Regulation of Chemokine Function: The Roles of GAG-Binding and Post-Translational Nitration
Abstract
:1. Introduction
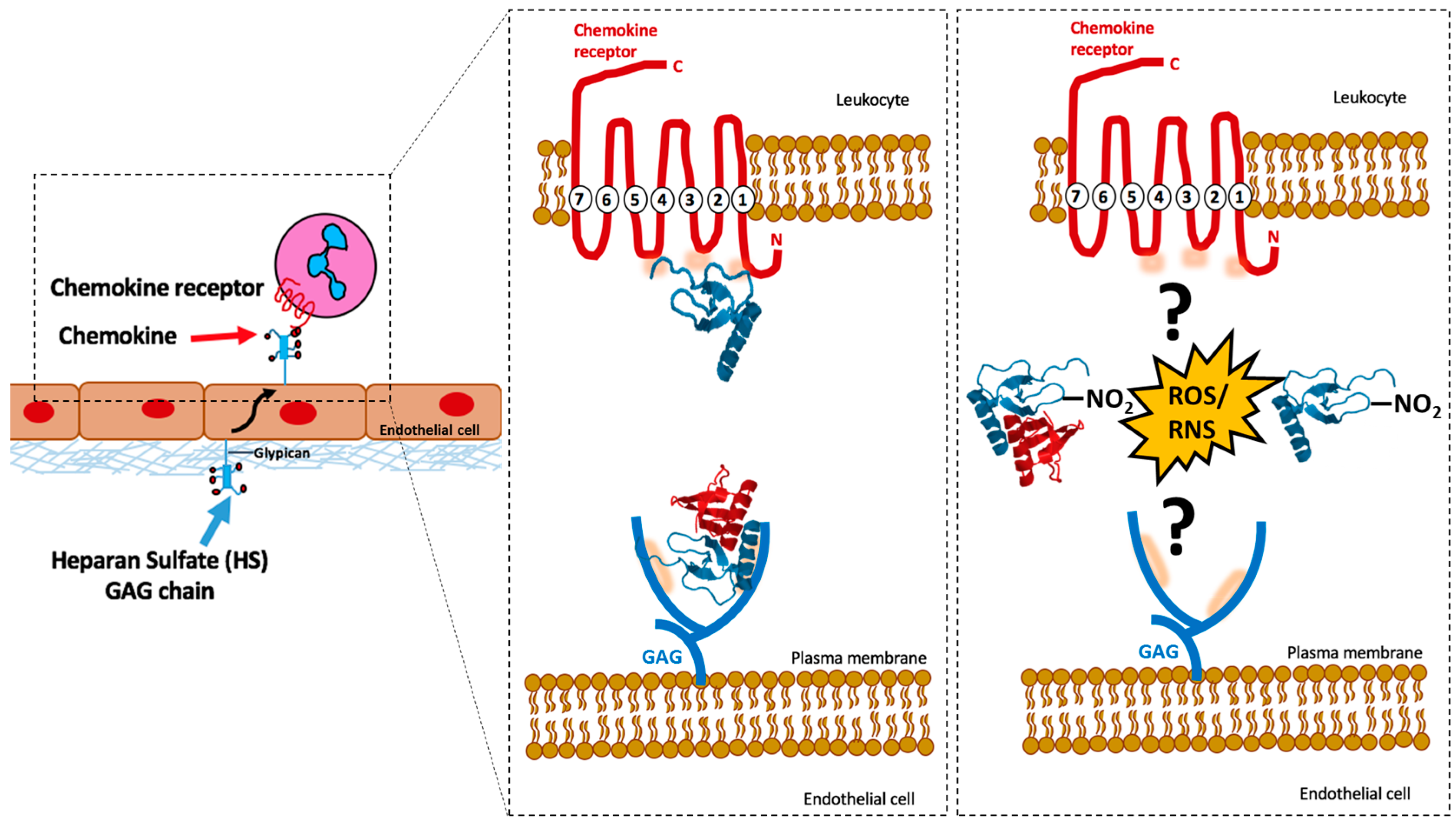
2. Chemokine and Chemokine Receptor Interactions
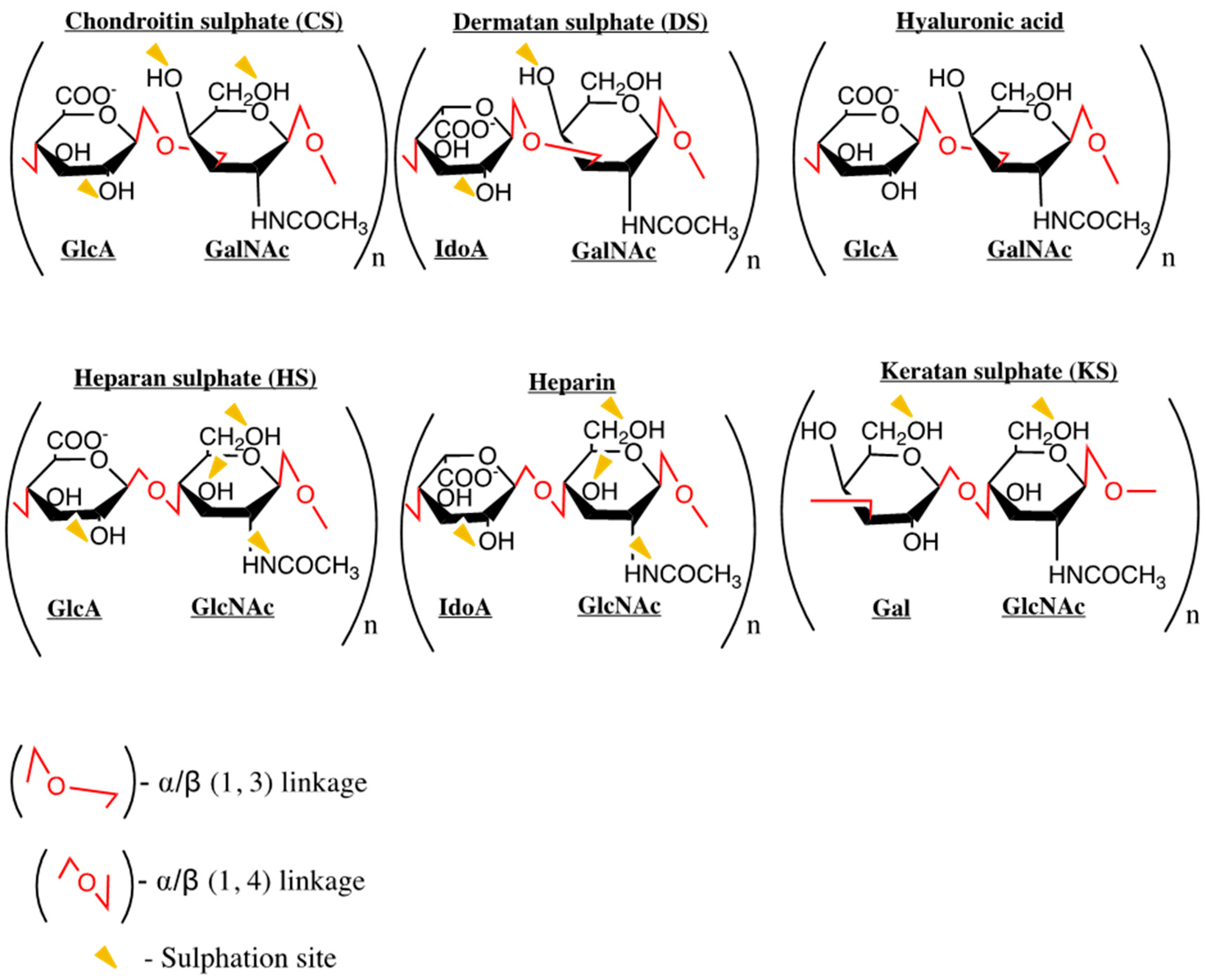
3. Chemokines and GAG Interactions
Common GAGs: Heparan Sulphate and Heparin
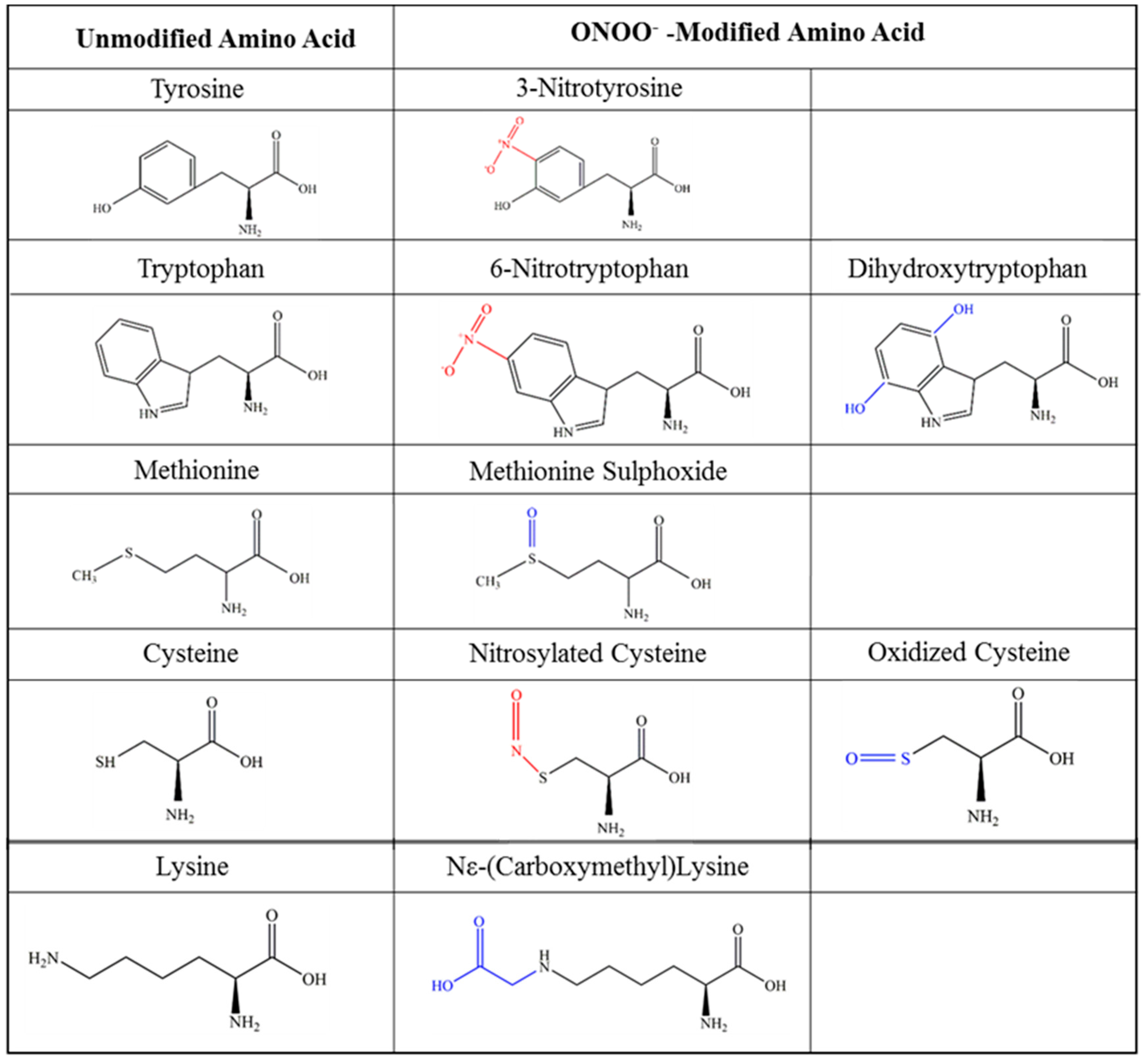
4. Post-Translational Modification of Chemokines
5. Nitration of Chemokines
5.1. Effects of Nitration: Detection of Chemokines
5.2. Effects of Nitration: Chemotaxis
5.3. Effects of Nitration: Receptor Binding
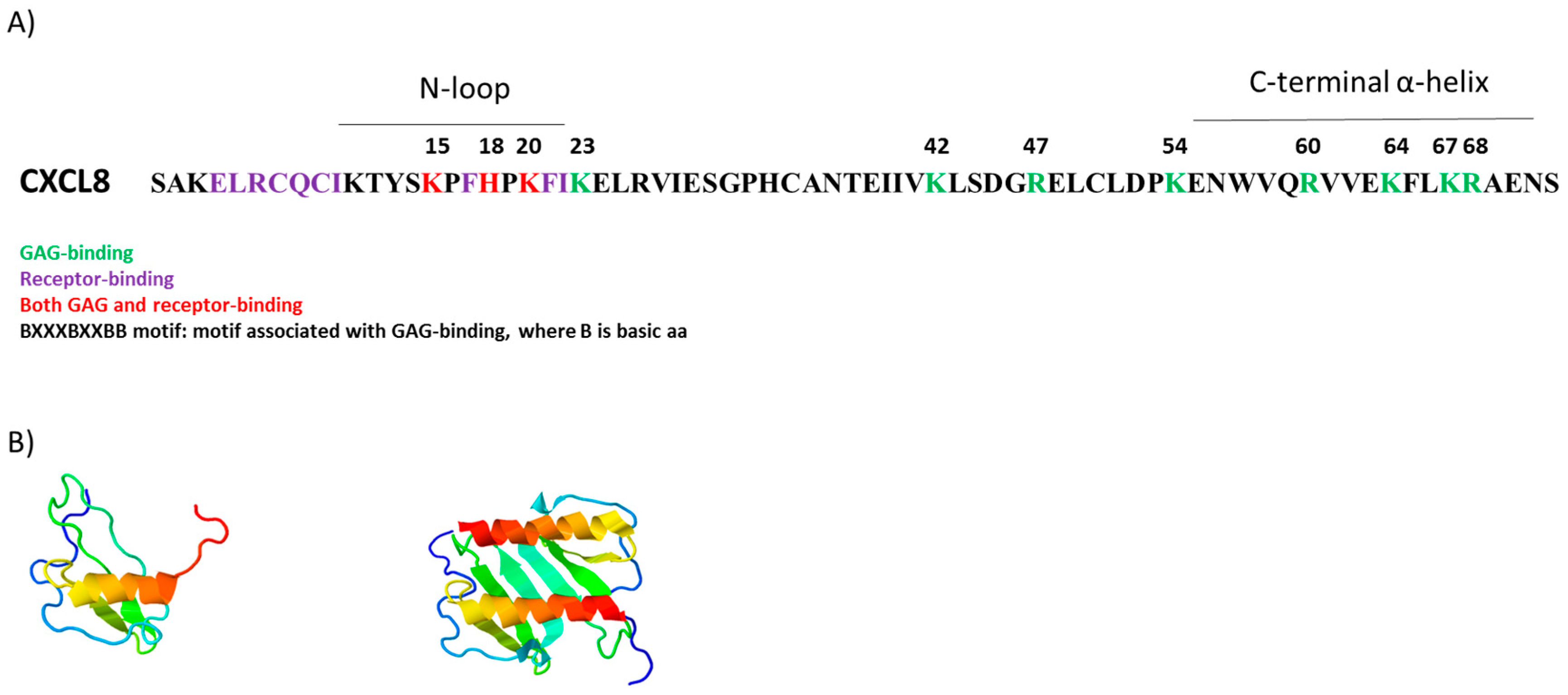
5.4. Effects of Nitration: GAG Binding
6. GAGs, Nitration and CXCL8 Function
6.1. Targeting CXCL8-GAG Interactions
6.2. Competitive Displacement of Chemokines
6.3. Mutants with Altered GAG Binding
6.4. Using Peptides to Block Chemokine-GAG Binding
6.5. Nitration and CXCL8 Function
7. Future Research Directions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACKR | Atypical chemokine receptor |
| ECM | Extracellular matrix |
| GAG | Glycosaminoglycan |
| Gal | Galactose |
| GalNAc | N-acetyl-galactosamine |
| GlcA | Glucuronic acid |
| GlcNAc | N-acetyl-glucosamine |
| GlcNS | Glucosamine |
| GPCR | G-protein coupled receptor |
| HS | Heparan sulphate |
| HS2ST | 2-O-sulphotransferases |
| HS6ST | 6-O-sulphotransferases |
| HS3ST | 3-O-sulphotransferases |
| IdoA | Iduronic acid |
| LPS | Lipopolysaccharide |
| MMPs | Matrix metalloproteinases |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NDSTs+ | N-deacetylase/N-sulphotranferases |
| NO | Nitric oxide |
| O2− | Superoxide anion |
| ONOO− | Peroxynitrite |
| PTM | Post-translational modifications |
| RNS | Reactive nitrogen species |
| SULF1/2 | Sulphatases |
| vCKBP | Viral chemokine binding proteins |
| 3-NT | 3-Nitrotyrosine |
References
- Lo, D.J.; Weaver, T.A.; Kleiner, D.E.; Mannon, R.B.; Jacobson, L.M.; Becker, B.N.; Swanson, S.J.; Hale, D.A.; Kirk, A.D. Chemokines and their receptors in human renal allotransplantation. Transplantation 2011, 91, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Meloni, F.; Solari, N.; Miserere, S.; Morosini, M.; Cascina, A.; Klersy, C.; Arbustini, E.; Pellegrini, C.; Vigano, M.; Fietta, A.M. Chemokine redundancy in BOS pathogenesis. A possible role also for the CC chemokines: MIP3-β, MIP3-α, MDC and their specific receptors. Transpl. Immunol. 2008, 18, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.J.; Sparer, T.E.; Karlstad, M.D.; Burke, S.J. Pancreatic islet inflammation: An emerging role for chemokines. J. Mol. Endocrinol. 2017, 59, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Pirapakaran, T.; Luo, X.M. Chemokines and chemokine receptors in the development of lupus nephritis. Mediat. Inflamm. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Pathak, M.; Lal, G. Role of chemokine receptors and intestinal epithelial cells in the mucosal inflammation and tolerance. J. Leukoc. Biol. 2017, 101, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hauschild, R.; Schwarz, J.; Moussion, C.; de Vries, I.; Legler, D.F.; Luther, S.A.; Bollenbach, T.; Sixt, M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 2013, 339, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Kufareva, I.; Salanga, C.L.; Handel, T.M. Chemokine and chemokine receptor structure and interactions: Implications for therapeutic strategies. Immunol. Cell Biol. 2015, 93, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; de Andres, M.C.; Hashimoto, K.; Itoi, E.; Oreffo, R.O.C. Epigenetic regulation of interleukin-8, an inflammatory chemokine, in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Das, S.T.; Rajagopalan, L.; Guerrero-Plata, A.; Sai, J.; Richmond, A.; Garofalo, R.P.; Rajarathnam, K. Monomeric and dimeric CXCL8 are both essential for in vivo neutrophil recruitment. PLoS ONE 2010, 5, e11754. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.P.; Salanga, C.L.; Volkman, B.F.; Kawamura, T.; Handel, T.M. The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization. Glycobiology 2016, 26, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, A.E.I.; Handel, T.M.; Johnson, Z.; Lau, E.K.; LiWang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.N.C.; Kosco-Vilbois, M.H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Zweemer, A.J.M.; Toraskar, J.; Heitman, L.H.; Ijzerman, A.P. Bias in chemokine receptor signalling. Trends Immunol. 2014, 35, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zidar, D.A.; Violin, J.D.; Whalen, E.J.; Lefkowitz, R.J. Selective engagement of g protein coupled receptor kinases (GRKS) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 9649–9654. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, L.; Rajarathnam, K. Structural basis of chemokine receptor function—A model for binding affinity and ligand selectivity. Biosci. Rep. 2006, 26, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Bromley, S.K.; Mempel, T.R.; Luster, A.D. Orchestrating the orchestrators: Chemokines in control of T cell traffic. Nat. Immunol. 2008, 9, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Kleist, A.B.; Getschman, A.E.; Ziarek, J.J.; Nevins, A.M.; Gauthier, P.-A.; Chevigné, A.; Szpakowska, M.; Volkman, B.F. New paradigms in chemokine receptor signal transduction: Moving beyond the two-site model. Biochem. Pharmacol. 2016, 114, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, S.L. Promiscuous chemokine receptors and their redundant ligands play an enigmatic role during HIV-1 infection. Am. J. Respir. Cell Mol. Biol. 1999, 20, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Dewald, B.; Moser, B. Human chemokines: An update. Annu. Rev. Immunol. 1997, 15, 675–705. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, K.; van Assche, G.; Joossens, S.; Struyf, S.; Proost, P.; Rutgeerts, P.; Geboes, K.; van Damme, J. CXCR1-binding chemokines in inflammatory bowel diseases: Down-regulated IL-8/CXCL8 production by leukocytes in crohn’s disease and selective GCP-2/CXCL6 expression in inflamed intestinal tissue. Eur. J. Immunol. 2004, 34, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Bachelerie, F.; Graham, G.J.; Locati, M.; Mantovani, A.; Murphy, P.M.; Nibbs, R.; Rot, A.; Sozzani, S.; Thelen, M. New nomenclature for atypical chemokine receptors. Nat. Immunol. 2014, 15, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Loos, T.; Opdenakker, G.; van Damme, J.; Proost, P. Citrullination of CXCL8 increases this chemokine’s ability to mobilize neutrophils into the blood circulation. Haematologica 2009, 94, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.M.; Tarbell, J.M. Mechano-sensing and transduction by endothelial surface glycocalyx: Composition, structure, and function. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Handel, T.M.; Johnson, Z.; Crown, S.E.; Lau, E.K.; Sweeney, M.; Proudfoot, A.E. Regulation of protein function by glycosaminoglycans-as exemplified by chemokines. Annu. Rev. Biochem. 2005, 74, 385–410. [Google Scholar] [CrossRef] [PubMed]
- Mihov, D.; Spiess, M. Glycosaminoglycans: Sorting determinants in intracellular protein traffic. Int. J. Biochem. Cell Biol. 2015, 68, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sepuru, K.M.; Rajarathnam, K. CXCL1/MGSA is a novel glycosaminoglycan (GAG)-binding chemokine structural evidence for two distinct non-overlapping binding domains. J. Biol. Chem. 2016, 291, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Sepuru, K.M.; Nagarajan, B.; Desai, U.R.; Rajarathnam, K. Molecular basis of chemokine CXCL5-glycosaminoglycan interactions. J. Biol. Chem. 2016, 291, 20539–20550. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.G.; Triandafillou, C.G.; Huang, T.Y.; Zulueta, M.M.L.; Banerjee, S.; Dinner, A.R.; Hung, S.C.; Tang, W.J. Structural basis for oligomerization and glycosaminoglycan binding of CCL5 and CCL3. Proc. Natl. Acad. Sci. USA 2016, 113, 5000–5005. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Joseph, P.R.B.; Sawant, K.V.; Rajarathnam, K. Chemokine CXCL7 heterodimers: Structural insights, CXCR2 receptor function, and glycosaminoglycan interactions. Int. J. Mol. Sci. 2017, 18, 748. [Google Scholar] [CrossRef] [PubMed]
