Nfatc1 Is a Functional Transcriptional Factor Mediating Nell-1-Induced Runx3 Upregulation in Chondrocytes
Abstract
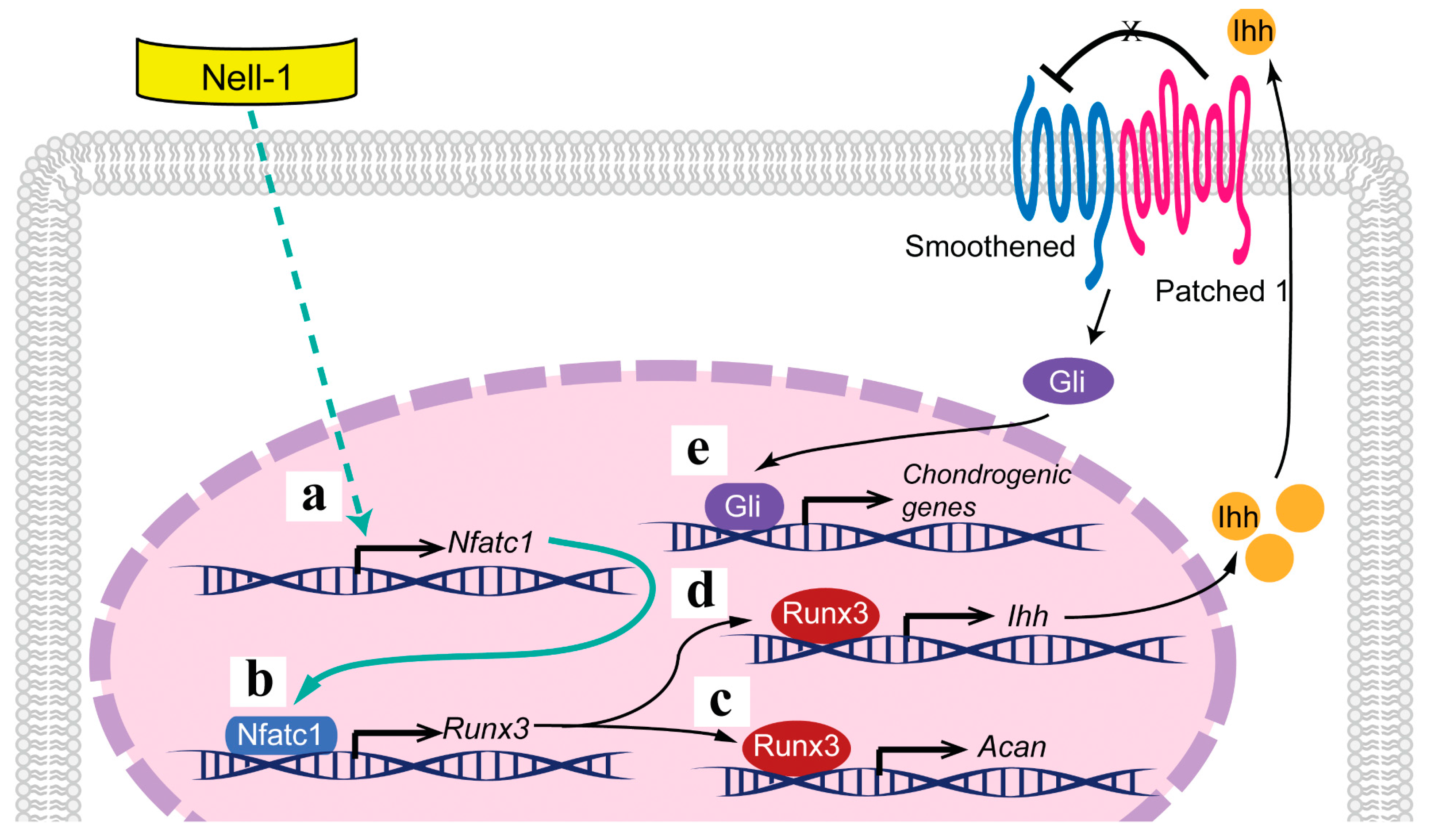
:1. Introduction
2. Results
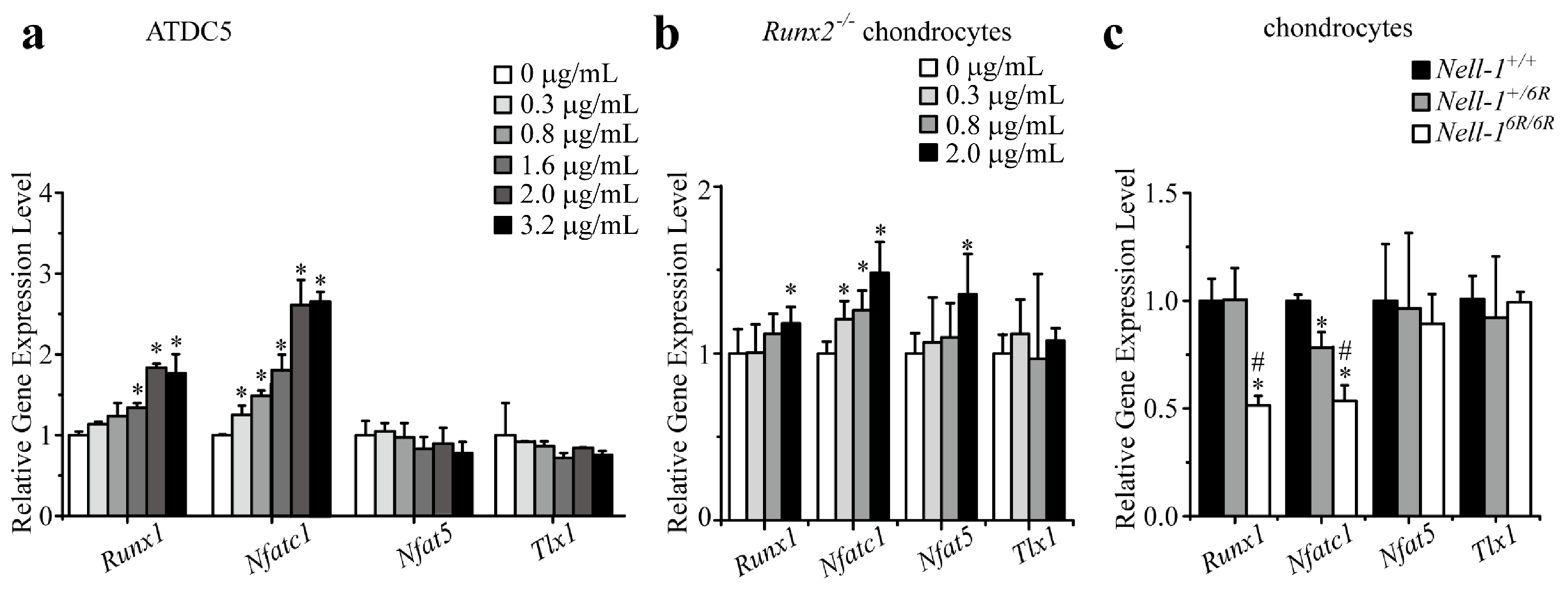
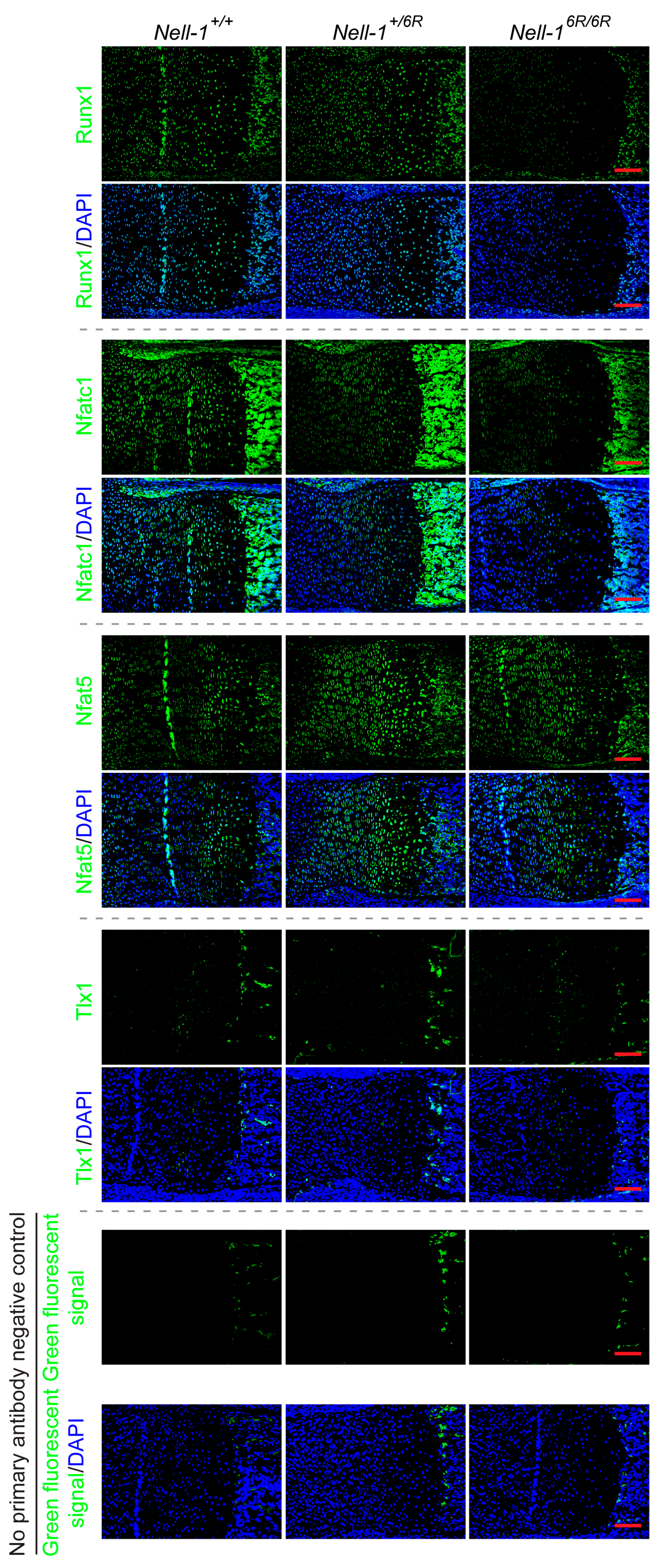
2.1. Runt-Related Transcription Factor 1 (Runx1) and Nfatc1 Were Selected as the Candidates That Bridge Nell-1 → Runx3 Signal Transduction in Chondrocytes
2.2. Knockdown Runx1 Failed to Demonstrate the Effects on the Nell-1-Mediated Runx3-Ihh Signaling
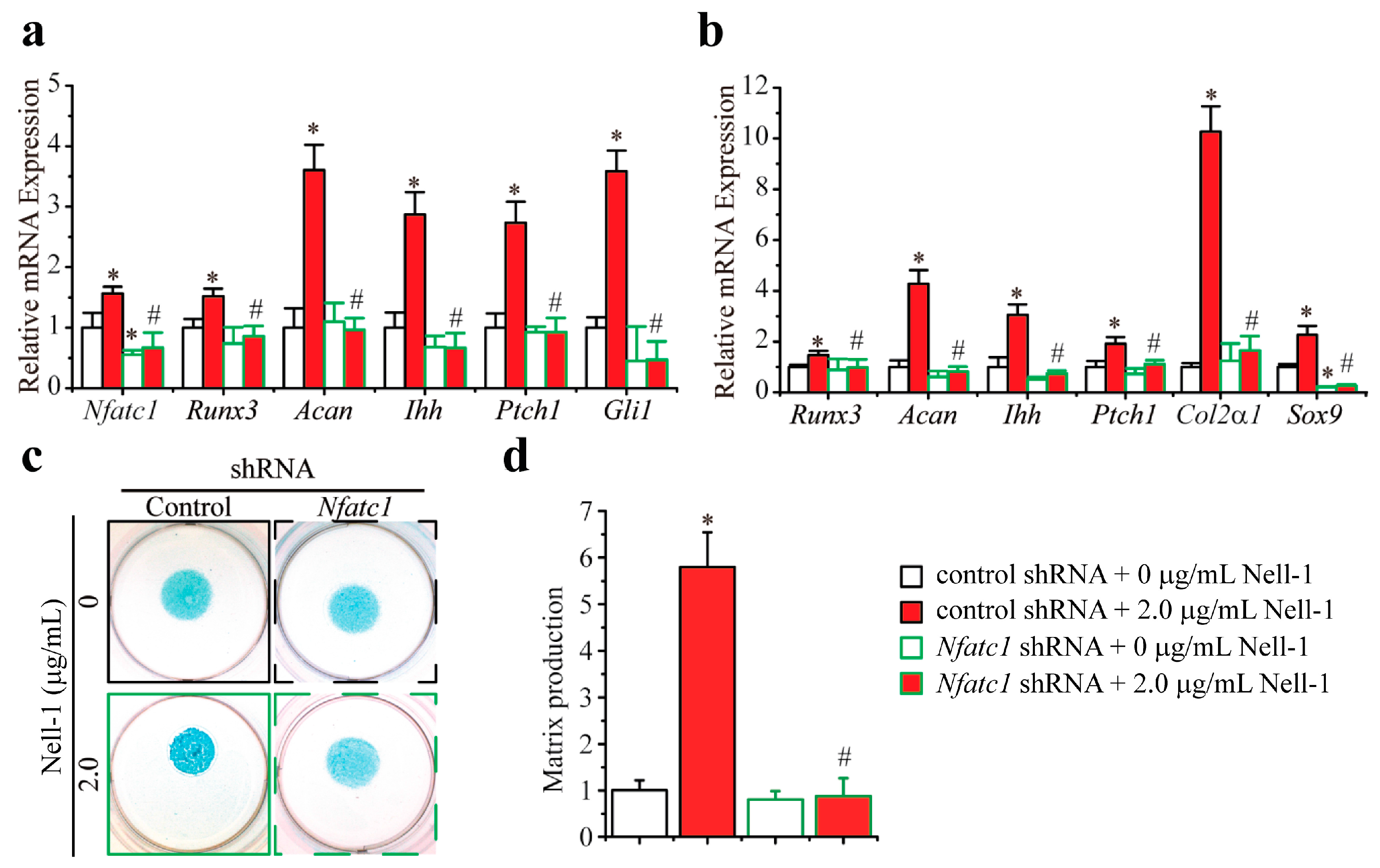
2.3. Nfatc1 Mediates Nell-1’s Role in Runx3-Ihh Signaling and Chondrogenic Differentiation
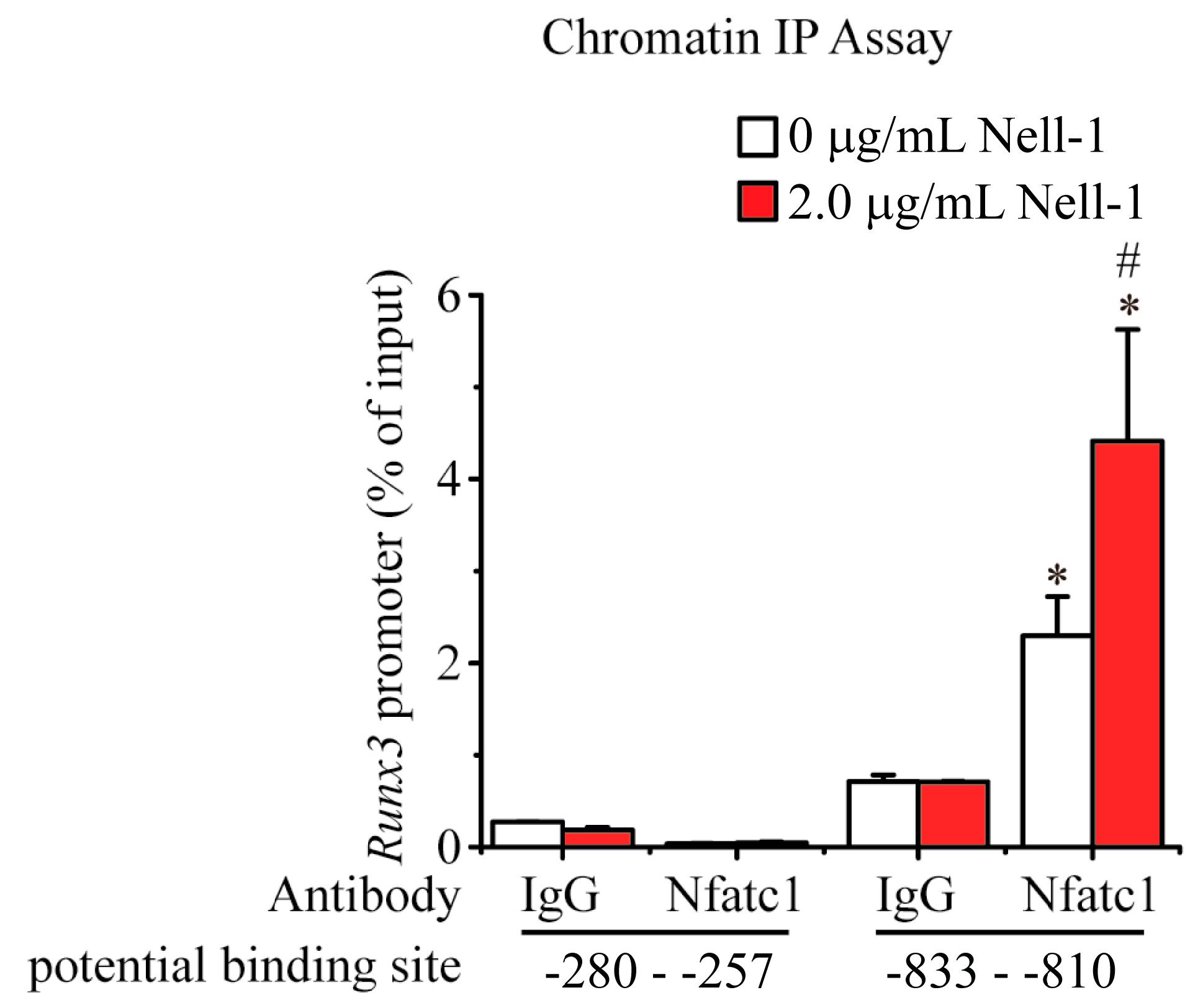
2.4. Nell-1 Enhances the Binding of Nfatc1 at the −833–−810 Region of Runx3 Promoter in Chondrocytes
3. Discussion
4. Materials and Methods
4.1. In Silico Promoter Analysis
4.2. Animal Maintenance
4.3. Immunofluorescence (IF) Staining
4.4. Cultivation of ATDC5 Cell Line
4.5. Mouse Primary Chondrocyte Isolation and Cultivation
4.6. RNAi
4.7. ChIP Assay
4.8. qPCR
4.9. Alcian Blue Staining and Quantification
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Acan | Aggrecan |
| ChIP | Chromatin immunoprecipitation |
| DAPI | 2-(4-amidinophenyl)-1H-indole-6-carboxamidine |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ENU | N-ethyl-N-nitrosourea |
| FBS | Fetal bovine serum |
| Gapdh | Glyceraldehyde 3-phosphate dehydrogenase |
| IF | Immunofluorescent |
| Ihh | Indian hedgehog |
| Nfat5 | Nuclear factor of activated T-cells 5 |
| Nfatc1 | Nuclear factor of activated T-cells 1 |
| Patched1 | Patched homology 1 |
| qPCR | Quantitative real-time PCR |
| RNAi | RNA interference |
| RT | Reverse transcription |
| Runx1 | Runt-related transcription factor 1 |
| Runx2 | Runt-related transcription factor 2 |
| Runx3 | Runt-related transcription factor 3 |
| Tlx1 | T-cell leukemia, homeobox1 |
References
