.H.; Jang, T.Y. Anti-IL-33 antibody has a therapeutic effect in a murine model of allergic rhinitis. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Willart, M.A.M.; Deswarte, K.; Pouliot, P.; Braun, H.; Beyaert, R.; Lambrecht, B.N.; Hammad, H. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J. Exp. Med. 2012, 209, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, X.Y.; Liu, T.; Yuan, B.H.; Zhang, B.B.; Hu, S.L.; Gu, H.B.; Jin, X.B.; Zhu, J.Y. Adenovirus-mediated delivery of soluble ST2 attenuates ovalbumin-induced allergic asthma in mice. Clin. Exp. Immunol. 2012, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Wu, Y.; Zhou, Y.; Zeng, L.; Huang, T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem. Biophys. Res. Commun. 2009, 386, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Matsumoto, K.; Gon, Y.; Maruoka, S.; Kujime, K.; Hayashi, S.; Takeshita, I.; Horie, T. p38 MAP kinase regulates TNF alpha-, IL-1 alpha- and PAF-induced RANTES and GM-CSF production by human bronchial epithelial cells. Clin. Exp. Allergy 2000, 30, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Ghosh, P.; Kusaba, H.; Buchholz, M.; Longo, D.L. Effect of promoter methylation on the regulation of IFN-gamma gene during in vitro differentiation of human peripheral blood T cells into a Th2 population. J. Immunol. 2003, 171, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- Chava, K.R.; Karpurapu, M.; Wang, D.; Bhanoori, M.; Kundumani-Sridharan, V.; Zhang, Q.; Ichiki, T.; Glasgow, W.C.; Rao, G.N. CREB-mediated IL-6 expression is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Mroz, R.M.; Holownia, A.; Chyczewska, E.; Drost, E.M.; Braszko, J.J.; Noparlik, J.; Donaldson, K.; Macnee, W. Cytoplasm-Nuclear Trafficking of Creb and Creb Phosphorylation at Ser133 During Therapy of Chronic Obstructive Pulmonary Disease. J. Physiol. Pharmacol. 2007, 58, 437–444. [Google Scholar] [PubMed]
- Korneev, K.V.; Kondakova, A.N.; Sviriaeva, E.N.; Mitkin, N.A.; Palmigiano, A.; Kruglov, A.A.; Telegin, G.B.; Drutskaya, M.S.; Sturiale, L.; Garozzo, D.; et al. Hypoacylated LPS from Foodborne Pathogen Campylobacter jejuni Induces Moderate TLR4-Mediated Inflammatory Response in Murine Macrophages. Front. Cell. Infect. Microbiol. 2018, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Mitkin, N.A.; Muratova, A.M.; Schwartz, A.M.; Kuprash, D.V. The A allele of the Single-Nucleotide Polymorphism rs630923 creates a Binding site for MEF2C resulting in reduced CXCR5 Promoter activity in B-cell lymphoblastic cell lines. Front. Immunol. 2016, 7, 515. [Google Scholar] [CrossRef] [PubMed]
- Mitkin, N.A.; Muratova, A.M.; Sharonov, G.V.; Korneev, K.V.; Sviriaeva, E.N.; Mazurov, D.; Schwartz, A.M.; Kuprash, D.V. p63 and p73 repress CXCR5 chemokine receptor gene expression in p53-deficient MCF-7 breast cancer cells during genotoxic stress. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Mitkin, N.A.; Muratova, A.M.; Korneev, K.V.; Pavshintsev, V.V.; Rumyantsev, K.A.; Vagida, M.S.; Uvarova, A.N.; Afanasyeva, M.A.; Schwartz, A.M.; Kuprash, D.V. Protective C allele of the single-nucleotide polymorphism rs1335532 is associated with strong binding of Ascl2 transcription factor and elevated CD58 expression in B-cells. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- Hasson, S.A.; Kane, L.A.; Yamano, K.; Huang, C.H.; Sliter, D.A.; Buehler, E.; Wang, C.; Heman-Ackah, S.M.; Hessa, T.; Guha, R.; et al. High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature 2013, 504, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Afanasyeva, M.A.; Britanova, L.V.; Korneev, K.V.; Mitkin, N.A.; Kuchmiy, A.A.; Kuprash, D.V. Clusterin is a potential lymphotoxin beta receptor target that is upregulated and accumulates in germinal centers of mouse spleen during immune response. PLoS ONE 2014, 9, e98349. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbacheva, A.M.; Korneev, K.V.; Kuprash, D.V.; Mitkin, N.A. The Risk G Allele of the Single-Nucleotide Polymorphism rs928413 Creates a CREB1-Binding Site That Activates IL33 Promoter in Lung Epithelial Cells. Int. J. Mol. Sci. 2018, 19, 2911. https://doi.org/10.3390/ijms19102911
Gorbacheva AM, Korneev KV, Kuprash DV, Mitkin NA. The Risk G Allele of the Single-Nucleotide Polymorphism rs928413 Creates a CREB1-Binding Site That Activates IL33 Promoter in Lung Epithelial Cells. International Journal of Molecular Sciences. 2018; 19(10):2911. https://doi.org/10.3390/ijms19102911
Chicago/Turabian StyleGorbacheva, Alisa M., Kirill V. Korneev, Dmitry V. Kuprash, and Nikita A. Mitkin. 2018. "The Risk G Allele of the Single-Nucleotide Polymorphism rs928413 Creates a CREB1-Binding Site That Activates IL33 Promoter in Lung Epithelial Cells" International Journal of Molecular Sciences 19, no. 10: 2911. https://doi.org/10.3390/ijms19102911
APA StyleGorbacheva, A. M., Korneev, K. V., Kuprash, D. V., & Mitkin, N. A. (2018). The Risk G Allele of the Single-Nucleotide Polymorphism rs928413 Creates a CREB1-Binding Site That Activates IL33 Promoter in Lung Epithelial Cells. International Journal of Molecular Sciences, 19(10), 2911. https://doi.org/10.3390/ijms19102911