1. Introduction
Higher plasma levels of saturated fatty acids (SFA), especially palmitic acid (C16:0) and myristic acid (C14:0), were independently associated with incident heart failure in both men and women in the Atherosclerosis Risk in Communities Study [
1]. Experimental animal studies suggest that dietary SFA may directly promote the development of heart failure by inducing lipotoxicity [
2]. However, most studies on the role of SFA in cardiac dysfunction have been based on obesogenic high-fat diets. Obesity-associated inflammation and systemic insulin resistance confound experimental investigations designed to study the causal role of SFA in cardiac dysfunction and heart failure. Indeed, systemic insulin resistance and associated chronic hyperinsulinemia may play a prominent direct role in the development of cardiac dysfunction. Whereas the insulin/phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt axis is involved in normal cardiac growth and physiological hypertrophy [
3,
4], chronic hyperinsulinemia stimulates angiotensin II signaling that is involved in pathological hypertrophy [
5]. Mismatch between cardiomyocyte size and vascularity may contribute to the transition from cardiac hypertrophy to heart failure [
6].
The effect of dietary SFA on the development of heart failure may be highly dependent on SFA chain length. The myristate-containing ceramide species C
14-ceramide has been implicated in the pathogenesis of lipotoxic cardiomyopathy in a milk-fat based diet model that was associated with an approximately 20% increase in body weight [
2,
7]. A cocoa butter derived high-fat diet (containing mainly palmitate (C16:0) and stearate (C18:0)) resulted in minor [
8] or no [
9] effects on body weight and did not have major effects on cardiac structure and function compared to a low-fat diet in sham-operated mice and in mice with pressure overload induced by transverse aortic constriction [
8,
9].
The objective of the current study was to evaluate whether coconut oil, which contains lauric acid (C12:0) and myristic acid (C14:0) as main fatty acids, modifies the development of pressure overload-induced cardiomyopathy induced by transverse aortic constriction (TAC) in C57BL/6 mice without inducing weight gain or systemic insulin resistance.
3. Discussion
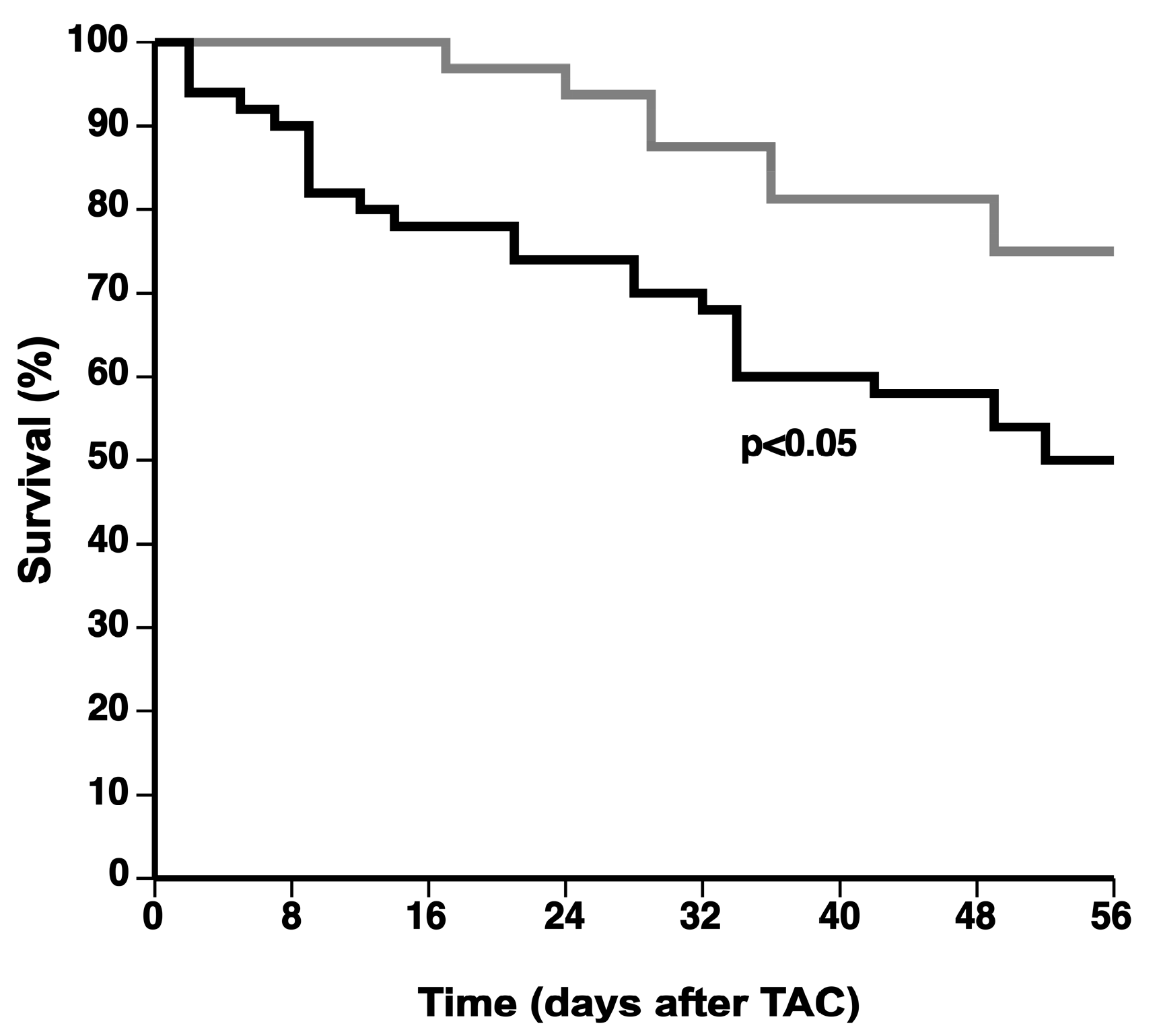
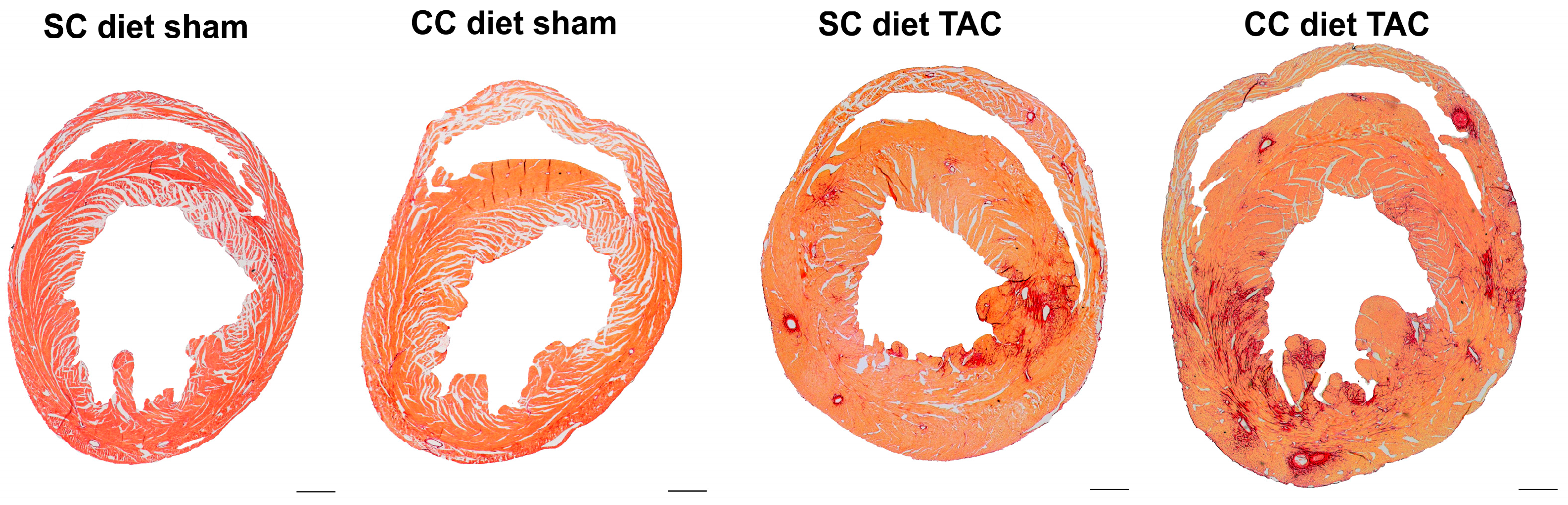
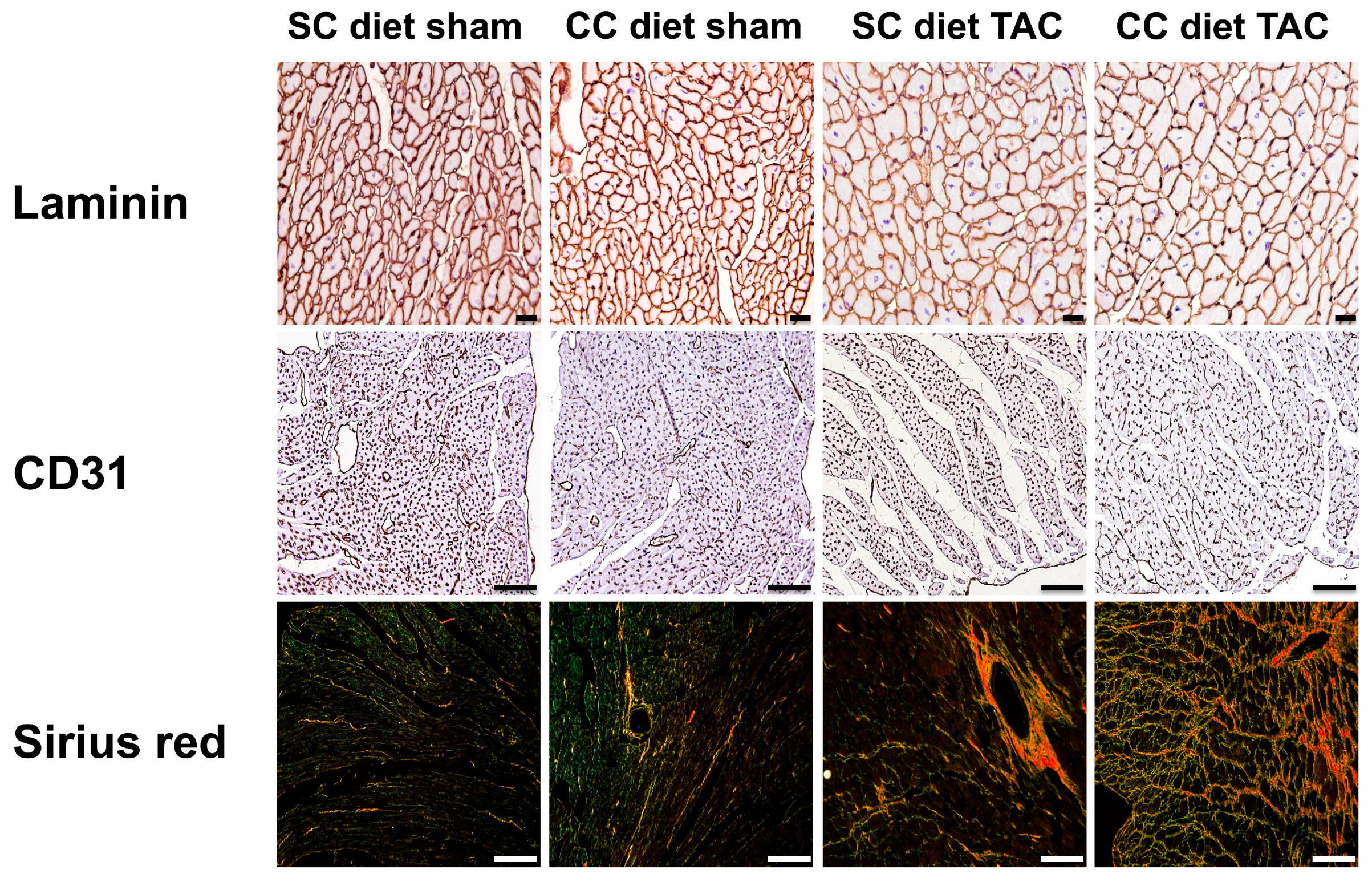
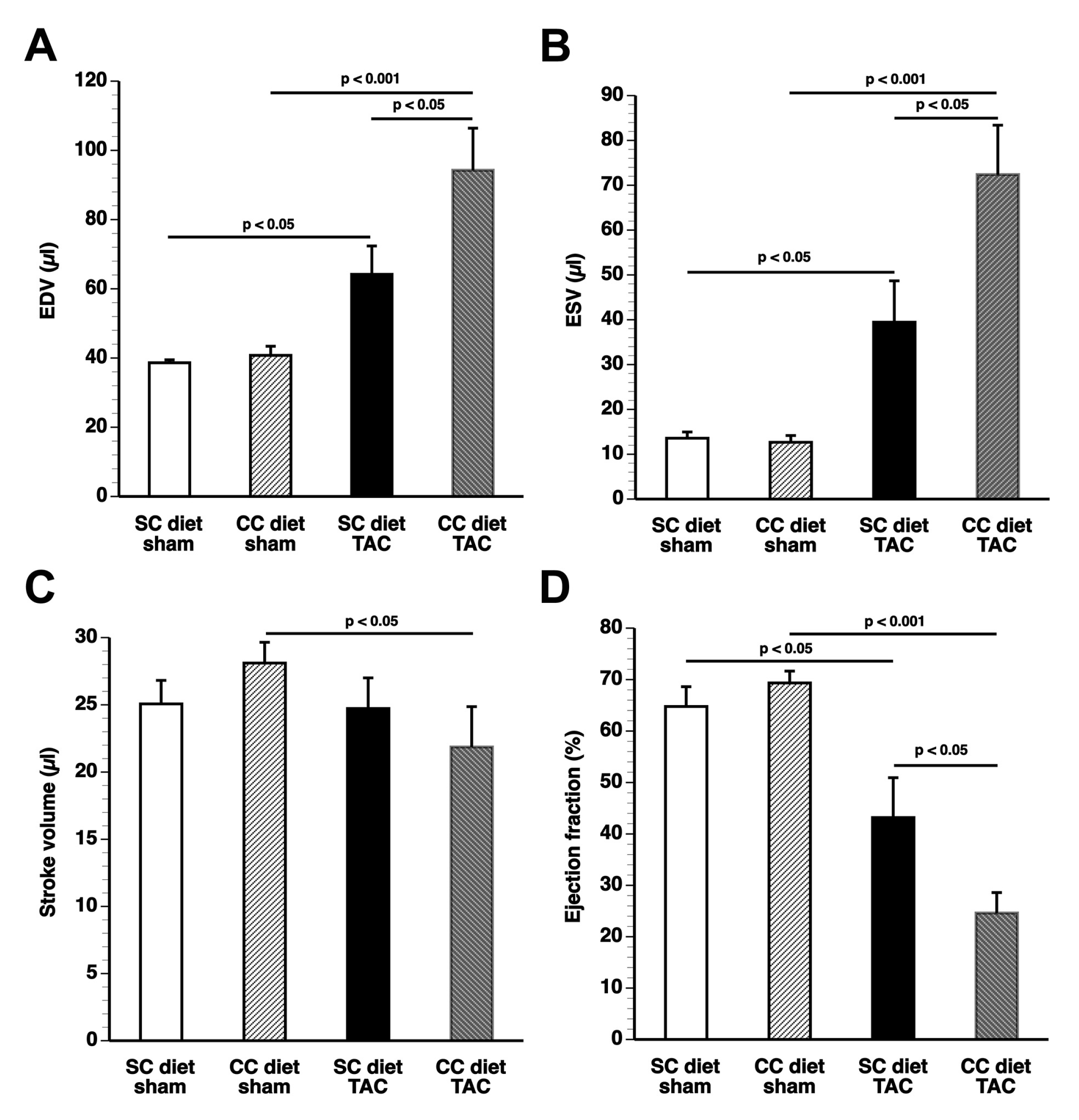
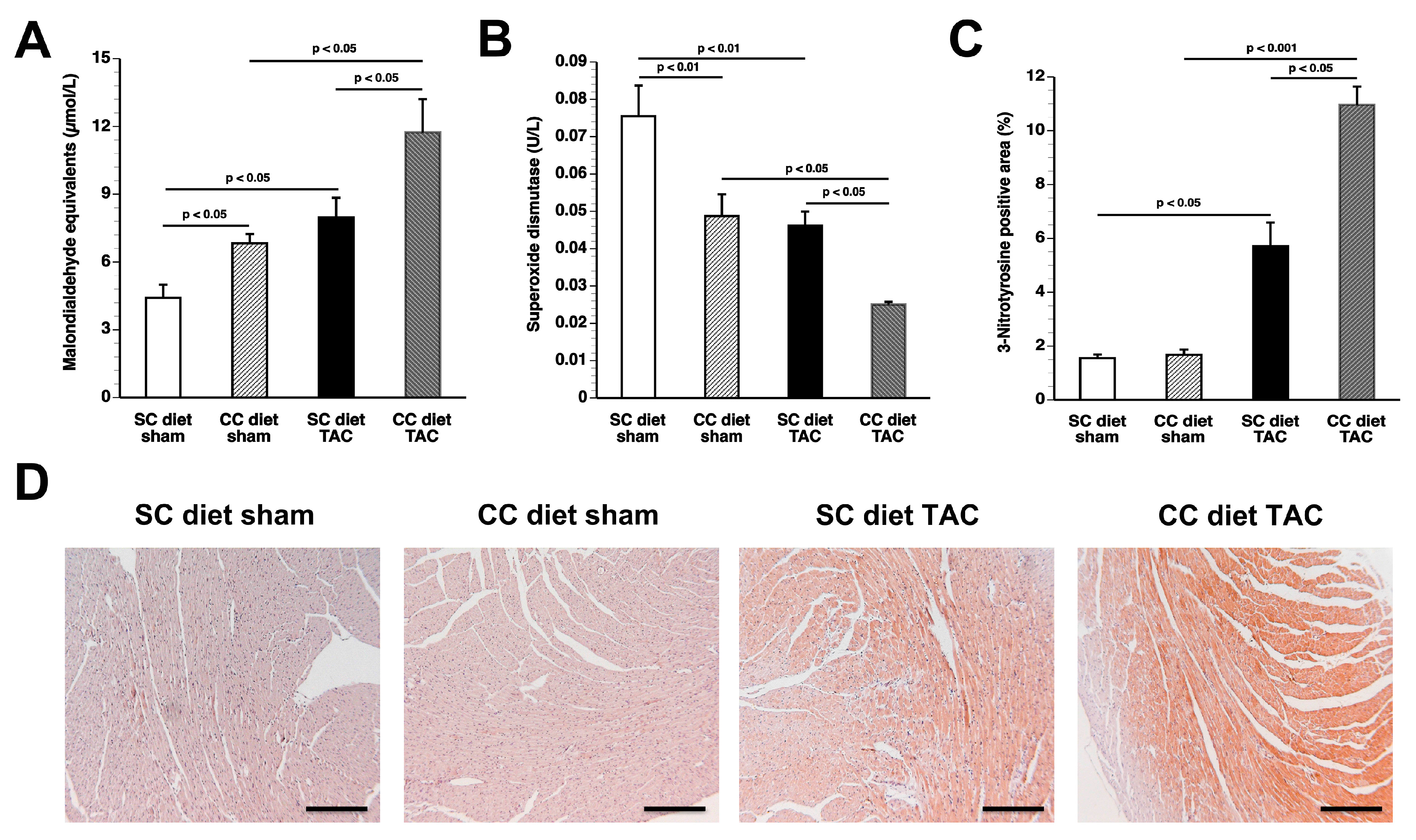
The main findings of the current study are that: (1) features of pathological hypertrophy following pressure overload in mice fed coconut oil are more prominent compared to SC diet mice as evidenced by striking myocardial fibrosis, a sharper decline in capillary density, and increased apoptosis in the myocardium; (2) the