Abstract
The incidence of chronic kidney disease (CKD) is increasing worldwide, with more than 26 million people suffering from CKD in the United States alone. More patients with CKD die of cardiovascular complications than progress to dialysis. Over 80% of CKD patients have hypertension, which is associated with increased risk of cardiovascular morbidity and mortality. Another common, perhaps underappreciated, feature of CKD is an overactive sympathetic nervous system. This elevation in sympathetic nerve activity (SNA) not only contributes to hypertension but also plays a detrimental role in the progression of CKD independent of any increase in blood pressure. Indeed, high SNA is associated with poor prognosis and increased cardiovascular morbidity and mortality independent of its effect on blood pressure. This brief review will discuss some of the consequences of sympathetic overactivity and highlight some of the potential pathways contributing to chronically elevated SNA in CKD. Mechanisms leading to chronic sympathoexcitation in CKD are complex, multifactorial and to date, not completely understood. Identification of the mechanisms and/or signals leading to sympathetic overactivity in CKD are crucial for development of effective therapeutic targets to reduce the increased cardiovascular risk in this patient group.
1. Introduction
More than 26 million people in the United States suffer from chronic kidney disease (CKD) with thousands of new cases diagnosed each year [1]. CKD has poor prognosis and health outcomes with very high health care costs. In the US alone, the cost of treatment for CKD has exceeded 49 billion dollars per year [2,3]. There are five stages of CKD defined by progressive decreases in renal function as quantified by estimated glomerular filtration rate (eGFR) and/or kidney damage (proteinuria, albuminuria). Stage 1 CKD constitutes kidney damage with normal or reduced eGFR (≥90 mL/min/1.73 m2). Stages 2, 3 and 4 show progressive renal dysfunction with an eGFR of 60–89, 30–59 and 15–29 mL/min/1.73 m2, respectively. Stage 5 CKD is renal failure or end-stage renal disease which is characterized by eGFR of <15 mL/min/1.73 m2 or dialysis. Moderate to severe CKD (stage 3–5) is associated with a significantly higher risk of cardiovascular (CV) morbidity and mortality [4,5]. In fact, over 50% of deaths in renal failure patients occur due to myocardial infarction, stroke, heart failure, and sudden cardiac death, the latter accounting for 25% of deaths in these patients alone [6,7,8]. There is growing evidence that sympathetic overactivity, a characteristic feature of CKD, is one of the major mechanisms leading to higher CV risk in this patient population [9,10]. This brief review will discuss some of the consequences of chronically elevated sympathetic nerve activity (SNA) and highlight some of the potential pathways contributing to elevated SNA in CKD.
A multitude of studies have shown elevated SNA in CKD [11,12,13,14,15]. Early observations of exaggerated SNA in renal failure patients came from studies showing significantly elevated plasma norepinephrine, the primary neurotransmitter released by sympathetic nerves [16,17]. Several studies have also used the technique of microneurography to directly record from post-ganglionic sympathetic nerve fibers innervating blood vessels that supply skeletal muscle (i.e., muscle sympathetic nerve activity (MSNA)). Converse et al. [11] was the first to report elevated resting MSNA in renal failure patients on hemodialysis (Figure 1). However, of note, sympathoexcitation is not confined to renal failure, but is also detectable in earlier stages of CKD [13,15,18,19]. Indeed, Grassi et al. [13] studied mild-to-moderate CKD patients who were divided into four groups based on their eGFR, with highest quartile of eGFR (95.4 ± 1.6 mL/min/1.73 m2) in group I and lowest quartile of eGFR (31.4 ± 1.8 mL/min/1.73 m2) in group IV. There was a significant and progressive increase in resting MSNA from the highest quartile to the lowest quartile of eGFR (Figure 2) [13]. Other studies have reported elevated MSNA even in patients with polycystic kidney disease and no impairment in renal function [18,19]. Collectively, these findings demonstrate two important points: (1) SNA is augmented early in CKD; and (2) SNA increases progressively with declining renal function.
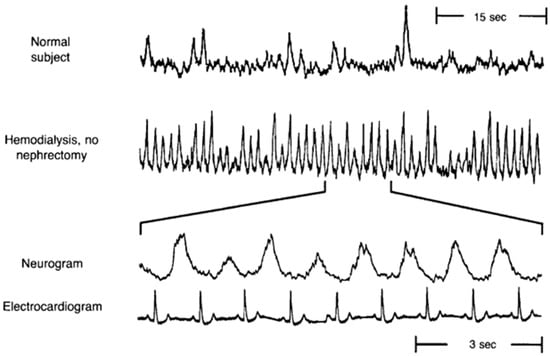
Figure 1.
Original record of muscle sympathetic nerve activity (MSNA) in a normal subject and a renal failure patient on hemodialysis, demonstrating significantly greater resting MSNA in the dialysis patient. The bottom two panels display a portion of the neurogram and simultaneous electrocardiogram from the patient on hemodialysis showing that the renal failure patient has a burst of MSNA with every cardiac cycle. (Modified from Converse et al. [11] with permission).
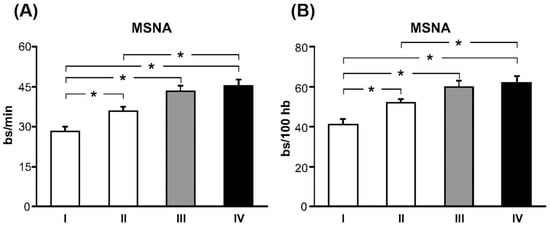
Figure 2.
Muscle sympathetic nerve activity (MSNA) expressed as burst frequency (bursts (bs)/min, A) and burst incidence (bs/100 heartbeats (hb), B) in subjects grouped according to their estimated glomerular filtration rate (eGFR), with the highest quartile of eGFR in group I and lowest quartile of eGFR in group IV. With decreasing eGFR, there is a significant and progressive increase in resting MSNA in chronic kidney disease (CKD) patients. * p < 0.05 significant difference between quartiles. (From Grassi et al. [13] with permission).
2. Consequences of Sympathetic Overactivity
Sympathetic overactivity is associated with various diseases and accelerates the progression of cardiovascular, metabolic and renal pathology [20,21,22]. The most common detrimental consequence of elevated SNA is hypertension [23,24,25], which is observed in 80% of CKD patients [26,27]. Hypertension, in turn, leads to structural and functional abnormalities such as left ventricular hypertrophy, increased vascular smooth muscle cell hyperplasia and hypertrophy, and endothelial dysfunction [28,29,30]. More importantly, chronic sympathoexcitation independent of hypertension has detrimental effects. For example, administration of sub-hypertensive doses of norepinephrine has been shown to cause myocardial cell hypertrophy and left ventricular hypertrophy [31,32,33]. Moreover, elevated SNA without increases in blood pressure has been shown to cause vascular smooth muscle cell hypertrophy and proliferation [34,35]. In addition, other deleterious effects of elevated SNA, independent of increased blood pressure, include, but are not limited to, exaggerated coronary vasoconstriction [36], arrythmogenicity [37], impaired renal function [38], glomerular podocyte injury [39], metabolic impairment [40], increased arterial stiffness [41,42,43], endothelial dysfunction [44,45,46] and subsequent development of atherosclerosis [47,48], all of which lead to increased risk of CV events.
On the other hand, reducing SNA without any lowering of blood pressure has been shown to be protective, in particular to the kidney [20]. In this regard, treatment of partially nephrectomized rats (an animal model of CKD) with non-hypotensive doses of the sympatholytic agent moxonidine, resulted in reduced glomerulosclerosis and urinary albumin excretion compared to untreated nephrectomized animals [20]. Furthermore, low doses of moxonidine that did not change blood pressure were shown to elicit an antialbuminuric effect in diabetic patients [49]. Collectively, these data provide strong evidence that sympathetic overactivity, independent of increases in blood pressure, can cause detrimental cardiovascular, metabolic, and renal effects. Although there is clear evidence of sympathetic overactivity in CKD, the mechanisms leading to chronic sympathoexcitation in CKD are complex, multifactorial and not completely understood. The remainder of this review will focus on some of the potential mechanisms contributing to elevated SNA in CKD.
