Systemic Chemokine Levels with “Gut-Specific” Vedolizumab in Patients with Inflammatory Bowel Disease—A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Clinical Findings of Inflammatory Bowel Disease-Patients after Treatment with Vedolizumab
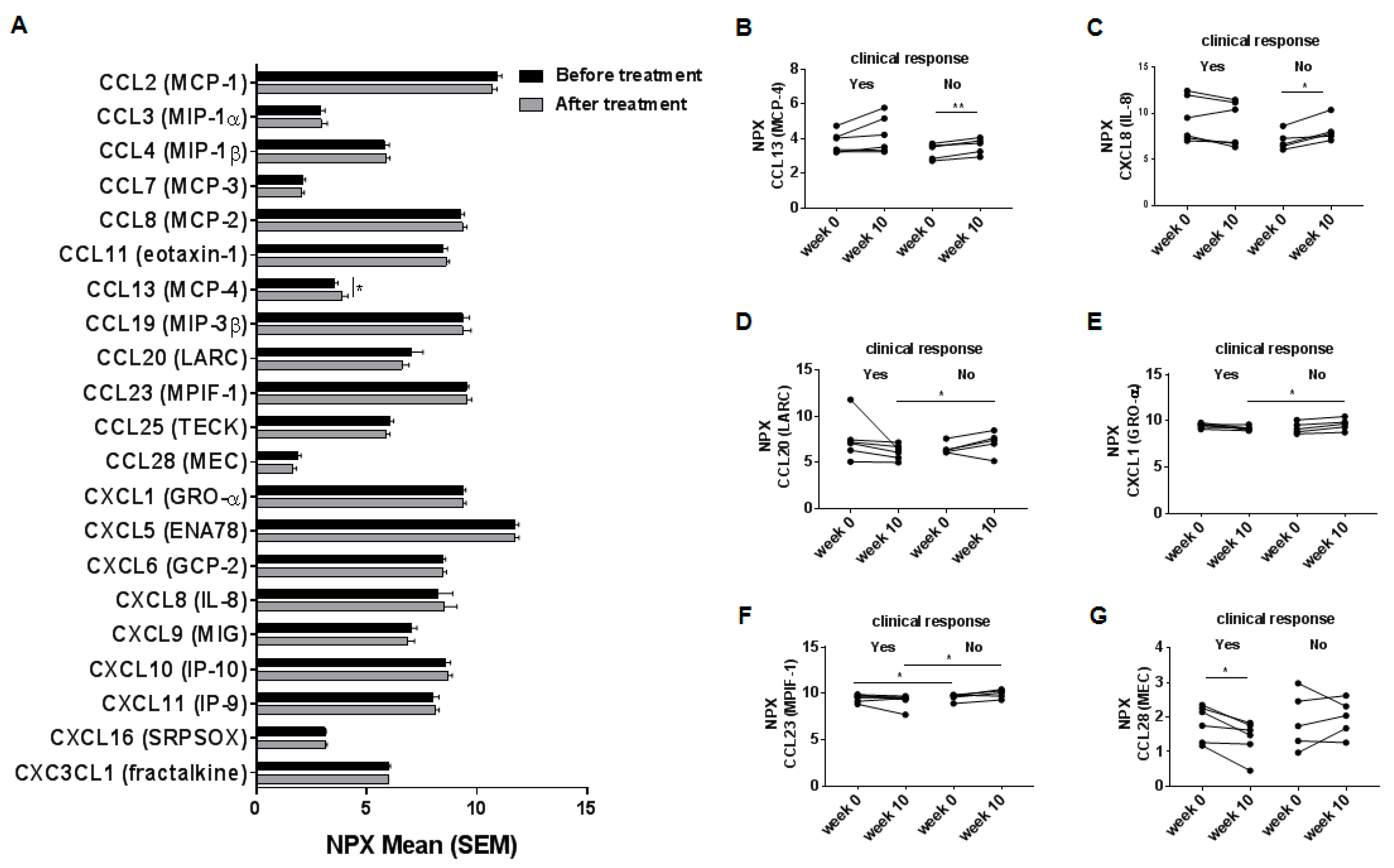
2.2. Systemic Chemokine Levels in Inflammatory Bowel Disease-Patients before and after Treatment with Vedolizumab
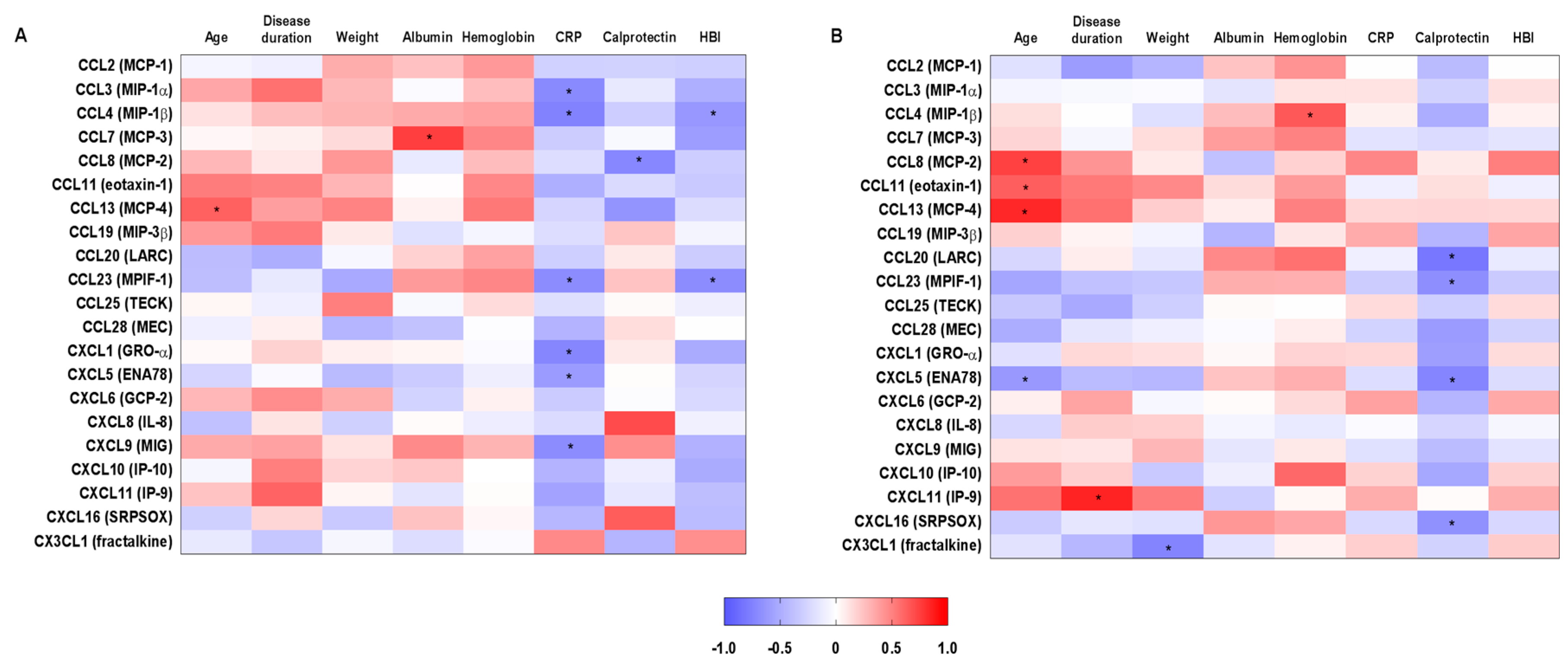
2.3. Correlations between Systemic Chemokine Levels and Clinical and Laboratory Parameters in Inflammatory Bowel Disease-Patients before and after Treatment with Vedolizumab
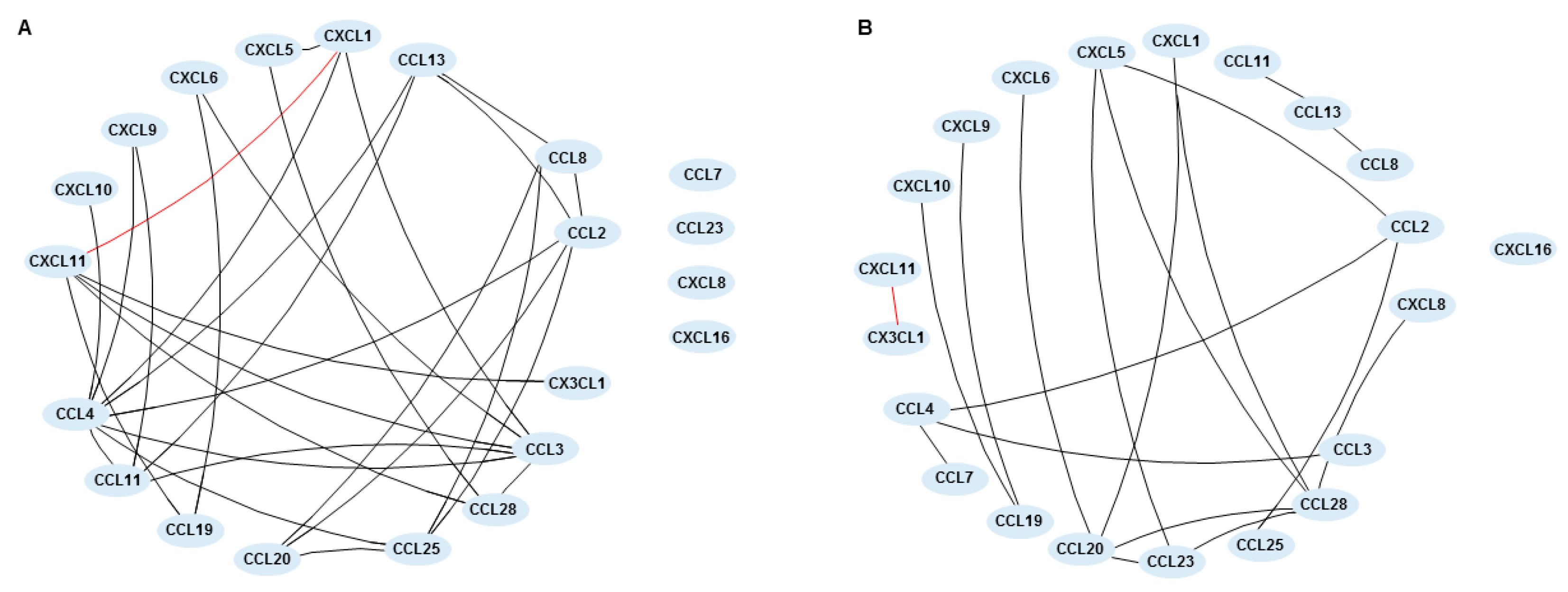
2.4. Chemokine Networks in Inflammatory Bowel Disease-Patients before and after Treatment with Vedolizumab
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Chemokine Quantification
4.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| HBI | Harvey-Bradshaw index |
| BMI | Body mass index |
| PGA | Physicians global assessment |
| CRP | C-reactive protein |
| TNF | Tumor necrosis factor |
| CCL | Chemokine (C–C motif) ligand |
| CXCL | Chemokine (C–X–C motif) ligand |
| CCR | C–C chemokine receptor |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| MAdCAM-1 | Mucosal addressin cell adhesion molecule-1 |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Arnone, B.; Buchman, A. Intestinal growth factors: Potential use in the treatment of inflammatory bowel disease and their role in mucosal healing. Inflamm. Bowel Dis. 2011, 17, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Boyapati, R.K.; Kalla, R.; Satsangi, J.; Ho, G.T. Biomarkers in search of precision medicine in IBD. Am. J. Gastroenterol. 2016, 111, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Ludwiczek, O.; Holzmann, S.; Moschen, A.R.; Weiss, G.; Enrich, B.; Graziadei, I.; Dunzendorfer, S.; Wiedermann, C.J.; Murzl, E.; et al. Increased expression of CCL20 in human inflammatory bowel disease. J. Clin. Immunol. 2004, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Singh, N.P.; Murphy, E.A.; Price, R.L.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine 2016, 77, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rollins, B.J. Chemokines. Blood 1997, 90, 909–928. [Google Scholar] [PubMed]