deterioration of cardiac function after TAC is significantly more pronounced in CC diet mice than in SC diet mice and results in more marked heart failure as evidenced by the higher lung weight and the prominent right ventricular hypertrophy; (3) pressure overload induces more marked metabolic remodeling in CC diet mice as evidenced by higher glucose uptake, higher PDH levels, and higher ACC levels; and (4) the effects of dietary coconut oil on structural, functional, and metabolic remodeling occur in the absence of weight gain, of systemic insulin resistance, and of cardiac steatosis.
Coconut oil is an edible oil that is present in the meat of the coconut and extracted from the fruit of the coconut tree (
Cocos nucifera). To exclude the possibility that the addition of 0.2% cholesterol to the diet modifies the effects of coconut oil, the effect of a 10% coconut oil diet without cholesterol on cardiac structure and function was evaluated. We did not observe any significant difference between 10% coconut oil-fed mice and CC diet mice (data not shown). Coconut oil predominantly contains medium-chain SFA: caproic acid (C6:0) 1%, caprylic acid (C8:0) 9%, capric acid (C10:0) 7%, and lauric acid (C12:0) 47% of the overall fatty acid composition [
10]. Medium-chain fatty acids do not rely on membrane transporters for their uptake into cells and mitochondria [
11,
12]. They are directly activated in the mitochondrial matrix by medium-chain acyl-CoA synthetase prior to β-oxidation. The currently available evidence suggests potential beneficial effects of medium-chain fatty acids on the myocardium [
11,
13,
14,
15,
16]. Therefore, it is rather unlikely that medium-chain fatty acids play a role in the coconut oil-induced pathological hypertrophy and remodeling. The large majority of lauric acid is transported directly to the liver via the portal vein whereas a minor part is reformed into new triglycerides and incorporated into chylomicrons, which enter the lymphatic system and reach the blood via the
ductus thoracicus [
17]. Lauric acid is rapidly oxidized in the liver and may induce the production of ketone bodies. Whereas ketone bodies may be used as fuel in advanced heart failure [
18,
19,
20], there is no evidence for a detrimental role of ketone bodies on the myocardium [
20].
Besides medium-chain fatty acids and the unsaturated fatty acids oleic acid (C18:1) (6%) and linoleic acid (C16:2, n-6) (1%), coconut oil further contains the long-chain SFA myristic acid (C14:0) (17%), palmitic acid (C16:0) (8%), and stearic acid (C18:0) (3%). The myristate-containing ceramide species C
14-ceramide has been implicated in the pathogenesis of lipotoxic cardiomyopathy [
2]. The potential pivotal role of myristic acid (C14:0) and palmitic acid (C16:0) is in line with epidemiological data [
1].