3. Mechanisms of Sympathetic Overactivity in Chronic Kidney Disease (CKD)
3.1. Renin–Angiotensin System
It is well known that the renin–angiotensin system is activated in CKD. Renin is secreted from the kidney and converts angiotensinogen to angiotensin I, which in turn is converted to angiotensin II (Ang II) by angiotensin-converting enzyme (ACE). Activation of renin–angiotensin system increases renin secretion ultimately leading to high circulating plasma concentrations of Ang II, a common feature of CKD [50,51,52]. Ang II is a potent vasoconstrictor with a multitude of peripheral and central actions as previously described in detail (for review see [38,53,54]). Briefly, in addition to causing direct peripheral vasoconstriction, Ang II also modulates peripheral SNA by potentiating norepinephrine release from sympathetic nerve terminals. Ang II also plays an important role in regulating sympathetic outflow from the brainstem. For example, microinjection of Ang II into the rostral ventrolateral medulla (RVLM) activates vasomotor sympathetic neurons resulting in elevated SNA [55,56,57] while microinjection of an Ang II receptor blocker, losartan, into the RVLM causes sympathoinhibition [57,58]. These findings and others [53,59,60] provide clear evidence for a direct role of Ang II in regulating central sympathetic outflow. Although Ang II receptor blockers and ACE inhibitors are a first-line choice of treatment in CKD patients, chronic treatment with these drugs only reduces MSNA but does not normalize it [15,16]. In other words, MSNA in CKD patients following chronic treatment with Ang II receptor blockers and ACE inhibitors is still higher than in healthy individuals. Therefore, mechanisms other than the renin–angiotensin system are also involved in causing sympathetic overactivity in CKD.
3.2. Renal Afferents
The kidneys are richly innervated by chemoreceptors and baroreceptors which send feedback to the brain to regulate sympathetic outflow and systemic blood pressure. Evidence from both animal and human studies indicates that neural signals originating from the kidney play a role in increasing sympathetic outflow in CKD [61,62]. Rats subjected to 5/6th nephrectomy, an animal model of CKD, develop hypertension with elevated norepinephrine turnover in various hypothalamic nuclei involved in the regulation of sympathetic outflow. Selective removal of afferent nerves (dorsal rhizotomy) in these animals prevents both the development of hypertension and increase in norepinephrine turnover in hypothalamic nuclei in the brain [61]. Furthermore, renal injury without a reduction in renal function also leads to augmented sympathetic outflow. Animal studies have shown that renal injury via intrarenal phenol injection, which does not decrease renal function, causes increases in renal SNA, plasma norepinephrine concentrations, blood pressure, and norepinephrine secretion in the posterior hypothalamus [62,63,64]. In addition, ligands such as urea and adenosine, which are elevated in CKD, can stimulate renal nerves, also contributing to increases in SNA [65,66]. Renal ischemia enhances adenosine production, which not only stimulates renal afferent nerves but also causes vasoconstriction of afferent arterioles leading to reduced GFR [67]. Interestingly, Hausberg et al. [68] observed that MSNA in renal transplant patients with intact native kidneys was similar to that in dialysis patients (stage 5 CKD). However, transplant patients who underwent bilateral native kidney nephrectomy exhibited a significant reduction in MSNA, to values not that different from control subjects. Thus, signals arising from the native kidney can contribute to heightened MSNA in renal disease. Moreover, recent studies have suggested reduced renalase levels as a potential contributing factor in elevated SNA in CKD [69,70,71]. Renalase is a monoamine oxidase produced by the kidneys that circulates in the blood in its inactive form prorenalase [70]. Once activated, renalase degrades catecholamines and can decrease blood pressure [70,72]. CKD and renal failure patients have significantly reduced levels of renalase, which would lead to less breakdown of catecholamines and contribute to the higher SNA in these patients [70].
3.3. Nitric Oxide Pathway
Another potential mechanism for chronic sympathoexcitation in CKD is reduced nitric oxide (NO) bioavailability. NO is produced by nitric oxide synthase (NOS) during oxidation of l-arginine to l-citrulline [73]. There are three isoforms of NOS: endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). While eNOS and nNOS are constitutively expressed in all cells and contribute to the regulation of vascular tone and blood pressure, iNOS is activated by macrophages and cytokines during inflammation. NO produced by eNOS diffuses into smooth muscle cells and causes vasodilation via stimulation of guanylate cyclase. Indeed, systemic NOS inhibition in healthy individuals causes an immediate increase in arterial blood pressure by reducing NO and endothelium-mediated vasodilation [74,75,76]. In addition to its action as a vasodilator, NO also plays a key role in maintaining vascular homeostasis by inhibiting platelet aggregation, atherogenesis, smooth muscle cell proliferation and leukocyte adhesion to the endothelium [77,78]. Thus, endothelium-derived NO not only regulates vascular tone and thereby, arterial blood pressure but it also plays an essential role in maintaining a healthy vasculature. While the role of peripheral NO in endothelium-mediated vasodilation and blood pressure regulation is well known, much less appreciated is the potential central effect of NO.
There is increasing evidence that suggests NO is a key signaling molecule involved in regulation of sympathetic outflow from the brainstem. Shapoval et al. [79] performed the first in vivo study to demonstrate a direct effect of central NO on sympathetic outflow. They showed that microinjection of the NOS inhibitor NG-monomethyl l-arginine (l-NMMA) directly into the RVLM of anesthetized animals increased renal SNA and consequently, blood pressure, while microinjections of l-arginine and sodium nitroprusside (NO donor) reduced renal SNA and blood pressure [79]. Microinjection of l-NMMA into the nucleus tractus solitarius (NTS) showed similar changes in renal SNA and blood pressure [80]. Tagawa and colleagues [81] used rat brainstem slices to show that l-arginine causes a dose-dependent increase in neuronal activity of ~40% of NTS neurons, and this response is attenuated by l-NMMA. Hemoglobin, which endogenously binds to NO, blocks the increase in neuronal activity evoked by l-arginine, suggesting that NO diffuses out into the extracellular space to excite the adjacent neurons from which the neural activity was measured. They further extended their findings by using methylene blue, a guanylate cyclase blocker, to investigate whether these effects of l-arginine were mediated by cyclic guanosine monophosphate (cGMP). Methylene blue inhibits the increase in neuronal activity in the NTS elicited by both l-arginine and sodium nitroprusside. These data indicate that NO produced from l-arginine in the NTS neurons diffuses out to nearby target neurons where it increases neuronal activity through cGMP. Other animal studies involved intravenous infusion, interacisternal injection or microinjection of NOS inhibitors into the paraventricular nucleus (PVN) and the NTS, demonstrating acute increases in renal SNA and blood pressure [74,80,82]. Furthermore, overexpression of NOS in the RVLM via adenovirus transfection results in elevated NO levels in the RVLM, which reduced urinary norepinephrine excretion along with a lowering of blood pressure [83,84]. Together, these studies indicate that centrally-derived NO is a key signaling molecule involved in the tonic restraint of sympathetic outflow from the brainstem.
