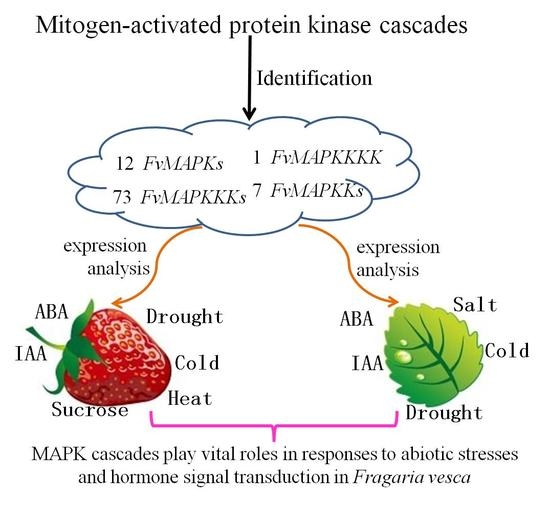
Identification and Analysis of Mitogen-Activated Protein Kinase (MAPK) Cascades in Fragaria vesca
Abstract
:1. Introduction
2. Results
2.1. Genome-Wide Identification of MAPK Cascade Genes in F. vesca
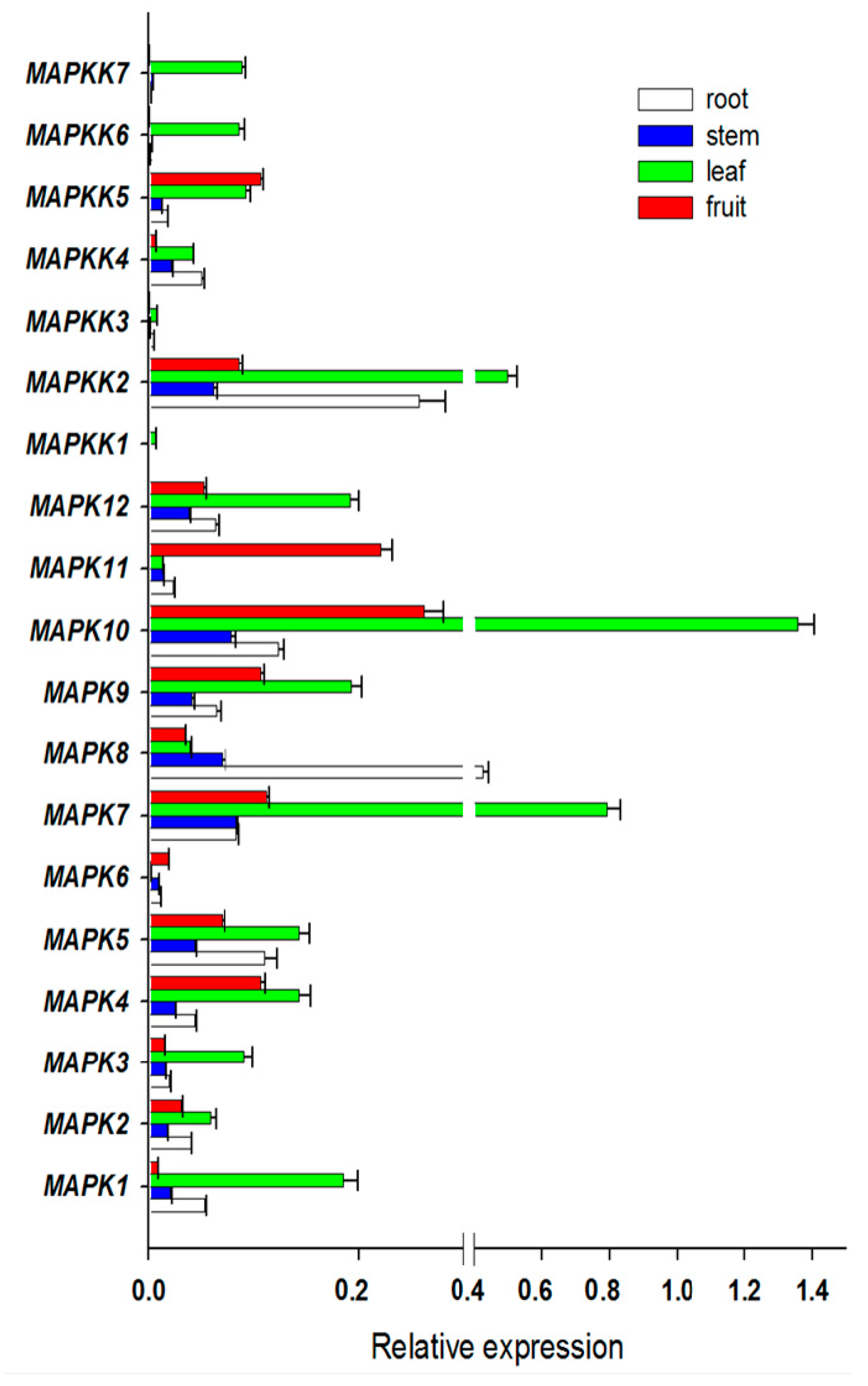
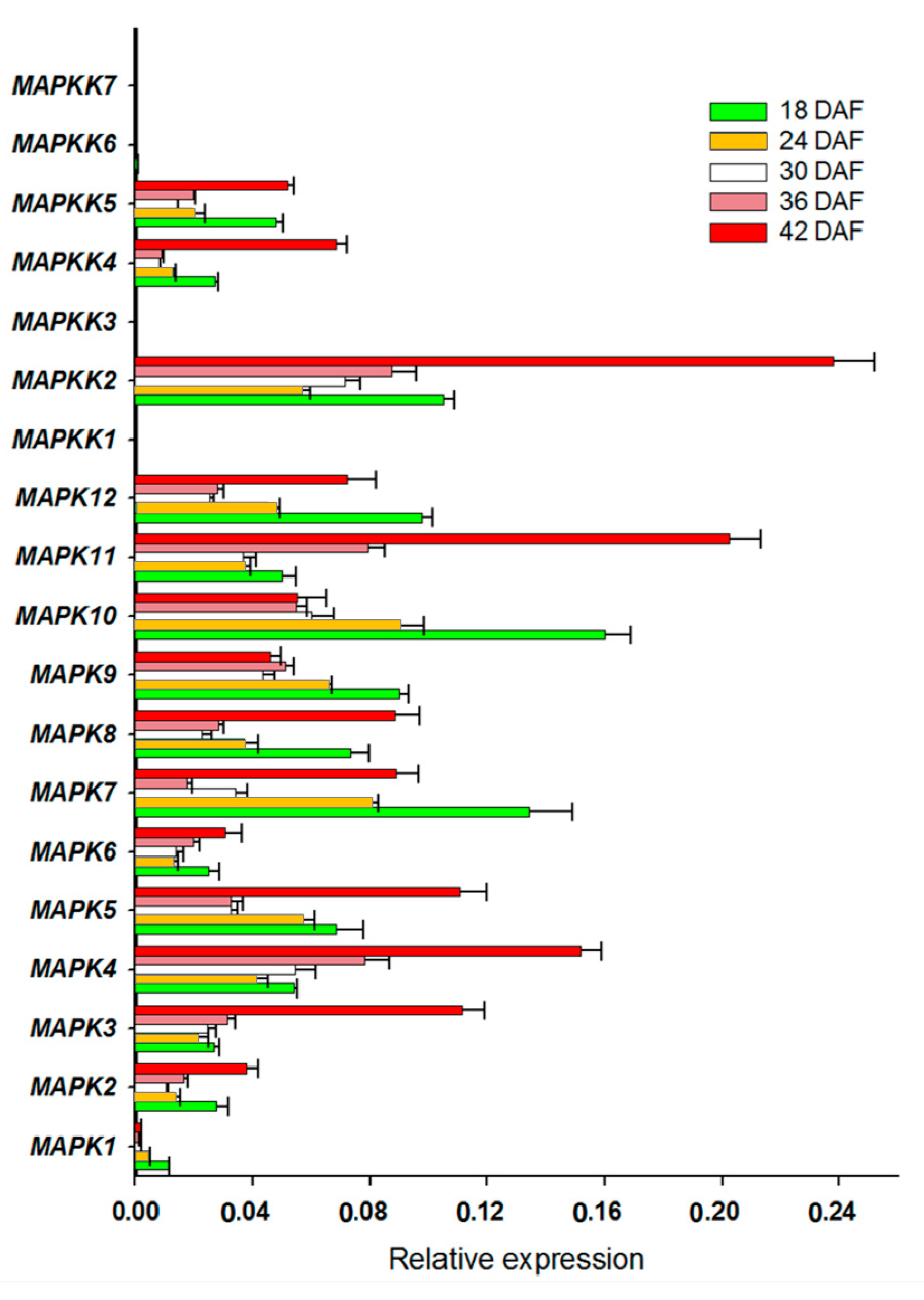
2.2. Expression Profiles of FvMAPKs and FvMAPKKs in Different Organs and Fruit Developmental Stages
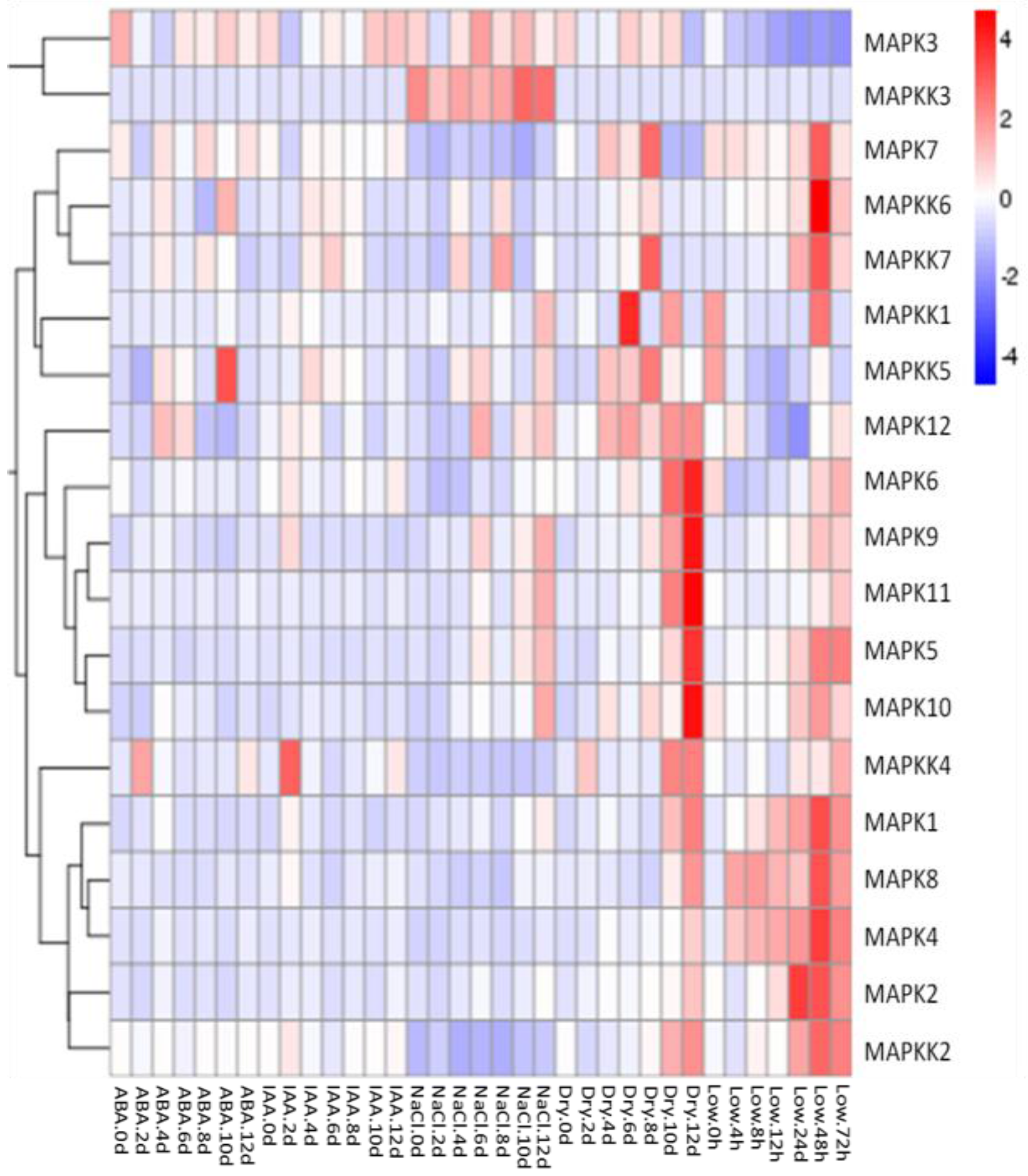
2.3. Gene Expression Profiles of FvMAPKs and FvMAPKKs in Response to Hormones and Abiotic Stresses in F. vesca Leaves
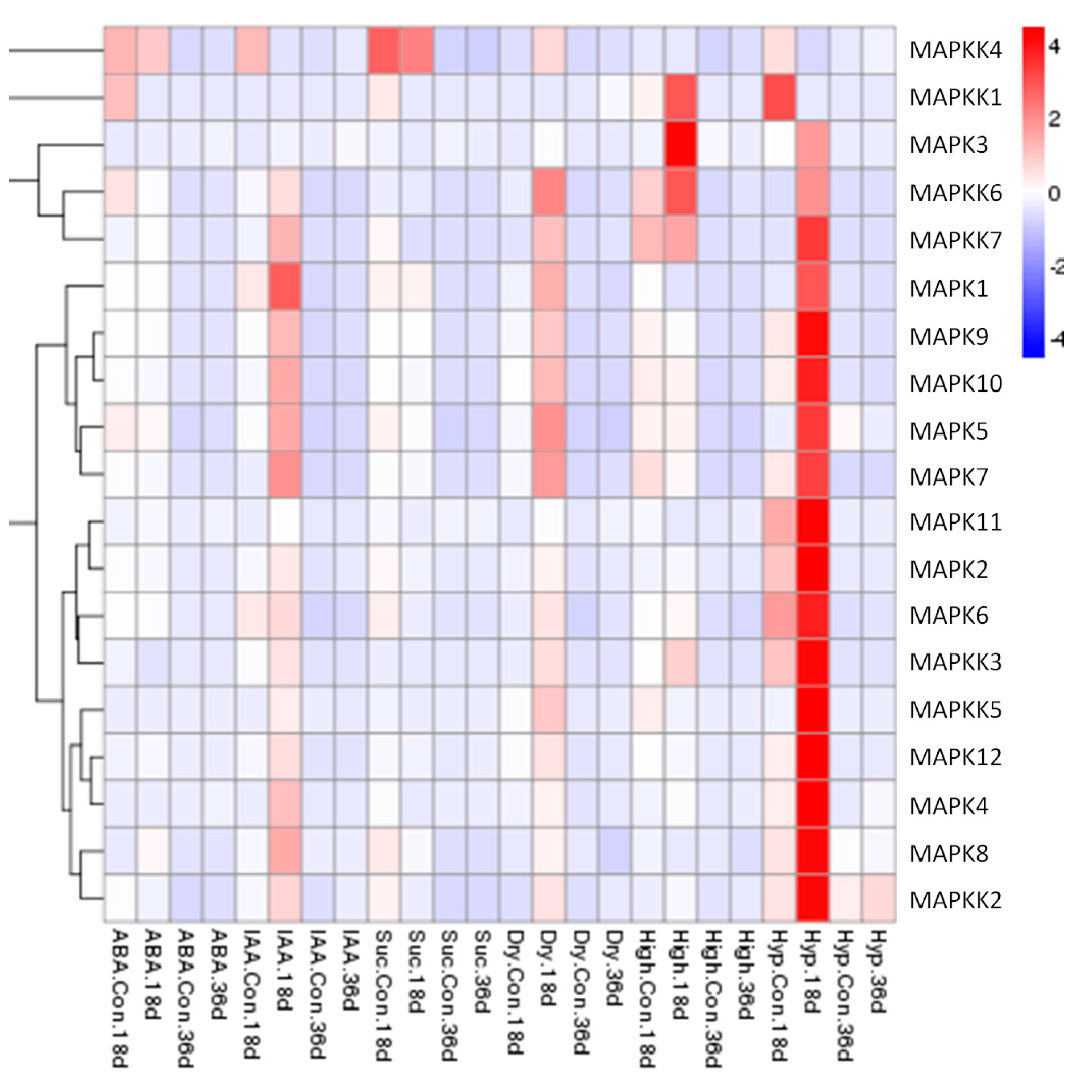
2.4. Expression Patterns of FvMAPK and FvMAPKK Genes in Response to Sucrose, Hormones and Abiotic Stresses in F. vesca Fruits
3. Discussion
4. Materials and Methods
4.1. Identification of Potential MAPK Cascade Gene Family Members in Strawberry
4.2. Phylogenetic Analysis of A. Thaliana and the Strawberry MAPK Cascade Genes
4.3. Plant Material and Fruit Pre-Treatments
4.4. The Processing of Strawberry Leaves under Different Stress and Hormone Treatments
4.5. RNA Extraction and Real-Time PCR Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baluška, F.; Mancuso, S. Signaling in Plants, Signaling and Communication in Plants; Springer-Verlag: New York, NY, USA; Berlin/Heidelberg, Germany, 2009; pp. 51–69. [Google Scholar]
- Hamel, L.P.; Nicole, M.C.; Sritubtim, S.; Morency, M.J.; Ellis, M.; Ehlting, J. Ancient signals: Comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Group, M.; Ichimura, K.; Shinozaki, K.; Tena, G.; Sheen, J.; Henry, Y. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar] [CrossRef]
- Chen, Z.; Gibson, T.B.; Robinson, F.; Silvestro, L.; Pearson, G.; Xu, B. MAP kinases. Chem. Rev. 2001, 101, 2449–2476. [Google Scholar] [CrossRef] [PubMed]
- Champion, A.; Picaud, A.; Henry, Y. Reassessing the MAP3K and MAP4K relationships. Trends Plant Sci. 2004, 9, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.; Ökrész, L.; Bögre, L.; Hirt, H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr. Opin. Plant Biol. 2002, 5, 415–424. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Arora, P.K.; Mohanta, N.; Parida, P.; Bae, H. Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants. BMC Genom. 2015, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Luo, T.; Fu, X.; Fan, Q.; Liu, J. Cloning and molecular characterization of a mitogen-activated protein kinase gene from Poncirus trifoliata whose ectopic expression confers dehydration/drought tolerance in transgenic tobacco. J. Exp. Bot. 2011, 62, 5191–5206. [Google Scholar] [CrossRef] [PubMed]