- Atreya, R.; Neurath, M.F. Chemokines in inflammatory bowel diseases. Dig. Dis. 2010, 28, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, K.A.; Targan, S.R. The role of chemokines and chemokine receptors in mucosal inflammation. Inflamm. Bowel Dis. 2000, 6, 303–313. [Google Scholar] [PubMed]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Pribila, J.T.; Quale, A.C.; Mueller, K.L.; Shimizu, Y. Integrins and T cell-mediated immunity. Annu. Rev. Immunol. 2004, 22, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Nieves, J. Strategies that target leukocyte traffic in inflammatory bowel diseases: Recent developments. Curr. Opin. Gastroenterol. 2015, 31, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Peyrin-Biroulet, L. Current, new and future biological agents on the horizon for the treatment of inflammatory bowel diseases. Therap. Adv. Gastroenterol. 2015, 8, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Wyant, T.; Fedyk, E.; Abhyankar, B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J. Crohn’s Colitis 2016, 10, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Lemann, M. Review article: Remission rates achievable by current therapies for inflammatory bowel disease. Aliment. Pharmacol. Therap. 2011, 33, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Eng. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Eng. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Arijs, I.; de Hertogh, G.; Lemmens, B.; van Lommel, L.; de Bruyn, M.; Vanhove, W.; Cleynen, I.; Machiels, K.; Ferrante, M.; Schuit, F.; et al. Effect of vedolizumab (anti-α4β7-integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut 2016. [Google Scholar] [CrossRef] [PubMed]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Bjorkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Rennel Dickens, E.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-plex pea immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef] [PubMed]
