Human Chorionic Gonadotrophin as a Possible Mediator of Leiomyoma Growth during Pregnancy: Molecular Mechanisms
Abstract
1. Introduction to Leiomyoma Pathophysiology
2. Pattern of Growth of Uterine Leiomyomas during Pregnancy
3. Sex Steroids in Pregnancy and Leiomyomas
3.1. Estrogens
3.2. Progesterone
4. Human Chorionic Gonadotropin in Pregnancy and Leiomyomas
4.1. Luteinizing Hormone/hCG Receptors in Myometrium and Leiomyomas
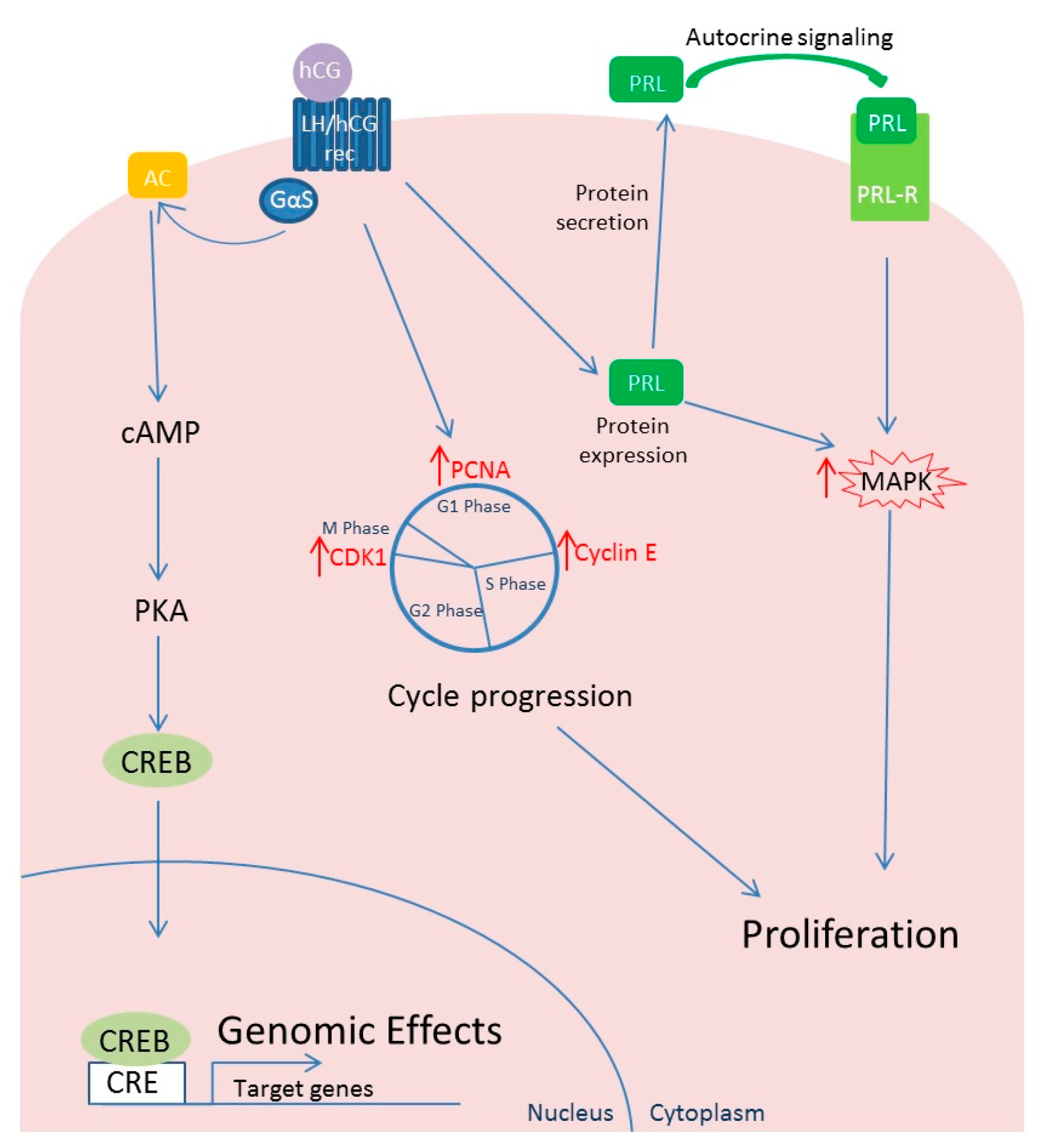
4.2. Effects of hCG on Leiomyoma Cells
4.3. Differential Behavior of LH and hCG in Relation to Leiomyoma Development
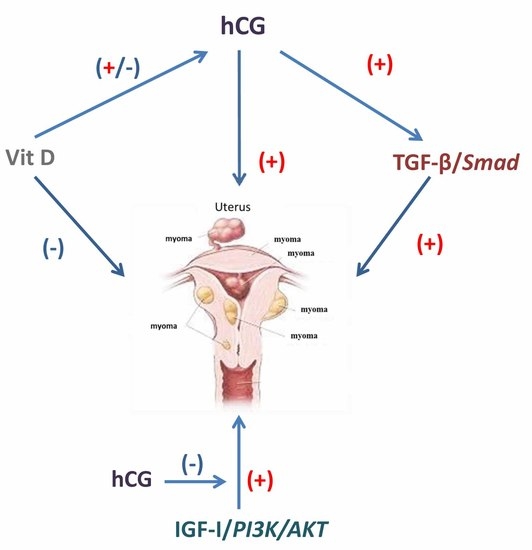
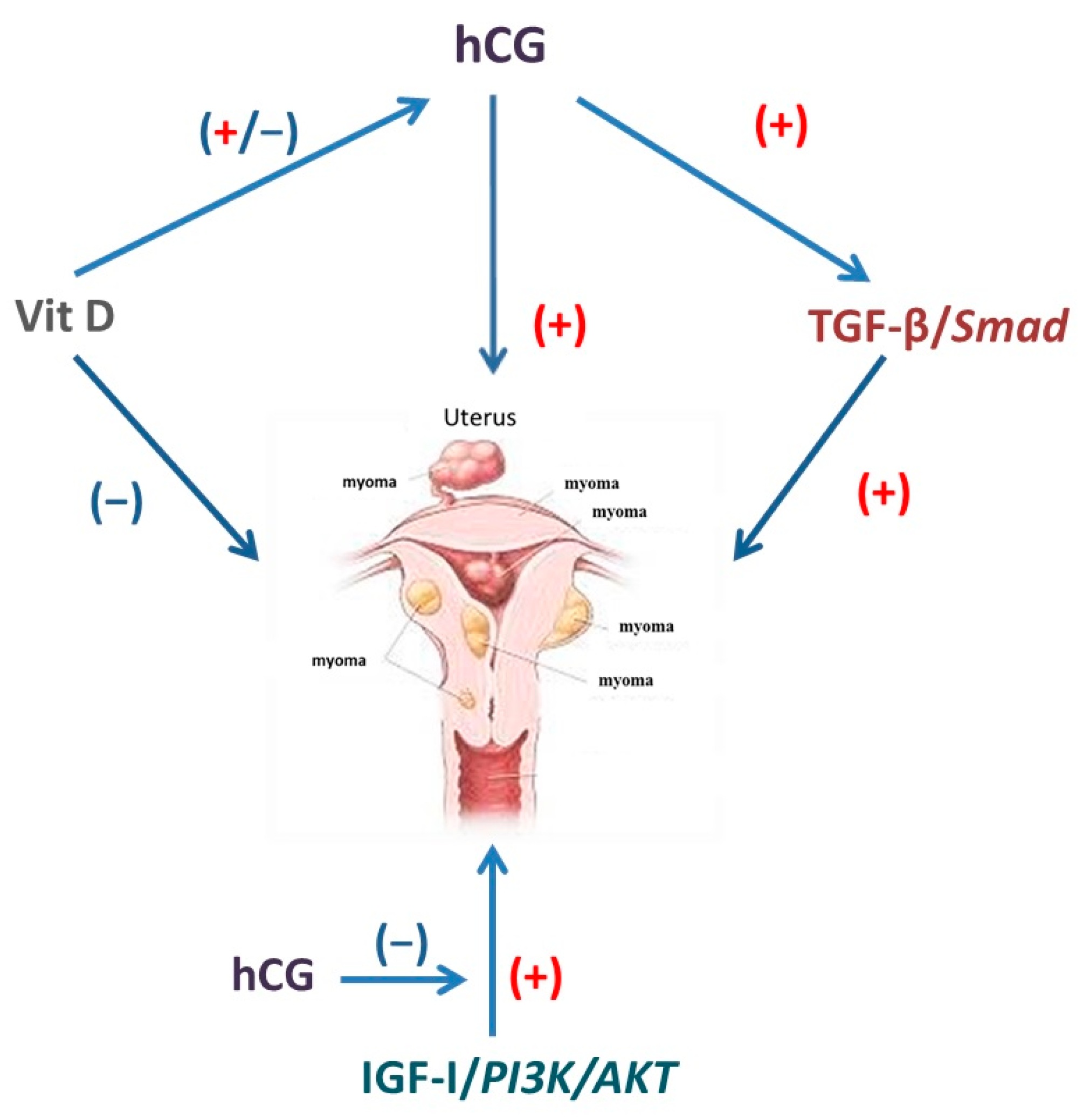
5. Cross-Talk between hCG and Other Pathways in Leiomyoma Development
5.1. Transforming Growth Factor (TGF)-β and hCG
5.2. Vitamin D and hCG
5.3. Insulin-Like Growth Factor-I and hCG
6. Conclusions and Prospects
Author Contributions
Conflicts of Interest
References
- Okolo, S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrovych, V.; Bereza, T.; Sajewicz, M.; Walocha, J.A.; Gil, K. Uterine fibroid: Common features of widespread tumor. Folia. Med. Cracov. 2015, 55, 61–75. [Google Scholar] [PubMed]
- Evans, P.; Brunsell, S. Uterine fibroid tumors: Diagnosis and treatment. Am. Fam. Physician. 2007, 75, 503–508. [Google Scholar]
- Somigliana, E.; Vercellini, P.; Daguati, R.; Pasin, R.; de Giorgi, O.; Crosignani, P.G. Fibroids and female reproduction: A critical analysis of the evidence. Hum. Reprod. Update 2007, 13, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Palmer, J.R.; Ruiz-Narvaez, E.; Reich, D.E.; Rosenberg, L. Is the observed association between dairy intake and fibroids in African Americans explained by genetic ancestry? Am. J. Epidemiol. 2013, 178, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Ruiz-Narvaez, E.A.; Palmer, J.R.; Cozier, Y.C.; Tandon, A.; Patterson, N.; Radin, R.G.; Rosenberg, L.; Reich, D. African ancestry and genetic risk for uterine leiomyomata. Am. J. Epidemiol. 2012, 176, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Terry, K.L.; de Vivo, I.; Hankinson, S.E.; Missmer, S.A. Reproductive characteristics and risk of uterine leiomyomata. Fertil. Steril. 2010, 94, 2703–2707. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Palmer, J.R.; Harlow, B.L.; Spiegelman, D.; Stewart, E.A.; Adams-Campbell, L.L.; Rosenberg, L. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: A prospective study. Am. J. Epidemiol. 2004, 159, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ciavattini, A.; di Giuseppe, J.; Stortoni, P.; Montik, N.; Giannubilo, S.R.; Litta, P.; Islam, M.S.; Tranquilli, A.L.; Reis, F.M.; Ciarmela, P. Uterine fibroids: Pathogenesis and interactions with endometrium and endomyometrial junction. Obstet. Gynecol. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Mehine, M.; Kaasinen, E.; Heinonen, H.R.; Mäkinen, N.; Kämpjärvi, K.; Sarvilinna, N.; Aavikko, M.; Vähärautio, A.; Pasanen, A.; Bützow, R.; et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc. Natl. Acad. Sci. USA 2016, 113, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.S. Modern management of uterine fibroids. Acta Obstet. Gynecol. Scand. 2008, 87, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Travlos, G.; Wilson, R.; Dunson, D.B.; Hill, M.C.; D’Aloisio, A.A.; London, S.J.; Schectman, J.M. Uterine leiomyomata in relation to insulin-like growth factor-I, insulin, and diabetes. Epidemiology 2009, 20, 604–610. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, A.; Mancuso, A.; Giacobbe, A.; Savasta, L.M.; de Dominici, R.; Dugo, N.; Dugo, C.; Vaiarelli, A. Uterine myomas during pregnancy: A longitudinal sonographic study. Ultrasound Obstet. Gynecol. 2011, 37, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Benaglia, L.; Cardellicchio, L.; Filippi, F.; Paffoni, A.; Vercellini, P.; Somigliana, E.; Fedele, L. The rapid growth of fibroids during early pregnancy. PLoS ONE 2014, 9, e85933. [Google Scholar] [CrossRef] [PubMed]
- Evans, J. Hyperglycosylated hCG: A Unique Human Implantation and Invasion Factor. Am. J. Reprod. Immunol. 2016, 75, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Neiger, R.; Sonek, J.D.; Croom, C.S.; Ventolini, G. Pregnangy-related changes in the size of uterine leiomyomas. J. Reprod. Med. 2006, 51, 671–674. [Google Scholar] [PubMed]