- Stulemeijer, I.J.; Stratmann, J.W.; Joosten, M.H. Tomato mitogen-activated protein kinases LeMPK1, LeMPK2, and LeMPK3 are activated during the Cf-4/Avr4-Induced hypersensitive response and have distinct phosphorylation specificities. Plant Physiol. 2007, 144, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Zaïdi, I.; Ebel, C.; Touzri, M.; Herzog, E.; Evrard, J.L.; Schmit, A. TMKP1 is a novel wheat stress responsive MAP Kinase phosphatase localized in the nucleus. Plant Mol. Biol. 2010, 73, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Khokhlatchev, A.V.; Canagarajah, B.; Wilsbacher, J.; Robinson, M.; Atkinson, M. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 1998, 93, 605–615. [Google Scholar] [CrossRef]
- Nadarajah, K.; Sidek, H. The green MAPKs. Asian J. Plant Sci. 2010, 9, 1–10. [Google Scholar] [CrossRef]
- Kumar, K.; Rao, K.P.; Biswas, D.K.; Sinha, A.K. Rice WNK1 is regulated by abiotic stress and involved in internal circadian rhythm. Plant Signal. Behav. 2011, 6, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Romeis, T. Protein kinases in the plant defence response. Curr. Opin. Plant Biol. 2001, 4, 407–414. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Tena, G.; Xiong, Y.; Sheen, J. Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature 2008, 451, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Cai, Z.; Guo, Y.; Gan, S. An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol. 2009, 150, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Tena, G.; Asai, T.; Chiu, W.-L.; Sheen, J. Plant mitogen-activated protein kinase signaling cascades. Curr. Opin. Plant Biol. 2001, 4, 392–400. [Google Scholar] [CrossRef]
- Xing, Y.; Cao, Q.Q.; Zhang, Q.; Shen, Y.Y.; Qin, L.; Zhang, J. MKK5 regulates high light-induced gene expression of Cu/Zn superoxide dismutase 1 and 2 in Arabidopsis. Plant Cell. Physiol. 2013, 54, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Chen, W.; Jia, W.; Zhang, J. Mitogen-activated protein kinase kinase 5 (MKK5)-mediated signalling cascade regulates expression of iron superoxide dismutase gene in Arabidopsis under salinity stress. J. Exp. Bot. 2015, 66, 5971–5981. [Google Scholar] [CrossRef] [PubMed]
- Teige, M.; Scheikl, E.; Eulgem, T.; Doczi, R.; Ichimura, K.; Shinozaki, K. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Jia, W.; Zhang, J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 2008, 54, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Jia, W.; Zhang, J. AtMKK1 and AtMPK6 are involved in abscisic acid and sugar signaling in Arabidopsis seed germination. Plant Mol. Biol. 2009, 70, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, H.; Zhang, A.; Ma, F.; Cao, J.; Jiang, M. A novel Mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. J. Integr. Plant Biol. 2010, 52, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, J.; Ye, N.; Cao, J.; Tan, M.; Zhang, J. ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize. J. Exp. Bot. 2010, 61, 4399–4411. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, M.; Kong, X.; Xing, X.; Liu, Y.; Sun, L. ZmMPK17, a novel maize group D MAP kinase gene, is involved in multiple stress responses. Planta 2012, 235, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, B.G.; Kwon, T.R.; Jeong, M.J.; Park, S.R.; Lee, J.W. Overexpression of the mitogen-activated protein kinase gene OsMAPK33 enhances sensitivity to salt stress in rice (Oryza sativa L.). J. Biosci. 