Corneal Fibroblasts as Sentinel Cells and Local Immune Modulators in Infectious Keratitis
Abstract
:1. Introduction
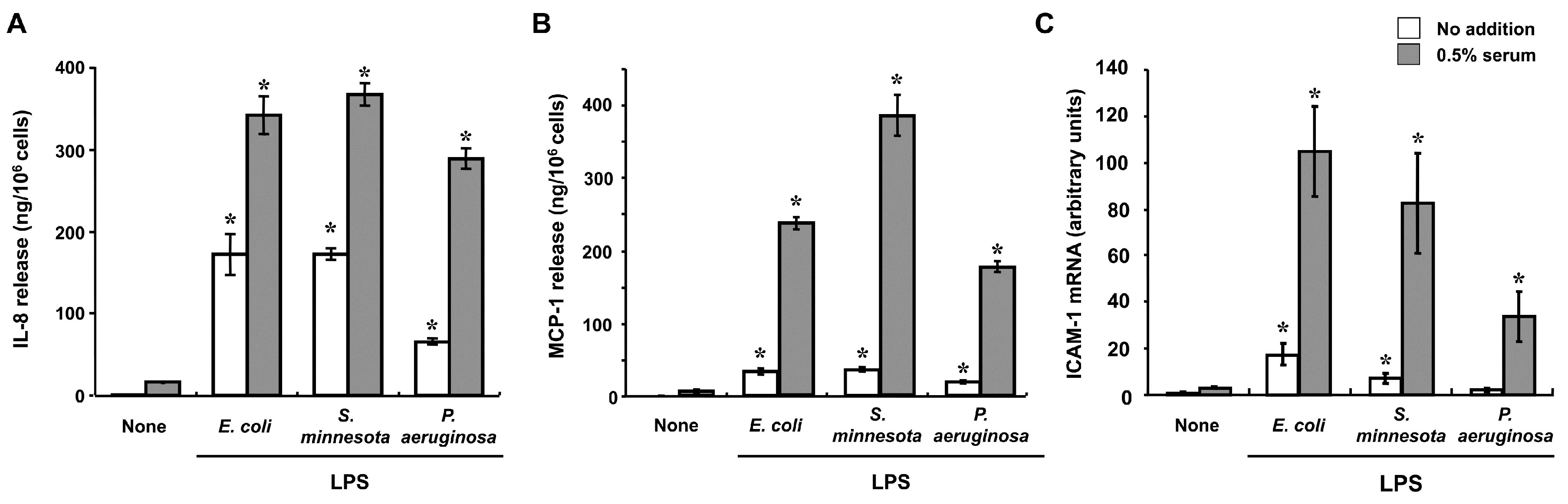
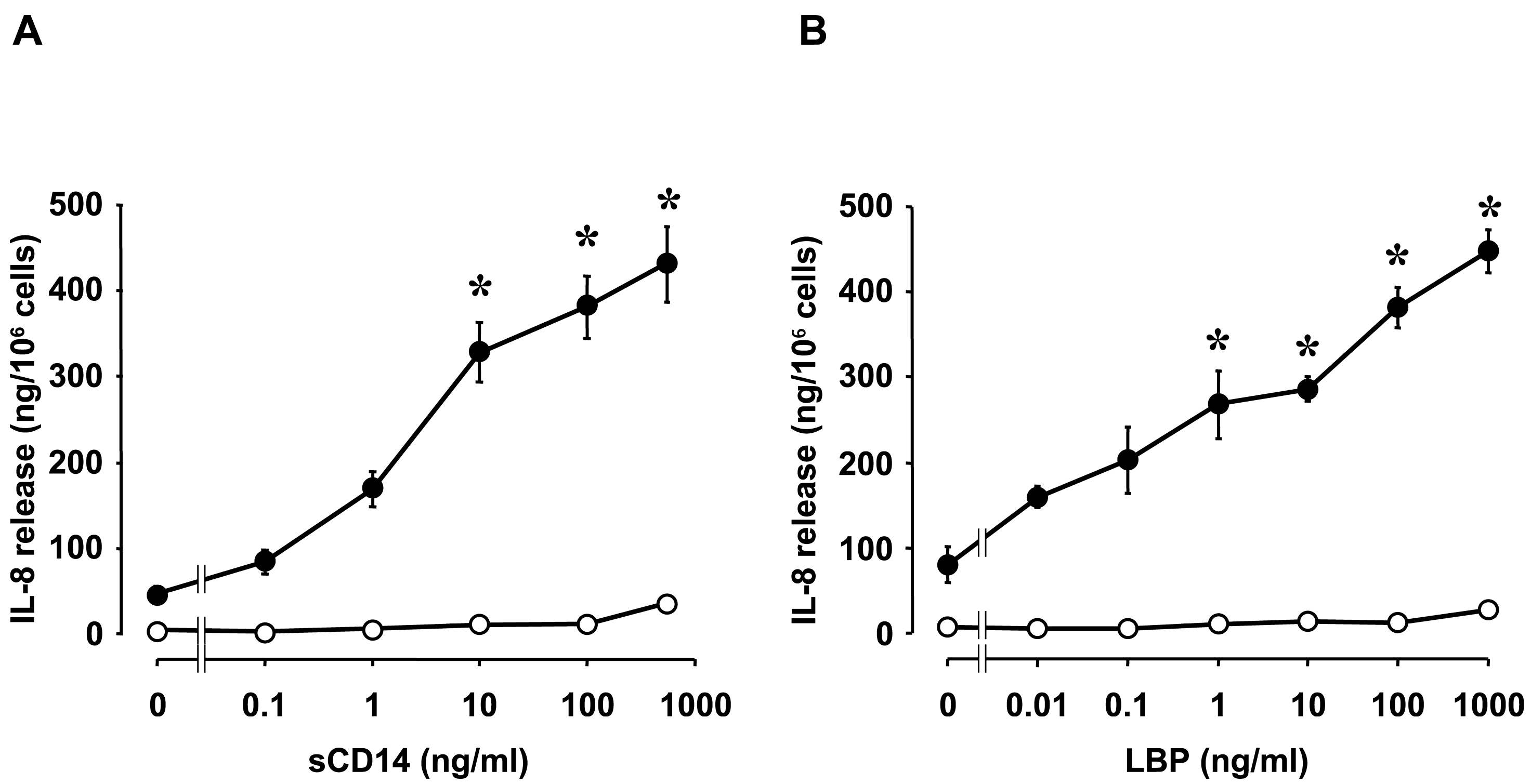
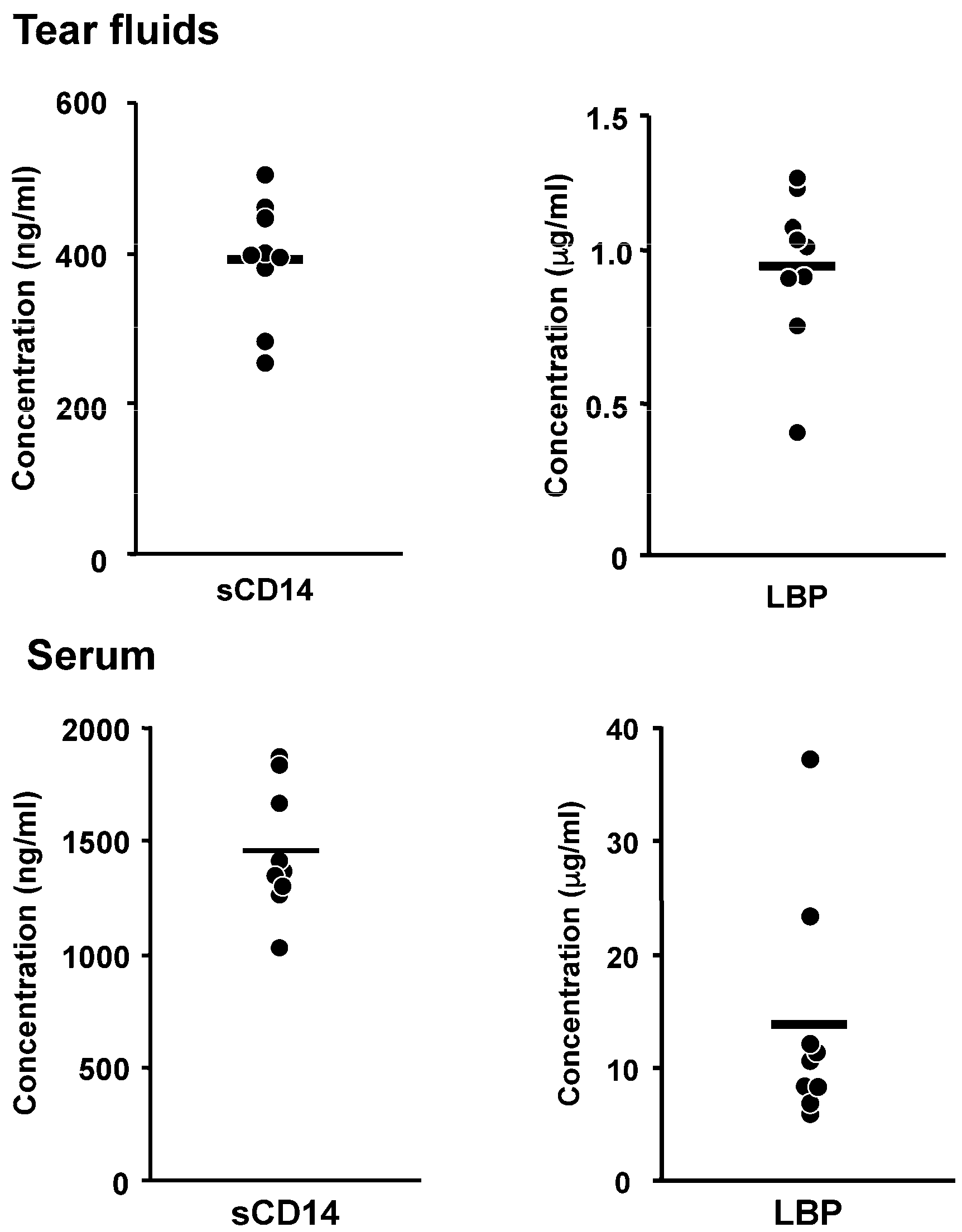
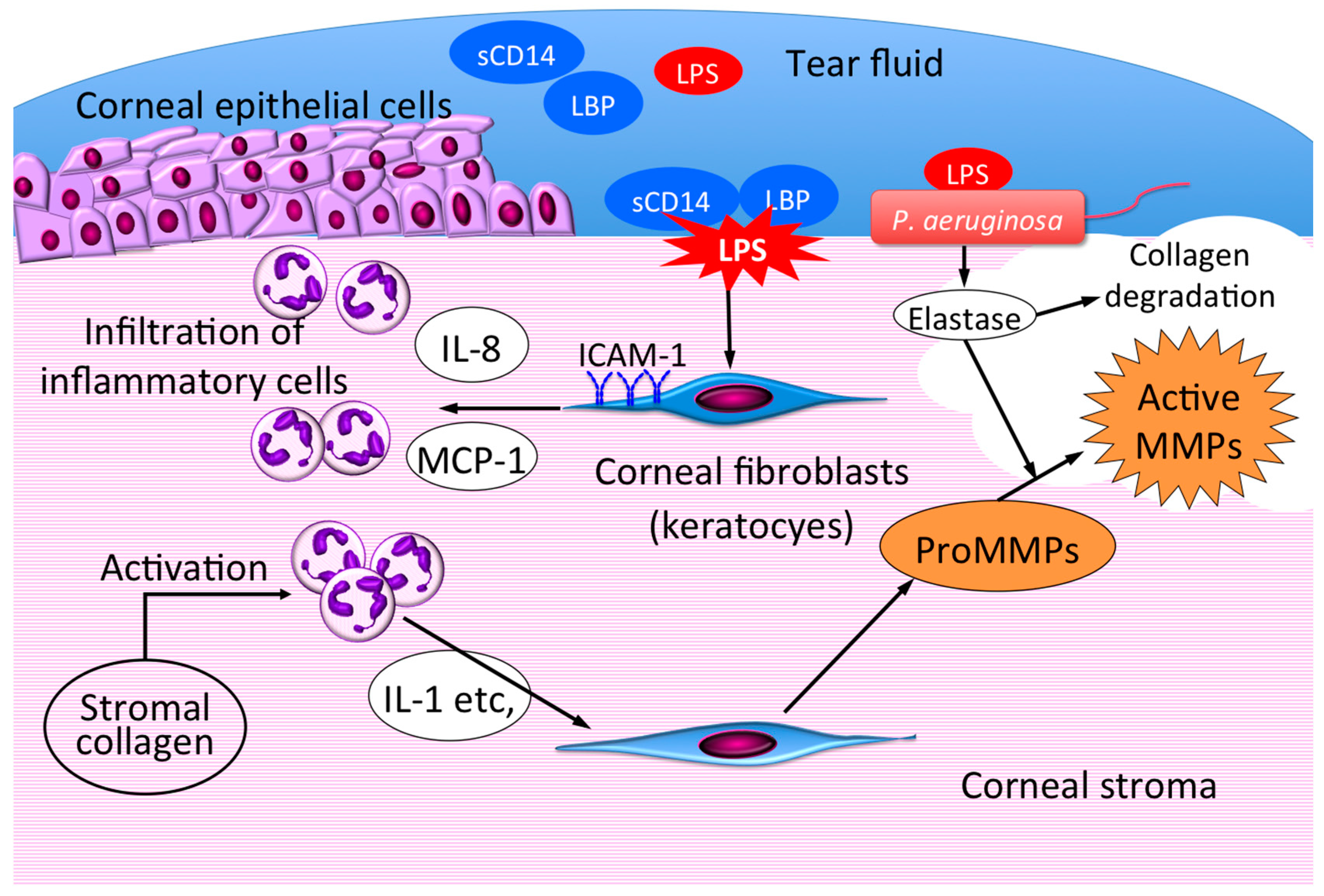
2. Innate Immune Responses to LPS in Corneal Cells
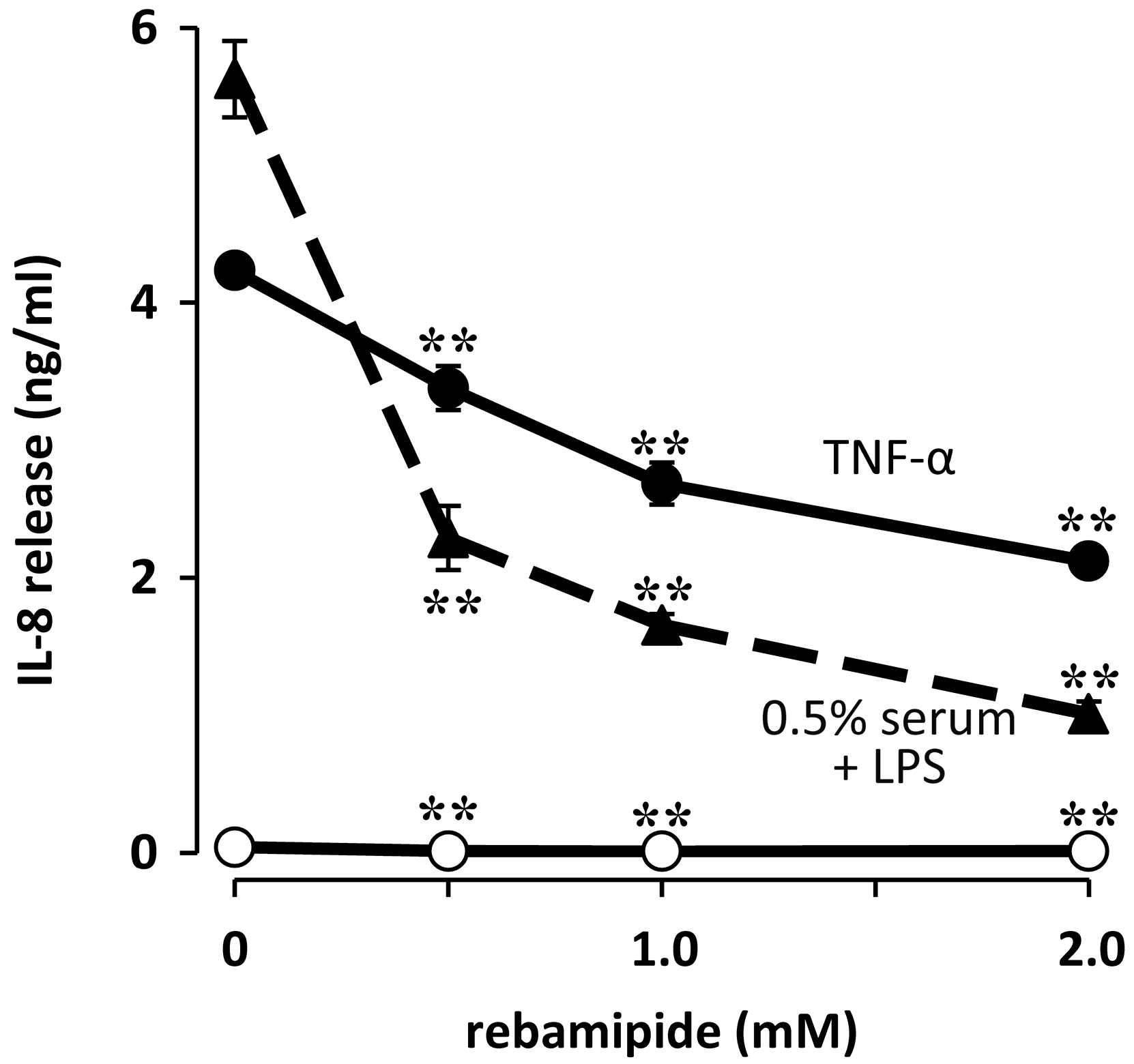
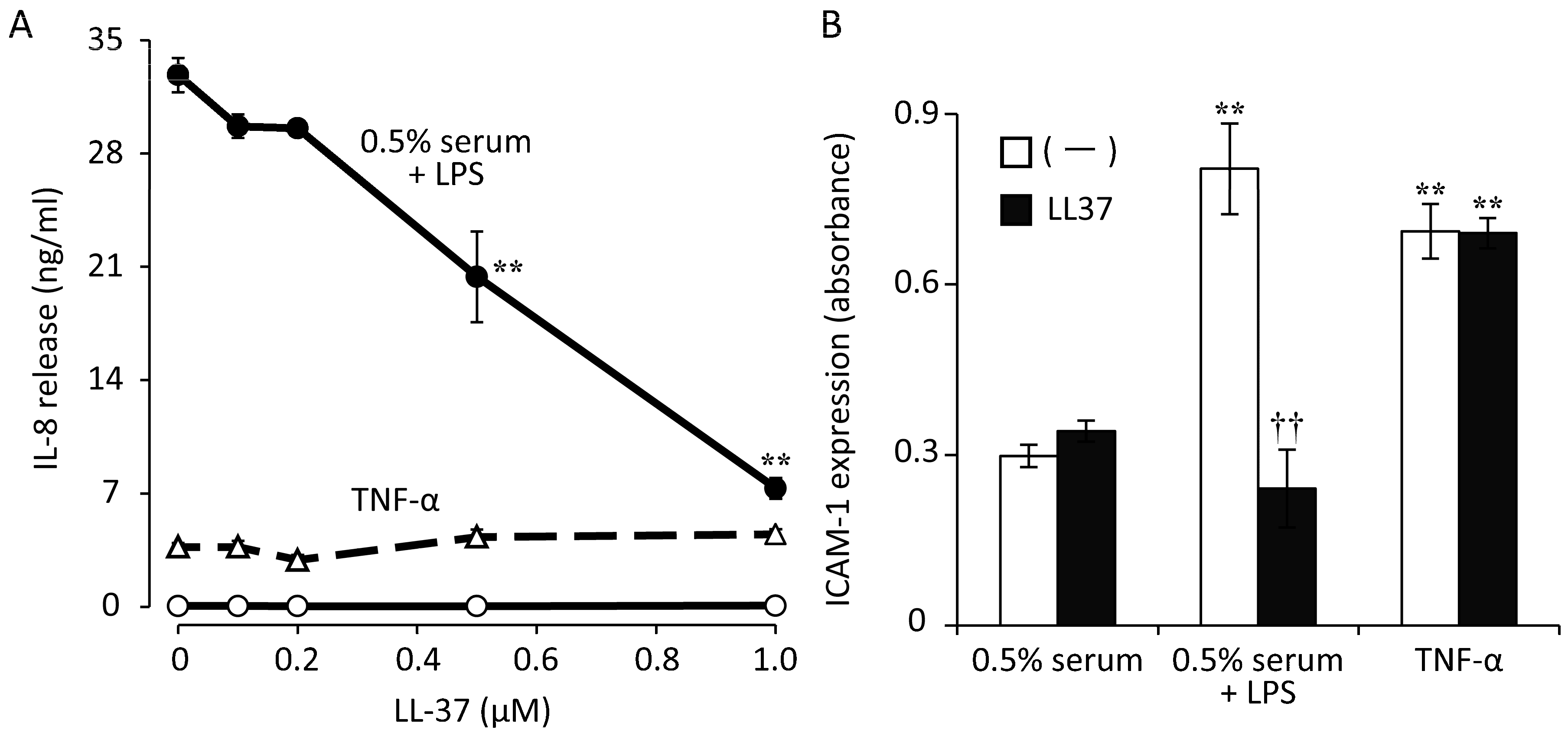
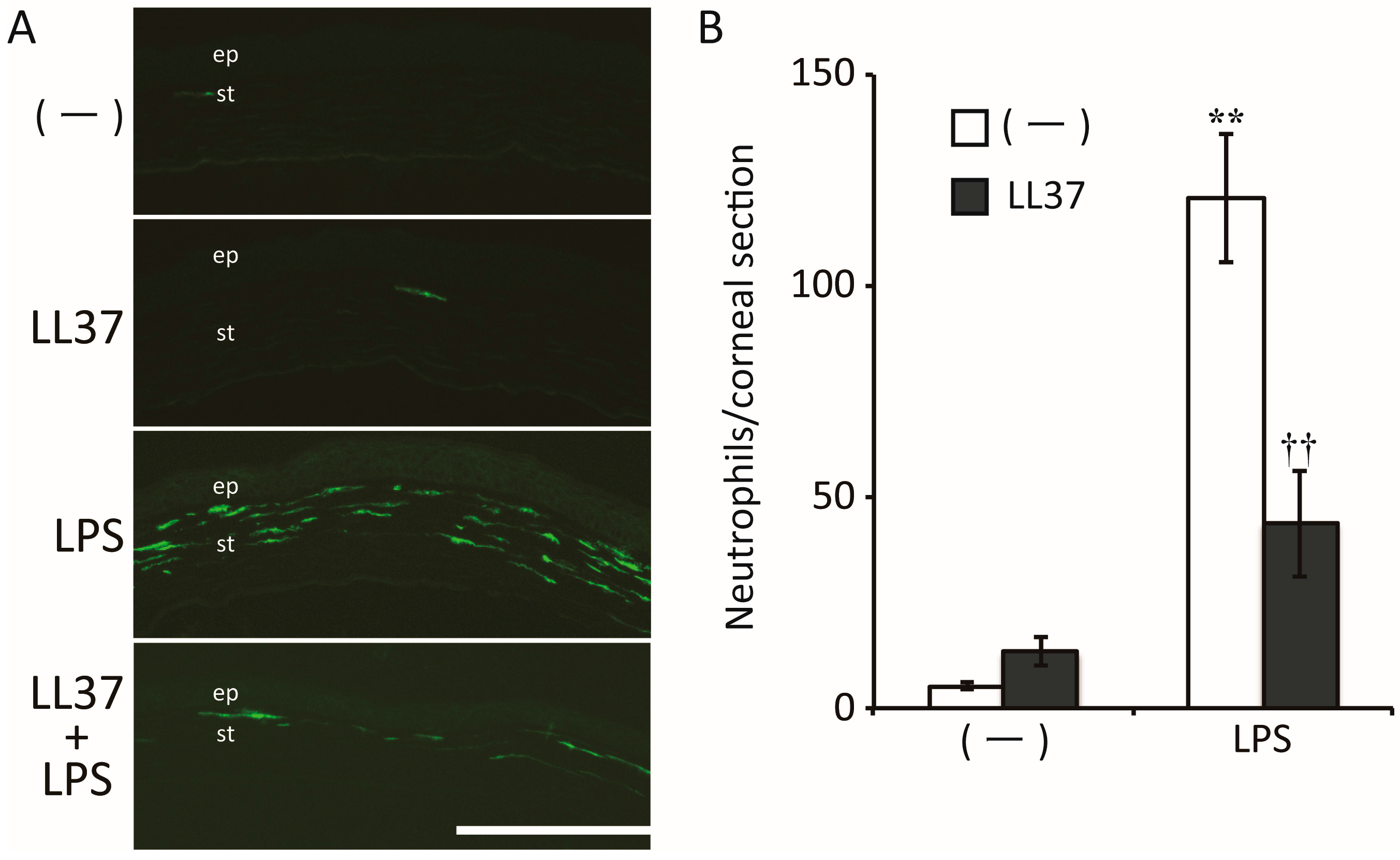
3. Therapeutic Intervention Targeting the Role of Corneal Fibroblasts in Infectious Keratitis
4. Conclusions
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| Th2 | T helper 2 |
| IL | Interleukin |
| TLR | Toll-like receptor |
| CD | Cluster of differentiation |
| MCP | Monocyte chemotactic protein |
| TNF-α | Tumor necrosis factor-α |
| ICAM-1 | Intercellular adhesion molecule-1 |
| LBP | Lipopolysaccharide binding protein |
| sCD14 | Soluble CD14 |
| mCD14 | Membrane-bound CD14 |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor-κB |
| uPA | Urokinase-type plasminogen activator |
References
- Green, M.; Apel, A.; Stapleton, F. Risk factors and causative organisms in microbial keratitis. Cornea 2008, 27, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D. Bacterial infections of the cornea (Pseudomonas aeruginosa). Chem. Immunol. Allergy 2007, 92, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Pachigolla, G.; Blomquist, P.; Cavanagh, H.D. Microbial keratitis pathogens and antibiotic susceptibilities: A 5-year review of cases at an urban county hospital in north Texas. Eye Contact Lens 2007, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, E.; Sun, Y.; Roy, S.; Karmakar, M.; Hise, A.G.; Szczotka-Flynn, L.; Ghannoum, M.; Chinnery, H.R.; McMenamin, P.G.; Rietsch, A. Host defense at the ocular surface. Int. Rev. Immunol. 