Aquaporin-4 Functionality and Virchow-Robin Space Water Dynamics: Physiological Model for Neurovascular Coupling and Glymphatic Flow
Abstract
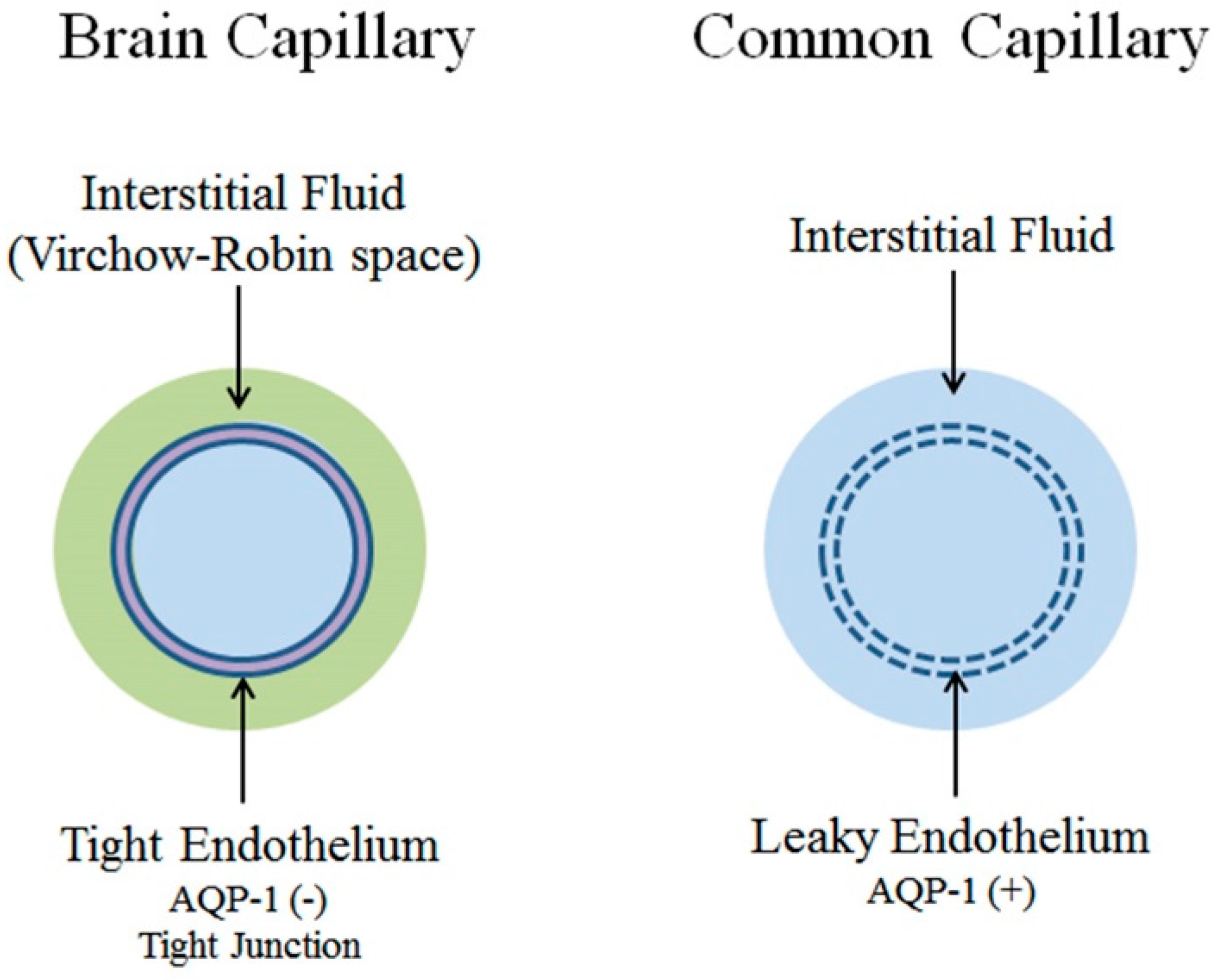
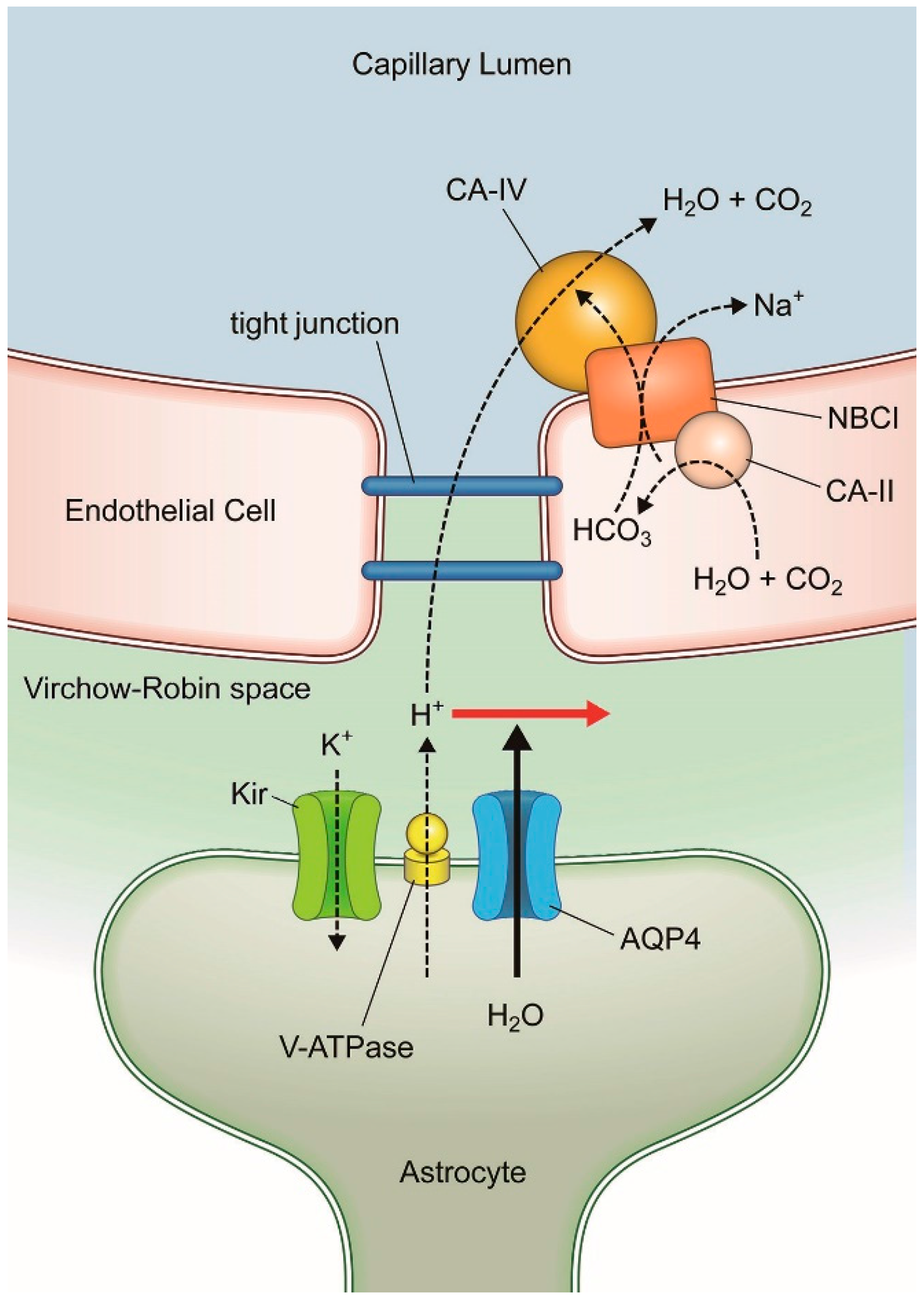
1. Introduction: Blood-Brain Barrier
2. Blood Flow Dynamics
2.1. Basics
2.2. Common Capillaries and Tissue Perfusion
2.3. Cerebral Autoregulation
2.4. Neurovascular Coupling
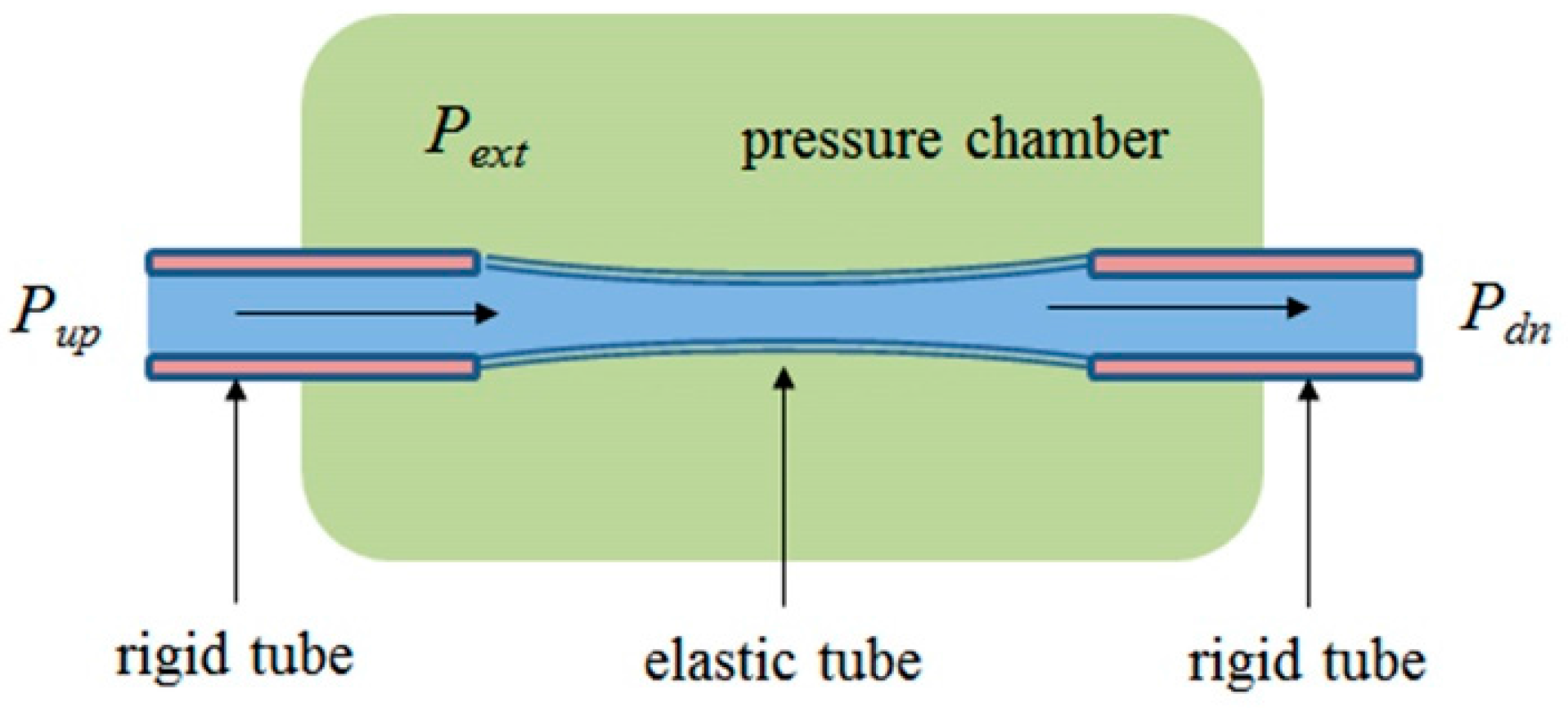
2.5. Flow and Pressure in a Starling Resistor
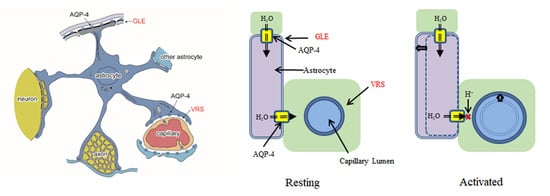
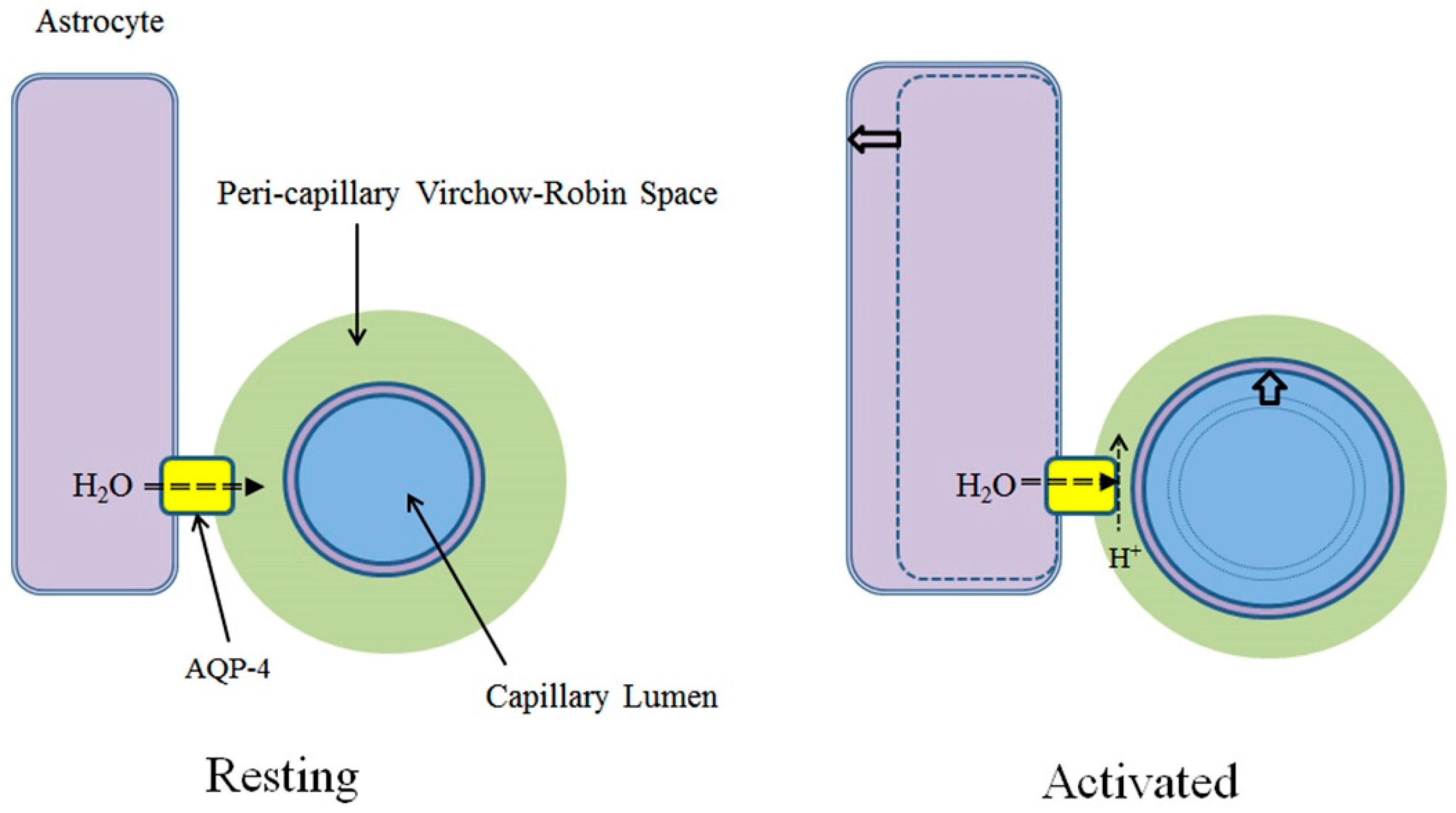
3. AQP-4 and Neurovascular Coupling
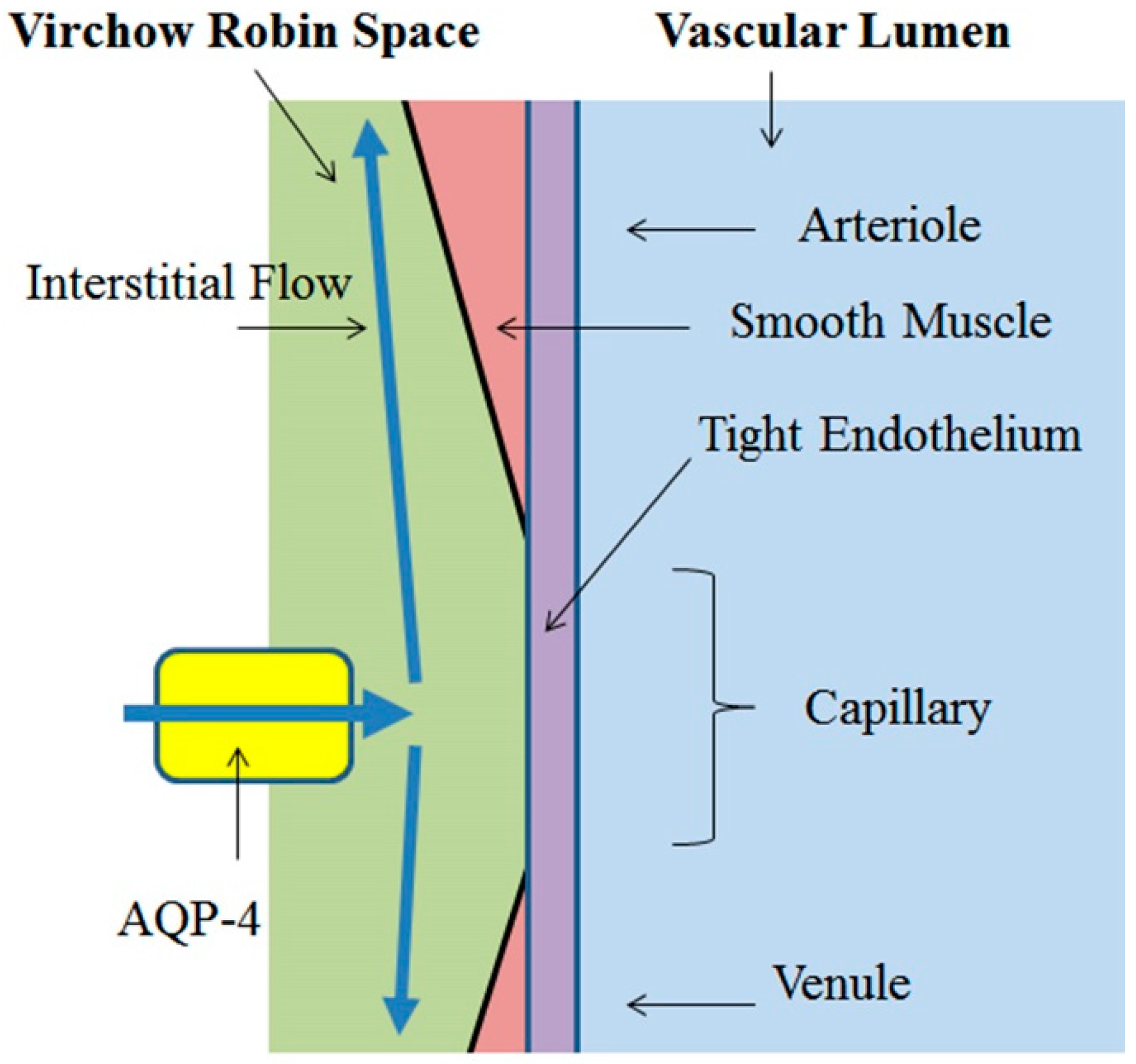
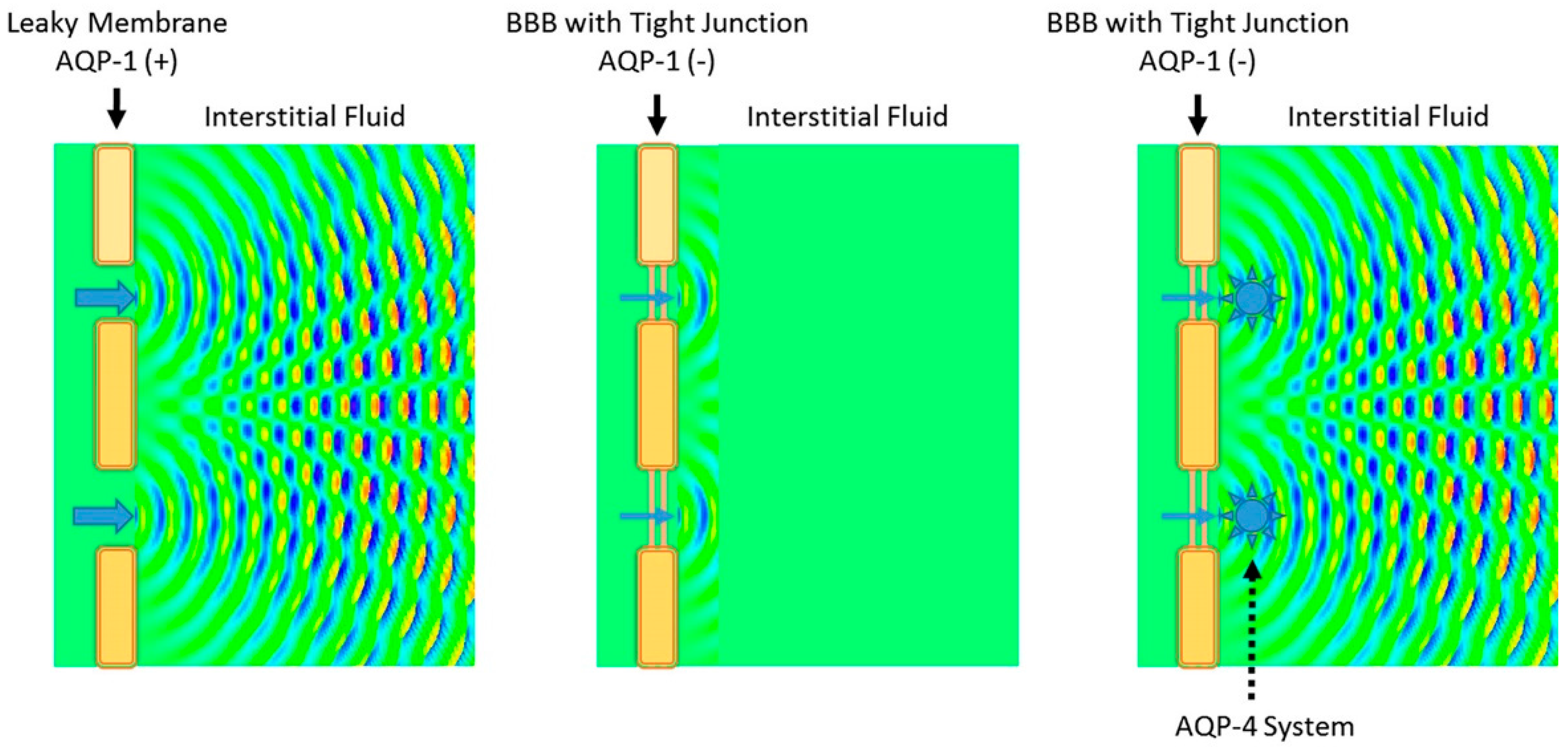
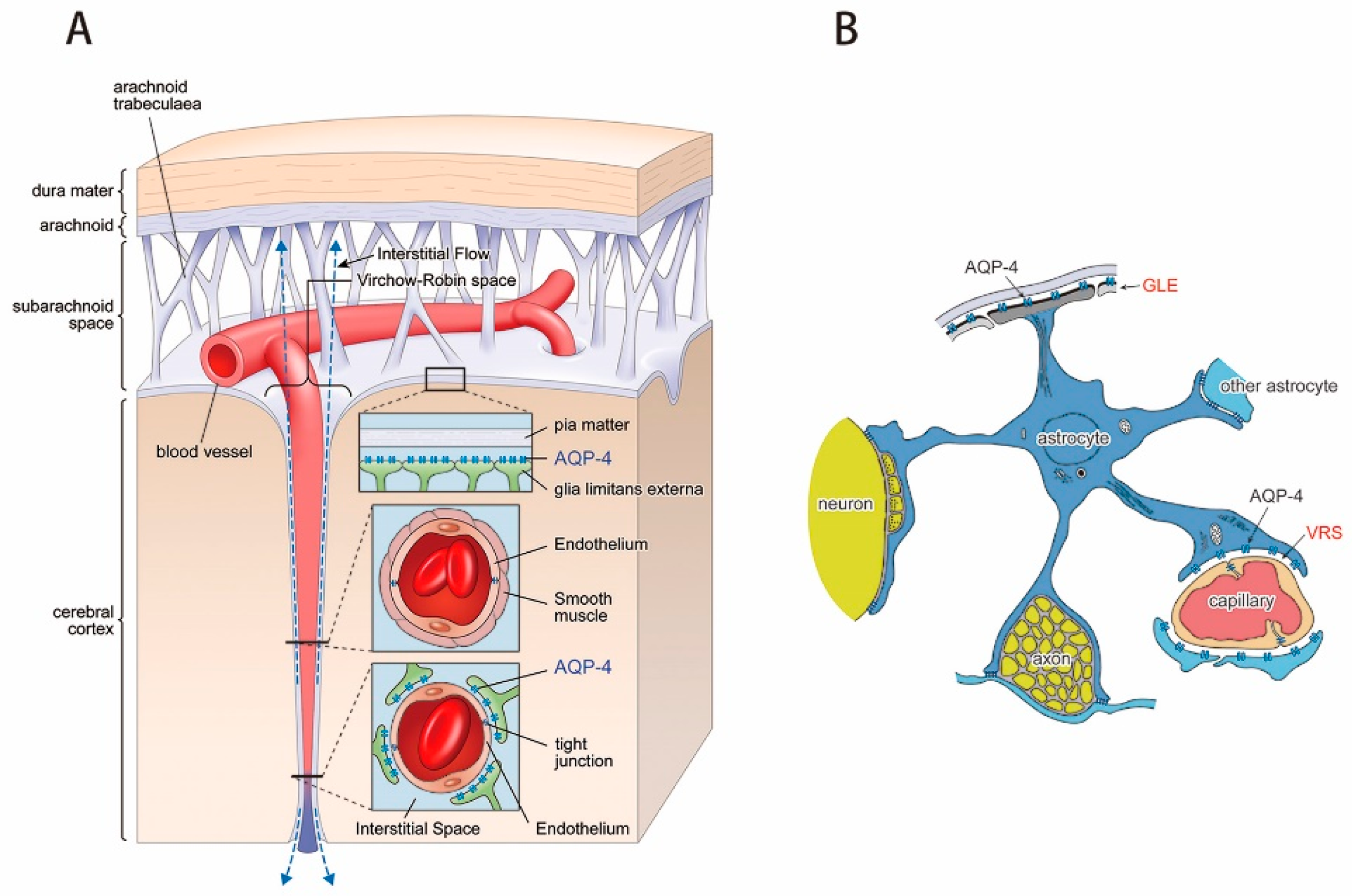
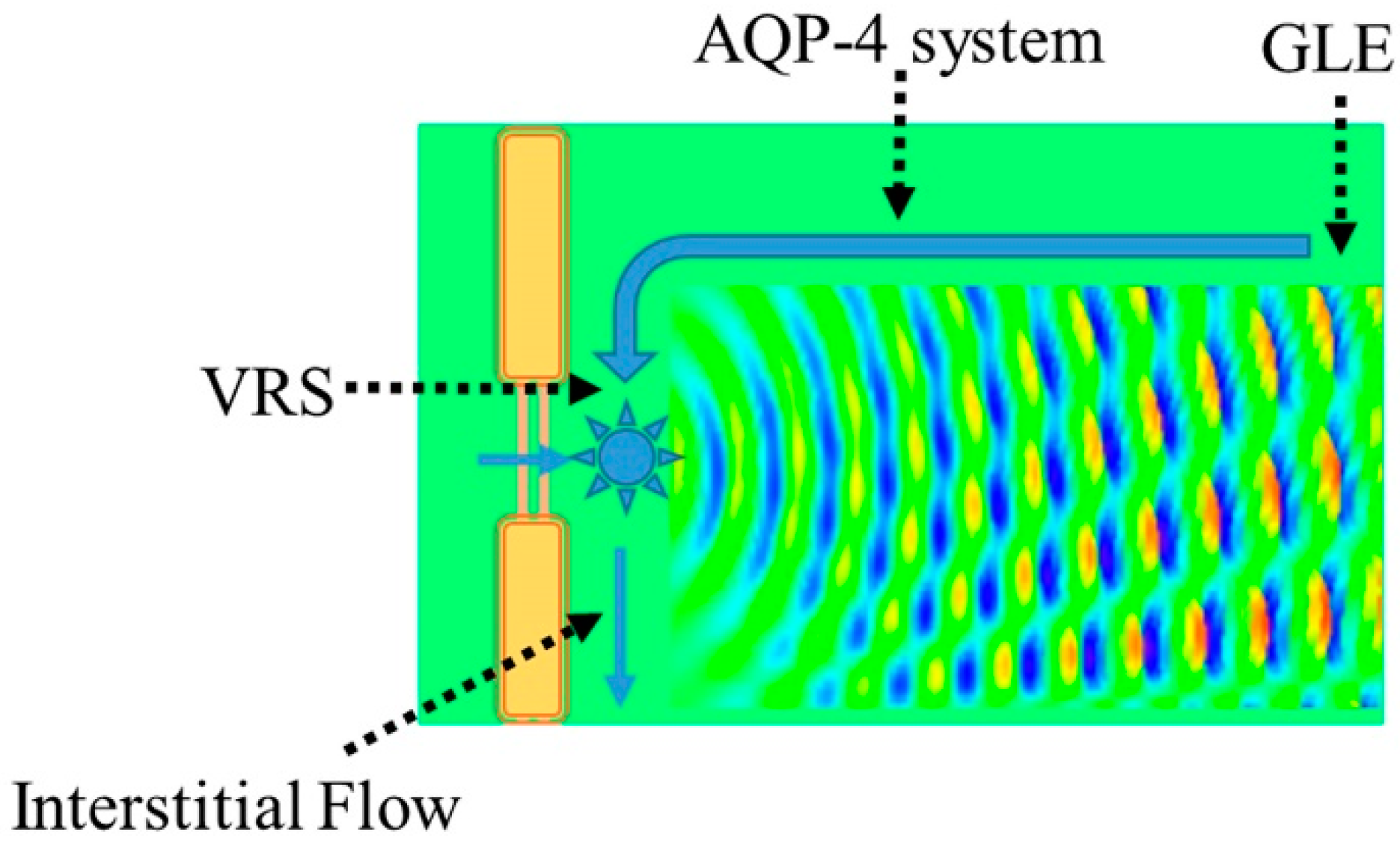
3.1. Virchow-Robin Space and Interstitial Flow
3.2. Neural Activities and AQP-4 Suppression
3.3. AQP-4 Suppressionand Neurovascular Coupling
4. AQP-4 and Interstitial Fluid Circulation
4.1. Interstitial Flow as Brain Lymphatic Equivalent–Glymphatic Flow
4.2. Glymphatic Flow and β-Amyloid Clearance
4.3. AQP-4 as Interstitial Fluid Circulator
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Reese, T.S.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Mack, A.F.; Wolburg, H. A novel look at astrocytes: Aquaporins, ionic homeostasis, and the role of the microenvironment for regeneration in the CNS. Neuroscientist 2013, 19, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Smith, B.L.; Christensen, E.I.; Agre, P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 1993, 90, 7275–7279. [Google Scholar] [CrossRef] [PubMed]