- Liu, C.F.; Samsa, W.E.; Zhou, G.; Lefebvre, V. Transcriptional control of chondrocyte specification and differentiation. Semin. Cell Dev. Biol. 2017, 62, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Siu, R.K.; Ting, K.; Wu, B.M. Effect of Nell-1 delivery on chondrocyte proliferation and cartilaginous extracellular matrix deposition. Tissue Eng. Part A 2010, 16, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Siu, R.K.; Zara, J.N.; Hou, Y.; James, A.W.; Kwak, J.; Zhang, X.; Ting, K.; Wu, B.M.; Soo, C.; Lee, M. Nell-1 promotes cartilage regeneration in an in vivo rabbit model. Tissue Eng. Part A 2012, 18, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, B.; Man, C.; Ma, Y.; Hu, J. NEL-like molecule-1-modified bone marrow mesenchymal stem cells/poly lactic-co-glycolic acid composite improves repair of large osteochondral defects in mandibular condyle. Osteoarthr. Cartil. 2011, 19, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Shannon, M.E.; Johnson, M.D.; Ruff, D.W.; Hughes, L.A.; Kerley, M.K.; Carpenter, D.A.; Johnson, D.K.; Rinchik, E.M.; Culiat, C.T. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum. Mol. Genet. 2006, 15, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, J.; Zheng, Z.; Lee, K.S.; Zhou, Y.; Chen, E.; Culiat, C.T.; Qiao, Y.; Chen, X.; Ting, K.; et al. Neural EGFL-like 1 is a downstream regulator of runt-related transcription factor 2 in chondrogenic differentiation and maturation. Am. J. Pathol. 2017, 183, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, Z.; Jiang, J.; Jiang, W.; Lee, K.; Berthiaume, E.A.; Chen, E.C.; Culiat, C.T.; Zhou, Y.-H.; Zhang, X.; et al. Neural EGFL like 1 (Nell-1) regulates cartilage maturation through runt-related transcription factor 3-mediated indian hedgehog signaling. Am. J. Pathol. 2017, in press. [Google Scholar]
- Chen, W.; Zhang, X.; Siu, R.K.; Chen, F.; Shen, J.; Zara, J.N.; Culiat, C.T.; Tetradis, S.; Ting, K.; Soo, C. Nfatc2 is a primary response gene of Nell-1 regulating chondrogenesis in ATDC5 cells. J. Bone Miner. Res. 2011, 26, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.A.; Yamamoto, H.; Fujita, T.; Furuichi, T.; Ito, K.; Inoue, K.; Yamana, K.; Zanma, A.; Takada, K.; Ito, Y.; et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of indian hedgehog. Genes Dev. 2004, 18, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Wuelling, M.; Vortkamp, A. Chondrocyte proliferation and differentiation. Endocr. Dev. 2011, 21, 1–11. [Google Scholar] [PubMed]