- Molin, C.J.; Westerberg, E.; Punga, A.R. Profile of upregulated inflammatory proteins in sera of myasthenia gravis patients. Sci. Rep. 2017, 7, 39716. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Eriksson, A.; Tran, B.; Assarsson, E.; Fredriksson, S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011, 39, e102. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Sandborn, W.J.; van Assche, G.; Lukas, M.; Xu, J.; James, A.; Abhyankar, B.; Lasch, K. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naive to or who have failed tumor necrosis factor antagonist therapy. Inflamm. Bowel Dis. 2017, 23, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Feagan, B.G.; Rutgeerts, P.; Colombel, J.F.; Sandborn, W.J.; Sy, R.; D’Haens, G.; Ben-Horin, S.; Xu, J.; Rosario, M.; et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014, 147, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Keshav, S.; Vanasek, T.; Niv, Y.; Petryka, R.; Howaldt, S.; Bafutto, M.; Racz, I.; Hetzel, D.; Nielsen, O.H.; Vermeire, S.; et al. A randomized controlled trial of the efficacy and safety of CCX282-b, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn’s disease. PLoS ONE 2013, 8, e60094. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Enriquez, E.; Garcia-Zepeda, E.A. The multiple faces of CCL13 in immunity and inflammation. Inflammopharmacology 2013, 21, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.H.; Lukacs, N.W. The role of chemokines and chemokine receptors in eosinophil activation during inflammatory allergic reactions. Braz. J. Med. Biol. Res. 2003, 36, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Soto, H.; Oldham, E.R.; Buchanan, M.E.; Homey, B.; Catron, D.; Jenkins, N.; Copeland, N.G.; Gilbert, D.J.; Nguyen, N.; et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J. Biol. Chem. 2000, 275, 22313–22323. [Google Scholar] [CrossRef] [PubMed]