- Poluri, K.M.; Joseph, P.R.B.; Sawant, K.V.; Rajarathnam, K. Molecular basis of glycosaminoglycan heparin binding to the chemokine CXCL1 dimer. J. Biol. Chem. 2013, 288, 25143–25153. [Google Scholar] [CrossRef] [PubMed]
- Crown, S.E.; Yu, Y.; Sweeney, M.D.; Leary, J.A.; Handel, T.M. Heterodimerization of CCR2 chemokines and regulation by glycosaminoglycan binding. J. Biol. Chem. 2006, 281, 25438–25446. [Google Scholar] [CrossRef] [PubMed]
- Gangavarapu, P.; Rajagopalan, L.; Kolli, D.; Guerrero-Plata, A.; Garofalo, R.P.; Rajarathnam, K. The monomer-dimer equilibrium and glycosaminoglycan interactions of chemokine CXCL8 regulate tissue-specific neutrophil recruitment. J. Leukoc. Biol. 2012, 91, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, A.E. Chemokines and glycosaminoglycans. Front. Immunol. 2015, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Mbemba, E.; Slimani, H.; Atemezem, A.; Saffar, L.; Gattegno, L. Glycans are involved in rantes binding to CCR5 positive as well as to CCR5 negative cells. Biochim. Biophys. Acta Biomembr. 2001, 1510, 354–366. [Google Scholar] [CrossRef]
- Brown, A.J.; Sepuru, K.M.; Rajarathnam, K. Structural basis of native CXCL7 monomer binding to CXCR2 receptor n-domain and glycosaminoglycan heparin. Int. J. Mol. Sci. 2017, 18, 508. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kett, W.C.; Severin, I.C.; Agyekum, I.; Duan, J.; Amster, I.J.; Proudfoot, A.E.I.; Coombe, D.R.; Woods, R.J. The interaction of heparin tetrasaccharides with chemokine CCL5 is modulated by sulfation pattern and PH. J. Biol. Chem. 2015, 290, 15421–15436. [Google Scholar] [CrossRef] [PubMed]
- Sawant, K.V.; Poluri, K.M.; Dutta, A.K.; Sepuru, K.M.; Troshkina, A.; Garofalo, R.P.; Rajarathnam, K. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ziarek, J.J.; Kleist, A.B.; London, N.; Raveh, B.; Montpas, N.; Bonneterre, J.; St-Onge, G.; DiCosmo-Ponticello, C.J.; Koplinski, C.A.; Roy, I. Structural basis for chemokine recognition by a g protein-coupled receptor and implications for receptor activation. Sci. Signal. 2017, 10, 5756. [Google Scholar] [CrossRef] [PubMed]
- Drury, L.J.; Ziarek, J.J.; Gravel, S.; Veldkamp, C.T.; Takekoshi, T.; Hwang, S.T.; Heveker, N.; Volkman, B.F.; Dwinell, M.B. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 17655–17660. [Google Scholar] [CrossRef] [PubMed]
- Lortat-Jacob, H.; Grosdidier, A.; Imberty, A. Structural diversity of heparan sulfate binding domains in chemokines. Proc. Natl. Acad. Sci. USA 2002, 99, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.R.B.; Mosier, P.D.; Desai, U.R.; Rajarathnam, K. Solution NMR characterization of chemokine CXCL8/IL-8 monomer and dimer binding to glycosaminoglycans: Structural plasticity mediates differential binding interactions. Biochem. J. 2015, 472, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Rot, A. Chemokine patterning by glycosaminoglycans and interceptors. Front. Biosci. 2009, 15, 645–660. [Google Scholar] [CrossRef]
- Ali, S.; Hardy, L.A.; Kirby, J.A. Transplant immunobiology: A crucial role for heparan sulfate glycosaminoglycans? Transplantation 2003, 75, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Hacker, U.; Nybakken, K.; Perrimon, N. Heparan sulphate proteoglycans: The sweet side of development. Nat. Rev. Mol. Cell Biol. 2005, 6, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, L.; Sheerin, N.S.; Kirby, J.A.; Ali, S. Mechanisms of renal graft chronic injury and progression to interstitial fibrosis. Curr. Transplant. Rep. 2015, 2, 259–268. [Google Scholar] [CrossRef]
