Thrombospondins: A Role in Cardiovascular Disease
Abstract
1. Introduction
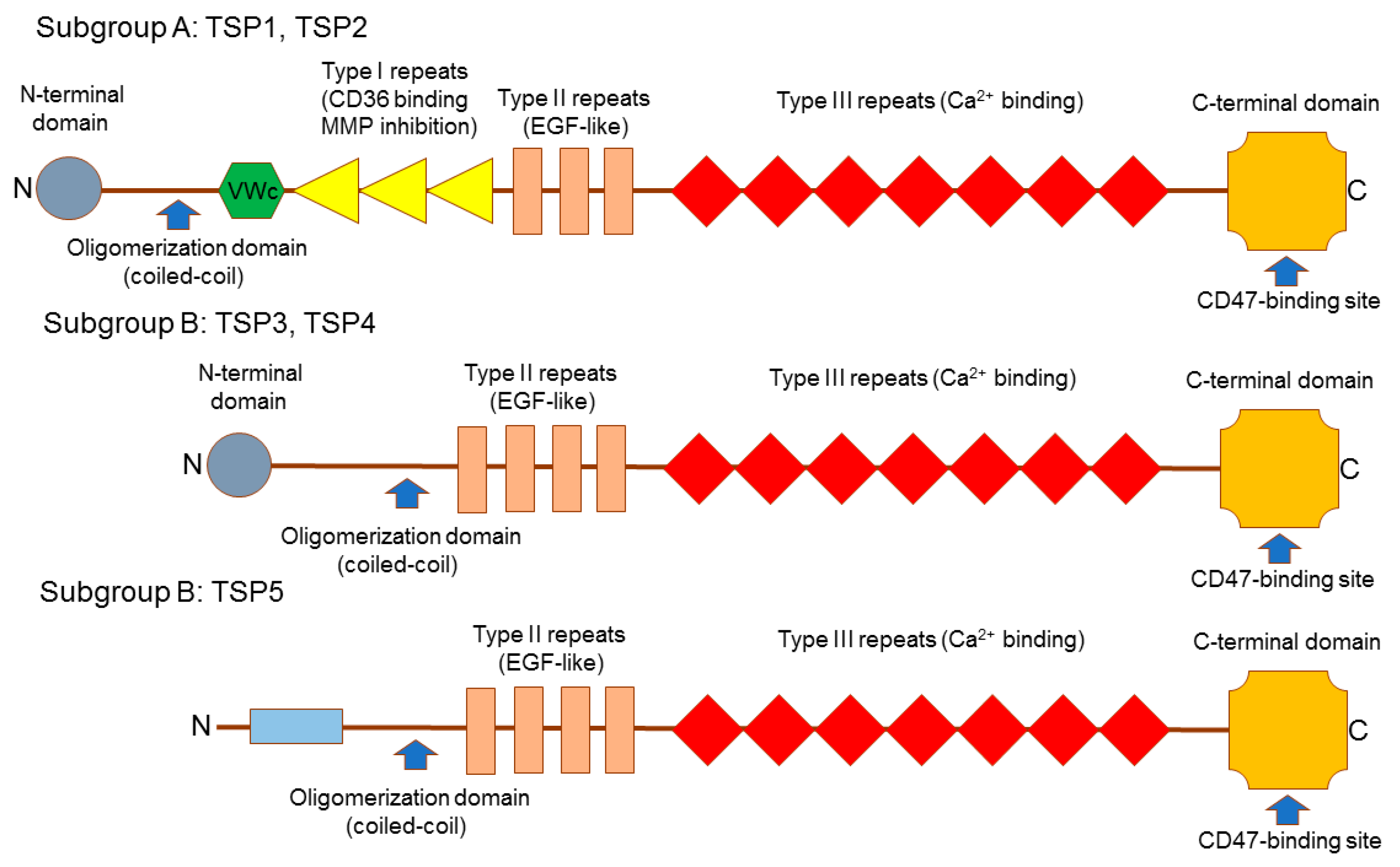
2. Thrombospondins Structure
3. Cardiac Integrity
4. TSPs in Angiogenesis
5. TSPs in Atherosclerotic Blood Vessels
6. TSPs in Myocardial Infarction
7. TMPs in Cardiac Hypertrophy
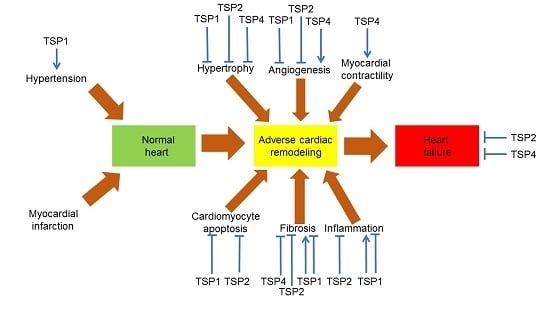
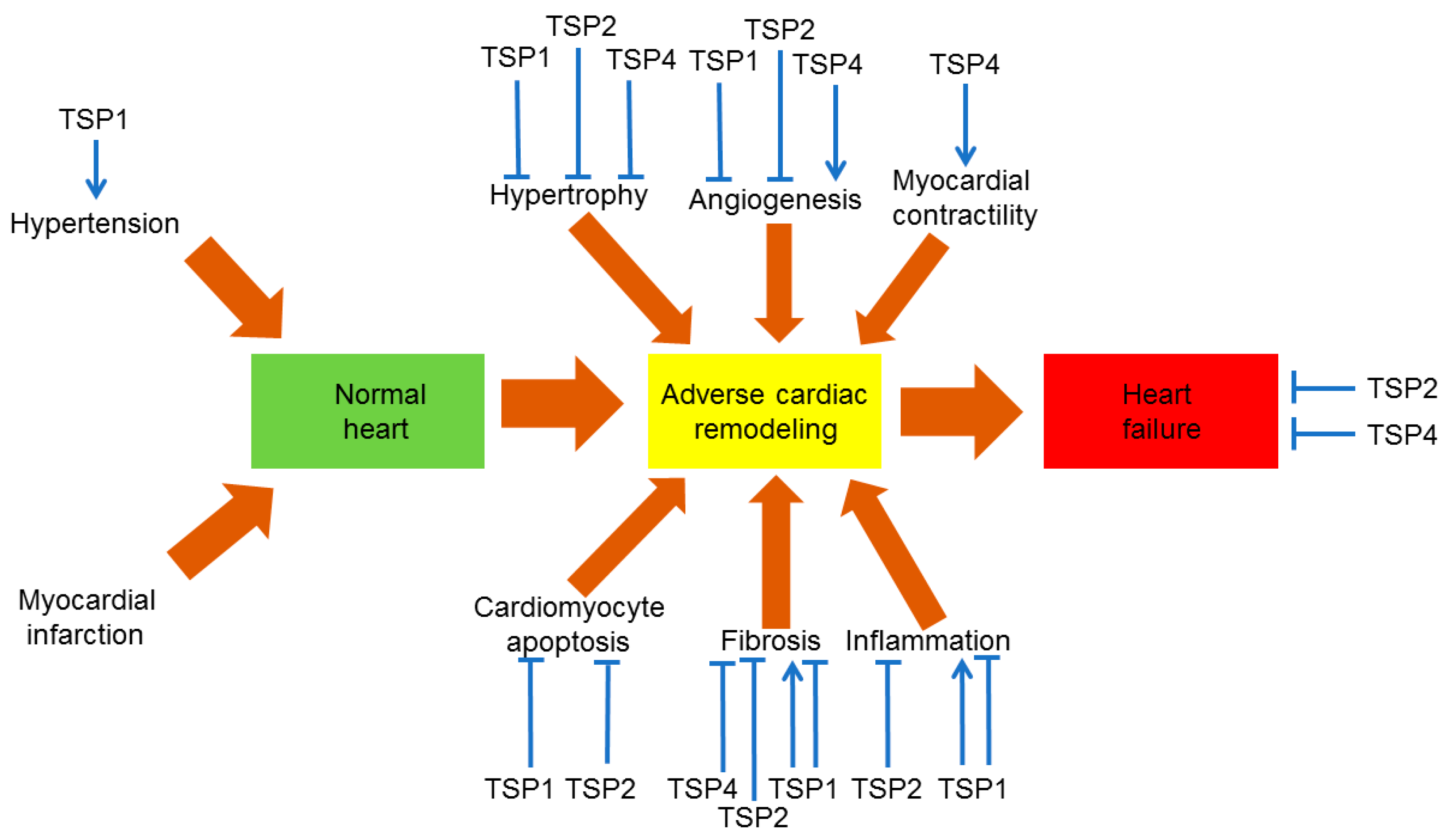
8. TSPs in Heart Failure
9. TSPs in Calcific Aortic Valve Disease
10. TSPs in other Pathologies
11. Therapeutic Potential of Thrombospondins
12. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N. Vascular extracellular matrix in atherosclerosis. Cardiol. Rev. 2013, 21, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, A.D.; Anssari-Benham, A.; Lee, D.A.; Taylor, P.M.; Chester, A.H.; Yacoub, M.H.; Screen, H.R. Anisotropic strain transfer through the aortic valve and its relevance to the cellular mechanical environment. Proc. Inst. Mech. Eng. H 2011, 225, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Okech, W.; Abberton, K.M.; Kuebel, J.M.; Hocking, D.C.; Sarelius, I.H. Extracellular matrix fibronectin mediates an endothelial cell response to shear stress via the heparin-binding, matricryptic RWRPK sequence of FNIII1H. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1063–H1071. [Google Scholar] [CrossRef] [PubMed]
- Casals, G.; Fernández-Varo, G.; Melgar-Lesmes, P.; Marfà, S.; Reichenbach, V.; Morales-Ruiz, M.; Jiménez, W. Factors involved in extracellular matrix turnover in human derived cardiomyocytes. Cell Physiol. Biochem. 2013, 32, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Luu, N.T.; Glen, K.E.; Egginton, S.; Rainger, G.E.; Nash, G.B. Integrin-substrate interactions underlying shear-induced inhibition of the inflammatory response of endothelial cells. Thromb. Haemost. 