- Chen, H.; Ghori-Javed, F.Y.; Rashid, H.; Adhami, M.D.; Serra, R.; Gutierrez, S.E.; Javed, A. Runx2 regulates endochondral ossification through control of chondrocyte proliferation and differentiation. J. Bone Miner. Res. 2014, 29, 2653–2665. [Google Scholar] [CrossRef] [PubMed]
- Wigner, N.A.; Soung do, Y.; Einhorn, T.A.; Drissi, H.; Gerstenfeld, L.C. Functional role of Runx3 in the regulation of aggrecan expression during cartilage development. J. Cell. Physiol. 2013, 228, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Ting, K.; Vastardis, H.; Mulliken, J.B.; Soo, C.; Tieu, A.; Do, H.; Kwong, E.; Bertolami, C.N.; Kawamoto, H.; Kuroda, S.; et al. Human Nell-1 expressed in unilateral coronal synostosis. J. Bone Miner. Res. 1999, 14, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kuroda, S.; Carpenter, D.; Nishimura, I.; Soo, C.; Moats, R.; Iida, K.; Wisner, E.; Hu, F.Y.; Miao, S.; et al. Craniosynostosis in transgenic mice overexpressing Nell-1. J. Clin. Investig. 2002, 110, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Carpenter, D.; Bokui, N.; Soo, C.; Miao, S.; Truong, T.; Wu, B.; Chen, I.; Vastardis, H.; Tanizawa, K.; et al. Overexpression of Nell-1, a craniosynostosis-associated gene, induces apoptosis in osteoblasts during craniofacial development. J. Bone Miner. Res. 2003, 18, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zara, J.; Siu, R.K.; Ting, K.; Soo, C. The role of Nell-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J. Dent. Res. 2010, 89, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ting, K.; Pathmanathan, D.; Ko, T.; Chen, W.; Chen, F.; Lee, H.; James, A.W.; Siu, R.K.; Shen, J.; et al. Calvarial cleidocraniodysplasia-like defects with enu-induced Nell-1 deficiency. J. Craniofac. Surg. 2012, 23, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Zhang, X.; Pathmanathan, D.; Soo, C.; Ting, K. Craniosynostosis-associated gene Nell-1 is regulated by Runx2. J. Bone Miner. Res. 2007, 22, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ting, K.; Bessette, C.M.; Culiat, C.T.; Sung, S.J.; Lee, H.; Chen, F.; Shen, J.; Wang, J.J.; Kuroda, S.; et al. Nell-1, a key functional mediator of Runx2, partially rescues calvarial defects in Runx2+/− mice. J. Bone Miner. Res. 2011, 26, 777–791. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Shen, J.; Velasco, O.; Asatrian, G.; Chung, C.G.; Khadarian, K.; Zhang, Y.; Chang, L.; Goyal, R.; Zhang, X.; et al. Systemic administration of Nell-1, a Wnt/β-catenin regulator, induces bone formation in osteoporotic mice via integrin β1. Presented at The Annual American Society of Bone and Mineral Research Meeting, Baltimore, MD, USA, October 2013. [Google Scholar]
- Kim, I.S.; Otto, F.; Zabel, B.; Mundlos, S. Regulation of chondrocyte differentiation by Cbfa1. Mech. Dev. 1999, 80, 159–170. [Google Scholar] [CrossRef]
- Hasebe, A.; Nakamura, Y.; Tashima, H.; Takahashi, K.; Iijima, M.; Yoshimoto, N.; Ting, K.; Kuroda, S.; Niimi, T. The C-terminal region of NELL1 mediates osteoblastic cell adhesion through integrin α3β1. FEBS Lett. 2012, 586, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; James, A.W.; Chung, J.; Lee, K.; Zhang, J.B.; Ho, S.; Lee, K.S.; Kim, T.M.; Niimi, T.; Kuroda, S.; et al. Nell-1 promotes cell adhesion and differentiation via integrin β1. J. Cell. Biochem. 2012, 113, 3620–3628. [Google Scholar] [CrossRef] [PubMed]
- Ranger, A.M.; Gerstenfeld, L.C.; Wang, J.; Kon, T.; Bae, H.; Gravallese, E.M.; Glimcher, M.J.; Glimcher, L.H. The nuclear factor of activated T cells (NFAT) transcription factor Nfatp (Nfatc2) is a repressor of chondrogenesis. J. Exp. Med. 2000, 191, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gardner, B.M.; Lu, Q.; Rodova, M.; Woodbury, B.G.; Yost, J.G.; Roby, K.F.; Pinson, D.M.; Tawfik, O.; Anderson, H.C. Transcription factor NFAT1 deficiency causes osteoarthritis through dysfunction of adult articular chondrocytes. J. Pathol. 2009, 219, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Rodova, M.; Lu, Q.; Li, Y.; Woodbury, B.G.; Crist, J.D.; Gardner, B.M.; Yost, J.G.; Zhong, X.B.; Anderson, H.C.; Wang, J. Nfat1 regulates adult articular chondrocyte function through its age-dependent expression mediated by epigenetic histone methylation. J. Bone Miner. Res. 2011, 26, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Sohn, P.; Cox, M.; Chen, D.; Serra, R. Molecular