- Bao, X.; Moseman, E.A.; Saito, H.; Petryanik, B.; Thiriot, A.; Hatakeyama, S.; Ito, Y.; Kawashima, H.; Yamaguchi, Y.; Lowe, J.B.; et al. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity 2010, 33, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Parish, C.R. The role of heparan sulphate in inflammation. Nat. Rev. Immunol. 2006, 6, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.J.; Ramdin, L.S.P.; Brooks, T.; Shute, J.K. Plasminogen activator inhibitor-1 supports IL-8-mediated neutrophil transendothelial migration by inhibition of the constitutive shedding of endothelial IL-8/heparan sulfate/syndecan-1 complexes. J. Immunol. 2003, 171, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Lipowsky, H.H.; Lescanic, A. Inhibition of inflammation induced shedding of the endothelial glycocalyx with low molecular weight heparin. Microvasc. Res. 2017, 112, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.M.; Ali, S.; Kirby, J.A. Endothelial inflammation: The role of differential expression of N-deacetylase/N-sulphotransferase enzymes in alteration of the immunological properties of heparan sulphate. J. Cell Sci. 2003, 116, 3591–3600. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Lever, R.; Page, C.P. Mast cell glycosaminoglycans. Glycoconj. J. 2016, 34, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Doster, A.; Schwarzig, U.; Zygmunt, M.; Rom, J.; Schuetz, F.; Fluhr, H. Unfractionated heparin selectively modulates the expression of CXCL8, CCL2 and CCL5 in endometrial carcinoma cells. Anticancer Res. 2016, 36, 1535–1544. [Google Scholar] [PubMed]
- Mortier, A.; Van Damme, J.; Proost, P. Regulation of chemokine activity by posttranslational modification. Pharmacol. Ther. 2008, 120, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.E.; Ali, S.; O’Boyle, G.; Kirby, J.A. Transplantation and inflammation: Implications for the modification of chemokine function. Immunology 2014, 143, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Loos, T.; Mortier, A.; Gouwy, M.; Ronsse, I.; Put, W.; Lenaerts, J.P.; Van Damme, J.; Proost, P. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: A naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood 2008, 112, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; Loos, T.; Mortier, A.; Schutyser, E.; Gouwy, M.; Noppen, S.; Dillen, C.; Ronsse, I.; Conings, R.; Struyf, S.; et al. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J. Exp. Med. 2008, 205, 2085–2097. [Google Scholar] [CrossRef] [PubMed]
- Struyf, S.; Noppen, S.; Loos, T.; Mortier, A.; Gouwy, M.; Verbeke, H.; Huskens, D.; Luangsay, S.; Parmentier, M.; Geboes, K.; et al. Citrullination of CXCL12 differentially reduces CXCR4 and CXCR7 binding with loss of inflammatory and anti-HIV-1 activity via CXCR4. J. Immunol. 2009, 182, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Gole, M.D.; Souza, J.M.; Choi, I.; Hertkorn, C.; Malcolm, S.; Foust, R.F.; Finkel, B.; Lanken, P.N.; Ischiropoulos, H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, 961–967. [Google Scholar]
- Greenacre, S.A.B.; Ischiropoulos, H. Tyrosine nitration: Localisation, quantification, consequences for protein function and signal transduction. Free Radic. Res. 2001, 34, 541–581. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, C.J.; Snyder, S.H. Nitric oxide, a novel biologic messenger. Cell 1992, 70, 705–707. [Google Scholar] [CrossRef]