2013, 109, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Von Bary, C.; Makowski, M.; Preissel, A.; Keithahn, A.; Warley, A.; Spuentrup, E.; Buecker, A.; Lazewatsky, J.; Cesati, R.; Onthank, D.; et al. MRI of coronary wall remodeling in a swine model of coronary injury using an elastin-binding contrast agent. Circ. Cardiovasc. Imaging 2011, 4, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Philip, J.L.; Xu, X.; Theccanat, T.; Abdur Razzaque, M.; Akhter, S.A. β-Arrestins regulate human cardiac fibroblast transformation and collagen synthesis in adverse ventricular remodeling. J. Mol. Cell Cardiol. 2014, 76, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Mujumdar, V.S.; Tyagi, S.C. Temporal regulation of extracellular matrix components in transition from compensatory hypertrophy to decompensatory heart failure. J. Hypertens. 1999, 17, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Sakamuri, S.S.; Takawale, A.; Basu, R.; Fedak, P.W.; Freed, D.; Sergi, C.; Oudit, G.Y.; Kassiri, Z. Differential impact of mechanical unloading on structural and nonstructural components of the extracellular matrix in advanced human heart failure. Transl. Res. 2016, 172, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P. Thrombospondins as matricellular modulators of cell function. J. Clin. Investig. 2001, 107, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Sato, I. Distribution of tenascin-C and -X, and soft X-ray analysis of the mandibular symphysis during mandible formation in the human fetus. Okajimas Folia Anat. Jpn. 2004, 81, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, L.; Niu, R.; Liao, H. Tenascin-R distinct domains modulate migration of neural stem/progenitor cells in vitro. In Vitro Cell Dev. Biol. Anim. 2009, 45, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for αVβ3 and alphaVbeta5 integrins and promotes cell motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar] [PubMed]
