1. Introduction
Unhealed chronic wounds that develop in patients with senile decay and/or metabolic disorders including diabetes cause serious problems for not only the individual, but also society. Fast healing and successful management of the wounds become even more important today. Repair mechanisms of wound healing have been well investigated [
1]; even in different organs, similar processes are involved, perhaps because of a fundamental response to injury in our bodies. Tissue response after wounding proceeds in a sequential manner [
1]; after wounding by damage or tissue loss, tissue-derived factors reduce blood flow by vasodilation and initiate inflammatory cell infiltrates, which may involve tissue remodeling and a protective response against tissue infection. Granulation tissue also starts to develop as a complemental tissue at the wound site [
2], in which extracellular matrix (ECM) maturation including the change in collagen type and angiogenesis/neovascularization occur. Granulation tissue is therefore important and works as a scaffold for tissue regeneration, such as epidermal regeneration in skin wounds.
After wounding, different growth factors are involved in the repair process [
3], which include transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF). These factors act to manifest biological roles in granulation tissue, i.e., proliferation, chemotaxis, collagen synthesis, transdifferentiating and angiogenesis/neovascularization. Granulation tissue also works for wound contraction, in which alpha-smooth muscle actin (αSMA)-expressing fibroblasts (myofibroblasts) play a central role. It is speculated that the origin of αSMA-expressing myofibroblasts is from local resident fibroblasts, bone marrow, endothelial-to-mesenchymal transition, epithelial-to-mesenchymal transition and tissue-specific mesenchymal stem cells [
4,
5]. Matured fat tissue also originates via dedifferentiation [
6]. TGF-β1 is a portent factor for wound contraction and ECM deposition through the function of αSMA expressing myofibroblasts [
5], by which ECM is remodeled in granulation tissue. At the end of wound healing, granulation tissue, including myofibroblasts, vanishes [
5], and wound healing reaches its completion.
Reverdin (1869) [
7] initially described the micrograft technique for wound healing, and to date, many applications with different graft sizes have been utilized [
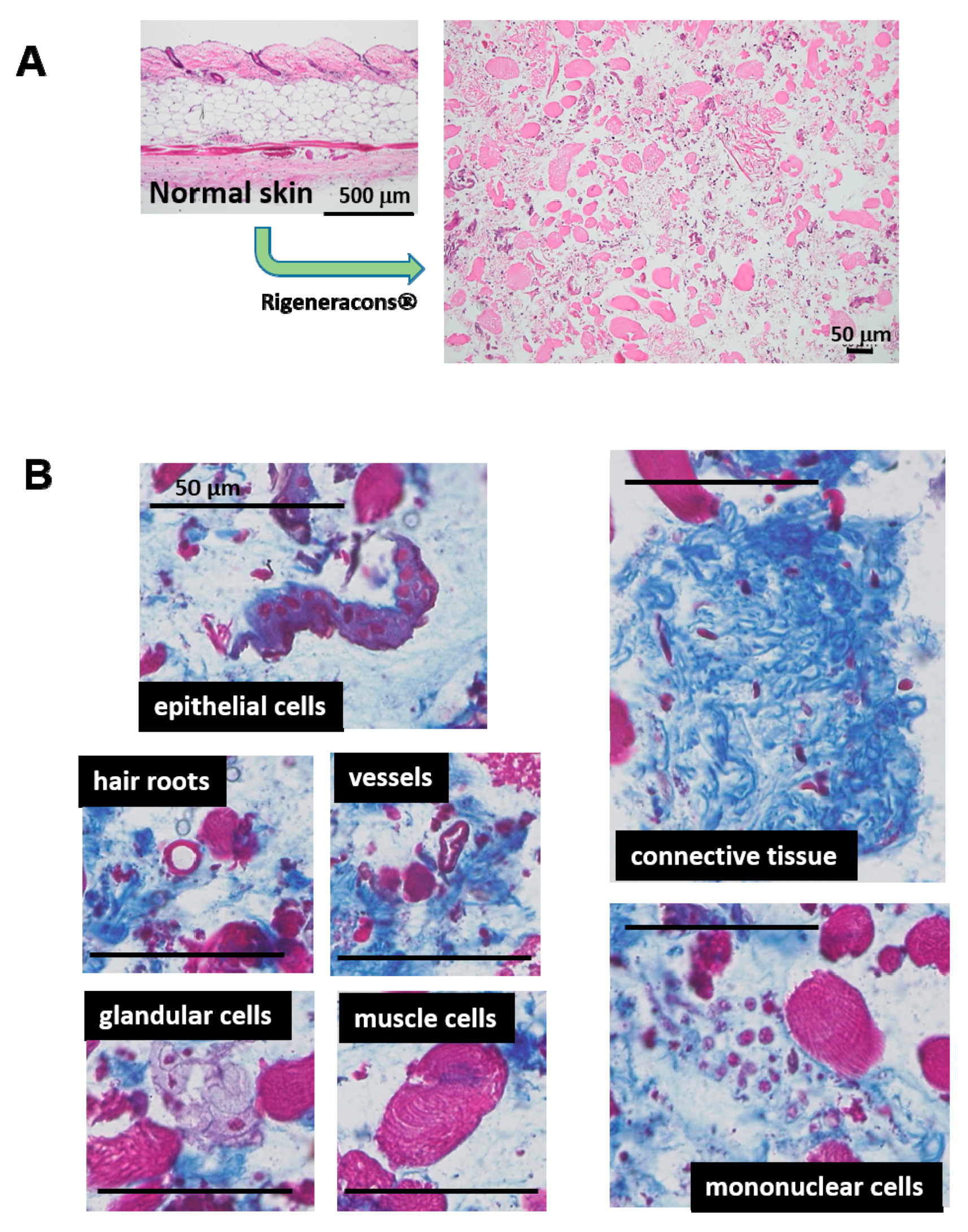
8]. Recently, an advanced and innovative micrograft technique has been established, namely the Rigenera protocol [
9]. This technique embraces a unique and advantageous concept; a normal autologous tissue is minced into less than 50 μm in diameter fragments by Rigeneracons
®. The resulting solution contains micro-tissue fragments, which also may include cellular niche and ECM in situ. Therefore, inherent cellular activity might be preserved after transplantation. Trovato et al. (2015) [
9] have shown that the obtained tissue solution is rich in progenitor cells for tissue regeneration. Regarding the wound healing study, an animal study using gingival connective tissue has been recently reported [
10]. Moreover, clinical trials using the technique have also been performed, such as dehiscent surgical wounds [
11], complex wound [
12], chronic ulcers [
13] and chronic scars [
14]. All of the results have demonstrated good outcomes. However, the pathophysiological mechanisms of wound healing accelerated by this technique are still unclear. Therefore, in the present study, we clarified the mechanism of action of micrograft in wound healing using our established mouse model [
15].