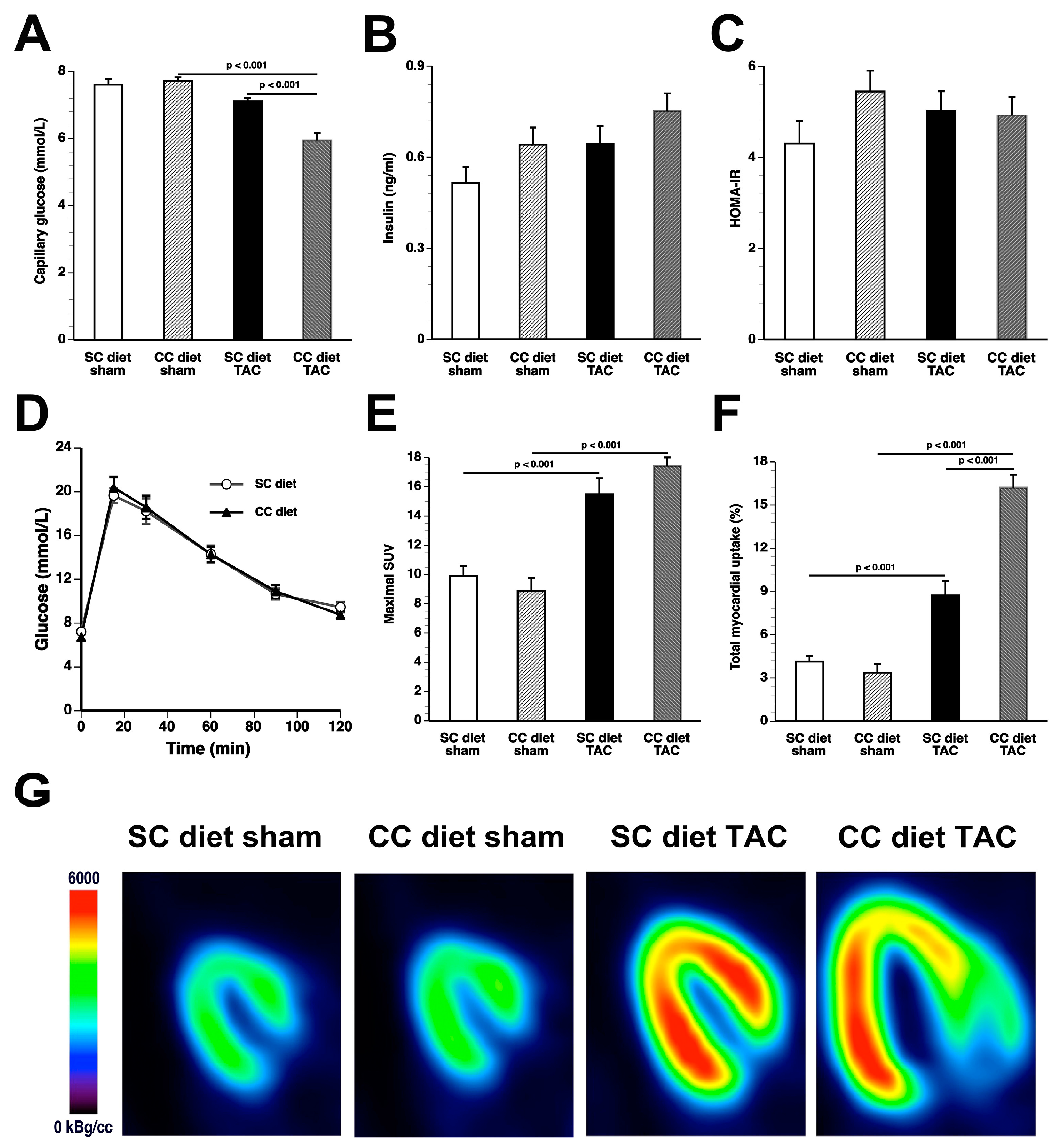
Whereas coconut oil did not alter myocardial triacylglycerols in sham mice, a significant decline of triacylglycerols was observed in CC diet TAC mice compared to SC diet TAC mice. Cardiac steatosis typically occurs in the presence of obesity, insulin resistance, and diabetes mellitus. The CC diet did not induce obesity, diabetes mellitus, or impaired glucose tolerance. Furthermore, based on HOMA-IR values, systemic insulin resistance was absent. Although the hyperinsulinemic-euglycemic clamp is the gold-standard method to assess insulin sensitivity, the application of this technique in mice is challenging because of issues such as high-cost, need for pump-infusion equipment, and considerable expertise [
21]. HOMA-IR values as surrogate measure provide a reasonable and reliable approximation of formal measures of insulin resistance when applied to rats and mice as they do in humans [
22,
23,
24].
The absence of cardiac steatosis does not exclude the presence of lipotoxic molecules in the heart. Triacylglycerols, the main lipid stored in lipid droplets, are themselves not thought to be harmful [
25]. Specific phospholipid and sphingolipid species, rather than diacylglycerols and triacylglycerols, may modulate the structural, metabolic, and functional effects of coconut oil. A significant decrease in phospholipids and a significant increase in sphingomyelin were present in CC diet TAC mice compared to SC diet TAC mice. Although these alterations may be the consequence of more prominent pathological remodeling and of more pronounced deterioration of cardiac function, specific lipid mediators are expected to be on the causal pathway between the CC diet and cardiac disease. Strikingly, the CC diet induced increased perivascular and interstitial fibrosis in sham mice and also resulted in deterioration of the isovolumetric relaxation in sham mice, indicating pressure overload-independent effects of the CC diet.