Initial studies performed to extrapolate these findings from direct central injections to systemic infusions of NOS inhibitors observed a reduction in SNA. Indeed, when NOS inhibitors were infused systemically in animals and humans, there was a rapid and large increase in blood pressure along with a decrease in SNA [85,86]. The rapid increase in blood pressure occurred due to inhibition of NO-mediated, endothelium-dependent vasodilation in the peripheral vasculature. This increase in blood pressure activated the arterial baroreflex resulting in a reflex-mediated decrease in SNA. Thus, baroreflex-mediated reductions in SNA masked any increases in sympathetic outflow that might have occurred due to reduced central NO. To eliminate the influence of the arterial baroreflex, the effect of systemic NOS inhibition was compared between barointact and barodenervated animals [74,87]. Administration of the NOS inhibitor l-nitroarginine methyl ester (l-NAME) in barointact animals caused a biphasic response in renal SNA, an initial decrease followed by an increase in renal SNA. In barodenervated animals, NOS inhibition caused a progressive and significant increase in renal SNA uncovering the role of central NO in restraining sympathetic outflow. Our laboratory has also provided evidence for NO in central sympathetic control in humans. To overcome the confounding inhibitory influence of the arterial baroreflex on SNA, we directly measured skin SNA, which is not under baroreceptor control. Systemic l-NAME infusion in healthy adults caused progressive and sustained increases in skin SNA [88].
Another consideration with systemic infusion of NOS inhibitors to examine effects of central NO on SNA is the time needed for the inhibitors to cross the blood brain barrier. In this regard, systemic infusion of l-NAME in animals with and without sympathectomy showed that both groups had a similar increase in blood pressure during the first hour of infusion primarily due to inhibition of peripheral endothelial NO production. Importantly, blood pressure in the sympathectomized animals after 8 h and after 6 days of l-NAME infusion was significantly lower than the control group, indicating a delayed but significant role of the sympathetic nervous system in the blood pressure-raising effect of NOS inhibition (Figure 3) [75]. In agreement with a time delay in sympathetic activation via systemic NOS inhibition, when phentolamine (α-adrenergic blocker) was infused immediately and 90 min after l-NAME infusion in humans, there was little effect on the initial hypertensive response but phentolamine significantly attenuated the subsequent further increase in blood pressure [76]. These results indicate that the initial increase in blood pressure occurred due to diminished peripheral NO causing inhibition of endothelium-mediated vasodilation while the later increase in blood pressure was caused by sympathetic adrenergic vasoconstriction. This would also explain the results observed in initial studies where NOS inhibition caused a reduction in SNA, as SNA was only measured for 40 min after l-NAME infusion [86], which may not have been long enough to observe central effects of systemic NOS inhibition. Taken together, these data indicate the role of central NO in tonically restraining sympathetic outflow from the brainstem in healthy rodents and humans. Thus, it is plausible that in CKD, reduced central NO concentrations potentially contribute to chronically elevated SNA in these patients.
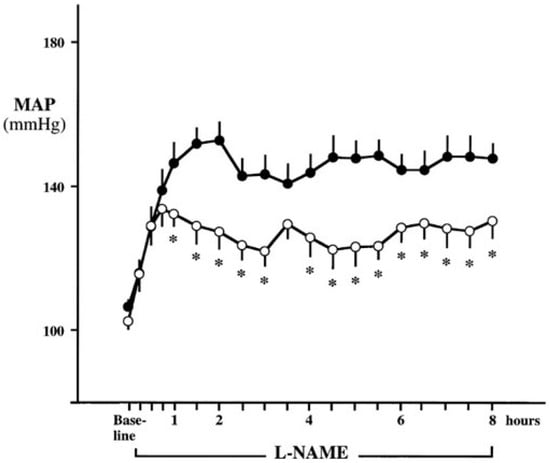
Figure 3.
Mean arterial pressure (MAP) responses during 8-h continuous systemic infusion of the nitric oxide synthase (NOS) inhibitor l-nitroarginine methyl ester (l-NAME) in rats with sympathectomy (open circles) and without sympathectomy (closed circles). Although no difference was observed in the initial increase in MAP (removal of endothelium-mediated dilation), sympathectomy attenuated the sustained hypertensive response to l-NAME, demonstrating a sympathetic contribution to the blood pressure raising effects of systemic NOS inhibition. * p < 0.05 vs. control. (From Sander et al. [75] with permission).
One of the major mechanisms causing reduced NO concentrations in CKD is elevated levels of asymmetric dimethylarginine (ADMA), the primary endogenous NOS inhibitor [89]. Plasma concentrations of ADMA are significantly elevated in mild CKD and increase progressively as renal function declines [14,90]. Importantly, numerous studies have shown ADMA to be a strong, independent predictor of future CV risk in CKD patients [10,91,92,93,94,95,96]. In fact, Zoccali et al. [94] reported that in renal failure patients, plasma ADMA concentrations are the second strongest predictor of all-cause and CV mortality (after age). ADMA concentrations also predict CV risk and mortality in earlier stages of CKD. For example, in a large cohort of stage 3 to 4 CKD patients, elevated ADMA was shown to have a strong association with CV disease and a modest association with all-cause and CV mortality [97]. While it is clear that high ADMA levels are associated with CV risk, to date the majority of work with ADMA has been correlational in nature with a focus on the well-known vascular endothelial properties of NO. Given the increasing functional evidence described above that indicates NO is also a key signaling molecule involved in the tonic restraint of central sympathetic outflow, a role for ADMA in contributing to sympathetic overactivity in CKD warrants consideration.
ADMA is produced in all cell types and can pass the blood–brain barrier [98,99]. It is produced by post-translational methylation of proteins via the enzyme arginine methyltransferase type I [95,99] and it is either metabolized by the dimethylarginine dimethylaminohydrolase (DDAH) enzyme or excreted by the kidneys [99,100]. Although reduced renal clearance of ADMA in CKD contributes to elevated plasma levels, the major pathway for elimination of ADMA is its metabolism by DDAH. It is estimated that the human body produces about 300 µmol of ADMA every day, of which 250 µmol is metabolized by DDAH and only a small amount is excreted by the kidneys [101]. In terms of sympathetic activation and ADMA, Augustyniak et al. [87] investigated the potential effects of ADMA on sympathetic outflow by systemic ADMA infusions in barointact and barodenervated animals. In barointact animals, ADMA infusion caused an initial decrease in renal SNA followed by an increase, causing renal SNA to go back to baseline values. In contrast, in barodenervated animals, systemic ADMA infusion caused a frank sympathoexcitation as indicated by ~50% increase in renal SNA at the end of ADMA infusion (Figure 4). Thus, elevated ADMA levels can cause inhibition of central NOS, thereby reducing NO bioavailability and increasing SNA. These data support the idea that elevated ADMA reduces central NO in CKD patients, potentially contributing to elevated sympathetic outflow from the brainstem (Figure 5). Along these lines, ADMA levels and resting muscle SNA were inversely related to eGFR in stage 2 to 4 CKD patients such that patients with higher ADMA levels had higher resting SNA and lower renal function (i.e., lower eGFR) [14].
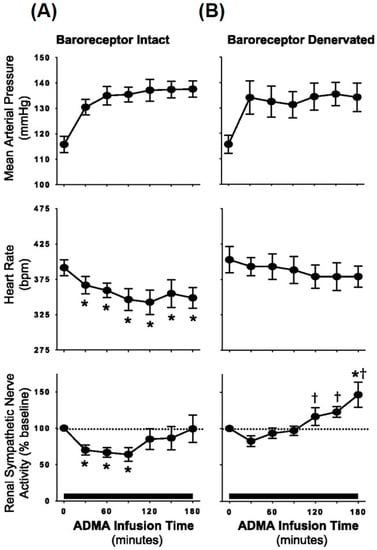
Figure 4.
Average mean arterial pressure, heart rate, and renal sympathetic nerve activity during continuous systemic infusion of asymmetric dimethylarginine (ADMA; an endogenous NOS inhibitor) in baroreceptor-intact (A) and baroreceptor-denervated animals (B). In comparison with barointact animals, barodenervated animals show a significant increase in renal SNA indicating frank sympathoexcitation in response to systemic ADMA infusion. * p < 0.05 vs. baseline; † p < 0.05 vs. baroreceptor intact. (From Augutyniak et al. [87] with permission.)