2011, 36, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.J.; Lee, S.K.; Kim, B.G.; Kwon, T.R.; Cho, W.S.; Park, Y.T. A rice (Oryza sativa L.) MAP kinase gene, OsMAPK44, is involved in response to abiotic stresses. Plant Cell Tissue Organ Cult. 2006, 85, 151–160. [Google Scholar] [CrossRef]
- Rao, K.P.; Richa, T.; Kumar, K.; Raghuram, B.; Sinha, A.K. In silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res. 2010, 17, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Katou, S.; Seto, H.; Gomi, K.; Ohashi, Y. The mitogen-activated protein kinases WIPK and SIPK regulate the levels of jasmonic and salicylic acids in wounded tobacco plants. Plant J. 2007, 49, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Kiegerl, S.; Cardinale, F.; Siligan, C.; Gross, A.; Baudouin, E.; Liwosz, A. SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 2000, 12, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, F.; Meskiene, I.; Ouaked, F.; Hirt, H. Convergence and divergence of stress-induced mitogen-activated protein kinase signaling pathways at the level of two distinct mitogen-activated protein kinase kinases. Plant Cell 2002, 14, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, C.; Wang, Y.; Ye, L.; Wu, J.; Chen, L. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genom. 2015, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Galletti, R.; Ferrari, S.; DeLorenzo, G. Arabidopsis MPK3 and MPK6 play different roles in basal and oligogalacturonide-or flagellin-induced resistance against Botrytis cinerea. Plant Physiol. 2011, 157, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Tang, D.; Innes, R.W. Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.R.; Kirti, P.B. A mitogen-activated protein kinase, AhMPK6 from peanut localizes to the nucleus and also induces defense responses upon transient expression in tobacco. Plant Physiol. Biochem. 2010, 48, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signalling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Reyna, N.S.; Yang, Y. Molecular analysis of the rice MAP kinase gene family in relation to Magnaporthe grisea infection. Mol. Plant Microbe. Interact. 2006, 19, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xue, Q. Computational identification and phylogenetic analysis of the MAPK gene family in Oryza sativa. Plant Physiol. Biochem. 2007, 45, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, D.P.; Misra, M.; Singh, P.; Sinha, A.K. Rice mitogen activated protein kinase kinase and mitogen activated protein kinase interaction network revealed by in-silico docking and yeast two-hybrid approaches. PLoS ONE 2013, 8, e65011. [Google Scholar] [CrossRef] [PubMed]
- Birsen, C.; Ozan, K. Mitogen-activated protein kinase cascades in Vitis vinifera. Front. Plant Sci. 2015, 6, 556. [Google Scholar]