3. Discussion
An innovative micrograft technique using autologous minced tissue less than 50 μm in diameter has recently been established [
9]. Many clinical trials including wound healing were already reported [
11,
12,
13,
14]; most of the results seem favorable. However, its mechanisms of action are still not clear. In the present investigation, the micrograft technique was examined using a mouse wound healing model, which we have established as a humanized wound in mice by splinting dermal tissue [
15]. The method has substantial merit to estimate regenerative epithelial healing, which is important for human wound healing.
Grafted tissue was obtained from normal skin tissue, which may contain not only different cell types from different tissues (connective tissue, epidermis, hair root, vessels, glands, and so on), but also skin-originated stem cells, including epithelial stem/progenitor and mesenchymal stem cells [
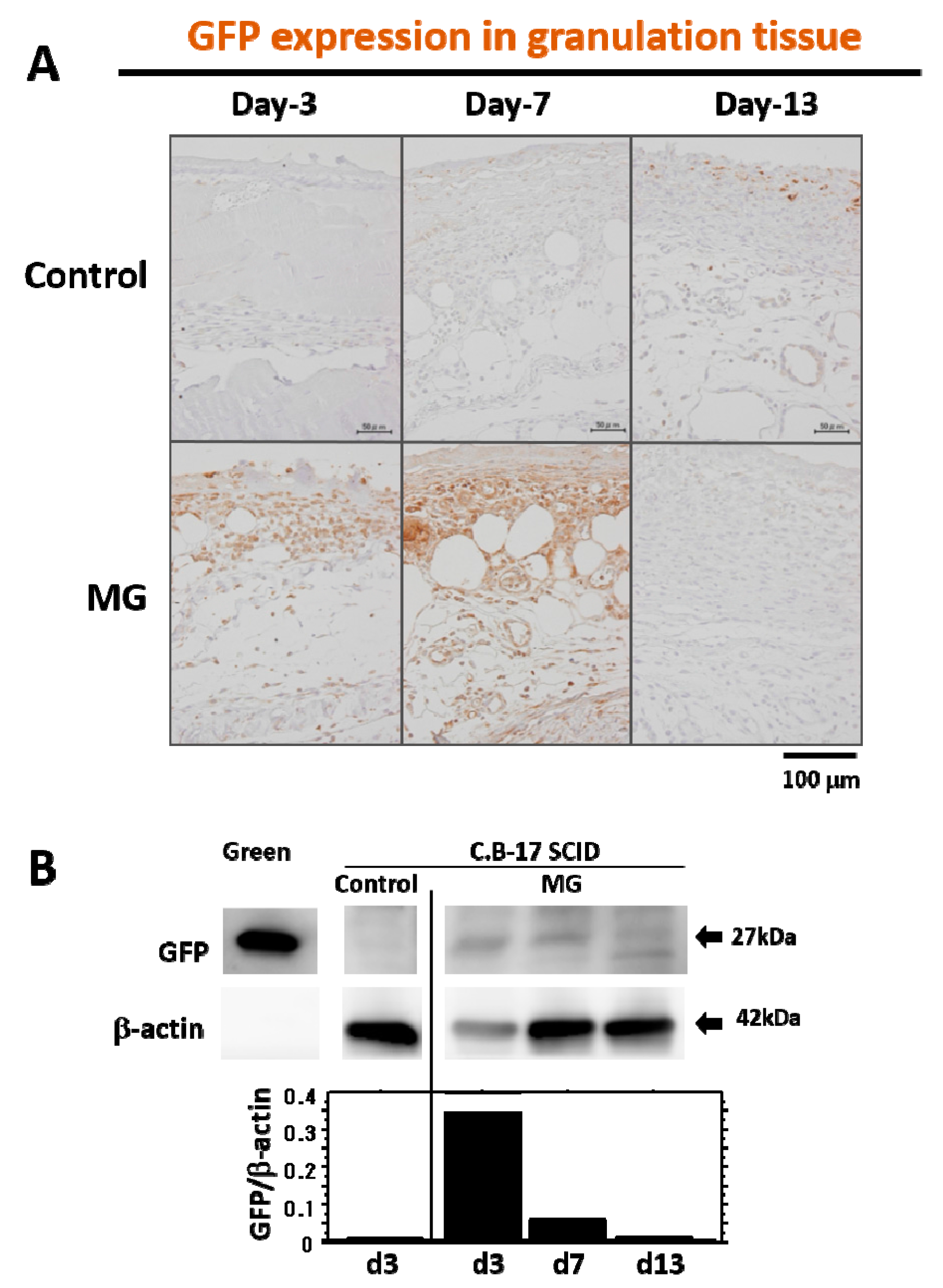
16]. In our tracing study of grafted tissue, the presence of GFP-expressing cells from the skin of green mice was limited in the early phase of granulation (four days after micrograft, seven days after wounding), but later on, no GFP-expressing cells were detected in matured granulation tissue. This indicates that grafted cells may be apoptosed due to completion of their initial roles for granulation tissue development, and they thus disappeared. Another possibility is that the GFP expression, which lasted for four days, may subsequently cease via alternative
GFP gene suppression by an unknown mechanism; such cells could induce the development of granulation tissue. Molecular mechanisms of grafted cells in wound healing are still unknown. However, unlike regeneration, granulation tissue is developed as a temporary foundation tissue that supports the growth of constitutional and/or regenerative cells already present in the tissue, which subsequently vanishes after completing its roles. Therefore, this inconsecutive existence of grafted cells in granulation tissue may be reasonable in physiology.
