GLUT10—Lacking in Arterial Tortuosity Syndrome—Is Localized to the Endoplasmic Reticulum of Human Fibroblasts
Abstract
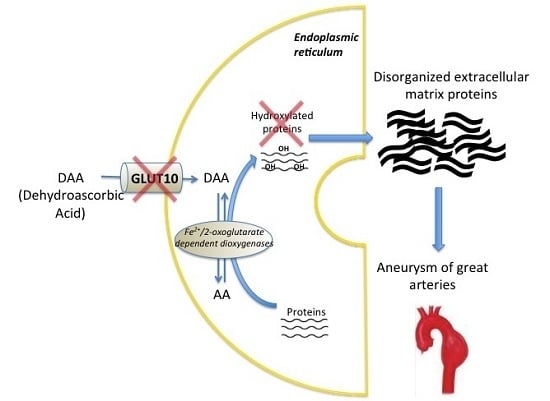
1. Introduction
2. Results
2.1. In Silico Prediction of Subcellular Localization of GLUT10
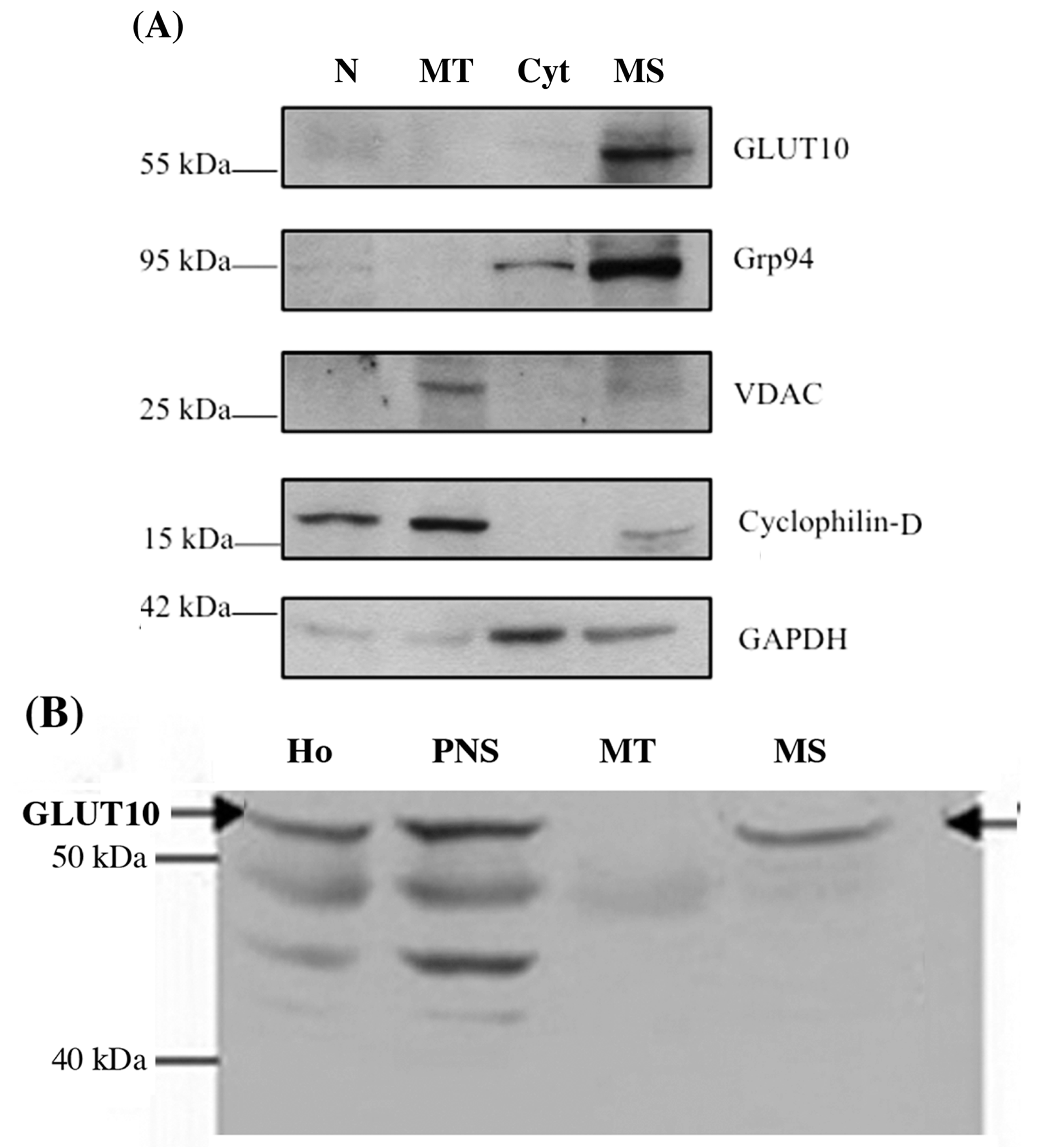
2.2. GLUT10 Is Predominantly Present in the Microsomal Fraction
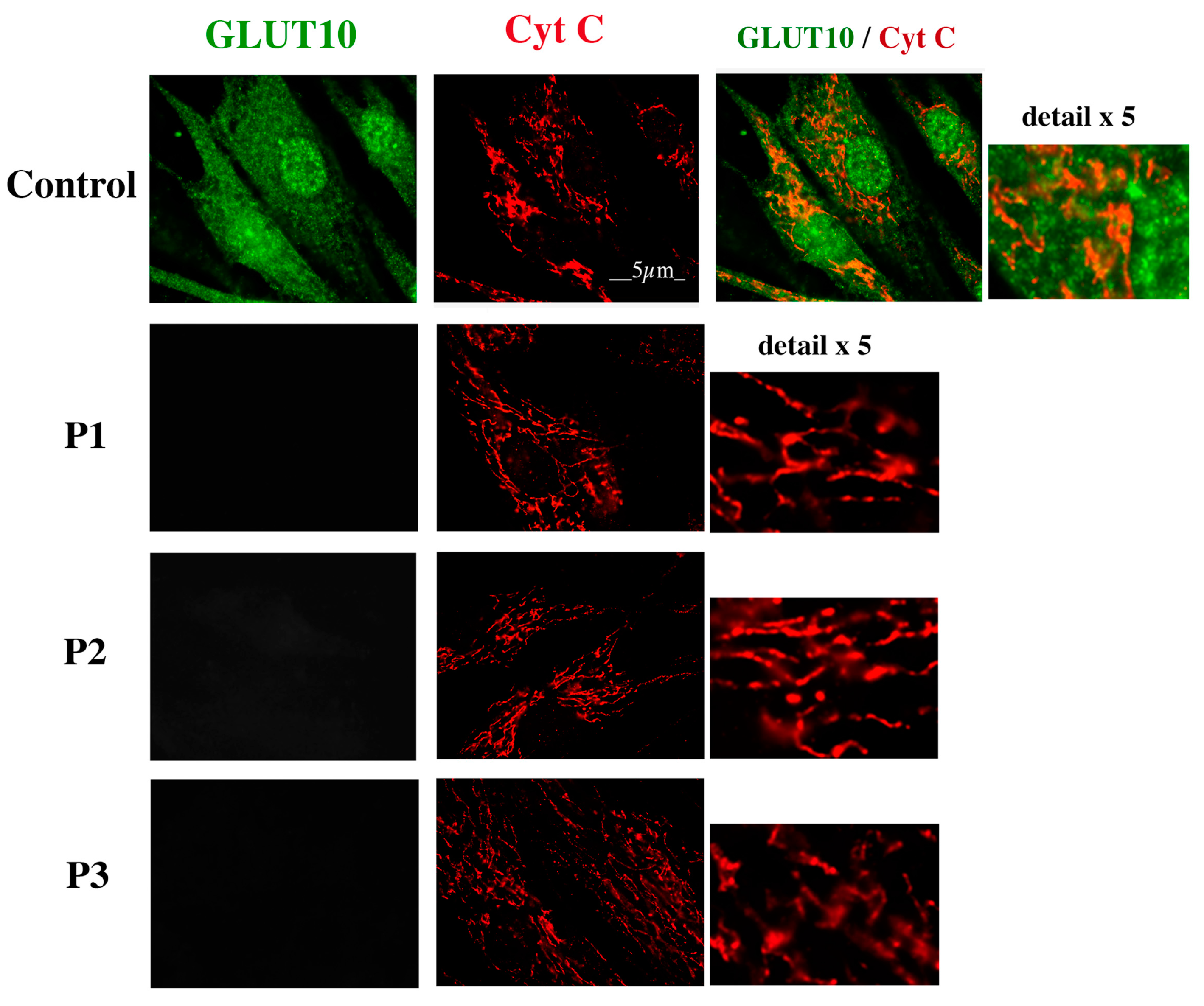
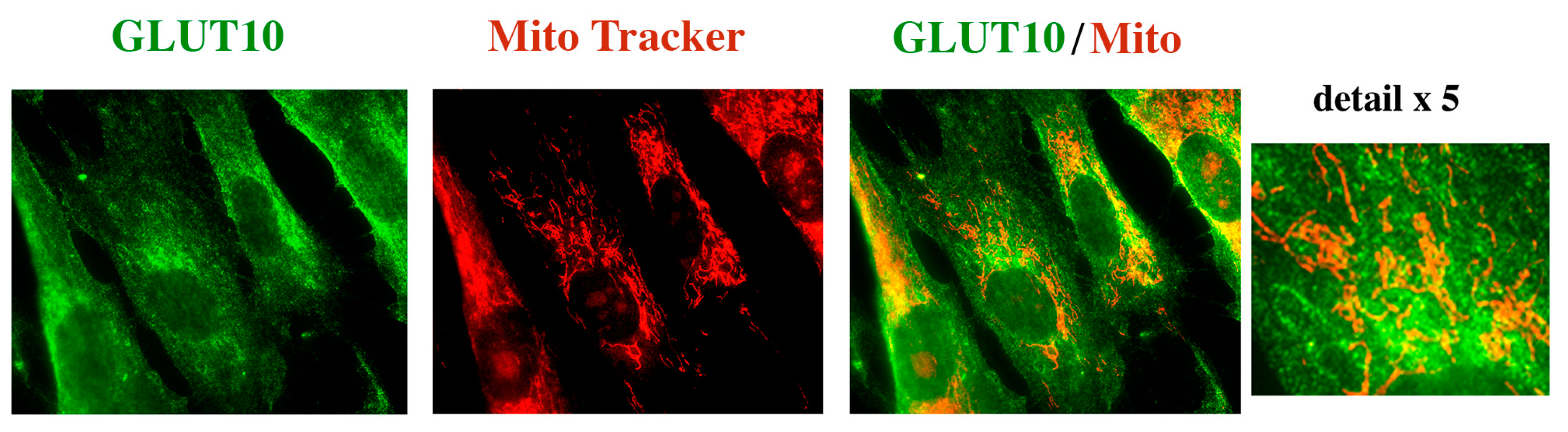
2.3. GLUT10 Immunofluorescence Reveals a Reticular Pattern in Control but Not in ATS Fibroblasts
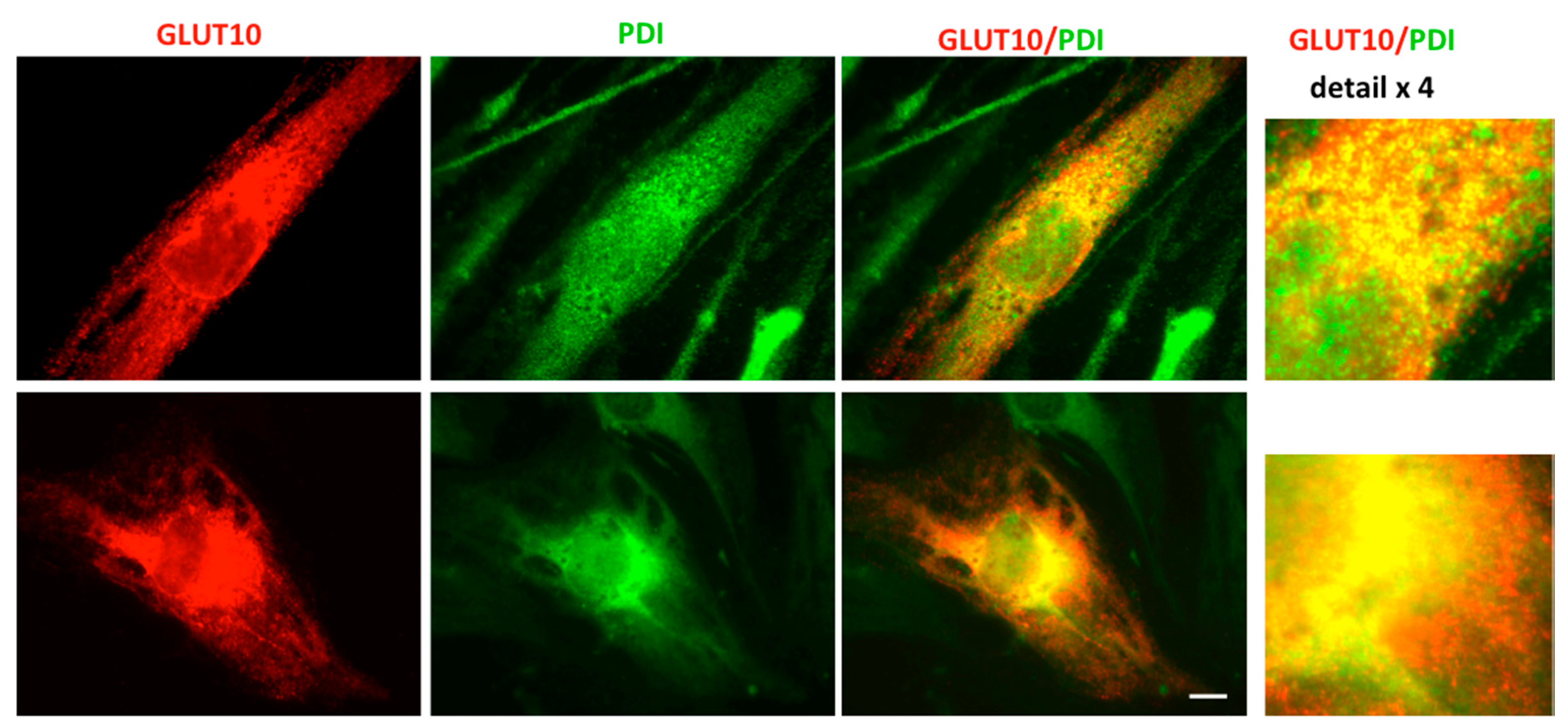
2.4. GLUT10 Co-Localizes with the ER Marker Protein PDI
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.2. Fibroblast Culture
4.3. Subcellular Fractionation of Human Fibroblasts
4.4. Preparation of Subcellular Fractions from Human Liver
4.5. Western Blot Analysis