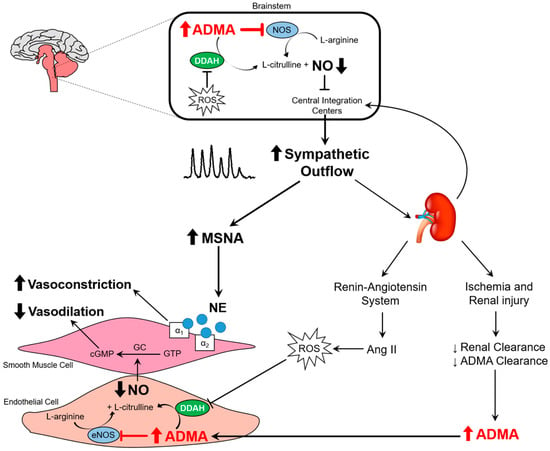
Figure 5.
Schematic illustration depicting the effects of elevated ADMA in the brainstem and the peripheral circulation. Reduced dimethylarginine dimethylaminohydrolase (DDAH) activity and renal clearance of ADMA leads to elevated plasma ADMA concentrations in chronic kidney disesase (CKD). Elevated ADMA in the brainstem inhibits NOS and reduces central nitric oxide (NO) production, contributing to higher central sympathetic outflow. This greater SNA results in peripheral vasoconstriction. When prolonged, the sympathetic overactivity leads to a host of other deleterious consequences as outlined in the text of the review. In the periphery, elevated ADMA inhibits NOS and decreases NO, thereby reducing endothelium-mediated vasodilation. Greater sympathetically-mediated vasoconstriction and lower endothelium-mediated vasodilation contributes to increased vascular tone and higher blood pressure. ROS, reactive oxygen species; eNOS, endothelial nitric oxide synthase; NE, norepinephrine. ⱶ- denotes inhibition. (See text for further details).
3.4. Oxidative Stress
CKD patients are reported to have higher oxidative stress [102,103,104,105,106], which could be another potential mechanism that results in chronic sympathoexcitation in CKD. Oxidative stress in the central nervous system has an important role in regulating sympathetic outflow from the brainstem [107]. Reactive oxygen species (ROS) such as superoxide ion, hydroxyl radical and hydrogen peroxide play an important role as intracellular messengers however, overproduction of ROS can be harmful. Various animal studies have indicated that elevated oxidative stress in the brain contributes to enhanced central sympathetic outflow, either directly or by scavenging NO [107,108,109,110,111]. Elevated levels of Ang II also contribute to overproduction of ROS as Ang II is a potent activator of nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase, the primary source of superoxide [112]. Reductions in oxidative stress in the brain using the superoxide dismutase (SOD; an enzyme that catalyzes superoxide ion) mimetic tempol or overexpression of SOD reverses the elevated central sympathetic outflow [113,114,115,116]. In addition, tempol administration through drinking water and systemic tempol infusion have both been shown to normalize SNA and reduce neuronal activity of presympathetic neurons in the PVN and the RVLM [117,118]. These studies provide clear evidence that oxidative stress can cause chronic sympathoexcitation. In addition to its direct effects, elevated oxidative stress also inhibits DDAH, the primary mechanism for breakdown of ADMA [96,119]. This may contribute to ADMA-induced increases in central sympathetic activation. Collectively, these studies indicate that oxidative stress is one of the mechanisms that increases sympathetic outflow from the brainstem and may be involved in elevated SNA in CKD.
4. Therapeutic Strategies
Sympathetic overactivity is a hallmark of CKD and therapeutic strategies to reduce this heightened sympathetic activation are needed. In this review we have identified some of the potential pathways contributing to elevated SNA in CKD that warrant consideration when discussing therapeutic targets to reduce SNA in this high risk population. Ang II receptor blockers and ACE inhibitors have been shown to lower resting MSNA and blood pressure but neither normalizes MSNA in CKD and thus, other therapeutic strategies are needed [15,16]. Sympatholytic agents such as moxonidine have been shown to be effective in reducing MSNA in patients [49] however, the side-effects limit their clinical application [120,121,122]. Statins are another potential therapeutic strategy that has been shown to reduce SNA and oxidative stress in addition to lowering cholesterol [123,124]. In fact, statins have been shown to downregulate Ang II receptors and upregulate nNOS in the brain [124]. Even short-term statin therapy has been shown to reduce sympathetic overactivity [125,126], making it a potentially beneficial therapy for CKD patients. Indeed, statin therapy in predialysis CKD patients reduced MSNA [127], delayed the start of dialysis [128], and decreased the risk of CV events and all-cause mortality [129,130]. In addition, reduction of oxidative stress is another potential mechanism for decreasing sympathetic overactivity in CKD. Although numerous animal studies have shown significant reductions in SNA after reducing oxidative stress, studies in patients have provided mixed results [131,132,133,134]. This could be, in part, due to the antioxidant used in the study (vitamin C vs. vitamin E), the duration of treatment (acute vs. chronic treatment), the dose of antioxidant and the efficacy of antioxidant in reducing oxidative stress. Furthermore, pioglitazone treatment may also be beneficial in CKD as it has been reported to reduce circulating ADMA levels [135,136]. Reductions in plasma ADMA concentrations can increase central NO and inhibit sympathetic outflow from the brainstem. Thus, pioglitazone treatment could be a promising therapy for reducing ADMA and sympathetic overactivity in CKD. In general, further research is warranted to identify viable pharmacological therapies to reduce sympathetic overactivity in CKD along with its deleterious consequences.
Aside from the aforementioned pharmacological strategies, there are therapeutic interventions such as renal denervation and carotid baroreflex stimulation that may also be used to reduce SNA in CKD. Renal denervation has been primarily utilized to treat resistant hypertension. Several studies in hypertensive patients and experimental animal disease models showed a significant reduction in blood pressure following renal denervation [137,138,139,140,141,142,143]. Interestingly, in a large controlled clinical trial (SYMPLICITY HTN-3), although hypertensive patients had a reduction in blood pressure following renal denervation, this decrease was not different from a sham control group [144]. Since bilateral nephrectomy in hemodialysis patients has been shown to reduce resting MSNA [11,68], renal denervation might be beneficial for the CKD population. Indeed, pilot studies have shown promising results: besides reducing blood pressure [145,146], renal denervation has also been shown to lower renin production, enhance GFR and reduce albuminuria [147,148]. Nevertheless, despite the advantages, some factors must be considered before performing renal denervation in CKD patients such as: contrast-induced nephropathy in CKD, non-optimal diameter of renal artery and complications due to low renal blood flow [146,149]. Another therapeutic intervention to potentially reduce sympathetic overactivity in CKD patients is carotid baroreceptor stimulation. With this intervention, a device is surgically implanted to chronically stimulate carotid baroreceptors. This technique has been shown to cause sympatho-inhibition and reduce blood pressure in hypertensive patients [150,151,152]. However, further studies are required to investigate the potential beneficial effects of chronic carotid baroreflex stimulation in CKD patients.
5. Conclusions
Chronic sympathoexcitation is a major contributor to increased CV risk and mortality in CKD. Despite recent advances in research, our understanding of the mechanisms causing sympathetic overactivity in CKD is still incomplete. As discussed in this review, various studies have proposed a role for the renin–angiotensin system, renal afferent stimulation, reduced NO concentrations due to elevated ADMA, and increased oxidative stress in contributing to chronic sympathoexcitation in CKD. Further research is needed to better clarify the individual and interactive roles of each of the aforementioned pathways in contributing to sympathetic overactivity in CKD patients.
Acknowledgments
The authors acknowledge the research support provided by NIH R01 HL127071.