- Lim, C.H.; Dedon, P.C.; Deen, W.M. Kinetic analysis of intracellular concentrations of reactive nitrogen species. Chem. Res. Toxicol. 2008, 21, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996, 9, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W.S. Thirty years of saying no. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Mount, P.F.; Power, D.A. Nitric oxide in the kidney: Functions and regulation of synthesis. Acta Physiol. 2006, 187, 433–446. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.A.; Cheng, R.Y.S.; Ridnour, L.A.; Basudhar, D.; Somasundaram, V.; McVicar, D.W.; Monteiro, H.P.; Wink, D.A. Inducible nitric oxide synthase in the carcinogenesis of gastrointestinal cancers. Antioxid. Redox Signal. 2016, 26, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Inauen, W.; Suzuki, M.; Granger, D.N. Mechanisms of cellular injury: Potential sources of oxygen free radicals in ischemia/reperfusion. Microcirc. Endothel. Lymphat. 1988, 5, 143–155. [Google Scholar]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Turko, I.V.; Murad, F. Protein nitration in cardiovascular diseases. Pharmacol. Rev. 2002, 54, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Gorman, S.L.; Zweier, J.L. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J. Biol. Chem. 1990, 265, 6656–6663. [Google Scholar] [PubMed]
- Gondouin, B.; Jourde-Chiche, N.; Sallee, M.; Dou, L.; Cerini, C.; Loundou, A.; Morange, S.; Berland, Y.; Burtey, S.; Brunet, P.; et al. Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels. Nephron 2015, 131, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Jung, H.; Kwak, K.H.; Yi, S.J.; Lim, J.A.; Park, S.H.; Park, J.M.; Kim, S.; Jee, D.L.; Lim, D.G. Inhibition of oxidative stress in renal ischemia-reperfusion injury. Anesth. Anal. 2017, 124, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Chun, K.S.; Na, Y.G.; Song, K.H.; Kim, S.I.; Lim, J.S.; Kim, G.H. Allopurinol protects against ischemia/reperfusion-induced injury in rat urinary bladders. Oxidative Med. Cell. Longev. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, W.F.; Paolocci, N.; John, M.E.S.; Skaf, M.W.; Stewart, G.C.; Xie, J.S.; Harrison, R.W.; Zeichner, J.; Mudrick, D.; Marbán, E.; et al. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ. Res. 2002, 90, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Cappola, T.P.; Kass, D.A.; Nelson, G.S.; Berger, R.D.; Rosas, G.O.; Kobeissi, Z.A.; Marbán, E.; Hare, J.M. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 2001, 104, 2407–2411. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Unno, Y.; Hayashi, M.C.; Masuda, S.; Hayase, F.; Kinae, N.; Horiuchi, S. Peroxynitrite induces formation of Nε-(carboxymethyl) lysine by the cleavage of amadori product and generation of glucosone and glyoxal from glucose. Diabetes 2002, 51, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Batthyány, C.; Bartesaghi, S.; Mastrogiovanni, M.; Lima, A.; Demicheli, V.; Radi, R. Tyrosine-nitrated proteins: Proteomic and bioanalytical aspects. Antioxid. Redox Signal. 2016, 26, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Gounarides, J.S.; Roos, E.S.; Wolin, M.S. Influence of peroxynitrite on energy metabolism and cardiac function in a rat ischemia-reperfusion model. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Fukuyama, N.; Iguchi, A.; Akimoto, H.; Ohmi, M.; Yokoyama, H.; Nakazawa, H.; Tabayashi, K. Quantitative analysis of cardiac 3-l-nitrotyrosine during acute allograft rejection in an experimental heart transplantation1. Transplantation 1999, 68, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Scopelliti, F.; Vulpis, E.; Tafani, M.; Villanova, L.; Verardo, R.; de Paulis, R.; Russo, M.A.; Frustaci, A. Increased oxidative stress contributes to cardiomyocyte dysfunction and death in patients with fabry disease cardiomyopathy. Hum. Pathol. 2015, 46, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Thuraisingham, R.C.; Nott, C.A.; Dodd, S.M.; Yaqoob, M.M. Increased nitrotyrosine staining in kidneys from patients with diabetic nephropathy. Kidney Int. 2000, 57, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Anwar, A.; Savage, R.S.; Thornalley, P.J.; Rabbani, N. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res. Ther. 2016, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, S.; Vivekanandan-Giri, A.; Locy, M.L.; Kulkarni, T.; Zhi, D.; Zeng, L.; Byun, J.; de Andrade, J.A.; Thannickal, V.J. Oxidative modifications of protein tyrosyl residues are increased in plasma of human subjects with interstitial lung disease. Am. J. Respir. Crit. Care Med. 2016, 193, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.; Orhan, H.; Sayal, A.; Özata, M.; Şahin, G.; Işımer, A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: Effects of glycemic control. Clin. Biochem. 2001, 34, 65–70. [Google Scholar] [CrossRef]
- Balafanova, Z.; Bolli, R.; Zhang, J.; Zheng, Y.; Pass, J.M.; Bhatnagar, A.; Tang, X.-L.; Wang, O.; Cardwell, E.; Ping, P. Nitric oxide (NO) induces nitration of protein kinase Cε (PKCΕ), facilitating PKCΕ translocation via enhanced PKCΕ–RACK2 interactions a novel mechanism of no-triggered activation of PKCΕ. J. Biol. Chem. 2002, 277, 15021–15027. [Google Scholar] [CrossRef] [PubMed]
- Molon, B.; Ugel, S.; Del Pozzo, F.; Soldani, C.; Zilio, S.; Avella, D.; De Palma, A.; Mauri, P.; Monegal, A.; Rescigno, M.; et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011, 208, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Simpson, K.L.; Grisham, M.B.; Koyama, S.; Robbins, R.A. Effects of reactive oxygen and nitrogen metabolites on rantes and IL-5-induced eosinophil chemotactic activity in vitro. Am. J. Pathol. 1999, 155, 591–598. [Google Scholar] [CrossRef]
- Janssens, R.; Mortier, A.; Boff, D.; Vanheule, V.; Gouwy, M.; Franck, C.; Larsen, O.; Rosenkilde, M.M.; Van Damme, J.; Amaral, F.A.; et al. Natural nitration of CXCL12 reduces its signaling capacity and chemotactic activity in vitro and abrogates intra-articular lymphocyte recruitment in vivo. Oncotarget 2016, 7, 62439–62459. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.R.; Kabakoff, R.C.; Hebert, C.A. Complete mutagenesis of the extracellular domain of interleukin-8 (IL-8) type a receptor identifies charged residues mediating IL-8 binding and signal transduction. J. Biol. Chem. 1994, 269, 19343–19348. [Google Scholar] [PubMed]
- Barker, C.E.; Thompson, S.; O’boyle, G.; Lortat-Jacob, H.; Sheerin, N.S.; Ali, S.; Kirby, J.A. CCL2 nitration is a negative regulator of chemokine-mediated inflammation. Sci. Rep. 2017, 7, 44384. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.A.; Legler, D.F. Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J. Leukoc. Biol. 2016, 99, 869–882. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.; Reyes-Aldasoro, C.C.; Candel, S.; Renshaw, S.A.; Mulero, V.; Calado, Â. CXCL8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J. Immunol. 2013, 190, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- Rot, A. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur. J. Immunol. 1993, 23, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Mehrad, B.; Keane, M.P.; Strieter, R.M. Chemokines as mediators of angiogenesis. Thromb. Haemost. 2007, 97, 755. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, A.A.; Holliday, M.J.; Isern, N.G.; Zhang, F.; Camilloni, C.; Huynh, C.; Vendruscolo, M.; Armstrong, G.; Eisenmesser, E.Z. The dynamics of interleukin-8 and its interaction with human CXC receptor I peptide. Protein Sci. 2014, 23, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Jayakumar, C.; Manicassamy, S.; Ramesh, G. CXCR2 knockout mice are protected against DSS-colitis-induced acute kidney injury and inflammation. Am. J. Physiol. Ren. Physiol. 2013, 305, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.W.; Raghuwanshi, S.K.; Grant, D.J.; Jala, V.R.; Rajarathnam, K.; Richardson, R.M. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J. Immunol. 2009, 183, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Fernando, H.; Chin, C.; Rösgen, J.; Rajarathnam, K. Dimer dissociation is essential for interleukin-8 (IL-8) binding to CXCR1 receptor. J. Biol. Chem. 2004, 279, 36175–36178. [Google Scholar] [CrossRef] [PubMed]
