Optimized Expression and Characterization of a Novel Fully Human Bispecific Single-Chain Diabody Targeting Vascular Endothelial Growth Factor165 and Programmed Death-1 in Pichia pastoris and Evaluation of Antitumor Activity In Vivo
Abstract
1. Introduction
2. Results
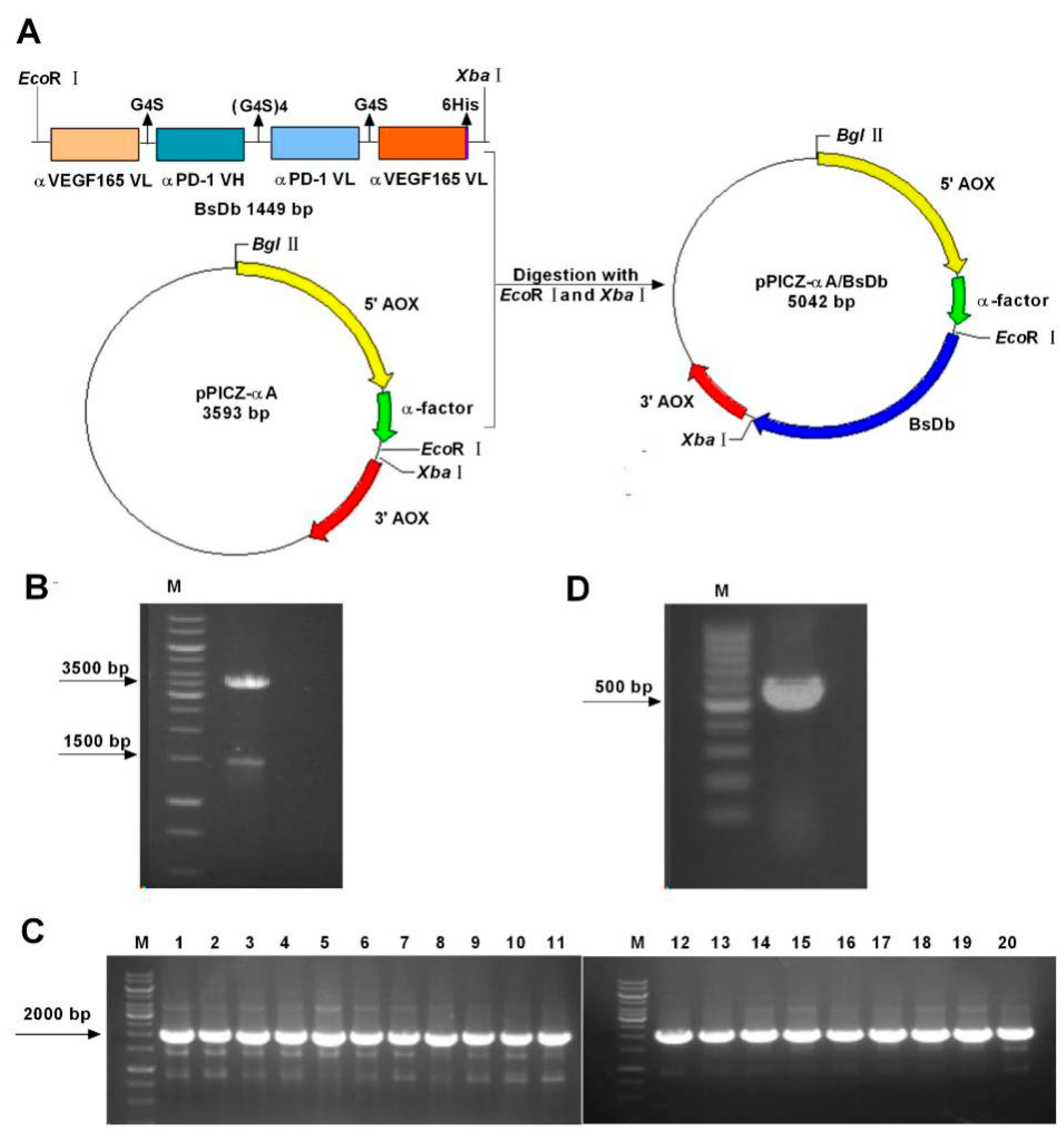
2.1. Construction and Transformation of the pPICZαA-Bispecific Single-Chain Diabody (BsDb) Expression Vector
2.2. Expression and Detection of Recombinant BsDb in Pichia pastoris
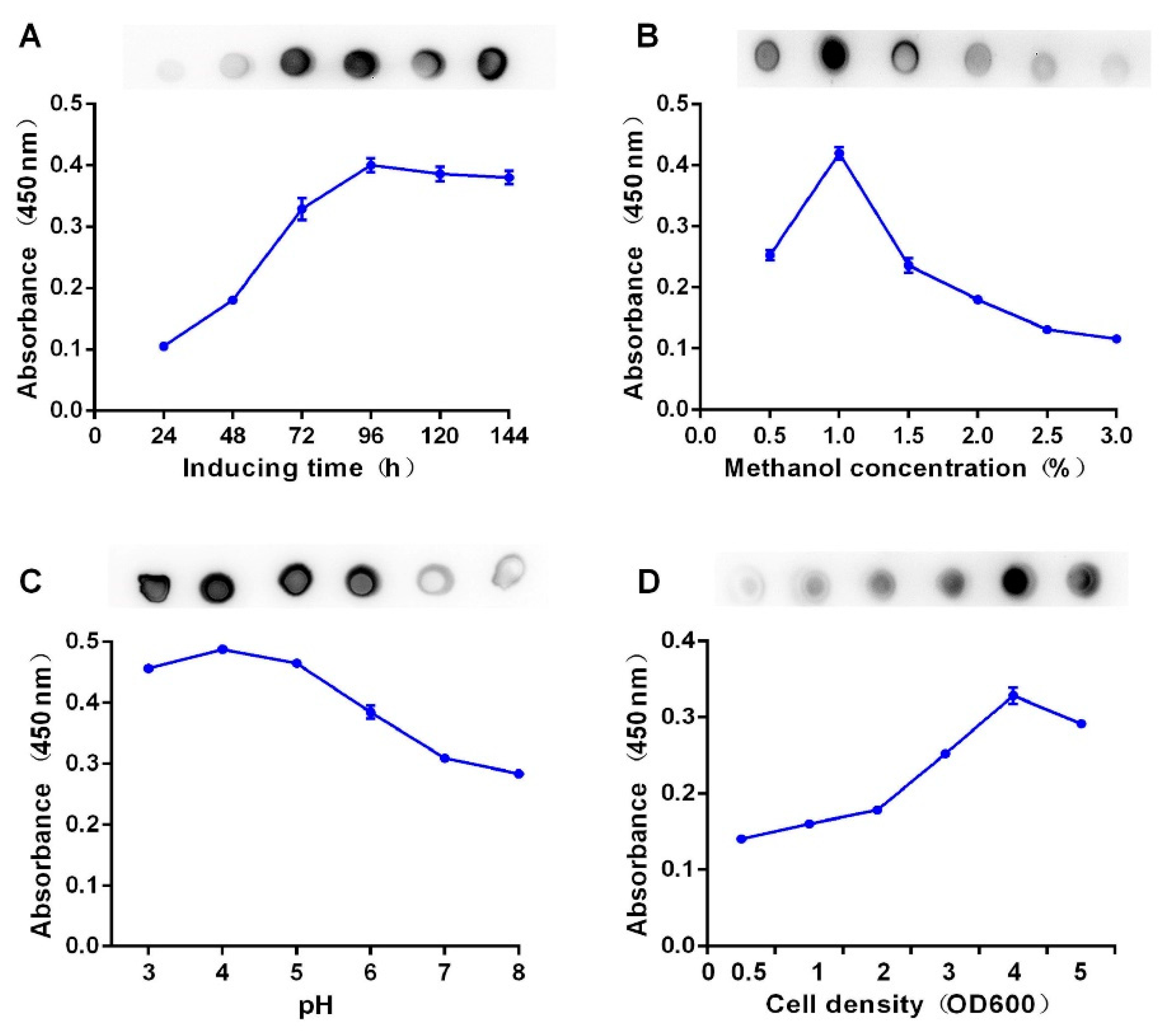
2.3. Optimized Expression of Recombinant BsDb
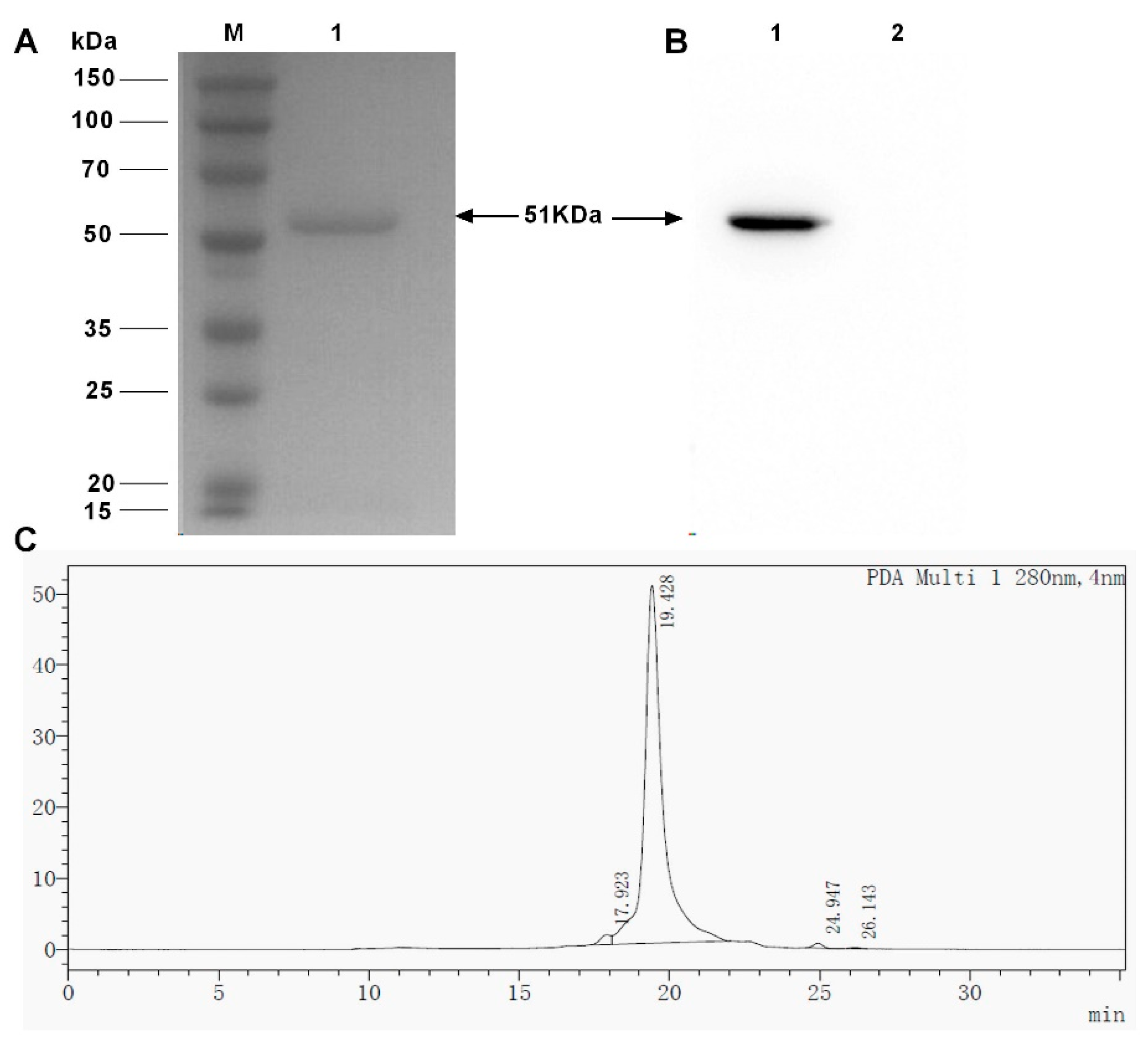
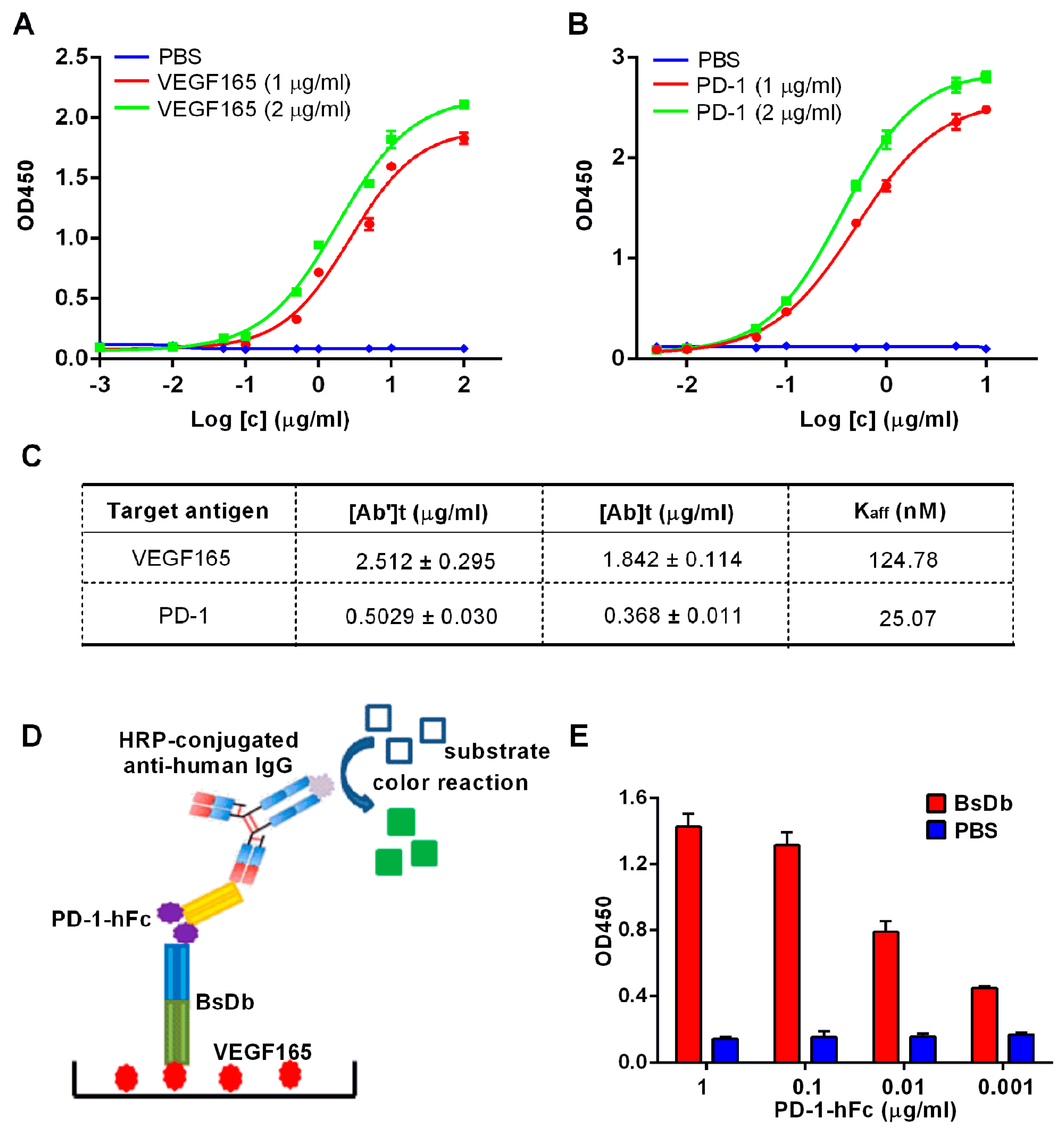
2.4. Obtain the Recombinant BsDb with High Purity and High Binding Abilities Using Nickel-Nitrilotriacetic Acid (Ni-NTA) Column Chromatography
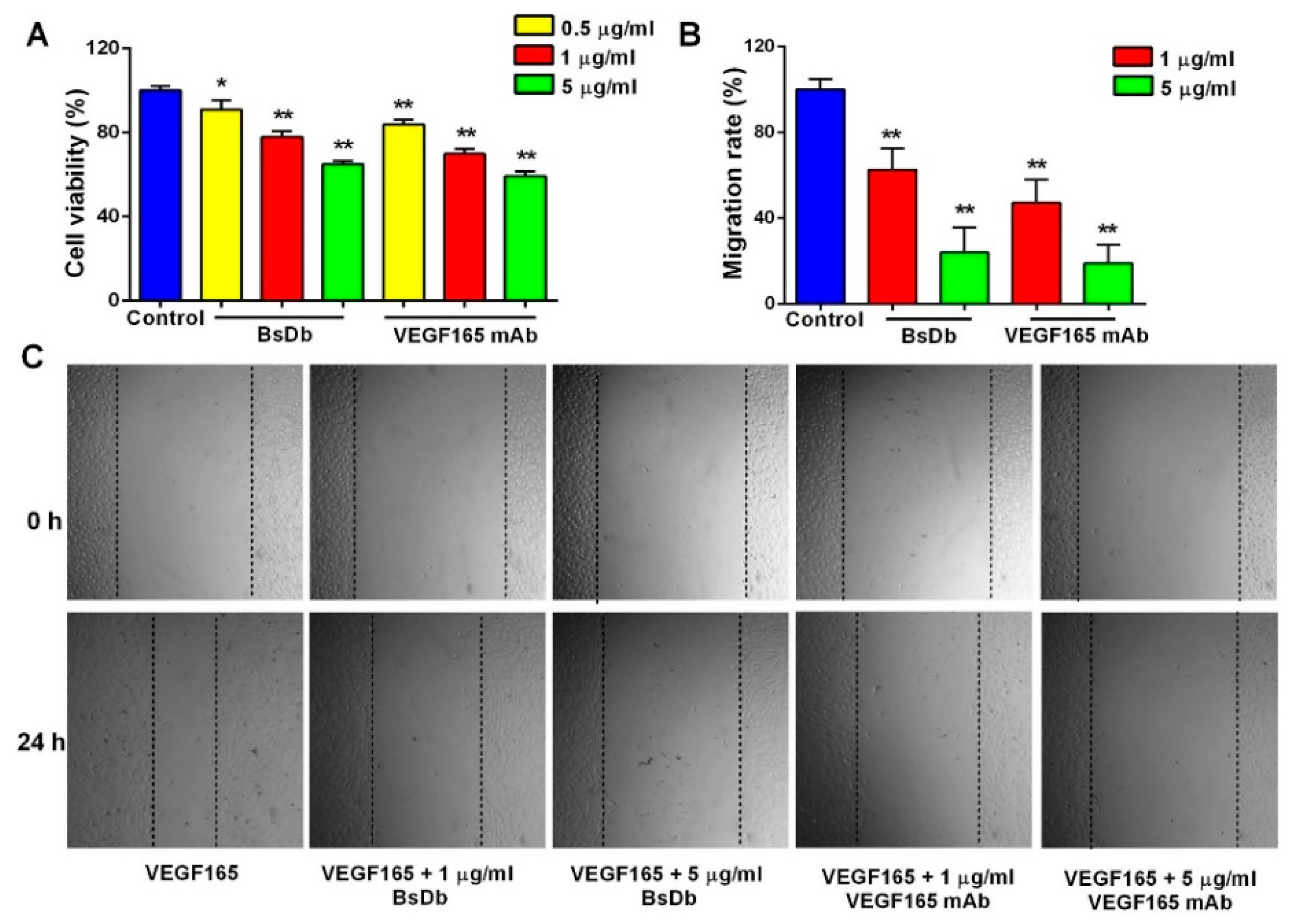
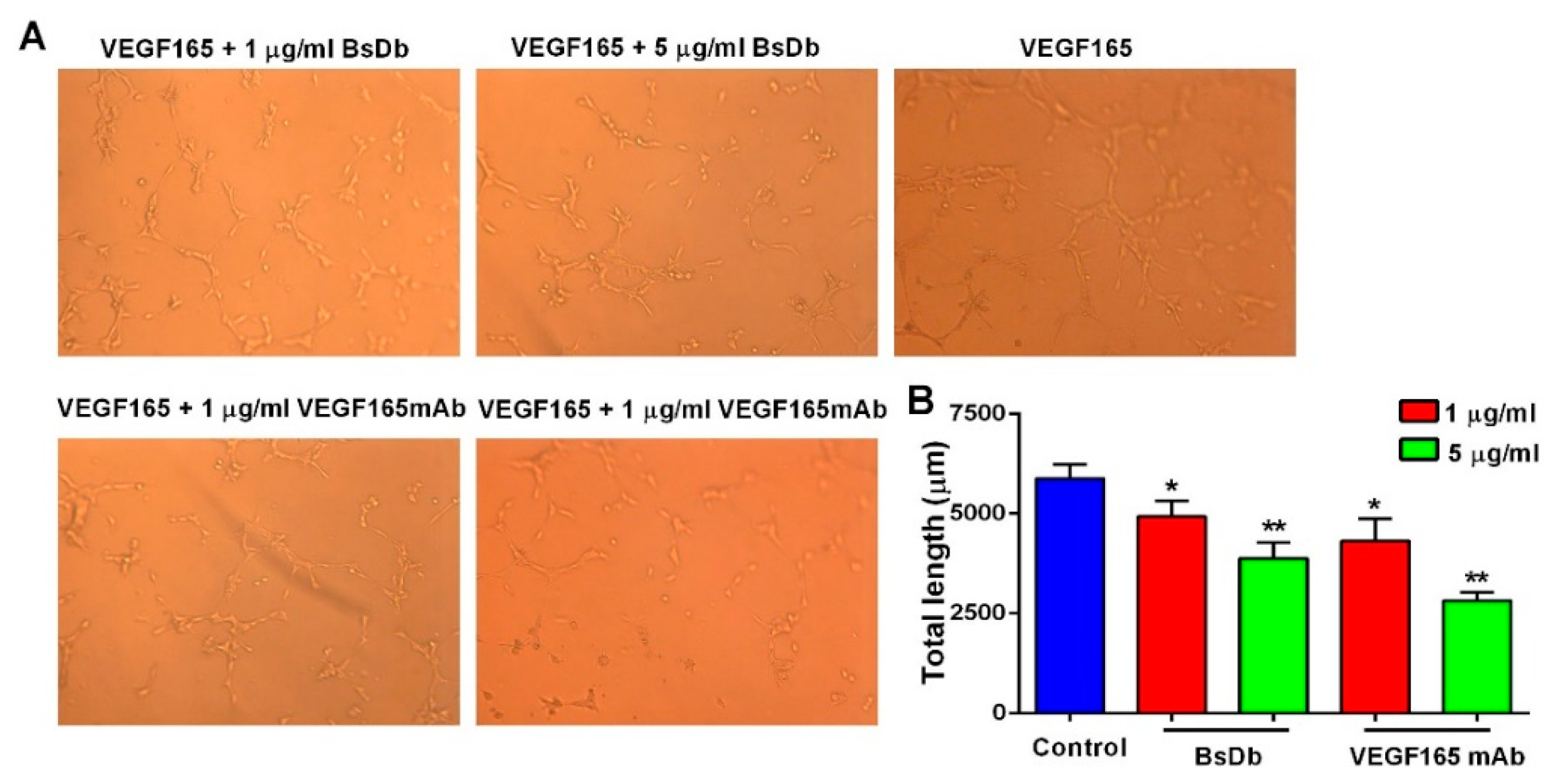
2.5. Inhibitory Activity of BsDb on Human Umbilical Vein Endothelial Cells (HUVECs) Proliferation, Migration, and Tube Formation
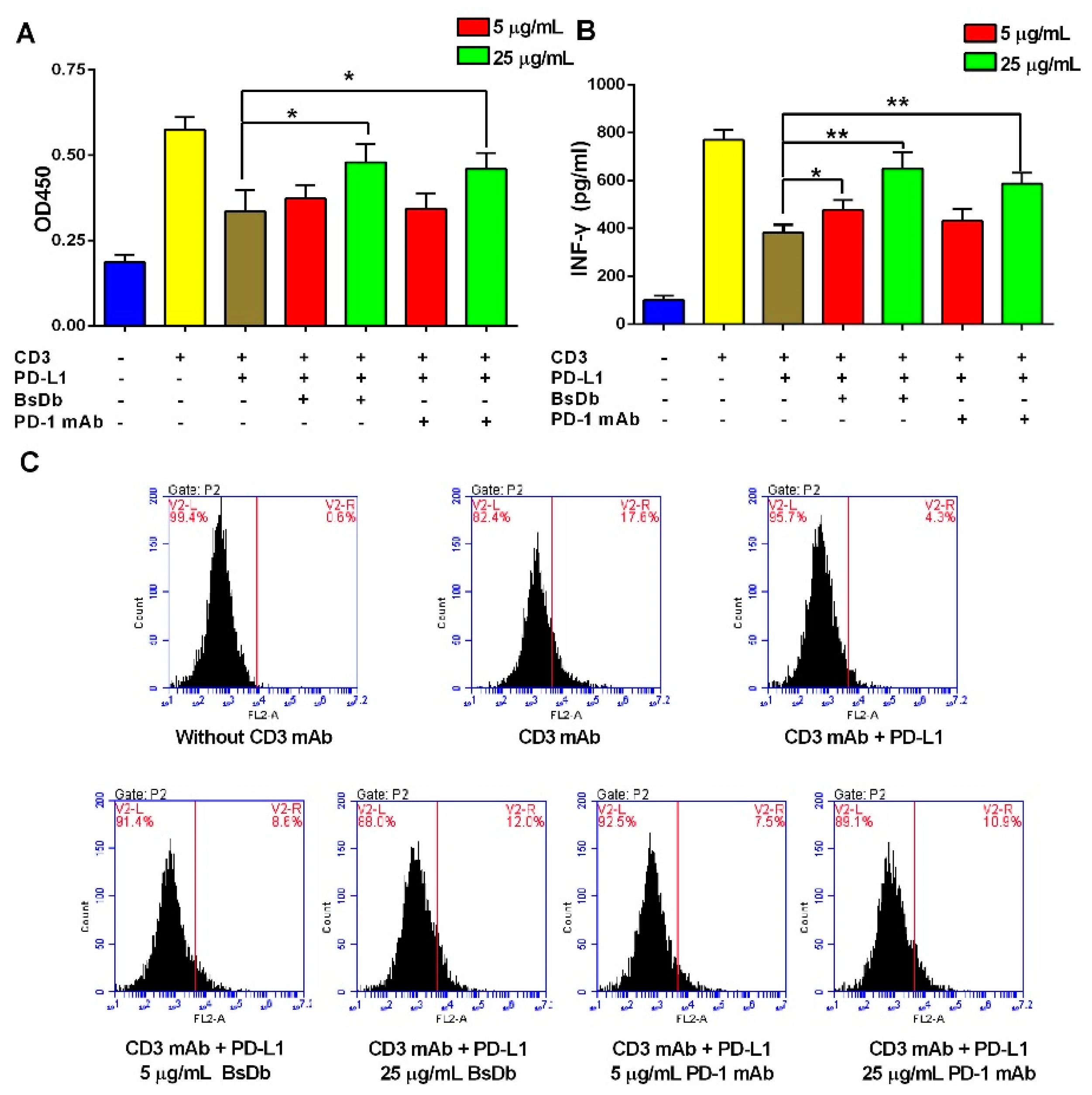
2.6. The Recombinant BsDb Improved T Cell Proliferation and Rescued T Cells Activation
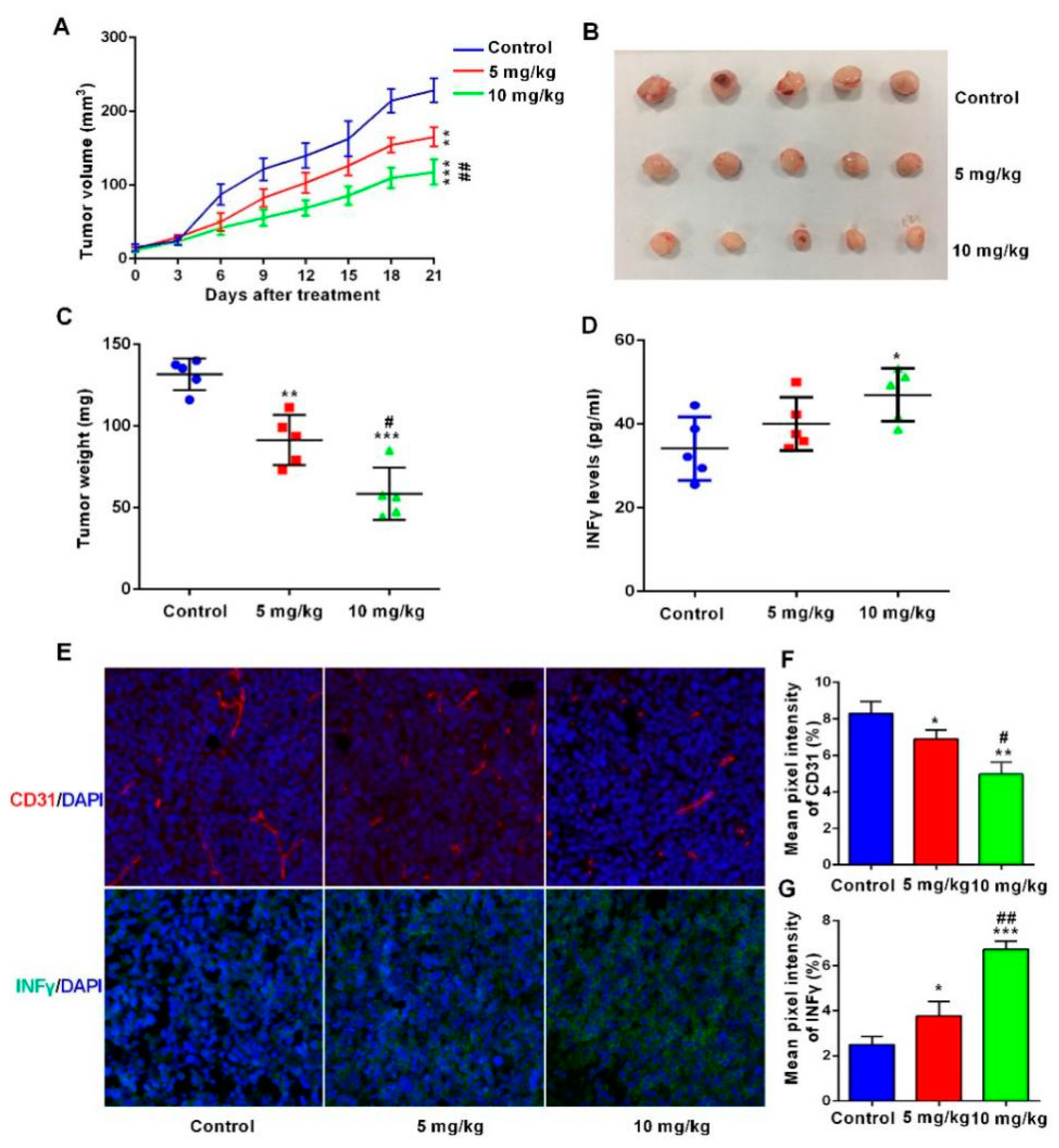
2.7. The BsDb Suppressed HT29 Xenograft Tumors Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Strains and Plasmids
4.2. Cell Culture
4.3. Construction of the BsDb Expression Vector
4.4. Transformation of Pichia pastoris and Selection of Recombinant Clones
4.5. Expression and Selection of High-Expressing Clones
4.6. Optimization of the Conditions for the Recombinant BsDb Expression
4.7. Purification of the Recombinant BsDb
4.8. SDS-PAGE and Western Blot
4.9. Measurement of Affinity by Indirect ELISA
4.10. Sandwich ELISA
4.11. HUVECs Proliferation Assay
4.12. HUVECs Migration Assay
4.13. HUVECs Tube Formation Assay
4.14. T Cell Proliferation Assay
4.15. T Cell Activation Assay
4.16. Evaluation Antitumor Activity of BsDb In Vivo