Author Contributions
Jasdeep Kaur contributed to concept generation, drafting of the manuscript, critical revision of the manuscript and final approval of the article; Benjamin E. Young contributed to concept generation, critical revision of the manuscript and final approval of the article; Paul J. Fadel contributed to concept generation, critical revision of the manuscript and final approval of the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACE | Angiotensin converting enzyme |
| ADMA | Asymmetric dimethylarginine |
| bs | Bursts |
| cGMP | Cellular guanosine monophosphate |
| CKD | Chronic kidney disease |
| CV | Cardiovascular |
| DDAH | Dimethylarginine dimethylaminohydrolase |
| eGFR | Estimated glomerular filtration rate |
| eNOS | Endothelial nitric oxide synthase |
| hb | Heartbeats |
| iNOS | Inducible nitric oxide synthase |
| l-NAME | l-NG-nitroarginine methyl ester |
| l-NMMA | NG-monomethyl l-arginine |
| MSNA | Muscle sympathetic nerve activity |
| NAD(P)H | Nicotinamide adenine dinucleotide phosphate |
| NE | Norepinephrine |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| nNOS | Neuronal nitric oxide synthase |
| NTS | Nucleus tractus solitarius |
| PVN | Paraventricular nucleus |
| RVLM | Rostral ventrolateral medulla |
| SNA | Sympathetic nerve activity |
| SOD | Superoxide dismutase |
References
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van, L.F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Li, Y.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Ayanian, J.; Bragg-Gresham, J.; Balkrishnan, R.; Chen, J.L.; Cope, E.; et al. US Renal Data System 2015 Annual Data Report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, A.A.; Segel, J.E.; Zhuo, X.; Hoerger, T.J.; Imai, K.; Williams, D. Medical costs of CKD in the Medicare population. J. Am. Soc. Nephrol. 2013, 24, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [PubMed]
- Herzog, C.A.; Asinger, R.W.; Berger, A.K.; Charytan, D.M.; Diez, J.; Hart, R.G.; Eckardt, K.U.; Kasiske, B.L.; McCullough, P.A.; Passman, R.S.; et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Roberts, P.R.; New, D.I.; Kalra, P.A. Sudden cardiac death in hemodialysis patients: An in-depth review. Am. J. Kidney Dis. 2011, 57, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Foley, R.N.; Gilbertson, D.T.; Chen, S.C. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int. Suppl. 2015, 5, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Parlongo, S.; Cutrupi, S.; Benedetto, F.A.; Tripepi, G.; Bonanno, G.; Rapisarda, F.; Fatuzzo, P.; Seminara, G.; et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 2002, 105, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Tripepi, G.; Maas, R.; Malatino, L.; Boger, R.; Zoccali, C. Analysis of the relationship between norepinephrine and asymmetric dimethyl arginine levels among patients with end-stage renal disease. J. Am. Soc. Nephrol. 2004, 15, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Converse, R.L., Jr.; Jacobsen, T.N.; Toto, R.D.; Jost, C.M.; Cosentino, F.; Fouad-Tarazi, F.; Victor, R.G. Sympathetic overactivity in patients with chronic renal failure. N. Engl. J. Med. 1992, 327, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Campese, V.M.; Nobakht, N.; Middlekauff, H.R. Differential distribution of muscle and skin sympathetic nerve activity in patients with end-stage renal disease. J. Appl. Physiol. 2008, 105, 1873–1876. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Quarti-Trevano, F.; Seravalle, G.; Arenare, F.; Volpe, M.; Furiani, S.; Dell’Oro, R.; Mancia, G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 2011, 57, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Ghiadoni, L.; Tripepi, G.; Bruno, R.M.; Mancia, G.; Zoccali, C. Sympathetic nerve traffic and asymmetric dimethylarginine in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.H.; Ligtenberg, G.; Oey, P.L.; Koomans, H.A.; Blankestijn, P.J. Enalapril and losartan reduce sympathetic hyperactivity in patients with chronic renal failure. J. Am. Soc. Nephrol. 2003, 14, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, G.; Blankestijn, P.J.; Oey, P.L.; Klein, I.H.; Dijkhorst-Oei, L.T.; Boomsma, F.; Wieneke, G.H.; van Huffelen, A.C.; Koomans, H.A. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N. Engl. J. Med. 1999, 340, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.N.; Vaziri, N.D.; Maksy, M. Plasma norepinephrine, epinephrine, and dopamine levels in end-stage renal disease. Effect of hemodialysis. Arch. Intern. Med. 1985, 145, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.H.; Ligtenberg, G.; Neumann, J.; Oey, P.L.; Koomans, H.A.; Blankestijn, P.J. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J. Am. Soc. Nephrol. 2003, 14, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.H.; Ligtenberg, G.; Oey, P.L.; Koomans, H.A.; Blankestijn, P.J. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J. Am. Soc. Nephrol. 2001, 12, 2427–2433. [Google Scholar] [PubMed]
- Amann, K.; Rump, L.C.; Simonaviciene, A.; Oberhauser, V.; Wessels, S.; Orth, S.R.; Gross, M.L.; Koch, A.; Bielenberg, G.W.; van Kats, J.P.; et al. Effects of low dose sympathetic inhibition on glomerulosclerosis and albuminuria in subtotally nephrectomized rats. J. Am. Soc. Nephrol. 2000, 11, 1469–1478. [Google Scholar] [PubMed]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. 2009, 148, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Dell’Oro, R.; Mancia, G. Sympathetic mechanisms, organ damage, and antihypertensive treatment. Curr. Hypertens. Rep. 2011, 13, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Abboud, F.M. The sympathetic system in hypertension. State-of-the-art review. Hypertension 1982, 4, 208–225. [Google Scholar] [PubMed]
- Smith, P.A.; Graham, L.N.; Mackintosh, A.F.; Stoker, J.B.; Mary, D.A. Relationship between central sympathetic activity and stages of human hypertension. Am. J. Hypertens. 2004, 17, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Cattaneo, B.M.; Seravalle, G.; Lanfranchi, A.; Mancia, G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 1998, 31, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S. Controlling the epidemic of cardiovascular disease in chronic renal disease: Where do we start? Am. J. Kidney Dis. 1998, 32, 5–13. [Google Scholar] [CrossRef]
- Coresh, J.; Wei, G.L.; McQuillan, G.; Brancati, F.L.; Levey, A.S.; Jones, C.; Klag, M.J. Prevalence of high blood pressure and elevated serum creatinine level in the United States: Findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch. Intern. Med. 2001, 161, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Feihl, F.; Liaudet, L.; Levy, B.I.; Waeber, B. Hypertension and microvascular remodelling. Cardiovasc. Res. 2008, 78, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Folkow, B. Physiological aspects of primary hypertension. Physiol. Rev. 1982, 62, 347–504. [Google Scholar] [PubMed]
- Katholi, R.E.; Couri, D.M. Left ventricular hypertrophy: Major risk factor in patients with hypertension: Update and practical clinical applications. Int. J. Hypertens. 2011. [Google Scholar] [CrossRef] [PubMed]
- Simpson, P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J. Clin. Investig. 1983, 72, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Raum, W.J.; Laks, M.M.; Garner, D.; Ikuhara, M.H.; Swerdloff, R.S. Norepinephrine increases β-receptors and adenylate cyclase in canine myocardium. Am. J. Physiol. 1984, 246, H31–H36. [Google Scholar] [PubMed]
- Laks, M.M.; Morady, F.; Swan, H.J. Myocardial hypertrophy produced by chronic infusion of subhypertensive doses of norepinephrine in the dog. Chest 1973, 64, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.D. Effect of sympathetic denervation on smooth muscle cell proliferation in the growing rabbit ear artery. Circ. Res. 1975, 37, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.D. Trophic effects of peripheral adrenergic nerves on vascular structure. Hypertension 1984, 6, 19–26. [Google Scholar] [CrossRef]