In a previously reported study, tissue-resident progenitor cells from different human tissues were harvested by this technique, such as from the skin [
13], periosteum, atrial appendage and lateral rectus muscle of the eyeball [
9]. Unlike cell transplantation, normal tissue solution prepared by this technique contained not only graftable cells, but also other ungraftable cells, mechanically degenerative cells and matrix proteins. This state might mimic tissue degeneration after wounding, in which important factors for tissue repair, such as TGF-β [
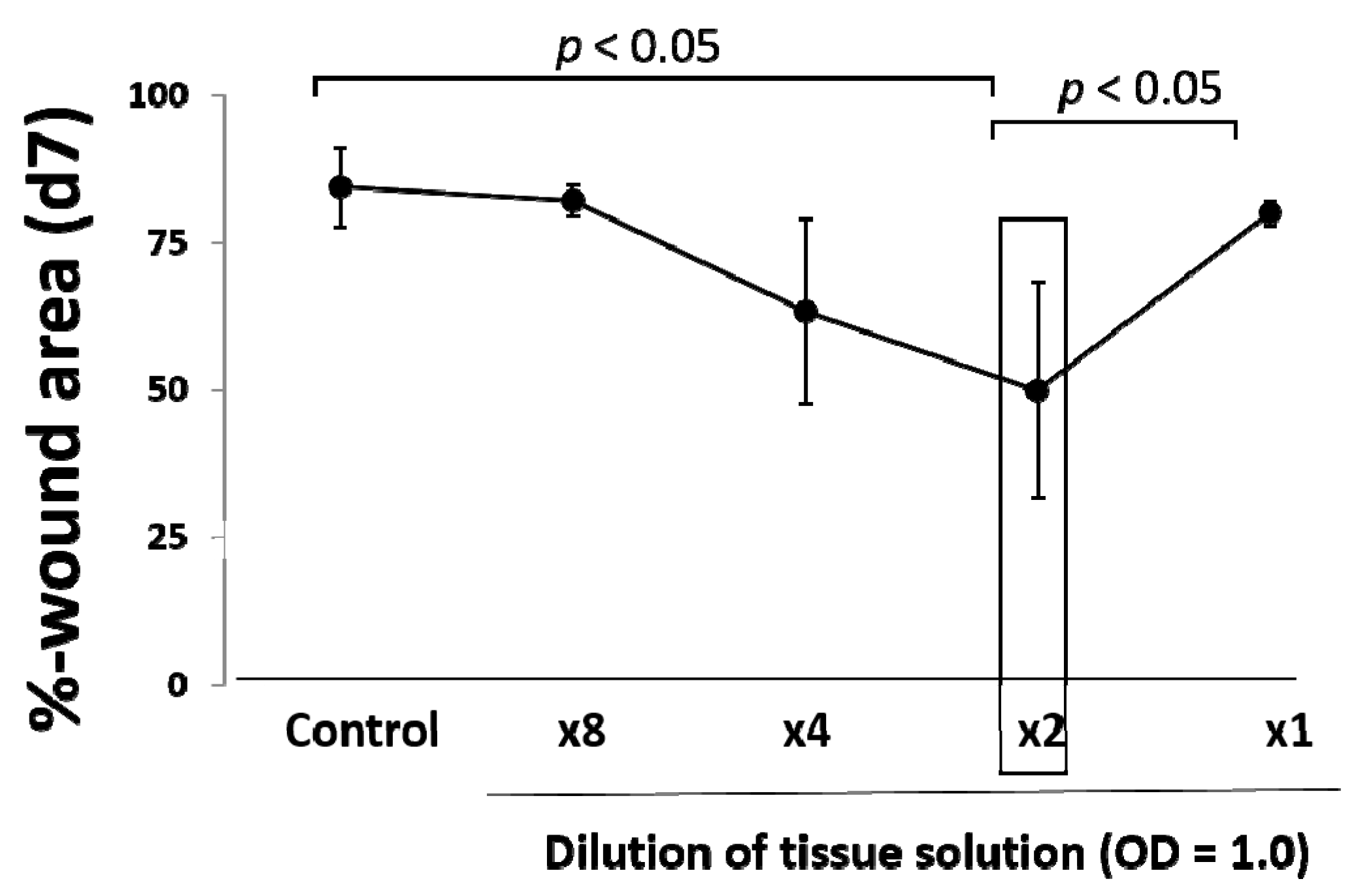
5], may be upregulated as seen in this study. In our results, an optimal amount of minced skin tissue for wound healing was obtained, whereas an excess amount was not favorable in outcome. This supports our hypothesis that the micrograft technique involves a mechanical tissue degenerative response, and excess degenerative manipulation thus causes imbalance in wound repair reaction, which leads to the delay of the wound healing.