2013, 32, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kumagai, N.; Fujitsu, Y.; Nishida, T. Fibroblasts as local immune modulators in ocular allergic disease. Allergol. Int. 2006, 55, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, N.; Fukuda, K.; Fujitsu, Y.; Yamamoto, K.; Nishida, T. Role of structural cells of the cornea and conjunctiva in the pathogenesis of vernal keratoconjunctivitis. Prog. Retin. Eye Res. 2006, 25, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T. Commanding roles of keratocytes in health and disease. Cornea 2010, 29 (Suppl. S1), S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Fujitsu, Y.; Kumagai, N.; Nishida, T. Characterization of the interleukin-4 receptor complex in human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2002, 43, 183–188. [Google Scholar]
- Fukuda, K.; Nishida, T.; Fukushima, A. Synergistic induction of eotaxin and VCAM-1 expression in human corneal fibroblasts by staphylococcal peptidoglycan and either IL-4 or IL-13. Allergol. Int. 2011, 60, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, N.; Fukuda, K.; Ishimura, Y.; Nishida, T. Synergistic induction of eotaxin expression in human keratocytes by TNF-α and IL-4 or IL-13. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1448–1453. [Google Scholar]
- Fukuda, K.; Fujitsu, Y.; Seki, K.; Kumagai, N.; Nishida, T. Differential expression of thymus- and activation-regulated chemokine (CCL17) and macrophage-derived chemokine (CCL22) by human fibroblasts from cornea, skin, and lung. J. Allergy Clin. Immunol. 2003, 111, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, N.; Fukuda, K.; Fujitsu, Y.; Nishida, T. Synergistic effect of TNF-α and either IL-4 or IL-13 on VCAM-1 expression by cultured human corneal fibroblasts. Cornea 2003, 22, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Tlr4: Central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 2000, 12, 20–26. [Google Scholar] [CrossRef]