- Dolman, D.; Drndarski, S.; Abbott, N.J.; Rattray, M. Induction of aquaporin 1 but not aquaporin 4 messenger RNA in rat primary brain microvessel endothelial cells in culture. J. Neurochem. 2005, 93, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. et Biophys. Acta 2009, 1788, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Yu, A.S.L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Milatz, S.; Krug, S.M.; Oelrich, B.; Schulzke, J.D.; Amasheh, S.; Günzel, D.; Fromm, M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010, 123, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.C.; Tristram-Nagle, S.; Nagle, J.F.; Zeidel, M.L. Structural determinants of water permeability through the lipid membrane. J. Gen. Physiol. 2008, 131, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T. The molecular mechanisms of neural flow coupling: A new concept. J. Neuroimage 2015, 25, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.E. Fluid Dynamics for Physicists; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Munde, P.B.; Khandekar, S.P.; Dive, A.M.; Upadhyaya, N.R. Pericytes in Health and Disease. Int. J. Oral Maxillofac. Pathol. 2014, 5, 2–7. [Google Scholar]
- Levick, J. Introduction to Cardiovascular Physiology, 5th ed.; Hodder Arnold: London, UK, 2010. [Google Scholar]
- Paulson, O.B.; Strandgaard, S.; Edvinsson, L. Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev. 1990, 2, 161–192. [Google Scholar] [PubMed]
- Heistad, D.D.; Kontos, H.A. The Cardiovascular System III. In Handbook of Physiology; Berne, R.M., Sperelakis, N., Eds.; American Physiological Society: Bethesda, MD, USA, 1979; pp. 137–182. [Google Scholar]