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BsDb | Bispecific single-chain diabody |
| BsAb | Bispecific antibody |
| VEGF165 | Vascular endothelial growth factor165 |
| PD-1 | Programmed death-1 |
| HUVECs | Human umbilical vein endothelial cells |
| PBMCs | Peripheral blood mononuclear cells |
| mAb | mAb |
| MTT | Methyl thiazolyl tetrazolium |
| ELISA | Enzyme-linked immune sorbent assay |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| HPLC | High performance liquid chromatography |
| MACS | Magnetic cell sorting |
| FCM | Flow cytometry |
| YPD | Yeast extract peptone dextrose medium |
| BMGY | Buffered minimal glycerol-complex medium |
| BMMY | Buffered minimal methanol-complex medium |
References
- Strohl, W.R. Current progress in innovative engineered antibodies. Protein Cell 2018, 9, 86–120. [Google Scholar] [CrossRef] [PubMed]
- Przepiorka, D.; Ko, C.W.; Deisseroth, A.; Yancey, C.L.; Candau-Chacon, R.; Chiu, H.J.; Gehrke, B.J.; Gomez-Broughton, C.; Kane, R.C.; Kirshner, S.; et al. FDA Approval: Blinatumomab. Clin. Cancer Res. 2015, 21, 4035–4039. [Google Scholar] [CrossRef] [PubMed]
- Godar, M.; de Haard, H.; Blanchetot, C.; Rasser, J. Therapeutic bispecific antibody formats: A patent applications review (1994–2017). Expert Opin. Ther. Pat. 2018, 28, 251–276. [Google Scholar] [CrossRef] [PubMed]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, F.; Wu, X.; Malik, A.K.; Zhong, C.; Baldwin, M.E.; Schanz, S.; Fuh, G.; Gerber, H.P.; Ferrara, N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 2007, 25, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.N.; Pu, J.J. PD-1 pathway and its clinical application: A 20 year journey after discovery of the complete human PD-1 gene. Gene 2018, 638, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, H.; Chen, B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J. Cancer 2017, 8, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D.; Suzman, D.L.; Blumenthal, G.; Mushti, S.; He, K.; Libeg, M.; Keegan, P.; Pazdur, R. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer with Progression on or after Platinum-Based Chemotherapy. Oncologist 2016, 21, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, W.; Yoneda, K.Y.; Moore, E.H.; Zhang, Y.; Pu, L.L.; Frampton, G.M.; Molmen, M.; Stephens, P.J.; Li, T. Severe nivolumab-induced pneumonitis preceding durable clinical remission in a patient with refractory, metastatic lung squamous cell cancer: A case report. J. Hematol. Oncol. 2017, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Pen, J.J.; Keersmaecker, B.D.; Heirman, C.; Corthals, J.; Liechtenstein, T.; Escors, D.; Thielemans, K.; Breckpot, K. Interference with PD-L1/PD-1 co-stimulation during antigen presentation enhances the multifunctionality of antigen-specific T cells. Gene Ther. 2014, 21, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kim, B.Y.S.; Chan, C.K.; Hahn, S.M.; Weissman, I.L.; Jiang, W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hodi, F.S.; Buchbinder, E.I. Inhibition of Immune Checkpoints and Vascular Endothelial Growth Factor as Combination Therapy for Metastatic Melanoma: An Overview of Rationale, Preclinical Evidence, and Initial Clinical Data. Front. Oncol. 2015, 5, 202. [Google Scholar] [CrossRef] [PubMed]
- Pircher, A.; Wolf, D.; Heidenreich, A.; Hilbe, W.; Pichler, R.; Heidegger, I. Synergies of Targeting Tumor Angiogenesis and Immune Checkpoints in Non-Small Cell Lung Cancer and Renal Cell Cancer: From Basic Concepts to Clinical Reality. Int. J. Mol. Sci. 2017, 18, 2291. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Jabouille, A.; Rivera, L.B.; Lodewijckx, I.; Missiaen, R.; Steri, V.; Feyen, K.; Tawney, J.; Hanahan, D.; Michael, I.P. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 2017, 9, aak9679. [Google Scholar] [CrossRef] [PubMed]