- Eksteen, B.; Miles, A.; Curbishley, S.M.; Tselepis, C.; Grant, A.J.; Walker, L.S.; Adams, D.H. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J. Immunol. 2006, 177, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Hieshima, K.; Ohtani, H.; Shibano, M.; Izawa, D.; Nakayama, T.; Kawasaki, Y.; Shiba, F.; Shiota, M.; Katou, F.; Saito, T.; et al. CCL28 Has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J. Immunol. 2003, 170, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Kelsall, B.L. Localization of distinct peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 2000, 191, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Berahovich, R.D.; Miao, Z.; Wang, Y.; Premack, B.; Howard, M.C.; Schall, T.J. Proteolytic activation of alternative CCR1 ligands in inflammation. J. Immunol. 2005, 174, 7341–7351. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A.; Al-Mofleh, I.A.; Al-Jebreen, A.M. Neutrophil chemokines GCP-2 and GRO-α in patients with inflammatory bowel disease. J. Dig. Dis. 2008, 9, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.P.; Kreider, B.L.; Li, Y.; Li, H.; Leung, K.; Salcedo, T.; Nardelli, B.; Pippalla, V.; Gentz, S.; Thotakura, R.; et al. Molecular and functional characterization of two novel human C–C chemokines as inhibitors of two distinct classes of myeloid progenitors. J. Exp. Med. 1997, 185, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Chintakuntlawar, A.V.; Chodosh, J. Chemokine CXCL1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J. Interferon Cytokine Res. 2009, 29, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Vries, M.H.; Wagenaar, A.; Verbruggen, S.E.; Molin, D.G.; Dijkgraaf, I.; Hackeng, T.H.; Post, M.J. CXCL1 Promotes arteriogenesis through enhanced monocyte recruitment into the peri-collateral space. Angiogenesis 2015, 18, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Chiba, T.; Nakamura, S.; Matsumoto, T. Changes in cytokine profile may predict therapeutic efficacy of infliximab in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2015, 30, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.K.; Strid, H.; Isaksson, S.; Bajor, A.; Lasson, A.; Ung, K.A.; Ohman, L. Response to infliximab therapy in ulcerative colitis is associated with decreased monocyte