- Mellander, S. Functional aspects of myogenic vascular control. J. Hypertens. 1989, 7, S21–S30. [Google Scholar]
- Osol, G.; Brekke, J.F.; McElroy-Yaggy, K.; Gokina, N.I. Myogenic tone, reactivity, and forced dilatation: A three-phase model of in vitro arterial myogenic behavior. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2260–H2267. [Google Scholar] [CrossRef] [PubMed]
- Busija, E.W.; Heistad, D.D. Factors involved in the physiological regulation of the cerebral circulation. Rev. Physiol. Biochem. Pharmcol. 1984, 101, 161–211. [Google Scholar]
- Talman, W.T.; Dragon, D.N. Neuronal nitric oxide mediates cerebral vasodilatation during acute hypertension. Brain Res. 2007, 1139, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Roland, P.E. Brain Activation; Wiley-Liss: New York, NY, USA, 1993. [Google Scholar]
- Nakada, T. Virchow-Robin space and aquaporin-4: New insights on an old friend. Croat. Med. J. 2014, 55, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Suzuki, K.; Kwee, I.L. Excess heat removal is likely to be the primary role of increase in regional cerebral blood flow associated with brain activation. In Proceedings of the Society of Neuroscience Annual Meeting, San Diego, CA, USA, 13–17 November 2010. [Google Scholar]
- Silver, I.A. Cellular microenvironment in relation to local blood flow. In Ciba Foundation Symposium 56–Cerebral Vascular Smooth Muscle and Its Control; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Whittaker, R.J.; Heil, M.; Jensen, O.E.; Waters, S. Predicting the onset of high-frequency elf-excited oscillations in elastic-walled tubes. Proc. R. Soc. A 2010, 466, 3635–3657. [Google Scholar] [CrossRef]