- Shulaev, V.; Sargent, D.J.; Crowhurst, R.N.; Mockler, T.C.; Folkerts, O.; Delcher, A.L. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 2011, 43, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Daigle, C.; Stafford-Richard, T.; Tebbji, F.; Lafleur, E.; Caron, S. Characterization of ScMAP4K1, a MAP kinase kinase kinase kinase involved in ovule, seed and fruit development in Solanum chacoense Bitt. Curr. Top. Plant. Biol. 2009, 10, 27–46. [Google Scholar]
- Bögre, L.; Calderini, O.; Binarova, P.; Mattauch, M.; Till, S.; Kiegerl, S. A MAP kinase is activated late in plant mitosis and becomes localized to the plane of cell division. Plant Cell 1999, 11, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hwa, C.M.; Yang, X.C. The AtMKK3 pathway mediates ABA and salt signaling in Arabidopsis. Acta. Physiol. Plantarum. 2008, 30, 277–286. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Irie, K.; Hirayama, T.; Hayashida, N.; Yamaguchi-Shinozaki, K.; Matsumoto, K. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Berberich, T.; Sano, H.; Kusano, T. Involvement of a MAP kinase, ZmMPK5, in senescence and recovery from low-temperature stress in maize. Mol. Gen. Genet. 1999, 262, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Doczi, R.; Brader, G.; Pettko-Szandtner, A.; Rajh, I.; Djamei, A.; Pitzschke, A. The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. Plant Cell 2007, 19, 3266–3279. [Google Scholar] [CrossRef] [PubMed]
- Mockaitis, K.; Howell, S.H. Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J. 2000, 24, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Roles of mitogen-activated protein kinase cascades in ABA signaling. Plant Cell Rep. 2012, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Wang, Y.; Liu, H.X.; Lei, L.; Yang, H.L. Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J. Biol. Chem. 2008, 283, 26996–27006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Klessig, D.F. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001, 6, 520–527. [Google Scholar] [CrossRef]
- Yuasa, T.; Ichimura, K.; Mizoguchi, T.; Shinozaki, K. Oxidative stress activates ATMPK6, an Arabidopsis homologue of MAP kinase. Plant Cell Physiol. 2001, 42, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.; Sanssené, J.; Rossard, S.; Courtois, J. Systemic induction of the defensin and phytoalexin pisatin pathways in pea (Pisum sativum) against Aphanomyces euteiches by acetylated and nonacetylated oligogalacturonides. Molecules 2017, 22, 1017. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
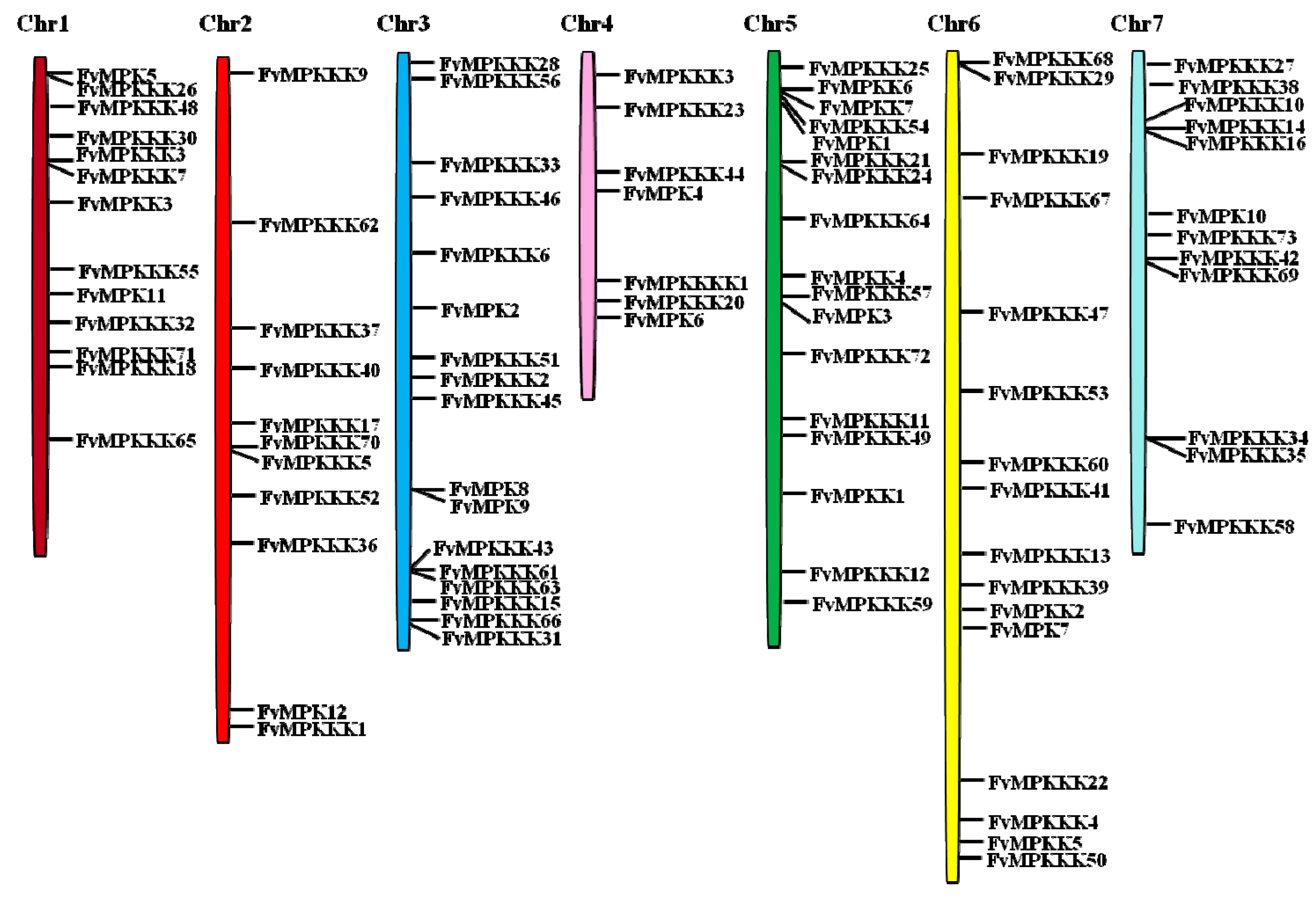

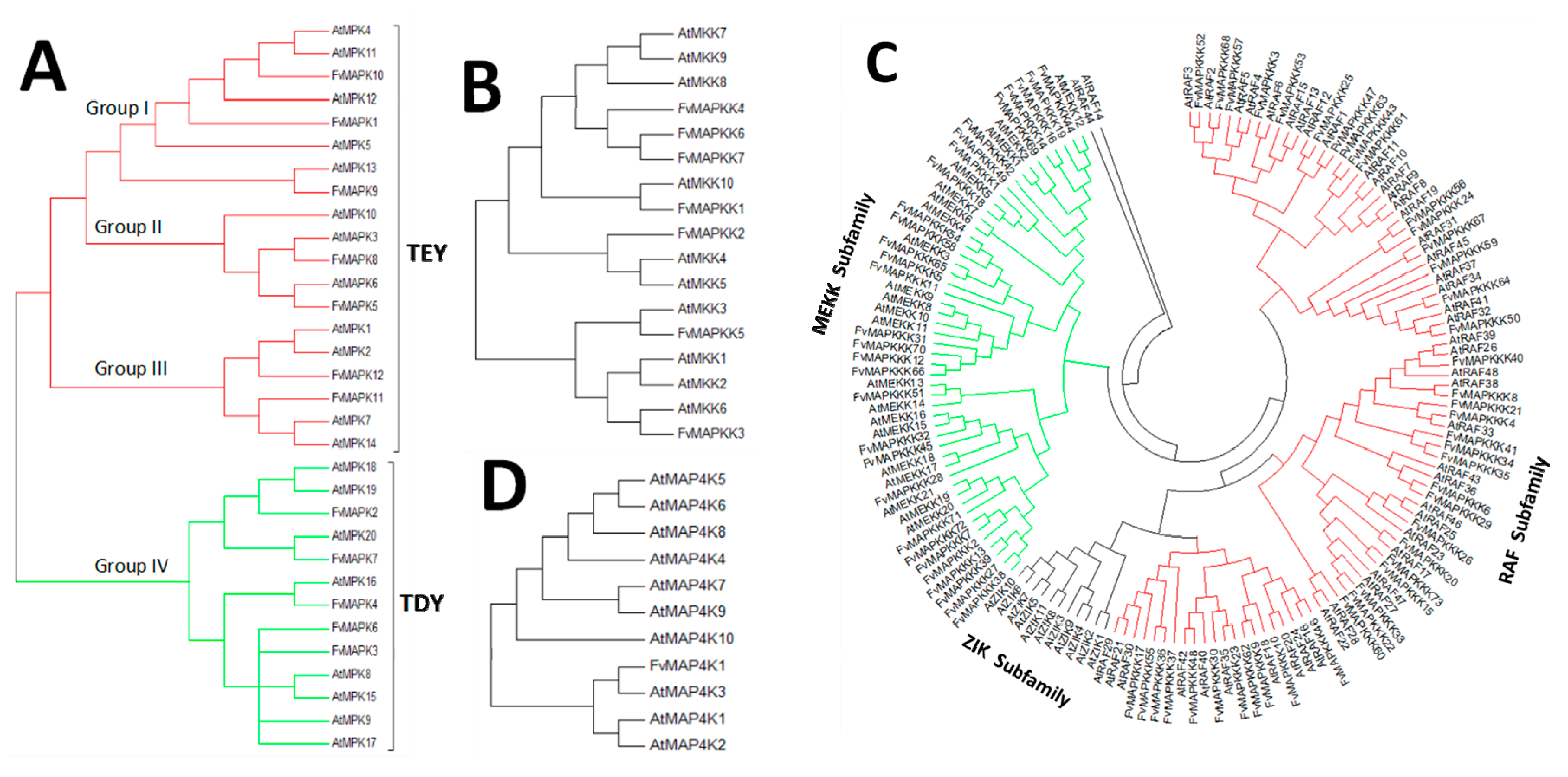

| Gene Name | Gene ID | Chr | Location | ORF (aa) | mW (kDa) | pI | Instability Index | Ai | GRAVY | Number of Exons |
|---|---|---|---|---|---|---|---|---|---|---|
| MAPKs | ||||||||||
| MAPK1 | 101290762 | 5 | 1784699..1788845 | 377 | 43.24 | 6.4 | 46.14 | 90.53 | −0.37 | 6 |