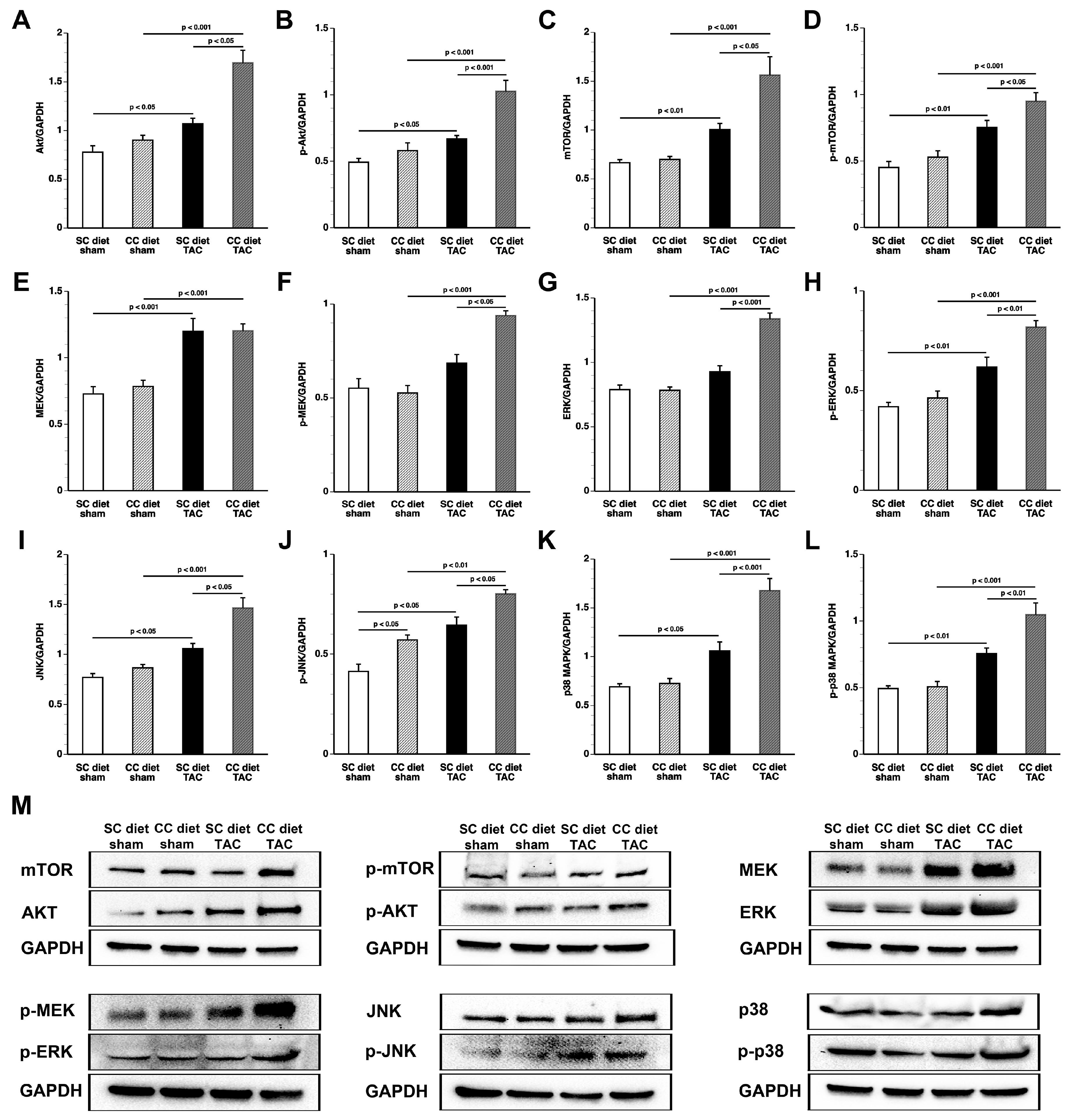
According to an often-proposed paradigm, the pressure-overloaded heart reverts toward a fetal-like metabolic profile, characterized by a decrease in fatty acid oxidation concomitant with an increased reliance on carbohydrates for oxidative energy metabolism [
26]. Glucose uptake was nearly doubled in CC diet TAC mice compared to SC diet TAC mice notwithstanding a similar degree of left ventricular hypertrophy. The mTOR pathway plays a key role in sensing and integrating multiple environmental signals [
27]. The mTOR complex 1 (mTORC1) promotes protein synthesis and cell growth, inhibits autophagy, and results in increased glucose oxidation and reduced fatty acid oxidation [
28,
29]. Increased mTOR and p-mTOR in CC diet TAC mice compared to SC diet TAC mice may be a critical mediator of many of the observed structural and metabolic effects.
In conclusion, the current study shows that coconut oil exerts profound effects on murine cardiac structure and function both in the presence and absence of pressure overload. Coconut oil induces oxidative stress and myocardial fibrosis even in the absence of pressure overload. Whether coconut oil has detrimental effects on cardiac structure and function in humans is unknown at present. However, claims of beneficial health effects of coconut oil are unsubstantiated. Data of the present study suggest that specific SFA may have direct detrimental effects that are independent of the effects on plasma cholesterol, on body weight, and on insulin sensitivity. Experimental dietary intervention studies with specific medium-chain triglycerides or long-chain triglycerides may unravel the specific culprits of the harmful effects of coconut oil.
4. Materials and Methods
4.1. In Vivo Experiments Evaluating the Effect of Coconut Oil on the Development of Pressure Overload-Induced Cardiomyopathy
All experimental procedures in animals were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee of the Catholic University of Leuven (Approval number: P154/2013, 1 October 2013). At the age of 12 weeks, female C57BL/6 mice, originally purchased from Taconic (Ry, Denmark) were fed standard chow (SC) diet (Sniff Spezialdiäten GMBH, Soest, Germany) or SC supplemented with 0.2% (w/w) cholesterol and 10% (v/w) coconut oil (CC diet) ad libitum. The experimental diet was maintained throughout the entire duration of the experiments. To induce pressure overload, transverse aortic constriction (TAC) was performed at the age of 17 weeks. Briefly, anesthesia was performed with a single intraperitoneal injection of sodium pentobarbital (Nembutal®, Ceva Sante Animale, Brussels, Belgium) at a dose of 40–70 mg/kg. Mice were put in supine position and temperature was maintained at 37 °C with a heating pad. A horizontal skin incision of 0.5 cm to 1 cm in length was made at the level of the suprasternal notch. A 2 mm to 3 mm longitudinal cut was performed in the proximal portion of the sternum and the thymus gland was retracted. This allowed visualization of the aortic arch under low-power magnification. A wire with a snare at the end was passed under the aorta between the origin of the right innominate artery and the left common carotid artery. A 7-0 silk suture (Ethicon, Johnson & Johnson, Livingston, Scotland, UK) was snared with the wire and pulled back around the aorta. Subsequently, a bent 27-gauge needle (BD Microlance®, BD, Franklin Lakes, NJ, USA) was placed next to the aortic arch and the suture was snugly tied around the needle and the aorta. Afterwards, the needle was quickly removed. The skin was closed and mice were allowed to recover on a warming pad until they were fully awake. The sham procedure was identical except that no constriction on the aorta was applied.