- Schmittnaegel, M.; Rigamonti, N.; Kadioglu, E.; Cassará, A.; Wyser, R.C.; Kiialainen, A.; Kienast, Y.; Mueller, H.J.; Ooi, C.H.; Laoui, D. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 2017, 9, aak9670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yun, D.J.; Chen, J.Q.; Zhao, J.Y.; Liu, S.G.; Cheng, G.X. Isolation and characterization of human anti-VEGF165 monoclonal antibody with anti-tumor efficacy from transgenic mice expressing human immunoglobulin loci. Cancer Lett. 2009, 273, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Cao, H.; He, W.; Gao, F.; Liu, Y.; Yin, L. Novel drug delivery liposomes targeted with a fully human anti-VEGF165 monoclonal antibody show superior antitumor efficacy in vivo. Biomed. Pharmacother. 2015, 73, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.D.; Beatty, B.G.; Vlahos, W.G. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J. Immunol. Methods 1987, 100, 173–179. [Google Scholar] [CrossRef]
- Ferla, R.; Bonomi, M.; Otvos, L., Jr.; Surmacz, E. Glioblastoma-derived leptin induces tube formation and growth of endothelial cells: Comparison with VEGF effects. BMC Cancer 2011, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Q.; Qiao, C.; Lin, Z.; Li, X.; Huang, Y.; Zhou, T.; Li, Y.; Shen, B.; Lv, M.; et al. Potent anti-angiogenesis and anti-tumor activity of a novel human anti-VEGF antibody, MIL60. Cell. Mol. Immunol. 2014, 11, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, N.; Zhou, J.; Ding, C.; Jin, Y.; Cui, X.; Pu, K.; Zhu, Y. Peptide Blocking of PD-1/PD-L1 Interaction for Cancer Immunotherapy. Cancer Immunol. Res. 2018, 6, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Kumagai, T.; Nagai, K.; Taki, S.; Shimomura, I.; Arai, K.; Ogata, H.; Okada, M.; Hayasaka, F.; Sanada, H.; et al. Domain order of a bispecific diabody dramatically enhances its antitumor