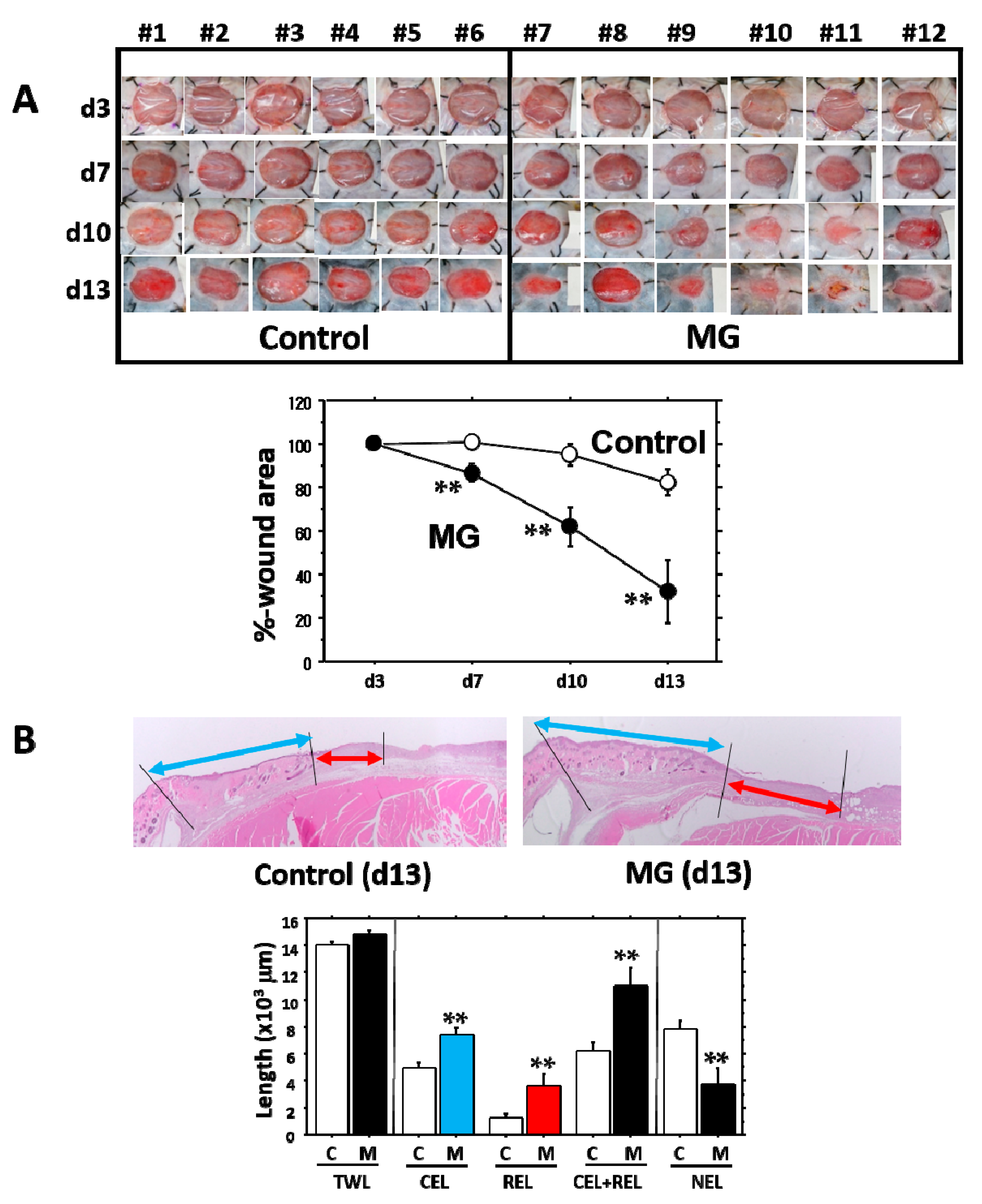
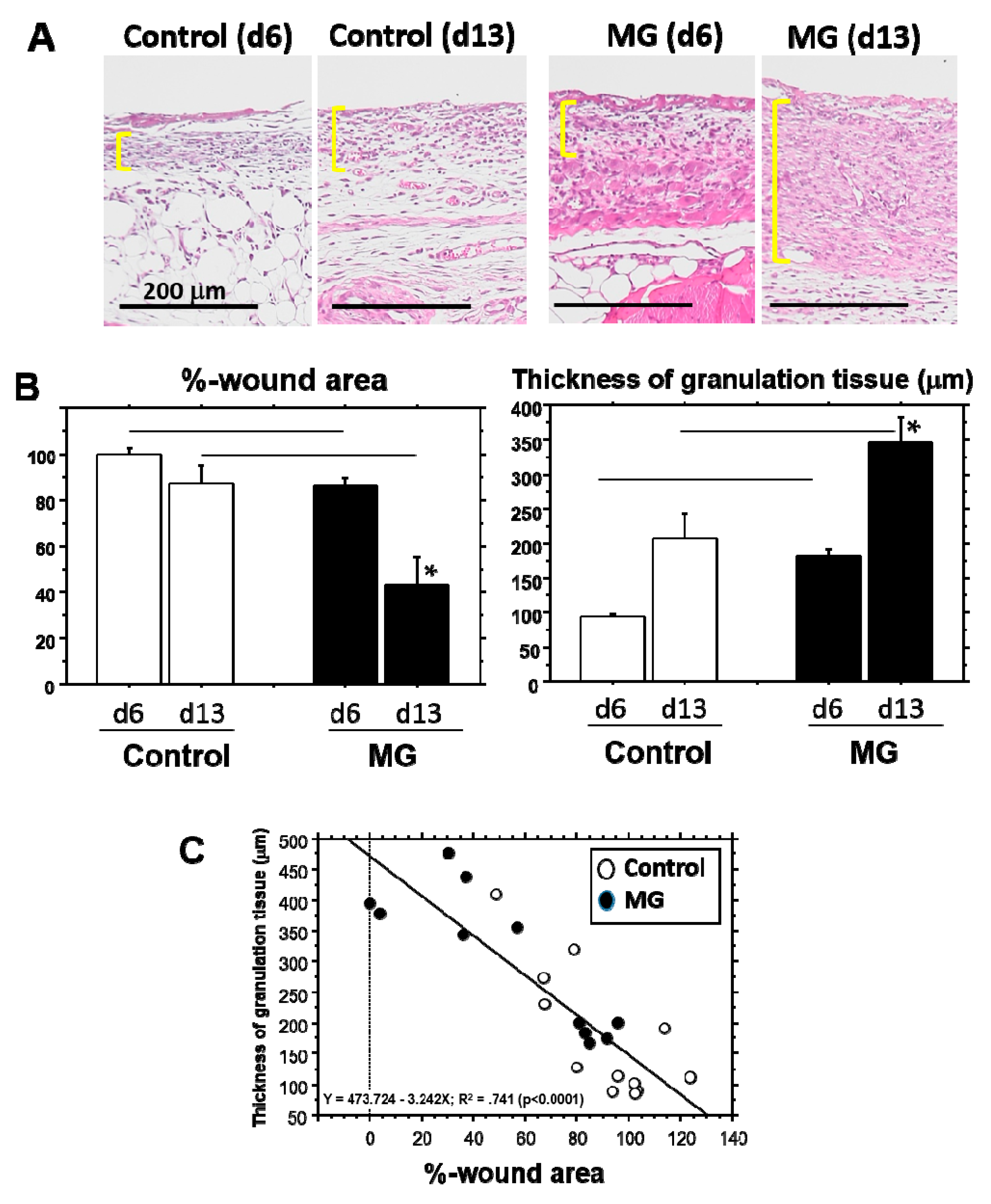
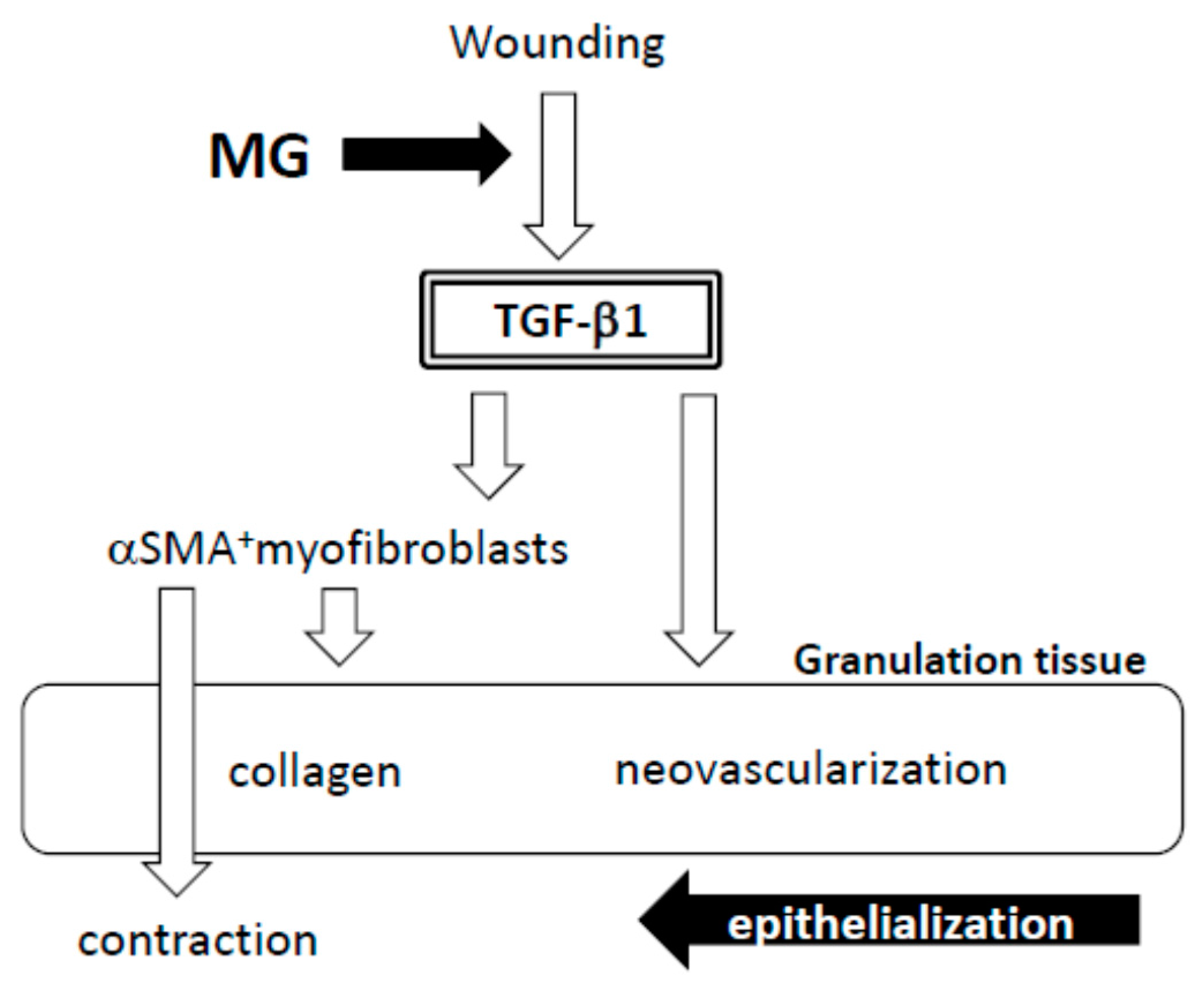
It is evident that the micrograft technique accelerated skin wound healing in mice. In the study, we looked at the epithelialization responses in skin wound healing, such as CEL for contractive epithelialization from the dermal marginal zone at the cutting edge and REL for regenerative epidermis [
15]. Interestingly, although CEL in the MG group showed about 1.3-times elongation versus the control group, REL elongation in the MG group greatly increased by more than three-times that of the control. This technique thereby may act primarily on regenerative healing, which is an important clinical target for human intractable ulcers. In regenerative epithelial healing, keratinocytes proliferate and migrate on granulation tissue. Both %-wound area and non-epithelialized granulation thickness were significantly greater in the MG than the control group. Remarkably, they were tightly correlated with each other. We thus focused on granulation tissue as an active supporting tissue for epidermis.