4.6. Construction of a Tagged-pG10 Expression Vector
4.7. Immunofluorescence Microscopical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| ATS | Arterial tortuosity syndrome |
| BSA | Bovine serum albumin |
| Cyt C | Cytochrome C |
| DAA | Dehydroascorbic acid |
| ER | Endoplasmic reticulum |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GLUT | Glucose transporter |
| hTERT | Human telomerase reverse transcriptase |
| KRR | Lys-Arg-Arg |
| MOPS | 3-(N-morpholino)propanesulfonic acid |
| NE | Nuclear envelope |
| PBS | Phosphate buffered saline |
| PDI | Protein disulfide isomerase |
| TGFβ | Transforming growth factor beta |
| VDAC | Voltage dependent anion channel |
References
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2016, 25, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Mychaleckyj, J.C.; Fossey, S.C.; Mihic, S.J.; Craddock, A.L.; Bowden, D.W. Sequence and functional analysis of GLUT10: A glucose transporter in the Type 2 diabetes-linked region of chromosome 20q12-13.1. Mol. Genet. Metab. 2001, 74, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Inoue, T.; Konishi, H. Increased gene expression of glucose transporters in the mouse brain after treatment with fluoxetine and pergolide. Drug Res. 2014, 64, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Pyla, R.; Poulose, N.; Jun, J.Y.; Segar, L. Expression of conventional and novel glucose transporters, GLUT1, -9, -10, and -12, in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2013, 304, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Németh, C.E.; Marcolongo, P.; Gamberucci, A.; Fulceri, R.; Benedetti, A.; Zoppi, N.; Ritelli, M.; Chiarelli, N.; Colombi, M.; Willaert, A.; et al. Glucose transporter