- Hammoud, A.O.; Asaad, R.; Berman, J.; Treadwell, M.C.; Blackwell, S.; Diamond, M.P. Volume change of uterine myomas during pregnancy: Do myomas really grow? J. Minim. Invasive Gynecol. 2006, 13, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Muezzinoglu, B.; Corakci, A. Pathological characteristics and clinical outcome of uterine leiomyomas associated with pregnancy. Pathol. Res. Pract. 2011, 207, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Ciavattini, A.; Delli Carpini, G.; Clemente, N.; Moriconi, L.; Gentili, C.; di Giuseppe, J. Growth trend of small uterine fibroids and human chorionic gonadotropin serum levels in early pregnancy: An observational study. Fertil. Steril. 2016, 105, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Lev-Toaff, A.S.; Coleman, B.G.; Arger, P.H.; Mintz, M.C.; Arenson, R.L.; Toaff, M.E. Leiomyomas in pregnancy: Sonographic study. Radiology 1987, 164, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; Reiter, A.; Golan, D.; Paltiely, Y.; Sharf, M. Patterns of growth of uterine leio-myomas during pregnancy. A prospective longitudinal study. Br. J. Obstet. Gynaecol. 1988, 95, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Rosati, P.; Exacoustos, C.; Mancuso, S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J. Ultrasound Med. 1992, 11, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, E.; Ugur, M.G.; Kalayci, H.; Balat, O. Uterine myoma in pregnancy: Report of 19 patients. Clin. Exp. Obstet. Gynecol. 2009, 36, 182–183. [Google Scholar] [PubMed]
- Fritz, M.A.; Speroff, L. The endocrinology of pregnancy. In Clinical Gynecologic Endocrinology and Infertility; Lippincott Williams & Wilkins: London, UK, 2005; pp. 269–327. [Google Scholar]
- Lau, K.M.; To, K.F. Importance of Estrogenic Signalling and Its Mediated Receptors in Prostate Cancer. Int. J. Mol. Sci. 2016, 17, 1434. [Google Scholar] [CrossRef] [PubMed]
- Benassayag, C.; Leroy, M.J.; Rigourd, V.; Robert, B.; Honoré, J.C.; Mignot, T.M.; Vacher-Lavenu, M.C.; Chapron, C.; Ferré, F. Estrogen receptors (ERα/ERβ) in normal and pathological growth of the human myometrium: Pregnancy and leiomyoma. Am. J. Physiol. 1999, 276, E1112–E1118. [Google Scholar] [PubMed]
- Kovács, K.A.; Oszter, A.; Göcze, P.M.; Környei, J.L.; Szabó, I. Comparative analysis of cyclin D1 and oestrogen receptor (α and β) levels in human leiomyoma and adjacent myometrium. Mol. Hum. Reprod. 2001, 7, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Shinohara, M.; Kashimura, M.; Yoshida, K.; Okamura, Y. A comparative study of the estrogen receptor ratio in myometrium and uterine leiomyomas. Int. J. Gynaecol. Obstet. 1989, 29, 189–194. [Google Scholar] [CrossRef]
- Olmos Grings, A.; Lora, V.; Dias Ferreira, G.; Simoni Brum, I.; von Eye Corleta, H.; Capp, E. Protein expression of estrogens receptors α and β and aromatase in myometrium and uterine leiomyoma. Gynecol. Obstet. Investig. 2012, 73, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Barbarisi, A.; Petillo, O.; di Lieto, A.; Melone, M.A.; Margarucci, S.; Cannas, M.; Peluso, G. 17-β estradiol elicits an autocrine leiomyoma cell proliferation: Evidence for a stimulation of protein kinase-dependent pathway. J. Cell. Physiol. 2001, 186, 414–424. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Protic, O.; Stortoni, P.; Grechi, G.; Lamanna, P.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil. Steril. 2013, 100, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Wang, Z.; Shi, Z.; Li, D.; Wang, Y.; Zhu, Y.; Lin, W.; Gui, Y.; Zheng, X.L. Differential expression of G-protein-coupled estrogen receptor-30 in human myometrial and uterine leiomyoma smooth muscle. Fertil. Steril. 2013, 99, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Borahay, M.A.; Al-Hendy, A.; Kilic, G.S.; Boehning, D. Signalling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Mol. Med. 2015, 21, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.; Hernández-Losa, J.; Lyons, R.J.; Castellone, M.D.; Gutkind, J.S. Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J. Biol. Chem. 2005, 280, 35081–35084. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A. The Biology of Cancer; Garland Science: New York, NY, USA, 2007. [Google Scholar]