- Armitstead, J.P.; Bertram, C.D.; Jensen, O.E. A study of the bifurcation behaviour of a model of flow through a collapsible tube. Bull. Math. Biol. 1996, 58, 611–641. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. Ueber die Erweiterung kleinerer Gefaesse. Arch. Pathol. Anat. Physiol. Klin. Med. 1851, 3, 427–462. [Google Scholar] [CrossRef]
- Robin, C. Recherches sur quelques particularites de la structure des capillaires de l’encephale. J. Physiol. Homme. Animaux. 1859, 2, 537–548. [Google Scholar]
- Haj-Yasein, N.N.; Jensen, V.; Ostby, I.; Omholt, S.W.; Voipio, J.; Kaila, K.; Ottersen, O.P.; Hvalby, O.; Nagelhus, E.A. Aquaporin-4 regulates extracellular space volume dynamics during high-frequency synaptic stimulation: A gene deletion study in mouse hippocampus. Glia 2012, 60, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Tsujita, M.; Kwee, I.L.; Nakada, T. Water influx into cerebrospinal fluid (CSF) is primarily controlled by aquaporin-4, not by aquaporin-1: O-17 JJVCPE MRI Study in Knockout Mice. Neuroreport 2014, 25, 39–43. [Google Scholar] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Tsujita, M.; Huber, V.J.; Kwee, I.L.; Nakada, T. Inhibition of Aquaporin-4 significantly increases regional cerebral blood flow. NeuroReport 2013, 24, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Williamson, A.; Palomba, M.; Eid, T.; de Lanerolle, N.C.; Nagelhus, E.A.; Adams, M.E.; Froehner, S.C.; Agre, P.; Ottersen, O.P. Delayed K+ clearance associated with aquaporin-4 mislocalization: Phenotypic defects in brains of α-syntrophin-null mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13615–13620. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Hsu, M.S.; Seldin, M.M.; Arellano, J.L.; Binder, D.K. Decreased expression of the glial water channel aquaporin-4 in the intrahippocampal kainic acid model of epileptogenesis. Exp. Neurol. 2012, 235, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.L.; Sontheimer, H. Functional implications for Kir4.1 channels in glial biology: From K+ buffering to cell differentiation. Neurochemmistry 2008, 107, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Strohschein, S.; Hüttmann, K.; Gabriel, S.; Devin, K.; Binder, D.K.; Heinemann, U.; Steinhäuser, C. Impact of aquaporin-4 channels on K+ buffering and gap junction coupling in the hippocampus. Glia 2011, 59, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 2004, 129, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Koulakoff, A.; Roux, L.; Holcman, D.; Rouach, N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010, 11, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Skinner, M.A.; Shi, W.; Yu, Q.C.; Wildeman, A.G.; Chan, Y.M. The 16 kDa subunit of vacuolar H+-ATPase is a novel sarcoglycan-interacting protein. Biochim. Biophys. Acta 2007, 772, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Kurachi, Y. Distinct detergent-resistant membrane microdomains (lipid rafts) respectively harvest K(+) and water transport systems in brain astroglia. Eur. J. Neurosci. 2007, 26, 2539–2555. [Google Scholar] [CrossRef] [PubMed]