activation, reduced CCL2 expression and downregulation of tenascin c. J. Crohn’s Colitis 2015, 9, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Nieves, J.; Olson, T.; Bamias, G.; Bruce, A.; Solga, M.; Knight, R.F.; Hoang, S.; Cominelli, F.; Ley, K. l-selectin, α4β1, and α4β7 integrins participate in CD4+ T cell recruitment to chronically inflamed small intestine. J. Immunol. 2005, 174, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
| Variable | Week 0 (n = 11) | Week 10 (n = 11) | p-Value * |
|---|---|---|---|
| HBI | 9.0 (6.0) | 8.0 (8.0) | 0.220 |
| Albumin (g/L) | 37.0 (2.0) | 36.0 (6.0) | 0.944 |
| Hemoglobin (g/L) | 130.0 (35.0) | 131.0 (30.0) | 0.475 |
| Fecal calprotectin (µg/g) † | 2011.0 (5013.5) | 191.0 (978.2) | 0.069 |
| C-reactive protein (mg/L) | 3.0 (17.0) | 8.0 (8.0) | 0.754 |
| Leukocyte count (×109/L) | 6.3 (4.9) | 6.1 (2.4) | 0.593 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwicker, S.; Lira-Junior, R.; Höög, C.; Almer, S.; Boström, E.A. Systemic Chemokine Levels with “Gut-Specific” Vedolizumab in Patients with Inflammatory Bowel Disease—A Pilot Study. Int. J. Mol. Sci. 2017, 18, 1827. https://doi.org/10.3390/ijms18081827
Zwicker S, Lira-Junior R, Höög C, Almer S, Boström EA. Systemic Chemokine Levels with “Gut-Specific” Vedolizumab in Patients with Inflammatory Bowel Disease—A Pilot Study. International Journal of Molecular Sciences. 2017; 18(8):1827. https://doi.org/10.3390/ijms18081827
Chicago/Turabian StyleZwicker, Stephanie, Ronaldo Lira-Junior, Charlotte Höög, Sven Almer, and Elisabeth A. Boström. 2017. "Systemic Chemokine Levels with “Gut-Specific” Vedolizumab in Patients with Inflammatory Bowel Disease—A Pilot Study" International Journal of Molecular Sciences 18, no. 8: 1827. https://doi.org/10.3390/ijms18081827
APA StyleZwicker, S., Lira-Junior, R., Höög, C., Almer, S., & Boström, E. A. (2017). Systemic Chemokine Levels with “Gut-Specific” Vedolizumab in Patients with Inflammatory Bowel Disease—A Pilot Study. International Journal of Molecular Sciences, 18(8), 1827. https://doi.org/10.3390/ijms18081827