- Song, P.I.; Abraham, T.A.; Park, Y.; Zivony, A.S.; Harten, B.; Edelhauser, H.F.; Ward, S.L.; Armstrong, C.A.; Ansel, J.C. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2867–2877. [Google Scholar]
- Ueta, M.; Nochi, T.; Jang, M.H.; Park, E.J.; Igarashi, O.; Hino, A.; Kawasaki, S.; Shikina, T.; Hiroi, T.; Kinoshita, S.; et al. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J. Immunol. 2004, 173, 3337–3347. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.L.; Buret, A.G.; Olson, M.E.; Ceri, H.; Read, R.R.; Morck, D.W. Lipopolysaccharide entry in the damaged cornea and specific uptake by polymorphonuclear neutrophils. Infect. Immun. 2000, 68, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Howes, E.L.; Cruse, V.K.; Kwok, M.T. Mononuclear cells in the corneal response to endotoxin. Investig. Ophthalmol. Vis. Sci. 1982, 22, 494–501. [Google Scholar]
- Schultz, C.L.; Morck, D.W.; McKay, S.G.; Olson, M.E.; Buret, A. Lipopolysaccharide induced acute red eye and corneal ulcers. Exp. Eye Res. 1997, 64, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, N.; Fukuda, K.; Fujitsu, Y.; Lu, Y.; Chikamoto, N.; Nishida, T. Lipopolysaccharide-induced expression of intercellular adhesion molecule-1 and chemokines in cultured human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2005, 46, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Fukuda, K.; Nakamura, Y.; Kumagai, N.; Nishida, T. Inhibition by triptolide of chemokine, proinflammatory cytokine, and adhesion molecule expression induced by lipopolysaccharide in corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3796–3800. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.; Katsnelson, M.; Malak, H.A.; Greene, N.G.; Howell, S.J.; Hise, A.G.; Camilli, A.; Kadioglu, A.; Dubyak, G.R.; Pearlman, E. Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J. Immunol. 2015, 194, 1763–1775. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.; Sun, Y.; Hise, A.G.; Rietsch, A.; Pearlman, E. L-1β processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and caspase-1. J. Immunol. 2012, 189, 4231–4235. [Google Scholar] [CrossRef] [PubMed]
- Cendra, M.D.M.; Christodoulides, M.; Hossain, P. Signaling Mediated by Toll-Like Receptor 5 Sensing of Pseudomonas aeruginosa Flagellin Influences IL-1β and IL-18 Production by Primary Fibroblasts Derived from the Human Cornea. Front. Cell. Infect. Microbiol. 2017, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kumagai, N.; Yamamoto, K.; Fujitsu, Y.; Chikamoto, N.; Nishida, T. Potentiation of lipopolysaccharide-induced chemokine and adhesion molecule expression in corneal fibroblasts by soluble CD14 or LPS-binding protein. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3095–3101. [Google Scholar] [CrossRef] [PubMed]
- Seydel, U.; Labischinski, H.; Kastowsky, M.; Brandenburg, K. Phase behavior, supramolecular structure, and molecular conformation of lipopolysaccharide. Immunobiology 1993, 187, 191–211. [Google Scholar] [CrossRef]
- Galanos, C.; Luderitz, O.; Rietschel, E.T.; Westphal, O.; Brade, H.; Brade, L.; Freudenberg, M.; Schade, U.; Imoto, M.; Yoshimura, H.; et al. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 1985, 148, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hailman, E.; Lichenstein, H.S.; Wurfel, M.M.; Miller, D.S.; Johnson, D.A.; Kelley, M.; Busse, L.A.; Zukowski, M.M.