- Yu, L.; Saile, K.; Swartz, C.D.; He, H.; Zheng, X.; Kissling, G.E.; Di, X.; Lucas, S.; Robboy, S.J.; Dixon, D. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol. Med. 2008, 14, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Nierth-Simpson, E.N.; Martin, M.M.; Chiang, T.C.; Melnik, L.I.; Rhodes, L.V.; Muir, S.E.; Burow, M.E.; McLachlan, J.A. Human uterine smooth muscle and leiomyoma cells differ in their rapid 17β-estradiol signalling: Implications for proliferation. Endocrinology 2009, 150, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, M.J.; Philipp, E.E. Cancer of the Ovary. In The History of Obstetrics and Gynaecology; Parthenon Publishing: New York, NY, USA, 1994; Volume 9, pp. 581–592. [Google Scholar]
- Kawaguchi, K.; Fujii, S.; Konishi, I.; Nanbu, Y.; Nonogaki, H.; Mori, T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am. J. Obstet. Gynecol. 1989, 160, 637–641. [Google Scholar] [CrossRef]
- Brandon, D.D.; Bethea, C.L.; Strawn, E.Y.; Novy, M.J.; Burry, K.A.; Harrington, M.S.; Erickson, T.E.; Warner, C.; Keenan, E.J.; Clinton, G.M. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am. J. Obstet. Gynecol. 1993, 169, 78–85. [Google Scholar] [CrossRef]
- Murphy, A.A.; Kettel, L.M.; Morales, A.J.; Roberts, V.J.; Yen, S.S. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J. Clin. Endocrinol. Metab. 1993, 76, 513–517. [Google Scholar] [PubMed]
- Eisinger, S.H.; Meldrum, S.; Fiscella, K.; le Roux, H.D.; Guzick, D.S. Low-dose mifepristone for uterine leiomyomata. Obstet. Gynecol. 2003, 101, 243–250. [Google Scholar] [PubMed]
- Williams, A.R.; Critchley, H.O.; Osei, J.; Ingamells, S.; Cameron, I.T.; Han, C.; Chwalisz, K. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum. Reprod. 2007, 22, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Nisolle, M.; Gillerot, S.; Casanas-Roux, F.; Squifflet, J.; Berliere, M.; Donnez, J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum. Reprod. 1999, 14, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Englund, K.; Blanck, A.; Gustavsson, I.; Lundkvist, U.; Sjöblom, P.; Norgren, A.; Lindblom, B. Sex steroid receptors in human myometrium and fibroids: Changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J. Clin. Endocrinol. Metab. 1998, 83, 4092–4096. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, A.; Reis, F.M.; Lee, M.H.; Jiang, B.; Tosti, C.; Centini, G.; Shen, F.R.; Chen, Y.G.; Petraglia, F. Increased progesterone receptor expression in uterine leiomyoma: Correlation with age, number of leiomyomas, and clinical symptoms. Fertil. Steril. 2015, 104, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Ellmann, S.; Sticht, H.; Thiel, F.; Beckmann, M.W.; Strick, R.; Strissel, P.L. Estrogen and progesterone receptors: From molecular structures to clinical targets. Cell. Mol. Life Sci. 2009, 66, 2405–2426. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoven, E.; Wissink, S.; van der Saag, P.T.; van der Burg, B. Negative interaction between the RelA(p65) subunit of NF-λB and the progesterone receptor. J. Biol. Chem. 1996, 271, 6217–6224. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, A.M.; Bamberger, C.M.; Gellersen, B.; Schulte, H.M. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc. Natl. Acad. Sci. USA 1996, 93, 6169–6174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Buzzio, O.L.; Li, S.; Lu, Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: Interaction with progesterone receptor. Biol. Reprod. 2005, 73, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, A.V.; Sefton, E.C.; Berry, E.; Lu, Z.; Hardt, J.; Marsh, E.; Yin, P.; Clardy, J.; Chakravarti, D.; Bulun, S.; Kim, J.J. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J. Clin. Endocrinol. Metab. 2009, 94, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Sefton, E.C. The role of progesterone signalling in the pathogenesis of uterine leiomyoma. Mol. Cell. Endocrinol. 2012, 358, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Boonyaratanakornkit, V.; Scott, M.P.; Ribon, V.; Sherman, L.; Anderson, S.M.; Maller, J.L.; Miller, W.T.; Edwards, D.P. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol. Cell 2001, 8, 269–280. [Google Scholar] [CrossRef]