activity beyond structural format conversion: The case of the hEx3 diabody. Protein Eng. Des. Sel. 2013, 26, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.A.; Power, B.E.; Garrett, T.P.J.; Yazaki, P.J.; Shively, J.E.; Raubischek, A.A.; Wu, A.M.; Hudson, P.J. The Crystal Structure of an Anti-CEA scFv Diabody Assembled from T84.66 scFvs in VL-to-VH Orientation: Implications for Diabody Flexibility. J. Mol. Biol. 2003, 326, 341–351. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Z.; Xi, H.; Gu, T.; Yuan, R.; Chen, X.; Jiang, C.; Kong, W.; Wu, Y. A VL-linker-VH Orientation Dependent Single Chain Variable Antibody Fragment Against Rabies Virus G Protein with Enhanced Neutralizing Potency in vivo. Protein Pept. Lett. 2015, 23, 24–32. [Google Scholar] [CrossRef]
- Liu, M.Y.; Hu, X.P.; Xie, M.; Jiang, S.J.; Li, L.J.; Liu, D.X.; Yang, X.S. Secretory expression of a bispecific antibody targeting tumor necrosis factor and ED-B fibronectin in Pichia pastoris and its functional analysis. Biotechnol. Lett. 2014, 36, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein expression in Pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef] [PubMed]
- Zarei, N.; Vaziri, B.; Shokrgozar, M.A.; Mahdian, R.; Fazel, R.; Khalaj, V. High efficient expression of a functional humanized single-chain variable fragment (scFv) antibody against CD22 in Pichia pastoris. Appl. Microbiol. Biotechnol. 2014, 98, 10023–10039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Yang, J.K.; Mao, L.; Miao, L.H. Codon optimization, promoter and expression system selection that achieved high-level production of Yarrowia lipolytica lipase in Pichia pastoris. Enzyme Microb. Technol. 2015, 71, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Minning, S.; Serrano, A.; Ferrer, P.; Solá, C.; Schmid, R.D.; Valero, F. Optimization of the high-level production of Rhizopus oryzae lipase in Pichia pastoris. J. Biotechnol. 2001, 86, 59–70. [Google Scholar] [CrossRef]