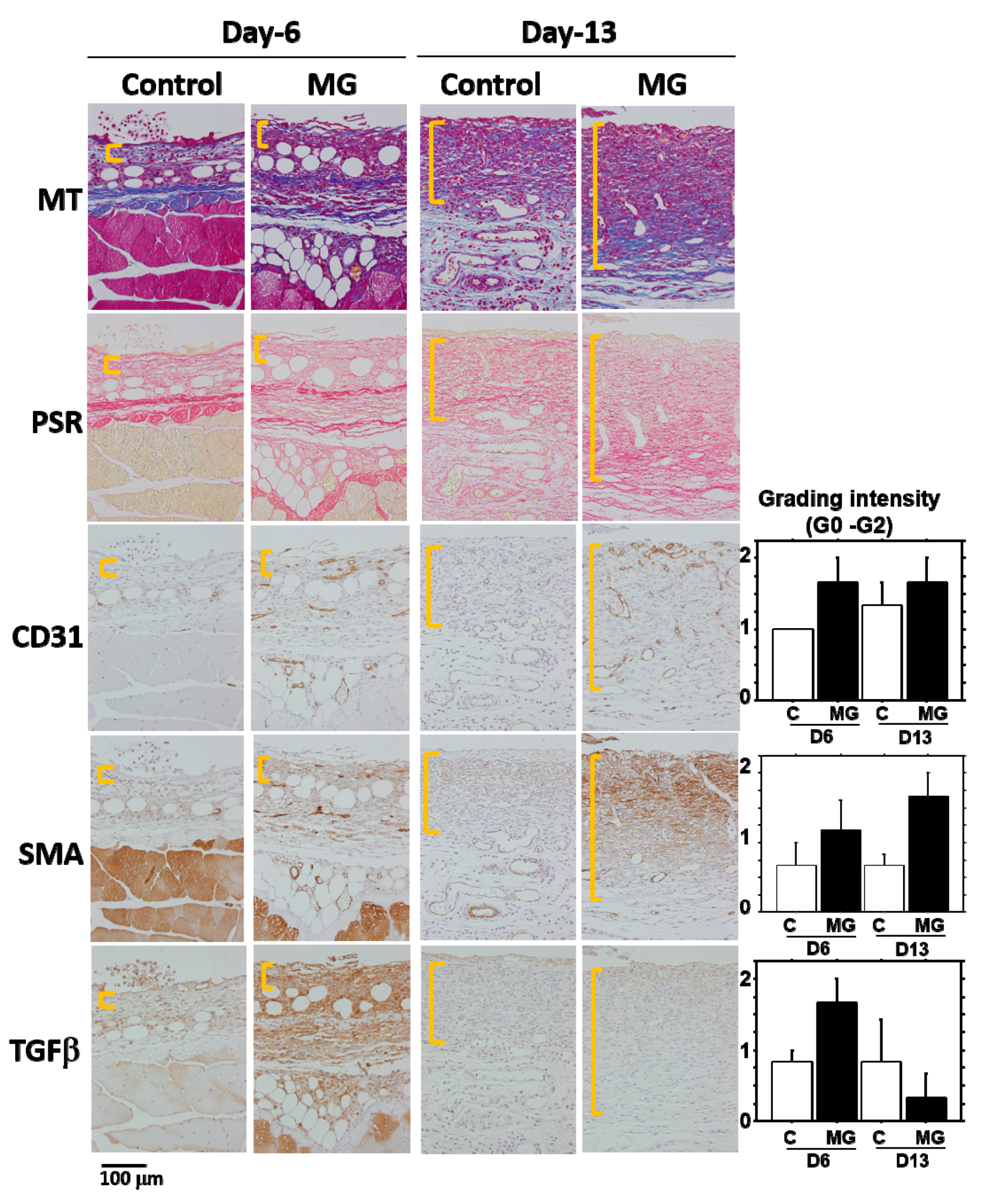
Granulation tissue is composed of infiltrated inflammatory cells, fibroblasts, newly-formed vessels and collagen matrix [
1]. In our study on Day 13, collagen deposition was accelerated in the MG group, especially in the deeper zone of granulation tissue. Moreover, PSR stain showed that the deeper zone contained materials emitted that are red/orange in color (rich in type I collagen [
17]), whereas the upper zone of the MG group and granulation tissue in the control group possessed green color (rich in type III collagen [
17]). It has been shown that collagen type III is deposited in newly-formed/immature granulation tissue; however, normal dermal collagen primarily contains collagen type I [
17]. Collagen matrix in granulation tissue could be more maturated in the MG group, which was also accompanied by an increase in neovascularization.
In the process of wound healing, wound contraction is another crucial step, in which αSMA-expressing myofibroblasts play a central role [
4]. The origin of myofibroblast is thought to be derived from local resident fibroblasts through proliferation, bone marrow, endothelial-to-mesenchymal and epithelial-to-mesenchymal transition [
4,
5] and possibly from matured fat tissue [
6]. When fibroblasts differentiate into myofibroblasts during tissue repair, αSMA is assembled in thick stress fibers [
18]. Fibroblast to myofibroblast differentiation is dependent on cellular adhesion on the surrounding ECM and the presence of TGF-β1 [
18,
19]. Many of the myofibroblasts appeared in granulation tissue in the MG group on Day 13. Furthermore, their distribution was limited in the immature zone of granulation tissue, indicating that granulation tissue acts not only as a replenisher tissue, but also as wound contraction tissue. These are perhaps some of the reasons for the acceleration of wound healing in the MG group.
A factor responsible for the emergence of αSMA-expressing myofibroblasts is believed to be TGF-β1 in wounds [
5,
20]. TGF-β also stimulates collagen production [
21] and neovascularization [
5] and acts as an inflammatory and anti-inflammatory cytokine in different phases of wound healing [
5]. In the present study, TGF-β1 was selectively accumulated in immature granulation tissue three days after the MG treatment (Day 6); and no accumulation was found on Day 13 in either of the groups. This suggests that the MG treatment may stimulate TGF-β1 production in early phase granulation, by which myofibroblast proliferation, neovascularization and collagen accumulation could be stimulated.