| MAPK2 | 101294541 | 3 | 11156420..11160717 | 609 | 69.37 | 9.26 | 32.32 | 83.92 | −0.44 | 10 |
| MAPK3 | 101295052 | 5 | 10188960..10193438 | 691 | 78.45 | 7.86 | 44.65 | 75.96 | −0.64 | 11 |
| MAPK4 | 101295411 | 4 | 13067191..13073043 | 561 | 63.82 | 8.73 | 36.24 | 77.4 | −0.48 | 10 |
| MAPK5 | 101297368 | 1 | 1081422..1084925 | 391 | 44.71 | 5.65 | 39.58 | 89.82 | −0.28 | 5 |
| MAPK6 | 101300335 | 4 | 18603202..18607991 | 580 | 66.33 | 7.07 | 38.18 | 80.53 | −0.55 | 12 |
| MAPK7 | 101306152 | 6 | 26819611..26825467 | 618 | 70.68 | 9.23 | 37.11 | 81.12 | −0.47 | 10 |
| MAPK8 | 101306313 | 3 | 19690549..19693371 | 371 | 42.71 | 5.62 | 39.46 | 93.32 | −0.26 | 6 |
| MAPK9 | 101308652 | 3 | 19870360..19873988 | 365 | 41.85 | 5.05 | 42.04 | 95.34 | −0.28 | 6 |
| MAPK10 | 101313449 | 7 | 4574723..4578939 | 373 | 42.67 | 6.08 | 43.9 | 89.38 | −0.33 | 6 |
| MAPK11 | 101313547 | 1 | 9041132..9043978 | 370 | 42.64 | 8.32 | 33.43 | 94.32 | −0.24 | 3 |
| MAPK12 | 101314433 | 2 | 32254545..32257169 | 372 | 42.64 | 6.09 | 38.46 | 95.13 | −0.25 | 3 |
| MAPKKs | ||||||||||
| MAPKK1 | 101296494 | 5 | 21018101..21019144 | 347 | 38.73 | 5.64 | 58.27 | 86.54 | −0.19 | 1 |
| MAPKK2 | 101298915 | 6 | 26457958..26460120 | 365 | 40.37 | 9.28 | 60.36 | 76.68 | −0.44 | 1 |
| MAPKK3 | 101299988 | 1 | 5116387..5119659 | 355 | 39.86 | 6.14 | 51.1 | 95.75 | −0.18 | 8 |
| MAPKK4 | 101300536 | 5 | 9858540..9860210 | 325 | 36.28 | 8.01 | 58.45 | 82.52 | −0.26 | 1 |
| MAPKK5 | 101302771 | 6 | 37368876..37372756 | 518 | 57.53 | 5.34 | 45.36 | 89.63 | −0.15 | 9 |
| MAPKK6 | 101309937 | 5 | 1450708..1451997 | 244 | 26.72 | 6.44 | 56.61 | 80.29 | −0.31 | 1 |
| MAPKK7 | 101310227 | 5 | 1459329..1461416 | 350 | 38.86 | 6.01 | 67.41 | 76.06 | −0.35 | 1 |
| MAPKKKs | ||||||||||
| MAPKKK1 | 101291312 | 2 | 32932997..32939667 | 714 | 78.16 | 9.58 | 56.43 | 66.79 | −0.55 | 10 |
| MAPKKK2 | 101291614 | 3 | 13958286..13959565 | 311 | 34.68 | 5.59 | 39.15 | 79.9 | −0.31 | 1 |
| MAPKKK3 | 101291671 | 1 | 4101379..4109515 | 973 | 108.54 | 7.03 | 45.49 | 81.31 | −0.42 | 15 |
| MAPKKK4 | 101291740 | 6 | 36971098..36974966 | 384 | 42.80 | 6.76 | 36.76 | 75.62 | −0.49 | 6 |
| MAPKKK5 | 101292378 | 2 | 18357934..18361624 | 278 | 31.77 | 9.66 | 43.47 | 96.12 | −0.04 | 8 |
| MAPKKK6 | 101292778 | 3 | 8405027..8408729 | 539 | 61.14 | 9.57 | 50.83 | 76.36 | −0.57 | 4 |
| MAPKKK7 | 101292844 | 1 | 4173320..4175043 | 265 | 29.70 | 5.77 | 46.62 | 83.47 | −0.40 | 1 |
| MAPKKK8 | 101293476 | 4 | 7105385..7111294 | 390 | 43.69 | 6.48 | 32.42 | 79.23 | −0.44 | 7 |
| MAPKKK9 | 101294141 | 2 | 2588978..2594536 | 1126 | 125.14 | 5.80 | 51.66 | 74.17 | −0.58 | 9 |
| MAPKKK10 | 101294311 | 7 | 2095625..2102937 | 1221 | 135.58 | 5.47 | 47.97 | 72.09 | −0.61 | 9 |
| MAPKKK11 | 101294663 | 5 | 16790800..16799279 | 511 | 55.61 | 8.61 | 52.95 | 79.77 | −0.45 | 8 |
| MAPKKK12 | 101294665 | 5 | 24653764..24659548 | 591 | 65.24 | 5.68 | 54.8 | 70.93 | −0.59 | 9 |