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 1994, 179, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Gioannini, T.L.; Teghanemt, A.; Zhang, D.; Levis, E.N.; Weiss, J.P. Monomeric endotoxin: Protein complexes are essential for TLR4-dependent cell activation. J. Endotoxin Res. 2005, 11, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Blais, D.R.; Vascotto, S.G.; Griffith, M.; Altosaar, I. LBP and CD14 secreted in tears by the lacrimal glands modulate the LPS response of corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4235–4244. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kumagai, N.; Nishida, T. Levels of soluble CD14 and lipopolysaccharide-binding protein in human basal tears. Jpn. J. Ophthalmol. 2010, 54, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszynski, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. Antibiotic-induced release of endotoxin: A reappraisal. Clin. Infect. Dis. 1992, 15, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.L.; Nagano, T.; Nakamura, M.; Kumagai, N.; Mishima, H.; Nishida, T. Galardin inhibits collagen degradation by rabbit keratocytes by inhibiting the activation of pro-matrix metalloproteinases. Exp. Eye Res. 1999, 68, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kumagai, N.; Fukuda, K.; Fujitsu, Y.; Nishida, T. Activation of corneal fibroblast-derived matrix metalloproteinase-2 by tryptase. Curr. Eye Res. 2006, 31, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fukuda, K.; Lu, Y.; Nakamura, Y.; Chikama, T.; Kumagai, N.; Nishida, T. Enhancement by neutrophils of collagen degradation by corneal fibroblasts. J. Leukoc. Biol. 2003, 74, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Hao, J.L.; Nakamura, M.; Kumagai, N.; Abe, M.; Nakazawa, T.; Nishida, T. Stimulatory effect of pseudomonal elastase on collagen degradation by cultured keratocytes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1247–1253. [Google Scholar]