- Kaptan, S.; Assentoft, M.; Schneider, H.P.; Fenton, R.A.; Deitmer, J.W.; MacAulay, N.; de Groot, B.L. H95 is a pH-dependent gate in aquaporin 4. Structure 2015, 23, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Magnotta, V.A.; Heo, H.Y.; Dlouhy, B.J.; Dahdaleh, N.S.; Follmer, R.L.; Thedens, D.R.; Welsh, M.J.; Wemmie, J.A. Detecting activity-evoked pH changes in human brain. Proc. Natl. Acad. Sci. USA 2012, 109, 8270–8273. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Tsujita, M.; Huber, V.J.; Kakita, A.; Shibuki, K.; Sakimura, K.; Kwee, I.L.; Nakada, T. Activity-dependent glial swelling is impaired in aquaporin-4 knockout mice. Neurosci. Res. 2009, 64, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Esiri, M.M.; Gay, D. Immunological and neuropathological significance of the Virchow-Robin space. J. Neurol. Sci. 1990, 100, 3–8. [Google Scholar] [CrossRef]
- Weller, R.O. Pathology of cerebrospinal fluid and interstitial fluid of the CNS: Significance for Alzheimer’s disease, prion disorders and multiple sclerosis. J. Neuropathol. Exp. Neurol. 1998, 57, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Papaiconomou, C. Cerebrospinal fluid transport: A lymphatic perspective. News Physiol. Sci. 2002, 17, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. Neurochem. Int. 2004, 45, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Djuanda, E.; Yow, H.Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Orešković, D.; Klarica, M. The formation of cerebrospinal fluid: Nearly a hundred years of interpretations and misinterpretations. Brain Res. Rev. 2010, 64, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer Disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Margaris, K.N.; Black, R.A. Modelling the lymphatic system: Challenges and opportunities. J. R. Soc. Interface 