profiling of the developing mouse axial skeleton: A role for Tgfbr2 in the development of the intervertebral disc. BMC Dev. Biol. 2010, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Canalis, E. Notch suppresses nuclear factor of activated T cells (NFAT) transactivation and Nfatc1 expression in chondrocytes. Endocrinology 2013, 154, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.P.; Tsang, K.; He, L.Z.; Garcia, R.A.; Ermann, J.; Mizoguchi, F.; Zhang, M.J.; Zhou, B.; Zhou, B.; Aliprantis, A.O. NFAT restricts osteochondroma formation from entheseal progenitors. JCI Insight 2016, 1, e86254. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, M.B.; Ritter, S.Y.; Wright, J.; Tsang, K.; Hu, D.; Glimcher, L.H.; Aliprantis, A.O. NFATc1 and NFATc2 repress spontaneous osteoarthritis. Proc. Natl. Acad. Sci. USA 2013, 110, 19914–19919. [Google Scholar] [CrossRef] [PubMed]
- Beier, F. NFATs are good for your cartilage. Osteoarthr. Cartil. 2014, 22, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Aliprantis, A.O.; Glimcher, L.H. NFATc1 in inflammatory and musculoskeletal conditions. Adv. Exp. Med. Biol. 2010, 658, 69–75. [Google Scholar] [PubMed]
- Klingenhoff, A.; Frech, K.; Quandt, K.; Werner, T. Functional promoter modules can be defected by formal models independent of overall nucleotide sequence similarity. Bioinformatics 1999, 15, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef]
- Rinchik, E.M.; Carpenter, D.A.; Selby, P.B. A strategy for fine-structure functional analysis of a 6- to 11-centimorgan region of mouse chromosome 7 by high-efficiency mutagenesis. Proc. Natl. Acad. Sci. USA 1990, 87, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Rinchik, E.M.; Carpenter, D.A.; Johnson, D.K. Functional annotation of mammalian genomic DNA sequence by chemical mutagenesis: A fine-structure genetic mutation map of a 1- to 2-cm segment of mouse chromosome 7 corresponding to human chromosome 11p14-p15. Proc. Natl. Acad. Sci. USA 2002, 99, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Y. ATDC5: An excellent in vitro model cell line for skeletal development. J. Cell. Biochem. 2013, 114, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- MacRae, V.E.; Davey, M.G.; McTeir, L.; Narisawa, S.; Yadav, M.C.; Millan, J.L.; Farquharson, C. Inhibition of phospho1 activity results in impaired skeletal mineralization during limb development of the chick. Bone 2010, 46, 1146–1155. [Google Scholar] [CrossRef] [PubMed]