- Rajarathnam, K.; Prado, G.N.; Fernando, H.; Clark-Lewis, I.; Navarro, J. Probing receptor binding activity of interleukin-8 dimer using a disulfide trap. Biochemistry 2006, 45, 7882–7888. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.M.C.; Clark-Lewis, I.; Alcami, A. The gammaherpesvirus chemokine binding protein binds to the N terminus of CXCL8. J. Virol. 2003, 77, 8588–8592. [Google Scholar] [CrossRef] [PubMed]
- Falsone, A.; Wabitsch, V.; Geretti, E.; Potzinger, H.; Gerlza, T.; Robinson, J.; Adage, T.; Teixeira, M.M.; Kungl, A.J. Designing CXCL8-based decoy proteins with strong anti-inflammatory activity in vivo. Biosci. Rep. 2013, 33. [Google Scholar] [CrossRef] [PubMed]
- Kuschert, G.S.V.; Hoogewerf, A.J.; Proudfoot, A.E.I.; Chung, C.-W.; Cooke, R.M.; Hubbard, R.E.; Wells, T.N.C.; Sanderson, P.N. Identification of a glycosaminoglycan binding surface on human interleukin-8. Biochemistry 1998, 37, 11193–11201. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Coombe, D.R.; Gill, S.E.; Kett, W.C.; Kajikawa, O.; Proudfoot, A.E.I.; Wells, T.N.C.; Parks, W.C.; Wight, T.N.; Martin, T.R.; et al. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J. Immunol. 2010, 184, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Al Faruque, H.; Kang, J.H.; Hwang, S.R.; Sung, S.; Alam, M.M.; Sa, K.H.; Nam, E.J.; Byun, Y.R.; Kang, Y.M. Stepwise inhibition of T cell recruitment at post-capillary venules by orally active desulfated heparins in inflammatory arthritis. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Kumasaka, K.; Browne, K.D.; Li, S.; St-Pierre, J.; Cognetti, J.; Marks, J.; Johnson, V.E.; Smith, D.H.; Pascual, J.L. Unfractionated heparin after TBI reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J. Trauma Acute Care Surg. 2016, 81, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Riffo-Vasquez, Y.; Somani, A.; Man, F.; Amison, R.; Pitchford, S.; Page, C.P. A non-anticoagulant fraction of heparin inhibits leukocyte diapedesis into the lung by an effect on platelets. Am. J. Respir. Cell Mol. Biol. 2016, 55, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Arimateia, D.S.; da Silva Brito, A.; de Azevedo, F.M.; de Andrade, G.P.V.; Chavante, S.F. Heparin fails to inhibit the leukocyte recruitment for an extended time following inflammatory stimulus. Pharm. Biol. 2015, 53, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Motos, V.; Kropp, K.A.; Viejo-Borbolla, A. Chemokine binding proteins: An immunomodulatory strategy going viral. Cytokine Growth Factor Rev. 2016, 30, 71–80. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, G.; Mellor, P.; Kirby, J.A.; Ali, S. Anti-inflammatory therapy by intravenous delivery of non-heparan sulfate-binding CXCL12. FASEB J. 2009, 23, 3906–3916. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.; Kosco-Vilbois, M.H.; Herren, S.; Cirillo, R.; Muzio, V.; Zaratin, P.; Carbonatto, M.; Mack, M.; Smailbegovic, A.; Rose, M.; et al. Interference with heparin binding and oligomerization creates a novel anti-inflammatory strategy targeting the chemokine system. J. Immunol. 2004, 173, 5776–5785. [Google Scholar] [CrossRef] [PubMed]