2012, 9, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Suzuki, Y.; Kwee, I.L.; Nakada, T. Water influx into cerebrospinal fluid is significantly reduced in senile plaque bearing transgenic mice, supporting β-amyloid clearance hypothesis of Alzheimer disease. Neurological Res. 2014, 36, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nakamura, Y.; Yamada, K.; Igarashi, H.; Kasuga, K.; Yokoyama, Y.; Ikeuchi, T.; Nishizawa, M.; Kwee, I.L.; Nakada, T. Reduced CSF water influx in Alzheimer’s disease supporting the β-amyloid clearance hypothesis. PLoS ONE 2015, 10, e0123708. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Mastrototaro, M.; Rossi, A.; Pisani, F.; Tortorella, C.; Ruggieri, M.; Lia, A.; Trojano, M.; Frigeri, A.; Svelto, M. Aquaporin-4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies. Glia 2009, 57, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Salam, E.A.; Abdel-Meguid, I.E.; Shatla, R.; Korraa, S. Evaluation of neural damage in Duchenne muscular dystrophy patients. Acta Myol. 2014, 33, 13–18. [Google Scholar] [PubMed]
- Suzuki, Y.; Higuchi, S.; Aida, I.; Nakajima, T.; Nakada, T. Abnormal distribution of GABAA receptors in brain of Duchenne muscular dystrophy patients. Muscle Nerve 2017, 55, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D. Amyloid-β impairs synaptic inhibition via GABA (A) receptor endocytosis. J. Neurosci. 2015, 35, 9205–9210. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E.; Yasumura, T.; Hudson, C.S.; Agre, P.; Nielsen, S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl. Acad. Sci. USA 1998, 95, 11981–11986. [Google Scholar] [CrossRef] [PubMed]