- Shimomura, Y.; Matsuo, H.; Samoto, T.; Maruo, T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J. Clin. Endocrinol. Metab. 1998, 83, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Nakago, S.; Kurachi, O.; Wang, J.; Takekida, S.; Matsuo, H.; Maruo, T. Progesterone down-regulates insulin-like growth factor-I expression in cultured human uterine leiomyoma cells. Hum. Reprod. 2004, 19, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Zhao, Y.; Tarnuzzer, R.W.; Rong, H.; Williams, R.S.; Schultz, G.S.; Chegini, N. Suppression of transforming growth factor-β (TGFβ) and TGFβ receptor messenger ribonucleic acid and protein expression in leiomyomata in women receiving gonadotropin-releasing hormone agonist therapy. J. Clin. Endocrinol. Metab. 1996, 81, 3222–3230. [Google Scholar] [PubMed]
- Lee, B.S.; Nowak, R.A. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-β3 (TGFβ3) and altered responses to the antiproliferative effects of TGFβ. J. Clin. Endocrinol. Metab. 2001, 86, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A. hCG, the wonder of today’s science. Reprod. Biol. Endocrinol. 2012, 28. [Google Scholar] [CrossRef] [PubMed]
- Wide, L.; Lee, J.Y.; Rasmussen, C. A change in the isoforms of human chorionic gonadotropin occurs around the 13th week of gestation. J. Clin. Endocrinol. Metab. 1994, 78, 1419–1423. [Google Scholar] [PubMed]
- Maruo, T.; Matsuo, H.; Ohtani, T.; Hoshina, M.; Mochizuki, M. Differential modulation of chorionic gonadotropin (CG) subunit messenger ribonucleic acid levels and CG secretion by progesterone in normal placenta and choriocarcinoma cultured in vitro. Endocrinology 1986, 119, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Saji, M.; Hidaka, A.; Moriya, N.; Okuno, A.; Kohn, L.D.; Cutler, G.B., Jr. A new constitutively activating point mutation in the luteinizing hormone/choriogonadotropin receptor gene in cases of male-limited precocious puberty. J. Clin. Endocrinol. Metab. 1995, 80, 1162–1168. [Google Scholar] [PubMed]
- Lashansky, G.; Saenger, P.; Fishman, K.; Gautier, T.; Mayes, D.; Berg, G.; di Martino-Nardi, J.; Reiter, E. Normative data for adrenal steroidogenesis in a healthy pediatric population: Age- and sex-related changes after adrenocorticotropin stimulation. J. Clin. Endocrinol. Metab. 1991, 73, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Ziecik, A.J.; Stanchev, P.D.; Tilton, J.E. Evidence for the presence of luteinizing hormone/human chorionic gonadotropin-binding sites in the porcine uterus. Endocrinology 1986, 119, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.D.; Odell, W.D. Identification of LH/hCG receptors in rabbit uterus. Proc. Soc. Exp. Biol. Med. 1988, 189, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Reshef, E.; Lei, Z.M.; Rao, C.V.; Pridham, D.D.; Chegini, N.; Luborsky, J.L. The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua. J. Clin. Endocrinol. Metab. 1990, 70, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Környei, J.L.; Lei, Z.M.; Rao, C.V. Human myometrial smooth muscle cells are novel targets of direct regulation by human chorionic gonadotropin. Biol. Reprod. 1993, 49, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Mizrachi, D.; Gurevich, M.; Shore, L.S.; Reed, J.; Chang, S.M.; Thatcher, W.W.; Fields, M.J. Expression of functional luteinizing hormone (LH) receptor and its messenger ribonucleic acid in bovine endometrium: LH augmentation of cAMP and inositol phosphate in vitro and human chorionic gonadotropin (hCG) augmentation of peripheral prostaglandin in vivo. Reprod. Biol. 2001, 1, 13–32. [Google Scholar] [PubMed]