Group assignment at the start of the study was performed at random. At the end of the study, data of all surviving mice were included in the analysis. Endpoint analyses were performed by investigators who were blinded to the group allocation of the animal. Unblinding of animal numbers corresponding to specific allocation groups was performed at completion of measurements.
4.2. In Vivo Hemodynamic Measurements
Invasive hemodynamic measurements were performed eight weeks after TAC or after sham operation. Mice were anesthetized by intraperitoneal administration of 1.4 g/kg urethane (Sigma, Steinheim, Germany). Body temperature was maintained with a heating pad and monitored with a rectal probe. An incision in the right carotid artery was made with a 26-gauge needle between a distal and proximal non-occlusive ligation of the artery. A 1.0 French Millar pressure catheter (SPR-67/NR; Millar instruments, Houston, TX, USA) was inserted and advanced to the left ventricle (LV). After stabilisation of the catheter, heart rate, maximal systolic LV pressure, minimal diastolic LV pressure, the peak rate of isovolumetric LV contraction (dP/dt
max), and the peak rate of isovolumetric LV relaxation (dP/dt
min) were measured. The end-diastolic LV pressure was calculated manually from the pressure in function of time curves. The time constant of isovolumetric LV pressure fall (tau) was calculated using the method of Weiss et al. [
30]. Arterial blood pressure measurements were obtained after withdrawal of the catheter from the LV to the ascending aorta. Data were registered with Powerlab Bridge Amplifier and Chart Software (sampling rate 2000 Hz; ADInstruments Ltd., Oxford, UK).
4.3. Blood Sampling
Blood was collected by puncture of the retro-orbital plexus. Anticoagulation was performed with 0.1 volume of 136 mmol/L trisodium citrate and plasma was immediately isolated by centrifugation at 1100× g for 10 min and stored at −20 °C.
4.4. Plasma Lipoprotein Analysis
Mouse lipoproteins were separated by density gradient ultracentrifugation in a swing-out rotor as described before [
31]. Fractions were stored at −20 °C until analysis. Non-HDL cholesterol was determined as the sum of cholesterol within very low-density lipoproteins (VLDL) (0.95 <
d < 1.006 g/mL), intermediate-density lipoproteins (IDL) (1.006 <
d < 1.019 g/mL), and low-density lipoproteins (LDL) (1.019 <
d < 1.05 g/mL) lipoprotein fractions. The cut-off value (
d = 1.05 g/mL) between LDL and high-density lipoproteins (HDL) for murine samples was chosen based on previous work by Camus et al. [
32]. Cholesterol in plasma and lipoprotein fractions was determined with commercially available enzymes (Roche Diagnostics, Basel, Switzerland). Precipath L (Roche Diagnostics) was used as a standard.
4.5. Quantification of Insulin and Homeostatic Model Assessment-Insulin Resistance (HOMA-IR)
Murine plasma insulin levels were quantified using the Insulin ELISA kit (Cayman Chemical, Ann Arbor, MI, USA). HOMA-IR values were calculated based on the formula HOMA-IR = (glucose × insulin)/22.5 where glucose is expressed in mmol/L and insulin in mU/L [
22,
24].
4.6. Glucose Tolerance Test
Glucose tolerance test was performed by intraperitoneal injection of glucose (2 g/kg) after 6 h of fasting as described by Hofmann et al. [
33] in SC diet mice and in CC diet mice at 13 weeks after the initiation of the diet. Tail blood glucose levels were measured with an Accu-Chek
® Active Glucometer (Roche Applied Science, Penzberg, Germany) before (0 min) and at 15, 30, 60, 90, and 120 min after injection.
4.7. Analysis of Lipid Peroxidation in Plasma
Measurement of Thiobarbituric Acid Reactive Substances (TBARS) used for quantification of lipid peroxidation was performed according to the instructions of the manufacturer (Cayman Chemical, Ann Arbor, MI, USA).
4.8. Quantification of Superoxide Dismutase
Superoxide dismutase activity was analyzed using the Superoxide Dismutase Assay kit (Cayman Chemical, Ann Arbor, MI, USA).
4.9. Myocardial Lipid Analysis
Major lipid classes (phospholipids, cholesterol, cholesteryl esters, triglycerides, free fatty acids and sphingomyelin) in the myocardium were analyzed in myocardial lipid extracts with classical (bio)chemical assays [
34,
35,
36,
37].
4.10. Quantification of Myocardial Protein Levels by Western Blot
Myocardial tissue samples were isolated 56 days after sham operation or TAC and immediately frozen in liquid nitrogen and stored at −80 °C. Tissues were placed in lysing matrix tubes (QBiogene/MP Biomedicals, Solon, OH, USA), mixed with 1 mL of protein extraction buffer containing 10 mmol/L imidazole, 300 mmol/L sucrose, 1 mmol/L dithiotreitol, 1 mmol/L sodium metabisulfite, 25 mmol/L sodium fluoride, 5 mmol/L sodium ethylenediaminetetraacetic acid, 5 mmol/L sodium pyrophosphate, 0.3 mmol/L phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail (Roche Diagnostics Belgium, Vilvoorde, Belgium) [
38], and homogenized using the FastPrep24 instrument (MP Biomedicals). Protein concentration was quantified using the Pierce BCA Protein Assay kit (Pierce Biotechnology Inc., Rockford, IL, USA). Equal amounts of proteins were separated on 4–20% Tris-Glycine gradient gels (Bio-Rad Laboratories N.V., Temse, Belgium) and blotted onto polyvinylidene difluoride membranes (Bio-Rad Laboratories N.V.). Membranes were incubated with primary antibodies (Cell Signaling Technologies, Beverly, MA, USA) against Akt, phospho (p)-Akt (Ser/Thr), mitogen-activated protein kinase (MAPK) kinase (MEK) 1/2, p-MEK 1/2 (Ser217/221), p38 MAPK, p-p38 MAPK (Thr180/Tyr182), mammalian or mechanistic target of rapamycin (mTOR), p-mTOR (Ser2481), acetyl-coenzyme A (acetyl-CoA) carboxylase (ACC), p-ACC (Ser79), AMP-activated protein kinase (AMPK)α, p-AMPKα (Thr172), c-Jun N-terminal kinase (JNK), also referred to as stress-activated protein kinase (SAPK)/JNK, p-JNK (Thr183/Tyr185), extracellular signal–regulated kinase (ERK) 1/2, p-ERK 1/2 (Thr202/Tyr204), Smad1, Smad2/3, p-Smad 1/5 (Ser463/465), Smad4, GLUT 4, pyruvate dehydrogenase (PDH), pyruvate dehydrogenase kinase, transforming growth factor (TGF)-β1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Protein expression was detected with Super signal west pico chemilumninescent reagents (Thermo Scientific, Rockford, IL, USA) and quantified using Image lab TM Analyzer software (Bio-Rad laboratories N.V.). All protein levels were normalized to the GAPDH protein level.