- Lu, Y.; Fukuda, K.; Liu, Y.; Kumagai, N.; Nishida, T. Dexamethasone inhibition of IL-1-induced collagen degradation by corneal fibroblasts in three-dimensional culture. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fukuda, K.; Seki, K.; Nakamura, Y.; Kumagai, N.; Nishida, T. Inhibition by triptolide of IL-1-induced collagen degradation by corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5082–5088. [Google Scholar] [CrossRef]
- Tanaka, H.; Fukuda, K.; Ishida, W.; Harada, Y.; Sumi, T.; Fukushima, A. Rebamipide increases barrier function and attenuates TNFα-induced barrier disruption and cytokine expression in human corneal epithelial cells. Br. J. Ophthalmol. 2013, 97, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Ishida, W.; Tanaka, H.; Harada, Y.; Fukushima, A. Inhibition by rebamipide of cytokine-induced or lipopolysaccharide-induced chemokine synthesis in human corneal fibroblasts. Br. J. Ophthalmol. 2014, 98, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, X.; Liu, K.; Gu, Q.; Yang, X. PAPep, a small peptide derived from human pancreatitis-associated protein, attenuates corneal inflammation in vivo and in vitro through the IKKα/β/IκBα/NF-κB signaling pathway. Pharmacol. Res. 2015, 102, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, X.; Wang, L.; Su, L.; Gu, Q.; Wei, F.; Liu, K. Inhibitory effect of a novel peptide, H-RN, on keratitis induced by LPS or poly(I:C) in vitro and in vivo via suppressing NF-κB and MAPK activation. J. Transl. Med. 2017, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hao, P.; Liu, G.; Wang, W.; Han, R.; Jiang, Z.; Li, X. Effects of 4-methylumbelliferone and high molecular weight hyaluronic acid on the inflammation of corneal stromal cells induced by LPS. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, K.; Kodama, A.; Yoshida, K.; Okada, K.; Mishima, H.; Aomatsu, K.; Matsuo, O.; Shimomura, Y. The roles of urokinase-type plasminogen activator in leukocyte infiltration and inflammatory responses in mice corneas treated with lipopolysaccharide. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5338–5350. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M. Defensins and other antimicrobial peptides at the ocular surface. Ocul. Surf. 2004, 2, 229–247. [Google Scholar] [CrossRef]
- Mohammed, I.; Said, D.G.; Dua, H.S. Human antimicrobial peptides in ocular surface defense. Prog. Retin. Eye Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Sanchez, J.I.; Garcia-Perez, B.E.; Munoz-Duarte, A.R.; Baltierra-Uribe, S.L.; Mejia-Lopez, H.; Lopez-Lopez, C.; Bautista-De Lucio, V.M.; Robles-Contreras, A.; Luna-Herrera, J. Defensin production by human limbo-corneal fibroblasts infected with mycobacteria. Pathogens 2013, 2, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, J.; Yu, F.S. Toll-like receptor 2-mediated expression of β-defensin-2 in human corneal epithelial cells. Microbes Infect. 2006, 8, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kumar, A.; Gui, J.F.; Yu, F.S. Staphylococcus aureus lipoproteins trigger human corneal epithelial innate response through toll-like receptor-2. Microb. Pathog. 2008, 44, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Mc, N.N.; Van, R.; Tuchin, O.S.; Fleiszig, S.M. Ocular surface epithelia express mRNA for human β defensin-2. Exp. Eye Res. 1999, 69, 483–490. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, K.; Ambati, B.; Yu, F.S. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4247–4254. [Google Scholar] [CrossRef]
- Brandt, C.R. Peptide therapeutics for treating ocular surface infections. J. Ocul. Pharmacol. Ther. 2014, 30, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Petkova, T.D.; Reins, R.Y.; Proske, R.J.; McDermott, A.M. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Ishida, W.; Harada, Y.; Fukuda, K.; Fukushima, A. Inhibition by the Antimicrobial Peptide LL37 of Lipopolysaccharide-Induced Innate Immune Responses in Human Corneal Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2016, 57, 30–39. [Google Scholar] [CrossRef]