- Neely, J.D.; Christensen, B.M.; Nielsen, S.; Agre, P. Heterotetrameric composition of aquaporin-4 water channels. Biochemistry 1999, 38, 11156–11163. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamada, K.; Suzuki, Y.; Kwee, I.L.; Nakada, T. 7.0 Tesla MRI reveals electrostatic environment of the glia limitans. In Proceedings of the Society of Neuroscience Annual Meeting, Chicago, IL, USA, 17–21 October 2010. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakada, T.; Kwee, I.L.; Igarashi, H.; Suzuki, Y. Aquaporin-4 Functionality and Virchow-Robin Space Water Dynamics: Physiological Model for Neurovascular Coupling and Glymphatic Flow. Int. J. Mol. Sci. 2017, 18, 1798. https://doi.org/10.3390/ijms18081798
Nakada T, Kwee IL, Igarashi H, Suzuki Y. Aquaporin-4 Functionality and Virchow-Robin Space Water Dynamics: Physiological Model for Neurovascular Coupling and Glymphatic Flow. International Journal of Molecular Sciences. 2017; 18(8):1798. https://doi.org/10.3390/ijms18081798
Chicago/Turabian StyleNakada, Tsutomu, Ingrid L. Kwee, Hironaka Igarashi, and Yuji Suzuki. 2017. "Aquaporin-4 Functionality and Virchow-Robin Space Water Dynamics: Physiological Model for Neurovascular Coupling and Glymphatic Flow" International Journal of Molecular Sciences 18, no. 8: 1798. https://doi.org/10.3390/ijms18081798
APA StyleNakada, T., Kwee, I. L., Igarashi, H., & Suzuki, Y. (2017). Aquaporin-4 Functionality and Virchow-Robin Space Water Dynamics: Physiological Model for Neurovascular Coupling and Glymphatic Flow. International Journal of Molecular Sciences, 18(8), 1798. https://doi.org/10.3390/ijms18081798