type 10-lacking in arterial tortuosity syndrome-facilitates dehydroascorbic acid transport. FEBS Lett. 2016, 590, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Coucke, P.J.; Willaert, A.; Wessels, M.W.; Callewaert, B.; Zoppi, N.; de Backer, J.; Fox, J.E.; Mancini, G.M.; Kambouris, M.; Gardella, R.; et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat. Genet. 2006, 38, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Drera, B.; Guala, A.; Zoppi, N.; Gardella, R.; Franceschini, P.; Barlati, S.; Colombi, M. Two novel SLC2A10/GLUT10 mutations in a patient with arterial tortuosity syndrome. Am. J. Med. Genet. 2007, 143, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, B.L.; Loeys, B.L.; Casteleyn, C.; Willaert, A.; Dewint, P.; de Backer, J.; Sedlmeier, R.; Simoens, P.; de Paepe, A.M.; Coucke, P.J. Absence of arterial phenotype in mice with homozygous slc2A10 missense substitutions. Genesis 2008, 46, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Castori, M.; Ritelli, M.; Zoppi, N.; Chiarelli, N.; Molisso, L.; Zaccagna, F.; Grammatico, P.; Colombi, M. Adult presentation of arterial tortuosity syndrome in a 51-year-old woman with the novel homozygous c.1411+1G > A mutation in the SLC2A10 gene. Am. J. Med. Genet. 2012, 158, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, B.; de Paepe, A.; Coucke, P. Arterial Tortuosity Syndrome. In Gene Reviews®; Internet; Pagon, R.A., Adam, M.P., Ardinger, H.H., Wallace, S.E., Amemiya, A., Bean, L.J.H., Bird, T.D., Dolan, C.R., Fong, C.T., Smith, R.J.H., et al., Eds.; University of Washington: Seattle, WA, USA, 2014; pp. 1993–2015. Available online: http://www.ncbi.nlm.nih.gov/books/NBK253404/ (accessed on 22 August 2017).