- Ziecik, A.J.; Kaczmarek, M.M.; Blitek, A.; Kowalczyk, A.E.; Li, X.; Rahman, N.A. Novel biological and possible applicable roles of LH/hCG receptor. Mol. Cell. Endocrinol. 2007, 269, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Stepien, A.; Shemesh, M.; Ziecik, A.J. Luteinising hormone receptor kinetic and LH-induced prostaglandin production throughout the oestrous cycle in porcine endometrium. Reprod. Nutr. Dev. 1999, 39, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Gawronska, B.; Stepien, A.; Ziecik, A.J. Effect of estradiol and progesterone on oviductal LH-receptors and LH-dependent relaxation of the porcine oviduct. Theriogenology 2000, 53, 659–672. [Google Scholar] [CrossRef]
- Singh, M.; Zuo, J.; Li, X.; Ambrus, G.; Lei, Z.M.; Yussman, M.A.; Sanfilippo, J.S.; Rao, C.V. Decreased expression of functional human chorionic gonadotropin/luteinizing hormone receptor gene in human uterine leiomyomas. Biol. Reprod. 1995, 53, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, A.; Nikaido, T.; Yoshizawa, T.; Itoh, K.; Kobayashi, Y.; Toki, T.; Konishi, I.; Fujii, S. HCG promotes proliferation of uterine leiomyomal cells more strongly than that of myometrial smooth muscle cells in vitro. Mol. Hum. Reprod. 2000, 6, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Smitz, J. Luteinizing hormone and human chorionic gonadotropin: Origins of difference. Mol. Cell. Endocrinol. 2014, 383, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Bailey, J.; Robson, S.C.; Europe-Finner, G.N. Differential expression of the adenylyl cyclase-stimulatory guanosine triphosphate-binding protein Gsα in the human myometrium during pregnancy and labor involves transcriptional regulation by cyclic adenosine 3′,5′-monophosphate and binding of phosphorylated nuclear proteins to multiple GC boxes within the promoter. J. Clin. Endocrinol. Metab. 2002, 87, 5675–5685. [Google Scholar] [PubMed]
- Bailey, J.; Phillips, R.J.; Pollard, A.J.; Gilmore, K.; Robson, S.C.; Europe-Finner, G.N. Characterization and functional analysis of cAMP response element modulator protein and activating transcription factor 2 (ATF2) isoforms in the human myometrium during pregnancy and labor: Identification of a novel ATF2 species with potent transactivation properties. J. Clin. Endocrinol. Metab. 2002, 87, 1717–1728. [Google Scholar] [PubMed]
- Stewart, E.A.; Floor, A.E.; Jain, P.; Nowak, R.A. Increased expression of messenger RNA for collagen type I, collagen type III, and fibronectin in myometrium of pregnancy. Obstet. Gynecol. 1995, 86, 417–422. [Google Scholar] [CrossRef]
- Nohara, A.; Ohmichi, M.; Koike, K.; Jikihara, H.; Kimura, A.; Masuhara, K.; Ikegami, H.; Inoue, M.; Miyake, A.; Murata, Y. Prolactin stimulates mitogen-activated protein kinase in human leiomyoma cells. Biochem. Biophys. Res. Commun. 1997, 238, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Sherr, C.J. Interaction of D-type cyclins with a novel myb-like transcription factor, DMP1. Mol. Cell. Biol. 1996, 16, 6457–6467. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Dobashi, Y.; Minehara, H.; Itoman, M.; Kameya, T. Involvement of cyclins in cell proliferation and their clinical implications in soft tissue smooth muscle tumors. Am. J. Pathol. 2000, 156, 2135–2147. [Google Scholar] [CrossRef]
- Rein, M.S.; Friedman, A.J.; Heffner, L.J. Decreased prolactin secretion by explant cultures of fibroids from women treated with a gonadotropin-releasing hormone agonist. J. Clin. Endocrinol. Metab. 1990, 70, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.A.; Rein, M.S.; Heffner, L.J.; Friedman, A.J.; Tashjian, A.H., Jr. Production of prolactin by smooth muscle cells cultured from human uterine fibroid tumors. J. Clin. Endocrinol. Metab. 1993, 76, 1308–1313. [Google Scholar] [PubMed]
- Santen, R.J.; Bardin, C.W. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J. Clin. Investig. 1973, 52, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Birken, S.; Maydelman, Y.; Gawinowicz, M.A.; Pound, A.; Liu, Y.; Hartree, A.S. Isolation and characterization of human pituitary chorionic gonadotropin. Endocrinology 1996, 137, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Galet, C.; Ascoli, M. The differential binding affinities of the luteinizing hormone (LH)/choriogonadotropin receptor for LH and choriogonadotropin are dictated by different extracellular domain residues. Mol. Endocrinol. 2005, 195, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Ascoli, M.; Fanelli, F.; Segaloff, D.L. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 2002, 23, 141–174. [Google Scholar] [CrossRef] [PubMed]