| In Silico Bioinformatics Predicted Mouse Runx3 Promoter Binding Transcriptional Factors That Expressed in Cartilage and Chondrocytes | Gene Expression Changes in ATDC5 Cells Due to the Nell-1 Treatment * | ||||||
|---|---|---|---|---|---|---|---|
| Matrix Family | Gene | Full Name | Strand | Sequence ** | Matrix Similarity | Fold-Change | p-Value |
| FKHD | Foxp1 | Forkhead box P1 | (−) | 5′-gggtcaaAACAgagggg-3′ | 1 | 1.14 | 5.7 × 10−6 |
| Foxp2 | Forkhead box P2 | (+) | 5′-cagcagtaAACAgagag-3′ | 0.994 | 0.79 | 1.1 × 10−2 | |
| Hnf3b/Foxa2 | Hepatic nuclear factor 3 beta | (+) | 5′-cagcagtaAACAgagag-3′ | 0.914 | 0.32 | 2.0 × 10−2 | |
| Foxo1 | Forkhead box protein O1 | (+) | 5′-aaaaagtcAACAcctcc-3′ | 0.9 | 0.73 | 7.9 × 10−5 | |
| Fhx/Foxj2 | Fork head homologous X binds DNA with a dual sequence specificity (FHXA and FHXB) | (+) | 5′-gtagccACAAgatcttc-3′ | 0.835 | 0.84 | 2.6 × 10−5 | |
| HAML | Runx1 | Runt-related transcription factor 1 | (+) | 5′-ctgtGTGGtccggac-3′ | 0.97 | 1.23 | 8.8 × 10−5 |
| HOMF | Hhex | Hematopoietically expressed homeobox, proline-rich homeodomain protein | (+) | 5′-aactaggtgttTAATtttg-3′ | 0.969 | 0.90 | 3.0 × 10−4 |
| (+) | 5′-ttcccaccattTAATgata-3′ | 0.952 | |||||
| Hmx3 | H6 homeodomain HMX3/Nkx5.1 transcription factor | (+) | 5′-cggaccccAAGTgcctcca-3′ | 0.897 | 2.99 | 0.19 *** | |
| (+) | 5′-ggctcaggAAGTgggggtg-3′ | 0.911 | |||||
| (+) | 5′-agccaaccAAGTgggtctg-3′ | 0.96 | |||||
| Msx-1 | Homeodomain proteins MSX-1 and MSX-2 | (+) | 5′-aggtgttTAATtttgcaac-3′ | 0.989 | 0.81 | 4.5 × 10−4 | |
| Hmx2 | Hmx2/Nkx5-2 homeodomain transcription factor | (−) | 5′-gtatcaTTAAatggtggga-3′ | 0.86 | 0.90 | 0.69 *** | |
| Tlx1/Hox11 | T-cell leukemia, homeobox 1 | (−) | 5′-tgggagcCGCTgagtgggt-3′ | 0.858 | 1.41 | 3.0 × 10−2 | |
| NFAT | Nfatc1 | Nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1, dimeric binding site | (−) | 5′-tttaccGTGGaacccagga-3′ | 0.826 | 1.55 | 5.3 × 10−4 |