- Ritelli, M.; Chiarelli, N.; Dordoni, C.; Reffo, E.; Venturini, M.; Quinzani, S.; Monica, M.D.; Scarano, G.; Santoro, G.; Russo, M.G.; et al. Arterial Tortuosity Syndrome: Homozygosity for two novel and one recurrent SLC2A10 missense mutations in three families with severe cardiopulmonary complications in infancy and a literature review. BMC Med. Genet. 2014, 15, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Huang, H.Y.; Chang, C.J.; Cheng, C.H.; Chen, Y.T. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: Mechanistic insight into arterial tortuosity syndrome. Hum. Mol. Genet. 2010, 19, 3721–3733. [Google Scholar] [CrossRef] [PubMed]
- Segade, F. Glucose transporter 10 and arterial tortuosity syndrome: The vitamin C connection. FEBS Lett. 2010, 584, 2990–2994. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, G.; Marcolongo, P.; Puskas, F.; Fulceri, R.; Mandl, J.; Benedetti, A. Dehydroascorbate and ascorbate transport in rat liver microsomal vesicles. J. Biol. Chem. 1998, 273, 2758–2762. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, G.; Benedetti, A.; Margittai, E.; Marcolongo, P.; Fulceri, R.; Németh, C.E.; Szarka, A. Subcellular compartmentation of ascorbate and its variation in disease states. Biochim. Biophys. Acta 2014, 1843, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Joost, H.G.; Thorens, B. The extended GLUT-family of sugar/polyol transport facilitators: Nomenclature, sequence characteristics, and potential function of its novel members (review). Mol. Membr. Biol. 2001, 18, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Tinel, N.; Terrenoire, C.; Romey, G.; Lazdunski, M.; Borsotto, M. p11, an annexin II subunit, an auxiliary protein associated with the background K+ channel, TASK-1. EMBO J. 2002, 21, 4439–4448. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, N.; Chiarelli, N.; Cinquina, V.; Ritelli, M.; Colombi, M. GLUT10 deficiency leads to oxidative stress and non-canonical αvβ3 integrin-mediated TGFβ signalling associated with extracellular matrix disarray in arterial tortuosity syndrome skin fibroblasts. Hum. Mol. Genet. 2015, 24, 6769–6787. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, G.; Marcolongo, P.; Burchell, A.; Benedetti, A. Heterogeneity of glucose transport in rat liver microsomal vesicles. Arch. Biochem. Biophys. 1998, 359, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Malhas, A.; Goulbourne, C.; Vaux, D.J. The nucleoplasmic reticulum: Form and function. Trends Cell. Biol. 2011, 21, 362–373. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, K.; Braakman, I. Protein quality control at the endoplasmic reticulum. Essays Biochem. 2016, 60, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Rizzuto, R.; Hajnoczky, G.; Su, T.P. MAM: More than just a housekeeper. Trends Cell Biol. 2009, 19, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Szarka, A.; Horemans, N.; Bánhegyi, G.; Asard, H. Facilitated glucose and dehydroascorbate transport in plant mitochondria. Arch Biochem. Biophys. 2004, 428, 73–80. [Google Scholar] [CrossRef] [PubMed]
- KC, S.; Cárcamo, J.M.; Golde, D.W. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005, 19, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, C.; Fiorani, M.; Cerioni, L.; Guidarelli, A.; Cantoni, O. Sodium-dependent transport of ascorbic acid in U937 cell mitochondria. IUBMB Life 2013, 65, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Montesino, C.; Roa, F.J.; Peña, E.; González, M.; Sotomayor, K.; Inostrosa, E.; Muñoz, C.; González, I.; Maldonado, M.; Soliz, C.; et al. Mitochondrial ascorbic acid transport is mediated by a low-affinity form of the sodium-coupled ascorbic acid transporter-2. Free Radic. Biol. Med. 2014, 70, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Szarka, A.; Balogh, T. In silico aided thoughts on mitochondrial vitamin C transport. J. Theor. Biol. 2015, 365, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, C.; Vissers, M.C. Ascorbate as a co-factor for Fe- and 2-oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front. Oncol. 2014, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Myllyharju, J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann. Med. 2008, 40, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Claros, M.G.; Vincens, P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 1996, 241, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Small, I.; Peeters, N.; Legeai, F.; Lurin, C. Predotar: A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 2004, 4, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Horton, P. PSORT: A program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 1999, 24, 34–36. [Google Scholar] [CrossRef]