| MAPKKK13 | 101294687 | 6 | 25086652..25087498 | 214 | 24.04 | 6.34 | 49.53 | 82.9 | −0.47 | 2 |
| MAPKKK14 | 101295466 | 7 | 2167754..2172789 | 690 | 76.10 | 6.23 | 37.12 | 84.51 | −0.24 | 15 |
| MAPKKK15 | 101295905 | 3 | 26006132..26009750 | 415 | 46.98 | 7.91 | 40.22 | 86.7 | −0.43 | 12 |
| MAPKKK16 | 101296046 | 7 | 2179758..2185318 | 678 | 75.20 | 5.52 | 39.59 | 87.45 | −0.22 | 15 |
| MAPKKK17 | 101296626 | 2 | 17778744..17784528 | 570 | 63.92 | 5.88 | 55.83 | 84.63 | −0.49 | 16 |
| MAPKKK18 | 101297008 | 1 | 11236088..11240295 | 625 | 68.99 | 9.27 | 67.35 | 68.82 | −0.54 | 11 |
| MAPKKK19 | 101297162 | 6 | 5243004..5248949 | 670 | 74.51 | 5.97 | 57.02 | 71.43 | −0.53 | 17 |
| MAPKKK20 | 101298508 | 4 | 17684149..17687733 | 434 | 49.07 | 7.79 | 36.92 | 84.06 | −0.48 | 11 |
| MAPKKK21 | 101298797 | 5 | 2958127..2962070 | 404 | 44.58 | 7.51 | 37.14 | 80.37 | −0.39 | 6 |
| MAPKKK22 | 101298822 | 6 | 35785690..35790732 | 457 | 51.78 | 6.10 | 44.73 | 84.07 | −0.46 | 13 |
| MAPKKK23 | 101299949 | 4 | 8553004..8558770 | 1092 | 121.56 | 5.55 | 43.15 | 72.31 | −0.59 | 9 |
| MAPKKK24 | 101299957 | 5 | 2999007..3002862 | 347 | 39.54 | 8.04 | 52.15 | 82.65 | −0.33 | 3 |
| MAPKKK25 | 101300060 | 5 | 1036941..1041266 | 697 | 78.06 | 6.49 | 44.46 | 77.91 | −0.58 | 14 |
| MAPKKK26 | 101300175 | 1 | 1133811..1138366 | 475 | 53.79 | 9.10 | 44.71 | 86.82 | −0.45 | 13 |
| MAPKKK27 | 101300188 | 7 | 404217..406592 | 344 | 37.94 | 5.76 | 36.34 | 88.46 | −0.07 | 1 |
| MAPKKK28 | 101300232 | 3 | 626166..628364 | 407 | 44.97 | 4.89 | 47.56 | 75.92 | −0.30 | 1 |
| MAPKKK29 | 101300748 | 6 | 105416..107101 | 426 | 47.79 | 8.88 | 45.4 | 80.61 | −0.45 | 1 |
| MAPKKK30 | 101302206 | 1 | 3358613..3363681 | 995 | 109.61 | 8.87 | 43.56 | 77.84 | −0.47 | 8 |
| MAPKKK31 | 101302247 | 3 | 26940964..26947028 | 717 | 79.93 | 5.70 | 43.53 | 77.98 | −0.33 | 10 |
| MAPKKK32 | 101302307 | 1 | 10029166..10031248 | 415 | 45.28 | 5.03 | 46.91 | 84.36 | −0.14 | 1 |
| MAPKKK33 | 101302624 | 3 | 4232964..4238052 | 458 | 51.96 | 7.59 | 49.66 | 90.72 | −0.41 | 12 |
| MAPKKK34 | 101302797 | 7 | 19633448..19634707 | 325 | 36.69 | 8.72 | 39.02 | 80.98 | −0.36 | 1 |
| MAPKKK35 | 101303378 | 7 | 19639527..19640520 | 322 | 36.61 | 8.80 | 40.45 | 87.17 | −0.42 | 1 |
| MAPKKK36 | 101303395 | 2 | 22992935..22998826 | 555 | 61.75 | 6.30 | 47.28 | 86.74 | −0.36 | 16 |
| MAPKKK37 | 101303762 | 2 | 14719795..14732427 | 554 | 62.13 | 5.37 | 42.28 | 87.27 | −0.39 | 16 |
| MAPKKK38 | 101303943 | 7 | 620946..625977 | 343 | 37.74 | 6.05 | 38.37 | 87.03 | −0.10 | 3 |
| MAPKKK39 | 101304212 | 6 | 25909877..25910863 | 328 | 36.85 | 8.61 | 42.91 | 89.45 | −0.25 | 1 |
| MAPKKK40 | 101304439 | 2 | 16018344..16021537 | 400 | 44.29 | 7.53 | 30.1 | 75.07 | −0.42 | 6 |
| MAPKKK41 | 101305170 | 6 | 21759476..21761269 | 309 | 34.91 | 4.67 | 38.89 | 94.4 | −0.13 | 4 |
| MAPKKK42 | 101305413 | 7 | 5759511..5762791 | 444 | 49.43 | 6.92 | 55.58 | 82.79 | −0.26 | 10 |