- Coutsos, M.; Sala-Mercado, J.A.; Ichinose, M.; Li, Z.; Dawe, E.J.; O’Leary, D.S. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Volders, P.G. Novel insights into the role of the sympathetic nervous system in cardiac arrhythmogenesis. Heart Rhythm 2010, 7, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- DiBona, G.F. Sympathetic nervous system and the kidney in hypertension. Curr. Opin. Nephrol. Hypertens. 2002, 11, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, K.; Noma, T.; Fujisawa, Y.; Ishihara, Y.; Arai, Y.; Nabi, A.H.; Suzuki, F.; Nagai, Y.; Nakano, D.; Hitomi, H.; et al. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation 2012, 125, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Jamerson, K.A.; Julius, S.; Gudbrandsson, T.; Andersson, O.; Brant, D.O. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension 1993, 21, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Salzer, D.A.; Medeiros, P.J.; Craen, R.; Shoemaker, J.K. Neurogenic-nitric oxide interactions affecting brachial artery mechanics in humans: Roles of vessel distensibility vs. diameter. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1181–R1187. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Lacolley, P.; Girerd, X.; Beck, L.; Safar, M.; Laurent, S. Sympathetic activation decreases medium-sized arterial compliance in humans. Am. J. Physiol. 1994, 267, H1368–H1376. [Google Scholar] [PubMed]
- Failla, M.; Grappiolo, A.; Emanuelli, G.; Vitale, G.; Fraschini, N.; Bigoni, M.; Grieco, N.; Denti, M.; Giannattasio, C.; Mancia, G. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. J. Hypertens. 1999, 17, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.; de, G.P.; Kooijman, M.; Smits, P.; Hopman, M.T. Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Hijmering, M.L.; Stroes, E.S.; Olijhoek, J.; Hutten, B.A.; Blankestijn, P.J.; Rabelink, T.J. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J. Am. Coll. Cardiol. 2002, 39, 683–688. [Google Scholar] [CrossRef]
- Santos, A.C.; Alves, M.J.; Rondon, M.U.; Barretto, A.C.; Middlekauff, H.R.; Negrao, C.E. Sympathetic activation restrains endothelium-mediated muscle vasodilatation in heart failure patients. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.R.; Manuck, S.B. Using ethological principles to study psychosocial influences on coronary atherosclerosis in monkeys. Acta Physiol. Scand. Suppl. 1997, 640, 96–99. [Google Scholar] [PubMed]
- Kaplan, J.R.; Manuck, S.B.; Clarkson, T.B.; Lusso, F.M.; Taub, D.M.; Miller, E.W. Social stress and atherosclerosis in normocholesterolemic monkeys. Science 1983, 220, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Strojek, K.; Grzeszczak, W.; Gorska, J.; Leschinger, M.I.; Ritz, E. Lowering of microalbuminuria in diabetic patients by a sympathicoplegic agent: Novel approach to prevent progression of diabetic nephropathy? J. Am. Soc. Nephrol. 2001, 12, 602–605. [Google Scholar] [PubMed]
- Anguiano, L.; Riera, M.; Pascual, J.; Valdivielso, J.M.; Barrios, C.; Betriu, A.; Clotet, S.; Mojal, S.; Fernandez, E.; Soler, M.J. Circulating angiotensin converting enzyme 2 activity as a biomarker of silent atherosclerosis in patients with chronic kidney disease. Atherosclerosis 2016, 253, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Bell, P.R.; Briggs, J.D.; Brown, J.J.; Fine, A.; Lever, A.F.; Morton, J.J.; Paton, A.M.; Robertson, J.I.; Tree, M.; et al. Changes of blood pressure, renin, and angiotensin after bilateral nephrectomy in patients with chronic renal failure. Br. Med. J. 1972, 4, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.N.; Rosenberg, M.E.; Hostetter, T.H. Role of the renin-angiotensin-aldosterone system in the progression of renal disease: A critical review. Semin. Nephrol. 1997, 17, 431–440. [Google Scholar] [PubMed]
- Reid, I.A. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am. J. Physiol. 1992, 262, E763–E778. [Google Scholar] [PubMed]
- Siddiqi, L.; Joles, J.A.; Grassi, G.; Blankestijn, P.J. Is kidney ischemia the central mechanism in parallel activation of the renin and sympathetic system? J. Hypertens. 2009, 27, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhu, D.N.; Yu, Z.; Wang, J.Q.; Sun, Z.J.; Yao, T. Expression of angiotensin II type 1 (AT1) receptor in the rostral ventrolateral medulla in rats. J. Appl. Physiol. 2002, 92, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Muratani, H.; Averill, D.B.; Ferrario, C.M. Effect of angiotensin II in ventrolateral medulla of spontaneously hypertensive rats. Am. J. Physiol. 1991, 260, 977–984. [Google Scholar]
- Hirooka, Y.; Potts, P.D.; Dampney, R.A. Role of angiotensin II receptor subtypes in mediating the sympathoexcitatory effects of exogenous and endogenous angiotensin peptides in the rostral ventrolateral medulla of the rabbit. Brain Res. 1997, 772, 107–114. [Google Scholar] [CrossRef]
- Allen, A.M. Blockade of angiotensin AT1-receptors in the rostral ventrolateral medulla of spontaneously hypertensive rats reduces blood pressure and sympathetic nerve discharge. J. Renin Angiotensin Aldosterone Syst. 2001, 2, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Moeller, I.; Jenkins, T.A.; Zhuo, J.; Aldred, G.P.; Chai, S.Y.; Mendelsohn, F.A. Angiotensin receptors in the nervous system. Brain Res. Bull. 1998, 47, 17–28. [Google Scholar] [CrossRef]
- Davisson, R.L. Physiological genomic analysis of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Campese, V.M.; Kogosov, E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension 1995, 25, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhong, H.; Yanamadala, V.; Campese, V.M. Renal injury caused by intrarenal injection of phenol increases afferent and efferent renal sympathetic nerve activity. Am. J. Hypertens. 2002, 15, 717–724. [Google Scholar] [CrossRef]
- Ye, S.; Ozgur, B.; Campese, V.M. Renal afferent impulses, the posterior hypothalamus, and hypertension in rats with chronic renal failure. Kidney Int. 1997, 51, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhong, H.; Yanamadala, S.; Campese, V.M. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension 2006, 48, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Katholi, R.E.; Whitlow, P.L.; Hageman, G.R.; Woods, W.T. Intrarenal adenosine produces hypertension by activating the sympathetic nervous system via the renal nerves in the dog. J. Hypertens. 1984, 2, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Recordati, G.; Moss, N.G.; Genovesi, S.; Rogenes, P. Renal chemoreceptors. J. Auton. Nerv. Syst. 1981, 3, 237–251. [Google Scholar] [CrossRef]
- Vallon, V.; Muhlbauer, B.; Osswald, H. Adenosine and kidney function. Physiol. Rev. 2006, 86, 901–940. [Google Scholar] [CrossRef] [PubMed]
- Hausberg, M.; Kosch, M.; Harmelink, P.; Barenbrock, M.; Hohage, H.; Kisters, K.; Dietl, K.H.; Rahn, K.H. Sympathetic nerve activity in end-stage renal disease. Circulation 2002, 106, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Desir, G.V.; Peixoto, A.J. Renalase in hypertension and kidney disease. Nephrol. Dial. Transplant. 2014, 29, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, G.; Wang, P.; Velazquez, H.; Yao, X.; Li, Y.; Wu, Y.; Peixoto, A.; Crowley, S.; Desir, G.V. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J. Clin. Investig. 2005, 115, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Desir, G.V. Renalase deficiency in chronic kidney disease, and its contribution to hypertension and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2008, 17, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, J.; Wang, P.; Velazquez, H.; Li, Y.; Wu, Y.; Desir, G.V. Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation 2008, 117, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Mungrue, I.N.; Bredt, D.S.; Stewart, D.J.; Husain, M. From molecules to mammals: What’s NOS got to do with it? Acta Physiol. Scand. 2003, 179, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, I.; Togashi, H.; Yoshioka, M.; Saito, H.; Yanagida, M.; Tamura, M.; Kobayashi, T.; Yasuda, H.; Gross, S.S.; Levi, R. NG-methyl-l-arginine, an inhibitor of l-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ. Res. 1992, 70, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Hansen, J.; Victor, R.G. The sympathetic nervous system is involved in the maintenance but not initiation of the hypertension induced by N(omega)-nitro-l-arginine methyl ester. Hypertension 1997, 30, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Chavoshan, B.; Victor, R.G. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension 1999, 33, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.O., III. Role of nitric oxide in cardiovascular disease: Focus on the endothelium. Clin. Chem. 1998, 44, 1809–1819. [Google Scholar] [PubMed]