| (+) | 5′-ggttccACGGtaaagccag-3′ | 0.816 | |||||
| (−) | 5′-atctccAAGGaaagaaagt-3′ | 0.827 | |||||
| (+) | 5′-ctttccTTGGagattttct-3′ | 0.877 | |||||
| Nfat5 | nuclear factor of activated T-cells 5 | (−) | 5′-ccaaGGAAagaaagtttcg-3′ | 0.844 | 1.52 | 6.0 × 10−5 | |
| SORY | Sox1 | SRY (sex determining region Y)-box 1, dimeric binding sites | (−) | 5′-gctGATTccccactcaggcagag-3′ | 0.795 | 0.43 | 0.14 *** |
| Sox21 | SRY (sex determining region Y)-box 21, dimeric binding sites | (−) | 5′-cctGCATgttggtcacacaacta-3′ | 0.783 | ND **** | ND **** | |
| (+) | 5′-attTAATgatactctgcacatag-3′ | 0.762 | |||||
| Sox2 | SRY-box containing gene 2, dimeric binding sites | (−) | 5′-gatCAAGggtgtgaatagagtcc | 0.701 | 0.90 | 8.7 × 10−6 | |
| Sox8 | SRY (sex determining region Y)-box 8, dimeric binding sites | (−) | 5′-aatGAAAggcagtactgacctgc-3′ | 0.777 | ND **** | ND **** | |
| (+) | 5′-cagGACTcccagtctcacagggt-3′ | 0.763 | |||||
| Hbp1 | high mobility group box transcription factor 1 | (−) | 5′-aatgaatgAATGaacgaggctca-3′ | 1 | 0.84 | 3.8 × 10−5 | |
| (−) | 5′-gatgaatgAATGaatgaacgagg-3′ | 1 | |||||
| (−) | 5′-ctggatgAATGaatgaatgaacg-3′ | 0.996 | |||||
| (−) | 5′-cagcctgGATGaatgaatgaatg-3′ | 0.847 | |||||
| Sox9 | SRY (sex determining region Y)-box 9 homodimer | (+) | 5′-acAGAAagcctaccttctctctc-3′ | 0.787 | 0.76 | 1.1 × 10−5 | |
| (+) | 5′-gaaccACAAggccaggccctcgc-3′ | 0.944 | |||||
| (−) | 5′-gcaCTATgtgcagagtatcatta-3′ | 0.733 | |||||
| Hmgiy/Hmga1 | HMGI(Y) high-mobility-group protein I (Y), architectural transcription factor organizing the framework of a nuclear protein-DNA transcriptional complex /High mobility group AT-Hook 1 | (−) | 5′-cacaAATTttcaacagcactatg-3′ | 0.935 | 0.56 | 1.5 × 10−3 | |
| (+) | 5′-tgaaAATTtgtggctagacattc-3′ | 0.935 | |||||
| Sox10 | SRY-box containing gene 10 | (−) | 5′-caGGAAtgtctagccacaaattt-3′ | 0.739 | 8.25 | 0.54 *** | |
| PAX | Pax1 | Pax1 paired domain protein, expressed in the developing vertebral column of mouse embryos | (−) | 5′-cTGTTttgttatatatatt-3′ | 0.667 | ND **** | ND **** |
| Gene | Primer Sequence |
|---|---|
| Acan | 5′-CCA GGC TCC ACC AGA TAC TC-3′ |
| 5′-TGC TCA TAG CCT GCC TCA TA-3′ | |