- Segaloff, D.L.; Ascoli, M. The Lutropin/Choriogonadotropin Receptor… 4 Years Later. Endocr. Rev. 1993, 14, 324–347. [Google Scholar] [PubMed]
- Casarini, L.; Lispi, M.; Longobardi, S.; Milosa, F.; La Marca, A.; Tagliasacchi, D.; Pignatti, E.; Simoni, M. LH and hCG Action on the Same Receptor Results in Quantitatively and Qualitatively Different Intracellular Signalling. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Chapekar, T.; Chhabra, Y.; Singh, P.; Sinha, S.; Luthra, K. Differential response to sustained stimulation by hCG & LH on goat ovarian granulosa cells. Indian J. Med. Res. 2014, 135, 331–340, Erratum in Indian J. Med. Res. 2014, 140, 700. [Google Scholar]
- Choi, J.; Smitz, J. Luteinizing hormone and human chorionic gonadotropin: Distinguishing unique physiologic roles. Gynecol. Endocrinol. 2014, 30, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Kesner, J.S.; Dunson, D.B. Luteinizing hormone in premenopausal women may stimulate uterine leiomyomata development. J. Soc. Gynecol. Investig. 2006, 13, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.A.; Haymond, S.; Parvin, C.A.; Gronowski, A.M.; Grenache, D.G. Diagnostic considerations in the measurement of human chorionic gonadotropin in aging women. Clin. Chem. 2005, 51, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Ciavattini, A.; Giannubilo, S.R.; Lamanna, P.; Fiorini, R.; Tranquilli, A.L.; Christman, G.M.; Castellucci, M. Management of leiomyomas in perimenopausal women. Maturitas 2014, 78, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Wrana, J.L.; Attisano, L.; Wieser, R.; Ventura, F.; Massagué, J. Mechanism of activation of the TGF-β receptor. Nature 1994, 370, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Attisano, L.; Wrana, J.L.; López-Casillas, F.; Massagué, J. TGF-β receptors and actions. Biochim. Biophys. Acta 1994, 1222, 71–80. [Google Scholar] [CrossRef]
- Andres, J.L.; Stanley, K.; Cheifetz, S.; Massagué, J. Membrane-anchored and soluble forms of betaglycan, a polymorphic proteoglycan that binds transforming growth factor-β. J. Cell. Biol. 1989, 109, 3137–3145. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.M.; Dou, Q.; Zhao, Y.; McLean, F.; Davis, J.; Chegini, N. The expression of transforming growth factor-β s and TGF-β receptor mRNA and protein and the effect of TGF-β s on human myometrial smooth muscle cells in vitro. Mol. Hum. Reprod. 1997, 3, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Vollenhoven, B.J.; Herington, A.C.; Healy, D.L. Epidermal growth factor and transforming growth factor-β in uterine fibroids and myometrium. Gynecol. Obstet. Investig. 1995, 40, 120–124. [Google Scholar] [CrossRef]
- Arslan, A.A.; Gold, L.I.; Mittal, K.; Suen, T.C.; Belitskaya-Levy, I.; Tang, M.S.; Toniolo, P. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: New evidence and a systematic review. Hum. Reprod. 2005, 20, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Quade, B.J.; Wang, T.Y.; Sornberger, K.; dal Cin, P.; Mutter, G.L.; Morton, C.C. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosom. Cancer 2004, 40, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N.; Luo, X.; Ding, L.; Ripley, D. The expression of Smads and transforming growth factor β receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol. Cell. Endocrinol. 2003, 209, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.B.; Yu, L.; Swartz, C.D.; Zheng, X.; Wang, L.; Castro, L.; Kissling, G.E.; Walmer, D.K.; Robboy, S.J.; Dixon, D. Human uterine leiomyoma-derived fibroblasts stimulate uterine leiomyoma cell proliferation and collagen type I production, and activate RTKs and TGF β receptor signalling in coculture. Cell Commun. Signal. 2010, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, X.; Chegini, N. Differential expression, regulation, and induction of Smads, transforming growth factor-β signal transduction pathway in leiomyoma, and myometrial smooth muscle cells and alteration by gonadotropin-releasing hormone analog. J. Clin. Endocrinol. Metab. 2003, 88, 1350–1361, Retraction in: J. Clin. Endocrinol. Metab. 2015, doi:10.1210/jc.2014-4201. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Souchelnytskyi, S.; Heldin, C.H. Smad regulation in TGF-β signal transduction. J. Cell. Sci. 2001, 114, 4359–4369. [Google Scholar] [PubMed]