- Cooke, J.P.; Dzau, V.J. Nitric oxide synthase: Role in the genesis of vascular disease. Annu. Rev. Med. 1997, 48, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Shapoval, L.N.; Sagach, V.F.; Pobegailo, L.S. Nitric oxide influences ventrolateral medullary mechanisms of vasomotor control in the cat. Neurosci. Lett. 1991, 132, 47–50. [Google Scholar] [CrossRef]
- Harada, S.; Tokunaga, S.; Momohara, M.; Masaki, H.; Tagawa, T.; Imaizumi, T.; Takeshita, A. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circ. Res. 1993, 72, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, T.; Imaizumi, T.; Harada, S.; Endo, T.; Shiramoto, M.; Hirooka, Y.; Takeshita, A. Nitric oxide influences neuronal activity in the nucleus tractus solitarius of rat brainstem slices. Circ. Res. 1994, 75, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Togashi, H.; Sakuma, I.; Yoshioka, M.; Kobayashi, T.; Yasuda, H.; Kitabatake, A.; Saito, H.; Gross, S.S.; Levi, R. A central nervous system action of nitric oxide in blood pressure regulation. J. Pharmacol. Exp. Ther. 1992, 262, 343–347. [Google Scholar] [PubMed]
- Kishi, T.; Hirooka, Y.; Sakai, K.; Shigematsu, H.; Shimokawa, H.; Takeshita, A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension 2001, 38, 896–901. [Google Scholar] [PubMed]
- Kishi, T.; Hirooka, Y.; Ito, K.; Sakai, K.; Shimokawa, H.; Takeshita, A. Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone spontaneously hypertensive rats. Hypertension 2002, 39, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Averill, D.B.; Khosla, M.C.; Ferrario, C.M. Role of nitric oxide and angiotensin II in the regulation of sympathetic nerve activity in spontaneously hypertensive rats. Hypertension 1993, 21, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Jacobsen, T.N.; Victor, R.G. Is nitric oxide involved in the tonic inhibition of central sympathetic outflow in humans? Hypertension 1994, 24, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, R.A.; Victor, R.G.; Morgan, D.A.; Zhang, W. l-NAME and ADMA-induced sympathetic neural activation in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R726–R732. [Google Scholar] [CrossRef] [PubMed]
- Young, C.N.; Fisher, J.P.; Gallagher, K.M.; Whaley-Connell, A.; Chaudhary, K.; Victor, R.G.; Thomas, G.D.; Fadel, P.J. Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J. Physiol. 2009, 587, 4977–4986. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wagner, L.; Schmidt, R.J.; Baylis, C. Circulating endothelial nitric oxide synthase inhibitory factor in some patients with chronic renal disease. Kidney Int. 2001, 59, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Boger, R.H.; Bode-Boger, S.M.; Frolich, J.C.; Haller, H.; Ritz, E.; Fliser, D. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J. Am. Soc. Nephrol. 2002, 13, 170–176. [Google Scholar] [PubMed]
- Ravani, P.; Tripepi, G.; Malberti, F.; Testa, S.; Mallamaci, F.; Zoccali, C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: A competing risks modeling approach. J. Am. Soc. Nephrol. 2005, 16, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Yamagishi, S.; Kaida, Y.; Okuda, S. Asymmetric dimethylarginine may be a missing link between cardiovascular disease and chronic kidney disease. Nephrology 2007, 12, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C. Asymmetric dimethylarginine (ADMA): A cardiovascular and renal risk factor on the move. J. Hypertens. 2006, 24, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Bode-Boger, S.; Mallamaci, F.; Benedetto, F.; Tripepi, G.; Malatino, L.; Cataliotti, A.; Bellanuova, I.; Fermo, I.; Frolich, J.; et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 2001, 358, 2113–2117. [Google Scholar] [CrossRef]
- Fliser, D.; Kronenberg, F.; Kielstein, J.T.; Morath, C.; Bode-Boger, S.M.; Haller, H.; Ritz, E. Asymmetric dimethylarginine and progression of chronic kidney disease: The mild to moderate kidney disease study. J. Am. Soc. Nephrol. 2005, 16, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Boger, R.H.; Bode-Boger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Terrin, N.; Wang, X.; Greene, T.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Sarnak, M.J.; Menon, V. Asymmetric dimethylarginine and mortality in stages 3 to 4 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.P.; Pazarentzos, E.; Fidanboylu, M.; Padilla, B.; Brown, R.; Thomas, S.A. The transporter and permeability interactions of asymmetric dimethylarginine (ADMA) and l-arginine with the human blood-brain barrier in vitro. Brain Res. 2016, 1648, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leiper, J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.T.; Leiper, J.M.; Vallance, P. The DDAH/ADMA/NOS pathway. Atheroscler. Suppl. 2003, 4, 33–40. [Google Scholar] [CrossRef]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Martinez Cantarin, M.P.; Whitaker-Menezes, D.; Lin, Z.; Falkner, B. Uremia induces adipose tissue inflammation and muscle mitochondrial dysfunction. Nephrol. Dial. Transplant. 2017, 32, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Morimoto, S.; Okigaki, M.; Seo, M.; Someya, K.; Morita, T.; Matsubara, H.; Sugiura, T.; Iwasaka, T. Decreased plasma level of vitamin C in chronic kidney disease: Comparison between diabetic and non-diabetic patients. Nephrol. Dial. Transplant. 2011, 26, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, Y.; Kishi, T.; Sakai, K.; Takeshita, A.; Sunagawa, K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R818–R826. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Sales, E.B.; Nishi, E.E.; Carillo, B.A.; Boim, M.A.; Dolnikoff, M.S.; Bergamaschi, C.T.; Campos, R.R. Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am. J. Hypertens. 2009, 22, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M.; Hirooka, Y.; Matsukawa, R.; Kishi, T.; Sunagawa, K. Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J. Hypertens. 2012, 30, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Kimura, Y.; Ito, K.; Shimokawa, H.; Takeshita, A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 2004, 109, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Campese, V.M.; Ye, S.; Zhong, H.; Yanamadala, V.; Ye, Z.; Chiu, J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Campese, V.M.; Shaohua, Y.; Huiquin, Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 2005, 46, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Hsu, K.S.; Huang, C.C.; Wang, L.L.; Ou, C.C.; Chan, J.Y. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ. Res. 2005, 97, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Mayhan, W.G.; Bidasee, K.R.; Zheng, H. Enhanced angiotensin II-mediated central sympathoexcitation in streptozotocin-induced diabetes: Role of superoxide anion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R311–R320. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.C.; Lazartigues, E.; Lang, J.A.; Sinnayah, P.; Ahmad, I.M.; Spitz, D.R.; Davisson, R.L. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ. Res. 2002, 91, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Chen, A.D.; Xu, Y.; Chen, Q.; Gao, X.Y.; Wang, W.; Zhu, G.Q. Long-term administration of tempol attenuates postinfarct ventricular dysfunction and sympathetic activity in rats. Pflug. Arch. 2009, 458, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.G.; Zhang, Z.H.; Yu, Y.; Felder, R.B. Systemically administered tempol reduces neuronal activity in paraventricular nucleus of hypothalamus and rostral ventrolateral medulla in rats. J. Hypertens. 2009, 27, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Ogawa, T.; Cooke, J.P. Novel mechanism for endothelial dysfunction: Dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 1999, 99, 3092–3095. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Pfeffer, M.A.; Rouleau, J.; Sharpe, N.; Swedberg, K.; Straub, M.; Wiltse, C.; Wright, T.J. Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON). Eur. J. Heart Fail. 2003, 5, 659–667. [Google Scholar] [CrossRef]