4. Materials and Methods
4.1. Animal Experiments
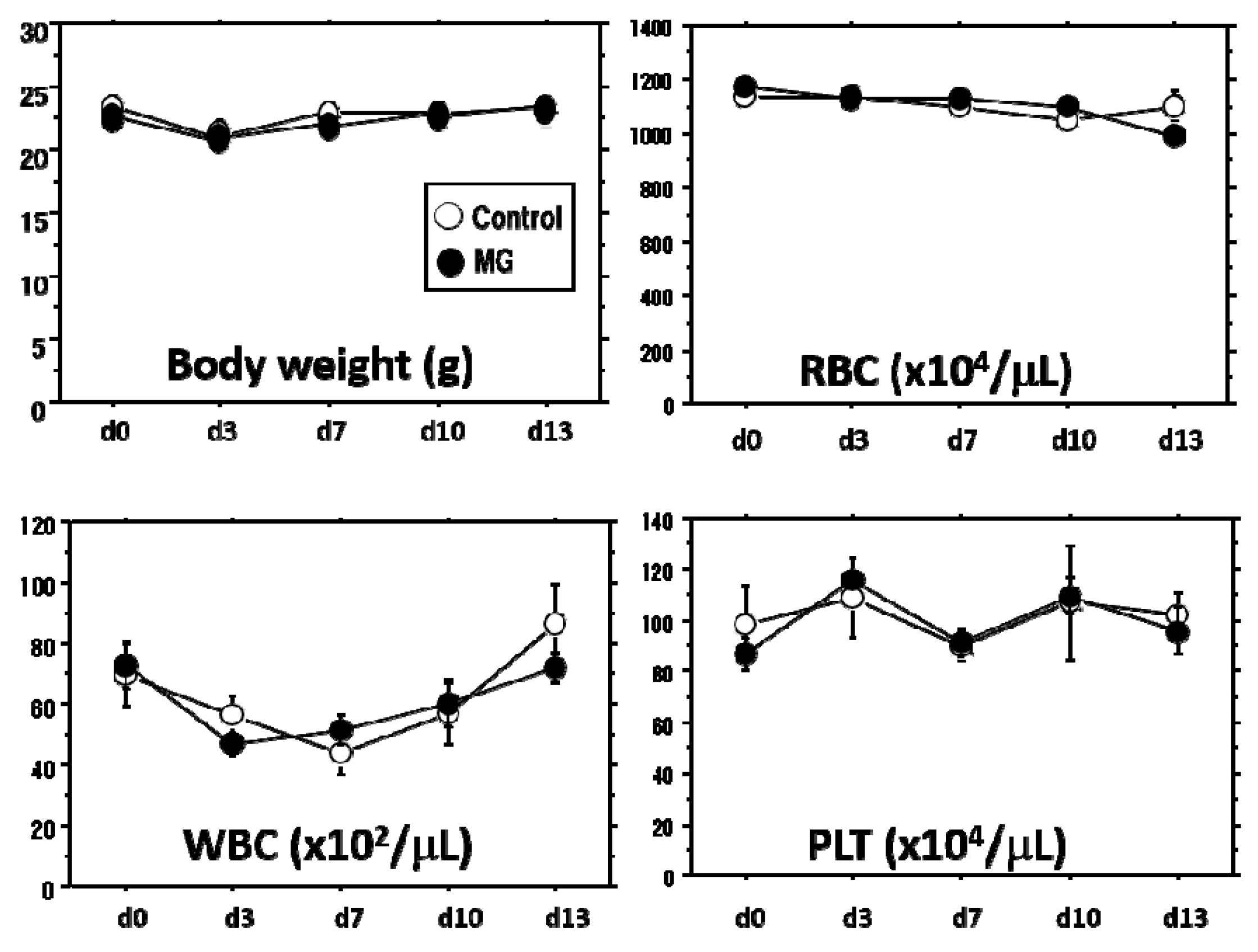
This animal study was approved by the Fukuoka University Animal Experiment Committee (No. 1507848: 3 July 2015). Study protocols were in compliance with the institution’s animal care guideline. Male C57BL/6 mice, Green mice (C57BL/6-Tg-CAG-EGFP) and CB-17SCID mice at a young age of 6–10 weeks were used in this study. All animals were purchased from Japan SLC Inc. (Shizuoka, Japan). All procedures were conducted under aseptic conditions, using autoclaves, ethylene oxide gas, 75% ethanol and povidone-iodine. Mice were anesthetized with isoflurane (Wako Pure Chemical Industries, Ltd., Osaka, Japan) or pentobarbital (Somnopentyl; KYORITSU SEIYAKU, Tokyo, Japan). Physiological checks including body weight and movement were regularly monitored. Hematological analyses, including red blood cell (RBC), white blood cell (WBC) and platelet (PLT) counts (Celltac-α, NIHON KOHDEN, Tokyo, Japan), were performed with blood collected from the orbital sinus with a heparinized 75-μL capillary (Hirschmann Laborgeräte GmbH & CO., Eberstadt, Germany). At the end of the study, the mice were sacrificed by lethal pentobarbital injection and arterial hemorrhage and wound tissue was obtained.
4.2. Skin Wounding
To create a humanized wound in mouse, our established splint method was used [
15]. In brief, mice were anesthetized with pentobarbital, and dorsal hair was removed with a commercial depilatory. A circular tattoo (1 cm in diameter) was made at the center of the lumbar area. The part of the skin was completely excised with scissors, and a doughnut-shaped plastic splint (outer diameter: 28 mm, inner hole diameter: 18 mm) was inserted beneath the skin near the wound defect and attached to the fascia with 6-stitch ligations. The splint was then fixed to the skin with surgical silk thread (6 stitches at regular intervals). Finally, the splinted wound was covered with a polyurethane film dressing (Tegaderm, SUMITOMO 3M, Tokyo, Japan). To prevent thread removal by the mice, they were dressed and fixed with a silicon-tight vest.