- Norian, J.M.; Malik, M.; Parker, C.Y.; Joseph, D.; Leppert, P.C.; Segars, J.H.; Catherino, W.H. Transforming growth factor β3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod. Sci. 2009, 16, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Chiu, P.C.; Hautala, L.; Salo, T.; Yeung, W.S.; Stenman, U.H.; Koistinen, H. Human chorionic gonadotropin and its free β-subunit stimulate trophoblast invasion independent of LH/hCG receptor. Mol. Cell. Endocrinol. 2013, 375, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Fournier, T.; Guibourdenche, J.; Evain-Brion, D. hCGs: Different sources of production, different glycoforms and functions. Placenta 2015, 36, S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A.; Butler, S. Hyperglycosylated hCG, hCGβ and Hyperglycosylated hCGβ: Interchangeable cancer promoters. Mol. Cell. Endocrinol. 2012, 349, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.A.; Ikram, M.S.; Mathieu, S.; Iles, R.K. The increase in bladder carcinoma cell population induced by the free β subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br. J. Cancer 2000, 82, 1553–1556. [Google Scholar] [PubMed]
- Berndt, S.; Blacher, S.; Munaut, C.; Detilleux, J.; Perrier d’Hauterive, S.; Huhtaniemi, I.; Evain-Brion, D.; Noël, A.; Fournier, T.; Foidart, J.M. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-β receptor activation. FASEB J. 2013, 27, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Lapthorn, A.J.; Harris, D.C.; Littlejohn, A.; Lustbader, J.W.; Canfield, R.E.; Machin, K.J.; Morgan, F.J.; Isaacs, N.W. Crystal structure of human chorionic gonadotropin. Nature 1994, 369, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Bläuer, M.; Rovio, P.H.; Ylikomi, T.; Heinonen, P.K. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil. Steril. 2009, 91, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Sharan, C.; Halder, S.K.; Thota, C.; Jaleel, T.; Nair, S.; Al-Hendy, A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil. Steril. 2011, 95, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 reduces TGF-β3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Sharan, C.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol. Reprod. 2012, 86, 116. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Halder, S.K.; Allah, A.S.; Roshdy, E.; Rajaratnam, V.; Al-Hendy, A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Womens Health 2013, 5, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Hill, M.C.; Schectman, J.M.; Hollis, B.W. Vitamin D and the risk of uterine fibroids. Epidemiology 2013, 24, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Paffoni, A.; Somigliana, E.; Vigano’, P.; Benaglia, L.; Cardellicchio, L.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Fedele, L. Vitamin D status in women with uterine leiomyomas. J. Clin. Endocrinol. Metab. 2013, 98, E1374–E1378. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Aguirre, A.; August, A.M.; Golos, T.G.; Kao, L.C.; Sakuragi, N.; Kliman, H.J.; Strauss, J.F., 3rd. 8-Bromo-adenosine 3′,5′-monophosphate regulates expression of chorionic gonadotropin and fibronectin in human cytotrophoblasts. J. Clin. Endocrinol. Metab. 1987, 64, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Lindell, C.M. Isolation and characterization of the human chorionic gonadotropin β subunit (CGβ) gene cluster: Regulation of transcriptionally active CG β gene by cyclic AMP. Mol. Cell. Biol. 1988, 8, 5100–5107. [Google Scholar] [CrossRef] [PubMed]
- Barrera, D.; Avila, E.; Hernández, G.; Méndez, I.; González, L.; Halhali, A.; Larrea, F.; Morales, A.; Díaz, L. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod. Biol. Endocrinol. 2008, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Genomatix. Available online: http://www.genomatix.de/ (accessed on 20 September 2017).