- Planitz, V. Crossover comparison of moxonidine and clonidine in mild to moderate hypertension. Eur. J. Clin. Pharmacol. 1984, 27, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Bristow, M.R.; Cohn, J.N.; Dargie, H.; Straub, M.; Wiltse, C.; Wright, T.J. Effects of sustained-release moxonidine, an imidazoline agonist, on plasma norepinephrine in patients with chronic heart failure. Circulation 2002, 105, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, W.; Li, Y.L.; Schultz, H.D.; Liu, D.; Cornish, K.G.; Zucker, I.H. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: Roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation 2005, 112, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, W.; Zucker, I.H. Simvastatin inhibits central sympathetic outflow in heart failure by a nitric-oxide synthase mechanism. J. Pharmacol. Exp. Ther. 2008, 326, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.H.; Fisher, J.P.; Vianna, L.C.; Kim, A.; Chockalingam, A.; Zimmerman, M.C.; Zucker, I.H.; Fadel, P.J. Statin therapy lowers muscle sympathetic nerve activity and oxidative stress in patients with heart failure. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, J.; Symonides, B.; Gaciong, Z.; Sinski, M. The effect of statins on sympathetic activity: A meta-analysis. Clin. Auton. Res. 2015, 25, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, L.; Joles, J.A.; Oey, P.L.; Blankestijn, P.J. Atorvastatin reduces sympathetic activity in patients with chronic kidney disease. J. Hypertens. 2011, 29, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.M.; Lin, M.S.; Hsu, J.T.; Hsiao, J.F.; Chang, S.T.; Pan, K.L.; Lin, C.L.; Lin, Y.S. Effects of statin therapy on cerebrovascular and renal outcomes in patients with predialysis advanced chronic kidney disease and dyslipidemia. J. Clin. Lipidol. 2017, 11, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Major, R.W.; Cheung, C.K.; Gray, L.J.; Brunskill, N.J. Statins and Cardiovascular Primary Prevention in CKD: A Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Messow, C.M.; Isles, C. Meta-analysis of statins in chronic kidney disease: Who benefits? QJM 2017. [Google Scholar] [CrossRef] [PubMed]
- Boaz, M.; Smetana, S.; Weinstein, T.; Matas, Z.; Gafter, U.; Iaina, A.; Knecht, A.; Weissgarten, Y.; Brunner, D.; Fainaru, M.; et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomised placebo-controlled trial. Lancet 2000, 356, 1213–1218. [Google Scholar] [CrossRef]
- Bruno, R.M.; Daghini, E.; Ghiadoni, L.; Sudano, I.; Rugani, I.; Varanini, M.; Passino, C.; Emdin, M.; Taddei, S. Effect of acute administration of vitamin C on muscle sympathetic activity, cardiac sympathovagal balance, and baroreflex sensitivity in hypertensive patients. Am. J. Clin. Nutr. 2012, 96, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.E.; El, M.S.; Lenders, J.W.; Bellersen, L.; Verheugt, F.W.; Smits, P.; Tack, C.J. High dose ascorbic acid does not reverse central sympathetic overactivity in chronic heart failure. J. Clin. Pharm. Ther. 2011, 36, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.F. Vitamin C supplementation in kidney failure: Effect on uraemic symptoms. Nephrol. Dial. Transplant. 2011, 26, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Yamagishi, S.; Mizoguchi, M.; Tahara, A.; Imaizumi, T. Pioglitazone decreases asymmetric dimethylarginine levels in patients with impaired glucose tolerance or type 2 diabetes. Rejuvenation Res. 2013, 16, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Wakino, S.; Hayashi, K.; Tatematsu, S.; Hasegawa, K.; Takamatsu, I.; Kanda, T.; Homma, K.; Yoshioka, K.; Sugano, N.; Saruta, T. Pioglitazone lowers systemic asymmetric dimethylarginine by inducing dimethylarginine dimethylaminohydrolase in rats. Hypertens Res. 2005, 28, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.D.; Krum, H.; Sobotka, P.A.; Schlaich, M.P.; Schmieder, R.E.; Bohm, M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The symplicity HTN-2 trial): A randomised controlled trial. Lancet 2010, 376, 1903–1909. [Google Scholar] [PubMed]
- Krum, H.; Schlaich, M.; Whitbourn, R.; Sobotka, P.A.; Sadowski, J.; Bartus, K.; Kapelak, B.; Walton, A.; Sievert, H.; Thambar, S.; et al. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet 2009, 373, 1275–1281. [Google Scholar] [CrossRef]
- Jacob, F.; Ariza, P.; Osborn, J.W. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2302–H2310. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, N.B.; Johnson, W.R.; Dwyer, T.M.; Alexander, B.T. Early renal denervation prevents development of hypertension in growth-restricted offspring. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Katholi, R.E.; Rocha-Singh, K.J. The role of renal sympathetic nerves in hypertension: Has percutaneous renal denervation refocused attention on their clinical significance? Prog. Cardiovasc. Dis. 2009, 52, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Hendel, M.D.; Collister, J.P. Renal denervation attenuates long-term hypertensive effects of Angiotensin II in the rat. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Lohmeier, T.E.; Hildebrandt, D.A.; Dwyer, T.M.; Barrett, A.M.; Irwin, E.D.; Rossing, M.A.; Kieval, R.S. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension 2007, 49, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Hering, D.; Mahfoud, F.; Walton, A.S.; Krum, H.; Lambert, G.W.; Lambert, E.A.; Sobotka, P.A.; Bohm, M.; Cremers, B.; Esler, M.D.; et al. Renal denervation in moderate to severe CKD. J. Am. Soc. Nephrol. 2012, 23, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Papademetriou, V.; Doumas, M.; Anyfanti, P.; Faselis, C.; Kokkinos, P.; Tsioufis, C. Renal nerve ablation for hypertensive patients with chronic kidney disease. Curr. Vasc. Pharmacol. 2014, 12, 47–54. [Google Scholar] [PubMed]
- Gattone, V.H.; Siqueira, T.M., Jr.; Powell, C.R.; Trambaugh, C.M.; Lingeman, J.E.; Shalhav, A.L. Contribution of renal innervation to hypertension in rat autosomal dominant polycystic kidney disease. Exp. Biol. Med. 2008, 233, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, M.G.; Maia, G.L.; de Queiroz Carreira, M.A.; Kiuchi, T.; Chen, S.; Andrea, B.R.; Graciano, M.L.; Lugon, J.R. Effects of renal denervation with a standard irrigated cardiac ablation catheter on blood pressure and renal function in patients with chronic kidney disease and resistant hypertension. Eur. Heart J. 2013, 34, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, M.P.; Bart, B.; Hering, D.; Walton, A.; Marusic, P.; Mahfoud, F.; Bohm, M.; Lambert, E.A.; Krum, H.; Sobotka, P.A.; et al. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int. J. Cardiol. 2013, 168, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Lohmeier, T.E.; Iliescu, R.; Dwyer, T.M.; Irwin, E.D.; Cates, A.W.; Rossing, M.A. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Heusser, K.; Tank, J.; Engeli, S.; Diedrich, A.; Menne, J.; Eckert, S.; Peters, T.; Sweep, F.C.; Haller, H.; Pichlmaier, A.M.; et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 2010, 55, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Bisognano, J.D.; Bakris, G.; Nadim, M.K.; Sanchez, L.; Kroon, A.A.; Schafer, J.; de Leeuw, P.W.; Sica, D.A. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: Results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J. Am. Coll. Cardiol. 2011, 58, 765–773. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).