| Col2α1 | 5′-GTC CTG AAG GTG CTC AAG GT-3′ |
| 5′-TTT GGC TCC AGG AAT ACC AT-3′ | |
| Gapdh | 5′-ATT CAA CGG CAC AGT CAA GG-3′ |
| 5′-GAT GTT AGT GGG GTC TCG CTC-3′ | |
| Gli1 | 5′-CCA AGC CAA CTT TAT GTC AGG G-3′ |
| 5′-AGC CCG CTT CTT TGT TAA TTT GA-3′ | |
| Ihh | 5′-CTC AGA CCG TGA CCG AAA TAA G-3′ |
| 5′-CCT TGG ACT CGT AAT ACA CCC AG-3′ | |
| Nfat5 | 5′-TGC TTT CTC AGC TTA CCA CGG-3′ |
| 5′-GTC CGC ACA ACA TAG GGC TC-3′ | |
| Nfatc1 | 5′-GGA GAG TCC GAG AAT CGA GAT-3′ |
| 5′-TTG CAG CTA GGA AGT ACG TCT-3′ | |
| Ptch 1 | 5′-TGC CAC AGC CCC TAA CAA AAA-3′ |
| 5′-ACC CAC AAT CAA CTC CTC CTG-3′ | |
| Runx1 | 5′-ACG ATG AAA ACT ACT CGG CAG-3′ |
| 5′-CTG AGG TCG TTG AAT CTC GCT-3′ | |
| Runx3 | 5′-CAG GTT CAA CGA CCT TCG ATT-3′ |
| 5′-GTG GTA GGT AGC CAC TTG GG-3′ | |
| Sox9 | 5′-ACG GCT CCA GCA AGA ACA AG-3′ |
| 5′-TTG TGC AGA TGC GGG TAC TG-3′ | |
| Tlx1 | 5′-CGG CTT GCC TAC AGT ACC C-3′ |
| 5′-CTG CGG TTA CTC TCC ATC CAG-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zheng, Z.; Zhang, X.; Asatrian, G.; Chen, E.; Song, R.; Culiat, C.; Ting, K.; Soo, C. Nfatc1 Is a Functional Transcriptional Factor Mediating Nell-1-Induced Runx3 Upregulation in Chondrocytes. Int. J. Mol. Sci. 2018, 19, 168. https://doi.org/10.3390/ijms19010168
Li C, Zheng Z, Zhang X, Asatrian G, Chen E, Song R, Culiat C, Ting K, Soo C. Nfatc1 Is a Functional Transcriptional Factor Mediating Nell-1-Induced Runx3 Upregulation in Chondrocytes. International Journal of Molecular Sciences. 2018; 19(1):168. https://doi.org/10.3390/ijms19010168
Chicago/Turabian StyleLi, Chenshuang, Zhong Zheng, Xinli Zhang, Greg Asatrian, Eric Chen, Richard Song, Cymbeline Culiat, Kang Ting, and Chia Soo. 2018. "Nfatc1 Is a Functional Transcriptional Factor Mediating Nell-1-Induced Runx3 Upregulation in Chondrocytes" International Journal of Molecular Sciences 19, no. 1: 168. https://doi.org/10.3390/ijms19010168
APA StyleLi, C., Zheng, Z., Zhang, X., Asatrian, G., Chen, E., Song, R., Culiat, C., Ting, K., & Soo, C. (2018). Nfatc1 Is a Functional Transcriptional Factor Mediating Nell-1-Induced Runx3 Upregulation in Chondrocytes. International Journal of Molecular Sciences, 19(1), 168. https://doi.org/10.3390/ijms19010168