- Sack, R.A.; Conradi, L.; Krumholz, D.; Beaton, A.; Sathe, S.; Morris, C. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: Angiogenin and other defense system constituents. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Stappenbeck, T.S.; Hong, C.V.; Gordon, J.I. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003, 4, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, K.W.; Joo, K.; Kim, J.C. Angiogenin ameliorates corneal opacity and neovascularization via regulating immune response in corneal fibroblasts. BMC Ophthalmol. 2016, 16, 57. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, K.; Ishida, W.; Fukushima, A.; Nishida, T. Corneal Fibroblasts as Sentinel Cells and Local Immune Modulators in Infectious Keratitis. Int. J. Mol. Sci. 2017, 18, 1831. https://doi.org/10.3390/ijms18091831
Fukuda K, Ishida W, Fukushima A, Nishida T. Corneal Fibroblasts as Sentinel Cells and Local Immune Modulators in Infectious Keratitis. International Journal of Molecular Sciences. 2017; 18(9):1831. https://doi.org/10.3390/ijms18091831
Chicago/Turabian StyleFukuda, Ken, Waka Ishida, Atsuki Fukushima, and Teruo Nishida. 2017. "Corneal Fibroblasts as Sentinel Cells and Local Immune Modulators in Infectious Keratitis" International Journal of Molecular Sciences 18, no. 9: 1831. https://doi.org/10.3390/ijms18091831
APA StyleFukuda, K., Ishida, W., Fukushima, A., & Nishida, T. (2017). Corneal Fibroblasts as Sentinel Cells and Local Immune Modulators in Infectious Keratitis. International Journal of Molecular Sciences, 18(9), 1831. https://doi.org/10.3390/ijms18091831