- Hernández-Sánchez, C.; Werner, H.; Roberts, C.T., Jr.; Woo, E.J.; Hum, D.W.; Rosenthal, S.M.; LeRoith, D. Differential regulation of insulin-like growth factor-I (IGF-I) receptor gene expression by IGF-I and basic fibroblastic growth factor. J. Biol. Chem. 1997, 272, 4663–4670. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.T., Jr. Control of insulin-like growth factor (IGF) action by regulation of IGF-I receptor expression. Endocr. J. 1996, 43, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Ghahary, A.; Murphy, L.J. Uterine insulin-like growth factor-I receptors: Regulation by estrogen and variation throughout the estrous cycle. Endocrinology 1989, 125, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Ghahary, A.; Chakrabarti, S.; Murphy, L.J. Localization of the sites of synthesis and action of insulin-like growth factor-I in the rat uterus. Mol. Endocrinol. 1990, 4, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Tommola, P.; Pekonen, F.; Rutanen, E.M. Binding of epidermal growth factor and insulin-like growth factor I in human myometrium and leiomyomata. Obstet. Gynecol. 1989, 74, 658–662. [Google Scholar] [PubMed]
- Chandrasekhar, Y.; Heiner, J.; Osuamkpe, C.; Nagamani, M. Insulin-like growth factor I and II binding in human myometrium and leiomyomas. Am. J. Obstet. Gynecol. 1992, 166, 64–69. [Google Scholar] [CrossRef]
- Gao, Z.; Matsuo, H.; Wang, Y.; Nakago, S.; Maruo, T. Up-regulation by IGF-I of proliferating cell nuclear antigen and Bcl-2 protein expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2001, 86, 5593–5599. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, K.D.; Howe, S.R.; Okubo, Y.; Fuchs-Young, R.; LeRoith, D.; Walker, C.L. Dysregulation of IGF-I signalling in uterine leiomyoma. J. Endocrinol. 2002, 172, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Iwase, A.; Harata, T.; Takigawa, S.; Suzuki, K.; Manabe, S.; Kikkawa, F. IGF1-induced AKT phosphorylation and cell proliferation are suppressed with the increase in PTEN during luteinization in human granulosa cells. Reproduction 2009, 137, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Oon, V.J.; Johnson, M.R. The regulation of the human corpus luteum steroidogenesis: A hypothesis? Hum. Reprod. Update 2000, 6, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, S.K.; Herring, A.H.; Savitz, D.A.; Olshan, A.F.; Fielding, J.R.; Hartmann, K.E.; Baird, D.D. Pregnancy-related fibroid reduction. Fertil. Steril. 2010, 94, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, S.K.; Hartmann, K.E.; Baird, D.D. Postpartum factors and natural fibroid regression. Am. J. Obstet. Gynecol. 2011, 204. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Somigliana, E.; Oneda, S.; Ossola, M.W.; Acaia, B.; Fedele, L. Decidualized ovarian endometriosis in pregnancy: A challenging diagnostic entity. Hum. Reprod. 2009, 24, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Enomoto, T.; Miyatake, T.; Fujita, M.; Yamamoto, R.; Kanagawa, T.; Shimizu, H.; Kimura, T. A retrospective analysis of ovarian endometriosis during pregnancy. Fertil. Steril. 2010, 94, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Benaglia, L.; Somigliana, E.; Calzolari, L.; Busnelli, A.; Cardellicchio, L.; Ragni, G.; Fedele, L. The vanishing endometrioma: The intriguing impact of pregnancy on small endometriotic ovarian cysts. Gynecol. Endocrinol. 2013, 29, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Vimal, M.V.; Budyal, S.; Kasliwal, R.; Jagtap, V.S.; Lila, A.R.; Bandgar, T.; Menon, P.; Shah, N.S. Vanishing tumor in pregnancy. Indian J. Endocrinol. Metab. 2012, 16, 1043–1046. [Google Scholar] [PubMed]
| Author, Year | n of Cases | I Trimester (%) | II Trimester (%) | III Trimester (%) | |
|---|---|---|---|---|---|
| Lev-Toaff et al. (1987) [20] | 71 | + | + (30); − (15) for small fibroids/* + (14); − (48) for large fibroids | − (35) for small fibroids/* − (59) for large fibroids | |
| Aharoni et al. (1988) [21] | 29 | NR | NS | NS | |
| Rosati et al. (1992) [22] | 36 | + (32) * | NS | NS | |
| Neiger et al. (2006) [16] | 72 | NR | NS | NS | |
| Hammoud et al. (2006) [17] | 107 | − (55); + (45) * | − (75); + (25) * | ||
| Ozturk et al. (2009) [23] | 19 | NR | NR | NR | |
| De Vivo et al. (2011) [13] | 38 | + (71) * | + (67) * | ||
| Benaglia et al. (2014) [14] | 25 | ++ * | NR | NR | |
| Ciavattini et al. (2016) [19] | 109 | ++ * | + * | NR | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarais, V.; Cermisoni, G.C.; Schimberni, M.; Alteri, A.; Papaleo, E.; Somigliana, E.; Vigano’, P. Human Chorionic Gonadotrophin as a Possible Mediator of Leiomyoma Growth during Pregnancy: Molecular Mechanisms. Int. J. Mol. Sci. 2017, 18, 2014. https://doi.org/10.3390/ijms18092014
Sarais V, Cermisoni GC, Schimberni M, Alteri A, Papaleo E, Somigliana E, Vigano’ P. Human Chorionic Gonadotrophin as a Possible Mediator of Leiomyoma Growth during Pregnancy: Molecular Mechanisms. International Journal of Molecular Sciences. 2017; 18(9):2014. https://doi.org/10.3390/ijms18092014
Chicago/Turabian StyleSarais, Veronica, Greta Chiara Cermisoni, Matteo Schimberni, Alessandra Alteri, Enrico Papaleo, Edgardo Somigliana, and Paola Vigano’. 2017. "Human Chorionic Gonadotrophin as a Possible Mediator of Leiomyoma Growth during Pregnancy: Molecular Mechanisms" International Journal of Molecular Sciences 18, no. 9: 2014. https://doi.org/10.3390/ijms18092014
APA StyleSarais, V., Cermisoni, G. C., Schimberni, M., Alteri, A., Papaleo, E., Somigliana, E., & Vigano’, P. (2017). Human Chorionic Gonadotrophin as a Possible Mediator of Leiomyoma Growth during Pregnancy: Molecular Mechanisms. International Journal of Molecular Sciences, 18(9), 2014. https://doi.org/10.3390/ijms18092014