- Duan, H.; Wang, H.; Ma, B.; Jiang, P.; Tu, P.; Ni, Z.; Li, X.; Li, M.; Ma, X.; Wang, B.; et al. Codon optimization and expression of irisin in Pichia pastoris GS115. Int. J. Biol. Macromol. 2015, 79, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Hearn, M.T. Expression of heterologous proteins in Pichia pastoris: A useful experimental tool in protein engineering and production. J. Mol. Recognit. 2005, 18, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Macauley-Patrick, S.; Fazenda, M.L.; McNeil, B.; Harvey, L.M. Heterologous protein production using the Pichia pastoris expression system. Yeast 2005, 22, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Ballmer-Hofer, K.; Caroline, A.C.H.; Schleier, T.; Avramovic, D. ScFvs as Allosteric Inhibitors of VEGFR-2: Novel Tools to Harness VEGF Signaling. Int. J. Mol. Sci. 2018, 19, 1334. [Google Scholar] [CrossRef] [PubMed]
- Stefano, J.E.; Bird, J.; Kyazike, J.; Cheng, A.W.-M.; Boudanova, E.; Dwyer, M.; Hou, L.; Qiu, H.; Matthews, G.; O’Callaghan, M.; et al. High-Affinity VEGF Antagonists by Oligomerization of a Minimal Sequence VEGF-Binding Domain. Bioconjug. Chem. 2012, 23, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Z.; Wang, Q.; Ge, Y.; Bai, L.; Wang, X.; Zhang, X. Generation and characterization of four novel monoclonal antibodies against human programmed death-1 molecule. Hybridoma 2010, 29, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Drees, J.J.; Augustin, L.B.; Mertensotto, M.J.; Schottel, J.L.; Leonard, A.S.; Saltzman, D.A. Soluble production of a biologically active single-chain antibody against murine PD-L1 in Escherichia coli. Protein Expr. Purif. 2014, 94, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Wu, Y.; Hou, W.H.; Wang, Y.X.; Yuan, Q.Y.; Wang, H.J.; Yu, M. A novel bispecific c-MET/PD-1 antibody with therapeutic potential in solid cancer. Oncotarget 2017, 8, 29067–29079. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Rodriguez, I.; Schalper, K.A.; Oñate, C.; Azpilikueta, A.; Rodriguez-Ruiz, M.E.; Morales-Kastresana, A.; Labiano, S.; Pérez-Gracia, J.L.; Martín-Algarra, S. Nivolumab and Urelumab Enhance Antitumor Activity of Human T Lymphocytes Engrafted in Rag2-/-IL2Rγnull Immunodeficient Mice. Cancer Res. 2016, 75, 3466–3478. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, M.; Wang, J.; Xu, Y.; Wang, Y.; Shao, X.; Yang, C.; Gao, Y.; Xiong, D. Improvement of tumor targeting and antitumor activity by a disulphide bond stabilized diabody expressed in Escherichia coli. Cancer Immunol. Immunother. 2009, 58, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Yamamoto, T.; Sakai, Y. Resistance to Anti-Angiogenic Therapy in Cancer-Alterations to Anti-VEGF Pathway. Int. J. Mol. Sci. 2018, 19, 1232. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Yoshimura, K.; Kuramasu, A.; Watanabe, Y.; Ito, H.; Kondo, T.; Oga, A.; Ito, H.; Yoshino, S.; Hazama, S. Combination Immunotherapy with 4-1BB Activation and PD-1 Blockade Enhances Antitumor Efficacy in a Mouse Model of Subcutaneous Tumor. Anticancer Res. 2015, 35, 129–136. [Google Scholar] [PubMed]
- Datta, M.; Via, L.E.; Kamoun, W.S.; Liu, C.; Chen, W.; Seano, G.; Weiner, D.M.; Schimel, D.; England, K.; Martin, J.D.; et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gu, T.J.; Jiang, C.L.; Yuan, R.S.; Zhang, H.F.; Hou, H.J.; Yu, X.H.; Chen, Y.; Zhang, Y.; Wu, Y.G. A novel disulfide-stabilized single-chain variable antibody fragment against rabies virus G protein with enhanced in vivo neutralizing potency. Mol. Immunol. 2012, 51, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Thudium, K.B.; Han, M.; Wang, X.T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2014, 2, 846–856. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lv, R.; Wang, K.; Huang, X.; Wu, W.; Yin, L.; Liu, Y. Cytoglobin exhibits anti-fibrosis activity on liver in vivo and in vitro. Protein J. 2011, 30, 437–446. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, C.; Mao, Y.; Wu, T.; Kang, N.; Zhao, M.; Di, R.; Li, X.; Ji, X.; Liu, Y. Optimized Expression and Characterization of a Novel Fully Human Bispecific Single-Chain Diabody Targeting Vascular Endothelial Growth Factor165 and Programmed Death-1 in Pichia pastoris and Evaluation of Antitumor Activity In Vivo. Int. J. Mol. Sci. 2018, 19, 2900. https://doi.org/10.3390/ijms19102900
Xiong C, Mao Y, Wu T, Kang N, Zhao M, Di R, Li X, Ji X, Liu Y. Optimized Expression and Characterization of a Novel Fully Human Bispecific Single-Chain Diabody Targeting Vascular Endothelial Growth Factor165 and Programmed Death-1 in Pichia pastoris and Evaluation of Antitumor Activity In Vivo. International Journal of Molecular Sciences. 2018; 19(10):2900. https://doi.org/10.3390/ijms19102900
Chicago/Turabian StyleXiong, Chenghao, Yingqing Mao, Tao Wu, Nannan Kang, Mingjun Zhao, Rongrong Di, Xiaoping Li, Xuemei Ji, and Yu Liu. 2018. "Optimized Expression and Characterization of a Novel Fully Human Bispecific Single-Chain Diabody Targeting Vascular Endothelial Growth Factor165 and Programmed Death-1 in Pichia pastoris and Evaluation of Antitumor Activity In Vivo" International Journal of Molecular Sciences 19, no. 10: 2900. https://doi.org/10.3390/ijms19102900
APA StyleXiong, C., Mao, Y., Wu, T., Kang, N., Zhao, M., Di, R., Li, X., Ji, X., & Liu, Y. (2018). Optimized Expression and Characterization of a Novel Fully Human Bispecific Single-Chain Diabody Targeting Vascular Endothelial Growth Factor165 and Programmed Death-1 in Pichia pastoris and Evaluation of Antitumor Activity In Vivo. International Journal of Molecular Sciences, 19(10), 2900. https://doi.org/10.3390/ijms19102900