| MAPKKK43 | 101305446 | 3 | 25233157..25239380 | 777 | 86.22 | 6.83 | 50.65 | 68.51 | −0.66 | 13 |
| MAPKKK44 | 101305461 | 4 | 12404607..12409591 | 677 | 74.37 | 7.24 | 47.43 | 74.93 | −0.44 | 17 |
| MAPKKK45 | 101305547 | 3 | 15016023..15017869 | 447 | 49.64 | 4.50 | 50.48 | 80.02 | −0.19 | 1 |
| MAPKKK46 | 101305739 | 3 | 5762142..5770103 | 1323 | 145.89 | 5.11 | 46.54 | 75.92 | −0.56 | 9 |
| MAPKKK47 | 101305774 | 6 | 13132618..13140647 | 845 | 93.61 | 5.77 | 44.72 | 81.34 | −0.37 | 16 |
| MAPKKK48 | 101305797 | 1 | 2243252..2248966 | 1403 | 152.80 | 5.25 | 47.01 | 73.02 | −0.52 | 10 |
| MAPKKK49 | 101307123 | 5 | 17495323..17497199 | 405 | 45.08 | 5.36 | 55.35 | 76.07 | −0.16 | 2 |
| MAPKKK50 | 101307418 | 6 | 37601874..37604741 | 346 | 38.84 | 7.21 | 42.97 | 84.91 | −0.24 | 6 |
| MAPKKK51 | 101307975 | 3 | 13141362..13142841 | 488 | 53.77 | 4.85 | 49.32 | 79.04 | −0.15 | 1 |
| MAPKKK52 | 101308436 | 2 | 20472173..20477216 | 927 | 101.52 | 5.36 | 43.52 | 83.95 | −0.32 | 13 |
| MAPKKK53 | 101308592 | 6 | 15883396..15899615 | 765 | 85.91 | 5.77 | 51.23 | 80.58 | −0.41 | 18 |
| MAPKKK54 | 101308868 | 5 | 1573420..1580407 | 902 | 98.41 | 9.44 | 60.15 | 64.01 | −0.60 | 12 |
| MAPKKK55 | 101309867 | 1 | 8084450..8090390 | 572 | 64.91 | 5.69 | 49.85 | 83.5 | −0.43 | 16 |
| MAPKKK56 | 101309911 | 3 | 949163..951911 | 374 | 42.66 | 8.96 | 46.65 | 81.1 | −0.42 | 3 |
| MAPKKK57 | 101310026 | 5 | 10174217..10182364 | 1034 | 112.94 | 5.53 | 48.91 | 81.21 | −0.44 | 13 |
| MAPKKK58 | 101310764 | 7 | 22365118..22371514 | 903 | 97.23 | 9.71 | 65.72 | 62.98 | −0.59 | 12 |
| MAPKKK59 | 101312454 | 5 | 26796369..26801049 | 390 | 44.24 | 5.57 | 43.6 | 83.49 | −0.40 | 6 |
| MAPKKK60 | 101312659 | 6 | 20974416..20976740 | 433 | 49.51 | 5.10 | 37.22 | 83.79 | −0.48 | 5 |
| MAPKKK61 | 101312816 | 3 | 25255805..25265535 | 794 | 89.06 | 6.09 | 50.14 | 76.49 | −0.45 | 17 |
| MAPKKK62 | 101312898 | 2 | 9859412..9866665 | 1192 | 130.83 | 5.15 | 48.2 | 80.05 | −0.44 | 9 |
| MAPKKK63 | 101313105 | 3 | 25269938..25276589 | 710 | 80.27 | 7.32 | 41.81 | 78.39 | −0.48 | 13 |
| MAPKKK64 | 101313212 | 5 | 5764644..5768035 | 350 | 39.78 | 6.43 | 46.69 | 83.09 | −0.30 | 7 |
| MAPKKK65 | 101313251 | 1 | 16064274..16069495 | 618 | 67.06 | 9.22 | 52.62 | 67.38 | −0.53 | 11 |
| MAPKKK66 | 101313299 | 3 | 26914066..26919397 | 239 | 27.17 | 9.23 | 30.57 | 96.69 | −0.21 | 11 |
| MAPKKK67 | 101315161 | 6 | 6707178..6711235 | 358 | 40.09 | 9.03 | 39.93 | 88.02 | −0.30 | 6 |
| MAPKKK68 | 101315443 | 6 | 93407..100195 | 918 | 100.48 | 5.91 | 44.43 | 77.24 | −0.45 | 13 |
| MAPKKK69 | 105349270 | 7 | 5764174..5768902 | 438 | 47.17 | 10.09 | 59.37 | 77.92 | −0.48 | 10 |
| MAPKKK70 | 105349796 | 2 | 18355556..18356787 | 219 | 24.73 | 8.73 | 42.83 | 89.45 | −0.21 | 4 |
| MAPKKK71 | 105351460 | 1 | 10823118..10824804 | 351 | 39.06 | 4.97 | 47.76 | 73.59 | −0.36 | 1 |