4.3. Micrograft Using the Rigenera Protocol
Rigenera protocol established [
9] was utilized. After removing the dorsal hair with a commercial depilatory, a circular tattoo (1 cm in diameter) was made on the back skin, and the marked skin was totally excised with scissors. The epidermal layer of the skin was then scraped away with a surgical knife, and the tissue was washed 2-times with saline solution. The tissue was dissected into small pieces (about 1 × 1 mm). The pieces of tissue were minced with the blade of Rigeneracons
® (Human Brain Wave S.r.L., Via Pinerolo, Torino, Italy). One milliliter of the tissue solution obtained through passages of the blade holes (50 μm in diameter) was used for measurement of absorbance determination (450 nm/550 nm) using a microplate reader (iMark, Bio-Rad Laboratories, Inc., Hercules, CA, USA). Tissue concentration was adjusted at OD = 1.0, and it was diluted up to 0.125-times with saline solution.
Wound surface manipulation was done as follows, unless otherwise stated. Wounds 3 days after wounding were exposed to 70% ethanol for 1 min to kill surficial cells on wounded tissue, mimicking a deterred clinical wound with necrotic tissue. After sealing the wounds with polyurethane film, 200 μL of the tissue solution using a syringe (24 G needle) were inoculated on the surface of wound by injection via peri-skin in which the tissue solution was filled in the closed space and spread over the wound surface (about 2.5 cm2). In the control group, saline solution alone was injected. A total of 13 days of study was performed.
4.4. Wound Tissue Evaluation
Macroscopic evaluation: Wound photos with scale indication were obtained from directly above with a digital camera (NEX-C3, Sony, Tokyo, Japan). The wound area was measured using a computer-assisted morphometric analyzer (VH Analyzer, VH-H1A5, KEYENCE Co., Osaka, Japan).
Microscopic evaluation: Dorsal skin tissue was dissected from the sacrificed mice. This tissue, together with the splint, was fixed in 10% buffered formaldehyde (pH 7.4) for several days. Two cross-cut tissue samples from each wound (about 5 mm thick) were excised (Sample-1 and Sample-2). Paraffin blocks were prepared by using a tissue processor (Tissue-Tec VIP Premier, SAKURA, Nagano, Japan), following which, 4-μm-thick tissue sections were cut with a microtome (RM2235, Leica Biosystems, Nußloch, Germany). For the frozen section, the Kawamoto method was used [
22]. After fixing with 5% neutral formalin, tissue was embedded in super cryo-embedding medium (Leica Microsystem GmbH, Wetzlar, Germany) and then quickly frozen in liquid nitrogen. After adhesion of cryofilm (Leica Microsystem GmbH, Wetzlar, Germany) on the frozen block, a 4 µm-thick section was cut with a cryostat (CM350: Leica Microsystem GmbH, Wetzlar, Germany).
4.5. Histological Examination
The paraffin section was stained with hematoxylin and eosin (HE), Masson’s trichrome (MT) and Picrosirius red (PSR) [
17,
23]. In immunohistochemical analysis, GFP antibody (Abcam plc, Tokyo, Japan), anti-mouse αSMA antibody (Abcam plc, Tokyo, Japan), anti-mouse CD31 antibody (Dianova GmbH, Hamburg, Germany), anti-mouse neutrophil antibody (NIME-R14: Abcam plc, Tokyo, Japan) and anti-mouse TGF-β1 antibody (Abcam plc, Tokyo, Japan). An EnVision Kit (DAKO Japan Inc., Tokyo, Japan) was used for visualization. Semi-quantitative evaluation was performed: negative (Grade 0), slightly positive (Grade 0.5), positive (Grade 1) and strongly positive (Grade 2).
4.6. Western Blotting
Just after excision of granulation tissue in wound and sub-epidermal tissue in normal skin, tissue was sunk in RIPA lysis buffer (Merck Millipore Co., Darmstadt, Germany) containing an EDTA-free protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA) and then homogenized using BioMasher (Nippi. Inc., Tokyo, Japan). Tissue lysates were then applied to a 4–15% gradient SDS-polyacrylamide gel and electrophoresed. Proteins separated in the gel were blotted onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Hercules, CA, USA). After blocking with 5% skim milk (NACALAI TESQUE, Inc., Kyoto, Japan) in Tris buffer-saline (pH 7.6) (TBS) containing 0.1% Tween-20 (TBS-T), the membrane was reacted with primary antibodies, including anti-GFP antibody (Abcam plc, Tokyo, Japan) and anti-beta-actin antibody (Abcam plc, Tokyo, Japan). After rinsing three times with TBS-T, the membrane was incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Cell Signaling Technology Inc., MA, USA). After washing, the membrane was incubated in ECL detection system (GE Healthcare Japan K.K., Tokyo, Japan) and luminescence was visualized by LAS-4000 mini (FUJIFILM Medical Co., Ltd., Tokyo, Japan). Densitometry was performed on targeted bands.
4.7. Morphometrical Analysis
Wound area percentage (WA%): Macroscopic wound evaluation was performed during the study. To obtain an accurate evaluation of wound area regression, the WA% at the time of measurement was calculated as follows: WA% = wound area at the time of measurement/wound area on Day 3 × 100.
Epithelial length from wound edge: Total epithelialization length (TEL), defined as the length of epithelial growth from the dermal cutting edge after wounding, was measured according to the previous method [
15]. Two types of epithelial lengths were measured: the contracted epithelium length (CEL) and the regenerative epithelium length (REL). The TEL was the sum of the CEL + REL. The CE was characterized by normal dermal structures with hair follicles and a dermal-muscular coat, whereas the RE grown on granulation tissue did not have these characteristics.
Granulation thickness: Granulation tissue formed beneath the non-epithelialized zone was evaluated. A zonal granulation tissue (about 2000 µm in length) on the wound was selected; the length and area of the granulation tissue were morphometrically measured. The thickness of the granulation tissue was calculated by the following formula: Thickness (µm) = Area (µm2)/Length (µm).
4.8. Statistical Analysis
Values were expressed as the mean ± standard error. Linear regression analysis with least-squares estimation were done by a computer-assisted statistics program (StatView 5.0, SAS Institute Inc., Tokyo, Japan). A p-value of <0.05 was considered statistically significant.