4.11. Histological and Morphometric Analysis
After hemodynamic analysis, mice were perfused via the abdominal aorta with phosphate-buffered saline (PBS) and hearts were arrested in diastole by CdCl
2 (100 μL; 0.1 mol/L), followed by perfusion fixation with 1% paraformaldehyde in phosphate buffered saline. After dissection, hearts were post-fixated overnight in 1% paraformaldehyde, embedded in paraffin, and 6 μm thick cross-sections at 130 μm spaced intervals were made extending from the apex to the basal part of the left ventricle. Left ventricle (LV) remodeling was assessed by morphometric analysis on mosaic images of Sirius red-stained heart cross-sections using Axiovision 4.6 software (Zeiss, Zaventem, Belgium). Anterior wall thickness and septal wall thickness were determined. All geometric measurements were computed in a blinded fashion from representative tissue sections of 4 separate regions and the average value was used to represent that animal for statistical purposes [
39,
40].
To measure collagen content in the interstitium, Sirius Red staining was performed as described by Junqueira et al. [
41]. Sirius Red polarization microscopy on a Leica RBE microscope with KS300 software (Zeiss) was used to quantify thick tightly packed mature collagen fibers as orange-red birefringent and loosely packed less cross-linked and immature collagen fibers as yellow-green birefringent. Collagen positive area was normalized to the LV remote area and was expressed as percentage. Any perivascular fibrosis was excluded from this analysis. Perivascular fibrosis was quantified as the ratio of the fibrosis area surrounding the vessel to the total vessel area. Two mid-ventricular sections were studied per animal [
42].
Cardiomyocyte hypertrophy was analyzed on paraffin sections stained with rabbit anti-mouse laminin (Sigma; 1/50) by measuring the cardiomyocyte cross-sectional area (μm
2) of at least 200 randomly selected cardiomyocytes in the LV myocardium. Capillary density in the myocardium was determined on CD31 stained sections using rat anti-mouse CD31 antibodies (BD; 1/500). Relative vascularity in the myocardium was determined as ((capillary density (number/mm
2)/cardiomyocyte density (number/mm
2))/cardiomyocyte cross-sectional area (μm
2)) [
6]. Two mid-ventricular cross-sections were analyzed per mouse [
39,
40].
Immunostaining for 3-nitrotyrosine was performed with rabbit anti-nitrotyrosine antibodies (Merck Millipore, Overijse, Belgium; dilution 1/250).
Apoptosis was quantified on deparaffinized tissue sections using SignalStain
® cleaved caspase-3 IHC detection kit (Cell Signaling Technologies, Beverly, MA, USA), which utilizes a polyclonal rabbit antibody to the neoepitope peptide at the end of cleaved caspase-3 [
42].
4.12. Evaluation of Cardiac Glucose Metabolism by Micro-Positron Emission Tomography (Micro-PET)
Glucose uptake in the myocardium and in the skeletal muscle was quantified by micro-PET using (
18F)-fluorodeoxyglucose (FDG) as a tracer. Imaging was performed 60 min after tracer administration. Animals were anesthetized by inhalation of 2% isoflurane in 100% oxygen and underwent static imaging for 10 min on a micro-PET Focus 220 scanner (Concorde Microsystems, Knoxville, TN, USA). Images were reconstructed with ordered subset expectation maximization algorithm with 6 iterations (OSEM3D 6i) and analyzed with PMOD v.3.4 (Pmod Technologies, Zurich, Switzerland). To exclude any effect of diurnal variability, micro-PET data acquisition was consistently performed within the same 2 h time frame of the day. The standardized uptake value (SUV) in a specific volume of interest is the ratio between the uptake in this volume versus the average uptake in the whole body. The simultaneous quantification of skeletal SUVs was performed since myocardial glucose metabolism is not necessarily parallel to skeletal and whole-body glucose metabolism [
43].
4.13. Statistical Analysis
All data are expressed as means ± standard error of the means (SEM). Parameters between four groups were compared by one-way analysis of variance followed by Bonferroni multiple comparisons post-test for comparing sham groups, TAC groups, and sham versus respective TAC groups using GraphPad Instat (GraphPad Software, San Diego, CA, USA). When the assumption of sampling from populations with identical standard deviations was not met, a logarithmic transformation was performed. When the assumption of sampling from populations with Gaussian distributions was not met, a Kruskal–Wallis test was performed followed by Dunn’s multiple comparisons post-test. Parameters between two groups were compared using Student’s t test. When indicated, a logarithmic transformation or a non-parametric Mann–Whitney test was performed. The assumption of Gaussian distribution was tested using the Kolmogorov–Smirnov method. Kaplan–Meier survival curves were analyzed by log-rank test using Prism4 (GraphPad Software). A two-sided p-value of less than 0.05 was considered statistically significant.