| MAPKKK72 | 105351574 | 5 | 13162396..13164156 | 298 | 33.63 | 6.84 | 50.69 | 82.08 | −0.43 | 1 |
| MAPKKK73 | 105353223 | 7 | 5144013..5149034 | 682 | 77.82 | 8.15 | 44.6 | 87.76 | −0.40 | 13 |
| MAPKKKKs | ||||||||||
| MAP4K1 | 101308962 | 4 | 17222313..17230075 | 821 | 90.20 | 5.21 | 47.82 | 71.06 | −0.54 | 18 |
| Gene | Sequences 5′→3′ | Annealing Temperature |
|---|---|---|
| MAPK1 | F: TAGCAAGAACAACATCCGAGAC | 54 °C |
| R: GCTCCAGGCGACATATTAGG | ||
| MAPK2 | F: ACACCTACACTAGAGACCATC | 54 °C |
| R: TGCCTTCCGTTCCATTCAT | ||
| MAPK3 | F: CCTTGGTGGACGGTGTTC | 54 °C |
| R: GGTGGTGGTGGATTGTGG | ||
| MAPK4 | F: CTTATCTTGAGGAGCACTATGGAA | 54 °C |
| R: CGTAATACTGAGCCGACAACT | ||
| MAPK5 | F: GCGGCAGATTCATCCAGTA | 54 °C |
| R: TGACAATGCTCCTCAGATAGA | ||
| MAPK6 | F: GCATCTCCACTTCTACATCTTCA | 54 °C |
| R: TGCCAATCACTTCTTGTATCTCA | ||
| MAPK7 | F: ACCGAAGGACGCTTGTTAG | 54 °C |
| R: AGCAGCAGCAGAACCAAT | ||
| MAPK8 | F: CATCGTCTGCTCGGTGTT | 54 °C |
| R: GCTCGGCTTCAAGTCTCTAT | ||
| MAPK9 | F: CCACCAGATAAGGAGAACTTCAAT | 54 °C |
| R: CGATACCAACGAGTGACAACA | ||
| MAPK10 | F: AGTGATAATGCCCGAAGATATGT | 54 °C |
| R: GGATTGAACTTGACCGACTC | ||
| MAPK11 | F: GAGAGGAGCATACGGTGTTG | 54 °C |
| R: AGTTAGCATTGATGAGCAGGTT | ||
| MAPK12 | F: CTGTGCTGCGATAACTATGGA | 54 °C |
| R: GGTGTTGGAGTGCTTCAG | ||
| MAPKK1 | F: TGACTCTCCAGGCATTATTGAA | 54 °C |
| R: GCCACGCCAGAGATTACC | ||
| MAPKK2 | F: CTCCACAGACGGCACATC | 54 °C |
| R: GGCGGCTGAGACATACAAAT | ||
| MAPKK3 | F: GAAAGGAAGTGGTGGTGTAGT | 54 °C |
| R: GCAAGATATGGTTCAAGAATTGTCT | ||
| MAPKK4 | F: ACTGCTCCTCCTCGTCTT | 54 °C |
| R: CGTGCCTTGCTGTTTGA | ||
| MAPKK5 | F: GGATGAAGGACTTGGCAGAT | 54 °C |
| R: AACACCGTCTCCACATATAAGG | ||
| MAPKK6 | F: CCACCTCCACCACTTACG | 54 °C |
| R: GGCGATCTTGACCTCATTCT | ||
| MAPKK7 | F: CTCCACCACTTACGCTCTC | 54 °C |
| R: GACCTCGTTCTTGTTGTTCATT | ||
| Actin | F: TGGGTTTGCTGGAGATGAT | 54 °C |
| R: CAGTTAGGAGAACTGGGTGC |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Ren, S.; Han, Y.; Zhang, Q.; Qin, L.; Xing, Y. Identification and Analysis of Mitogen-Activated Protein Kinase (MAPK) Cascades in Fragaria vesca. Int. J. Mol. Sci. 2017, 18, 1766. https://doi.org/10.3390/ijms18081766
Zhou H, Ren S, Han Y, Zhang Q, Qin L, Xing Y. Identification and Analysis of Mitogen-Activated Protein Kinase (MAPK) Cascades in Fragaria vesca. International Journal of Molecular Sciences. 2017; 18(8):1766. https://doi.org/10.3390/ijms18081766
Chicago/Turabian StyleZhou, Heying, Suyue Ren, Yuanfang Han, Qing Zhang, Ling Qin, and Yu Xing. 2017. "Identification and Analysis of Mitogen-Activated Protein Kinase (MAPK) Cascades in Fragaria vesca" International Journal of Molecular Sciences 18, no. 8: 1766. https://doi.org/10.3390/ijms18081766
APA StyleZhou, H., Ren, S., Han, Y., Zhang, Q., Qin, L., & Xing, Y. (2017). Identification and Analysis of Mitogen-Activated Protein Kinase (MAPK) Cascades in Fragaria vesca. International Journal of Molecular Sciences, 18(8), 1766. https://doi.org/10.3390/ijms18081766