- Höglund, A.; Dönnes, P.; Blum, T.; Adolph, H.W.; Kohlbacher, O. MultiLoc: Prediction of protein subcellular localization using N-terminal targeting sequences, sequence motifs and amino acid composition. Bioinformatics 2006, 22, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- King, B.R.; Vural, S.; Pandey, S.; Barteau, A.; Guda, C. ngLOC: Software and web server for predicting protein subcellular localization in prokaryotes and eukaryotes. BMC Research Notes 2012, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Briesemeister, S.; Rahnenführer, J.; Kohlbacher, O. YLoc—an interpretable web server for predicting subcellular localization. Nucleic Acids Res. 2010, 38, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.S.; Chen, Y.C.; Lu, C.H.; Hwang, J.K. Prediction of protein subcellular localization. Proteins 2006, 64, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, R.; Fulceri, R.; Marcolongo, P.; Bánhegyi, G.; Zammarchi, E.; Stafford, K.; Burchell, A.; Benedetti, A. Glucose 6-phosphate transport in fibroblast microsomes from glycogen storage disease type 1b patients: Evidence for multiple glucose 6-phosphate transport systems. Biochem. J. 2001, 357, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Margittai, É.; Bánhegyi, G. Isocitrate dehydrogenase: A NADPH-generating enzyme in the lumen of the endoplasmic reticulum. Arch. Biochem. Biophys. 2008, 471, 184–190. [Google Scholar] [CrossRef] [PubMed]
| Location | Target P | Mitoprot | Predotar | PSORT II | MultiLoc/TargetLoc | ngLOC | yLoc | Cello |
|---|---|---|---|---|---|---|---|---|
| Probability of Location | ||||||||
| Plasma membrane | - | - | - | 43.5% | 0.12 | 14.46 | 99.8% | 4.855 |
| Endoplasmic reticulum | 0.982 | - | 0.89 | 39.1% | 0.63 | - | 0.1% | 0.008 |
| Extracellular space | - | - | - | 4.3% | 0.03 | - | 0.1% | 0.061 |
| Lysosome | - | - | - | - | 0.06 | - | 0.0% | 0.029 |
| Golgi apparatus | - | - | - | 4.3% | 0.14 | - | 0.0% | - |
| Peroxisome | - | - | - | - | 0.01 | - | 0.0% | 0.007 |
| Mitochondrion | 0.014 | 0.0097 | 0.00 | 4.3% | 0.0 | - | 0.0% | 0.009 |
| Cytoplasm | - | - | - | - | 0.0 | 40.73 | 0.0% | 0.010 |
| Nucleus | - | - | - | - | 0.0 | 8.909 | 0.0% | 0.004 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamberucci, A.; Marcolongo, P.; Németh, C.E.; Zoppi, N.; Szarka, A.; Chiarelli, N.; Hegedűs, T.; Ritelli, M.; Carini, G.; Willaert, A.; et al. GLUT10—Lacking in Arterial Tortuosity Syndrome—Is Localized to the Endoplasmic Reticulum of Human Fibroblasts. Int. J. Mol. Sci. 2017, 18, 1820. https://doi.org/10.3390/ijms18081820
Gamberucci A, Marcolongo P, Németh CE, Zoppi N, Szarka A, Chiarelli N, Hegedűs T, Ritelli M, Carini G, Willaert A, et al. GLUT10—Lacking in Arterial Tortuosity Syndrome—Is Localized to the Endoplasmic Reticulum of Human Fibroblasts. International Journal of Molecular Sciences. 2017; 18(8):1820. https://doi.org/10.3390/ijms18081820
Chicago/Turabian StyleGamberucci, Alessandra, Paola Marcolongo, Csilla E. Németh, Nicoletta Zoppi, András Szarka, Nicola Chiarelli, Tamás Hegedűs, Marco Ritelli, Giulia Carini, Andy Willaert, and et al. 2017. "GLUT10—Lacking in Arterial Tortuosity Syndrome—Is Localized to the Endoplasmic Reticulum of Human Fibroblasts" International Journal of Molecular Sciences 18, no. 8: 1820. https://doi.org/10.3390/ijms18081820
APA StyleGamberucci, A., Marcolongo, P., Németh, C. E., Zoppi, N., Szarka, A., Chiarelli, N., Hegedűs, T., Ritelli, M., Carini, G., Willaert, A., Callewaert, B. L., Coucke, P. J., Benedetti, A., Margittai, É., Fulceri, R., Bánhegyi, G., & Colombi, M. (2017). GLUT10—Lacking in Arterial Tortuosity Syndrome—Is Localized to the Endoplasmic Reticulum of Human Fibroblasts. International Journal of Molecular Sciences, 18(8), 1820. https://doi.org/10.3390/ijms18081820