- Severin, I.C.; Gaudry, J.-P.; Johnson, Z.; Kungl, A.; Jansma, A.; Gesslbauer, B.; Mulloy, B.; Power, C.; Proudfoot, A.E.I.; Handel, T. Characterization of the chemokine CXCL11-heparin interaction suggests two different affinities for glycosaminoglycans. J. Biol. Chem. 2010, 285, 17713–17724. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Strutzmann, E.; Teixeira, M.M.; Anders, H.J.; Diedrichs-Möhring, M.; Gerlza, T.; Wildner, G.; Russo, R.C.; Adage, T.; Kungl, A.J. Glycosaminoglycans are important mediators of neutrophilic inflammation in vivo. Cytokine 2017, 91, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Trinker, M.U.; Hecher, B.; Adage, T.; Ali, S.; Kungl, A.J. Glycosaminoglycan silencing by engineered CXCL12 variants. FEBS Lett. 2015, 589, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Gerlza, T.; Winkler, S.; Atlic, A.; Zankl, C.; Konya, V.; Kitic, N.; Strutzmann, E.; Knebl, K.; Adage, T.; Heinemann, A.; et al. Designing a mutant CCL2–HSA chimera with high glycosaminoglycan-binding affinity and selectivity. Protein Eng. Des. Sel. 2015, 28, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bedke, J.; Nelson, P.J.; Kiss, E.; Muenchmeier, N.; Rek, A.; Behnes, C.-L.; Gretz, N.; Kungl, A.J.; Gröne, H.-J. A novel CXCL8 protein-based antagonist in acute experimental renal allograft damage. Mol. Immunol. 2010, 47, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Vanheule, V.; Janssens, R.; Boff, D.; Kitic, N.; Berghmans, N.; Ronsse, I.; Kungl, A.J.; Amaral, F.A.; Teixeira, M.M.; Van Damme, J.; et al. The positively charged COOH-terminal glycosaminoglycan-binding CXCL9 (74–103) peptide inhibits CXCL8-induced neutrophil extravasation and monosodium urate crystal-induced gout in mice. J. Biol. Chem. 2015, 290, 21292–21304. [Google Scholar] [CrossRef] [PubMed]
- Greenacre, S.A.B.; Rocha, F.A.C.; Rawlingson, A.; Meinerikandathevan, S.; Poston, R.N.; Ruiz, E.; Halliwell, B.; Brain, S.D. Protein nitration in cutaneous inflammation in the rat: Essential role of inducible nitric oxide synthase and polymorphonuclear leukocytes. Br. J. Pharmacol. 2002, 136, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Peluffo, G.; Radi, R. Protein tyrosine nitration—Functional alteration or just a biomarker? Free Radic. Biol. Med. 2008, 45, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.W.; Shyamala, V.; Siani, M.A.; Gallegos, C.A.; Feucht, P.H.; Abbott, J.; Lapointe, G.R.; Moghadam, M.; Khoja, H.; Zakel, J.; et al. Receptor recognition and specificity of interleukin-8 is determined by residues that cluster near a surface-accessible hydrophobic pocket. J. Biol. Chem. 1996, 271, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Clark-Lewis, I.; Dewald, B.; Loetscher, M.; Moser, B.; Baggiolini, M. Structural requirements for interleukin-8 function identified by design of analogs and CXC chemokine hybrids. J. Biol. Chem. 1994, 269, 16075–16081. [Google Scholar] [PubMed]
- Alvarez, B.; Ferrer-Sueta, G.; Freeman, B.A.; Radi, R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J. Biol. Chem. 1999, 274, 842–848. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, S.; Martínez-Burgo, B.; Sepuru, K.M.; Rajarathnam, K.; Kirby, J.A.; Sheerin, N.S.; Ali, S. Regulation of Chemokine Function: The Roles of GAG-Binding and Post-Translational Nitration. Int. J. Mol. Sci. 2017, 18, 1692. https://doi.org/10.3390/ijms18081692
Thompson S, Martínez-Burgo B, Sepuru KM, Rajarathnam K, Kirby JA, Sheerin NS, Ali S. Regulation of Chemokine Function: The Roles of GAG-Binding and Post-Translational Nitration. International Journal of Molecular Sciences. 2017; 18(8):1692. https://doi.org/10.3390/ijms18081692
Chicago/Turabian StyleThompson, Sarah, Beatriz Martínez-Burgo, Krishna Mohan Sepuru, Krishna Rajarathnam, John A. Kirby, Neil S. Sheerin, and Simi Ali. 2017. "Regulation of Chemokine Function: The Roles of GAG-Binding and Post-Translational Nitration" International Journal of Molecular Sciences 18, no. 8: 1692. https://doi.org/10.3390/ijms18081692
APA StyleThompson, S., Martínez-Burgo, B., Sepuru, K. M., Rajarathnam, K., Kirby, J. A., Sheerin, N. S., & Ali, S. (2017). Regulation of Chemokine Function: The Roles of GAG-Binding and Post-Translational Nitration. International Journal of Molecular Sciences, 18(8), 1692. https://doi.org/10.3390/ijms18081692