- Hoersch, S.; Andrade-Navarro, M.A. Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol. Biol. 2010, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, D.; Kimura, N.; Yoshioka, M.; Mukai, M.; Kimura, T.; Okada, Y.; Yozu, R.; Shukunami, C.; Hiraki, Y.; Kudo, A.; et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Investig. 2010, 120, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Steitz, S.A.; Speer, M.Y.; McKee, M.D.; Liaw, L.; Almeida, M.; Yang, H.; Giachelli, C.M. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am. J. Pathol. 2002, 161, 2035–2046. [Google Scholar] [CrossRef]
- McKee, M.D.; Addison, W.N.; Kaartinen, M.T. Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton. Cell. Tissues Organs 2005, 181, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Midura, R.J.; Midura, S.B.; Su, X.; Gorski, J.P. Separation of newly formed bone from older compact bone reveals clear compositional differences in bone matrix. Bone 2011, 49, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lau, L.F. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 2009, 41, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; Gonzalez-Quesada, C.; Frangogiannis, N.G. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J. Mol. Cell. Cardiol. 2010, 48, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.B.; Lawler, J.; Mosher, D.F. Structures of thrombospondins. Cell Mol. Life Sci. 2008, 65, 672–686. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.C.; Lawler, J. The thrombospondins. Cold Spring Harb. Perspect. Biol. 2011, 3, a009712. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, P.; Chadalavada, C.; Bohrer, J.; Adams, J.C. Phylogenomic analysis of vertebrate thrombospondins, reveals fish-specific paralogues, ancestral gene relationships and a tetrapod innovation. BMC Evol. Biol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Bentley, A.A.; Adams, J.C. The evolution of thrombospondins and their ligand-binding activities. Mol. Biol. Evol. 2010, 27, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Ullrich, J.E.; Iozzo, R.V. Thrombospondins in physiology and disease: New tricks for old dogs. Matrix Biol. 2012, 31, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Rath, G.M.; Schneider, C.; Dedieu, S.; Rothhut, B.; Soula-Rothhut, M.; Ghoneim, C.; Sid, B.; Morjani, H.; El Btaouri, H.; Martiny, L. The C-terminal CD47/IAP-binding domain of thrombospondin-1 prevents camptothecin- and doxorubicin-induced apoptosis in human thyroid carcinoma cells. Biochim. Biophys. Acta 2006, 1763, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P.; Armstrong, L.C.; Hankenson, K.D.; Kyriakides, T.R.; Yang, Z. Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol. 2000, 19, 557–568. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Bornstein, P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb. Haemost. 2003, 90, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Schellings, M.W.; Van Almen, G.C.; Sage, E.H.; Heymans, S. Thrombospondins in the heart: Potential functions in cardiac remodeling. J. Cell Commun. Signal 2009, 3, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Frolova, E.G.; Pluskota, E.; Krukovets, I.; Burke, T.; Drumm, C.; Smith, J.D.; Blech, L.; Febbraio, M.; Bornstein, P.; Plow, E.F.; et al. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ. Res. 2010, 107, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, E.; Ruskoaho, H.; Rysä, J. Thrombospondins, potential drug targets for cardiovascular diseases. Basic Clin. Pharmacol. Toxicol. 2013, 112, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Muppala, S.; Xiao, R.; Krukovets, I.; Verbovetsky, D.; Yendamuri, R.; Habib, N.; Raman, P.; Plow, E.; Stenina-Adognravi, O. Thrombospondin-4 mediates TGF-β-induced angiogenesis. Oncogene 2017, (in press). [CrossRef] [PubMed]
- Topol, E.J.; McCarthy, J.; Gabriel, S.; Moliterno, D.J.; Rogers, W.J.; Newby, L.K.; Freedman, M.; Metivier, J.; Cannata, R.; O’Donnell, C.J.; et al. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation 2001, 104, 2641–2644. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Randell, E.; Renouf, J.; Sun, G.; Han, FY.; Younghusband, B.; Xie, Y.G. Gender dependent association of thrombospondin-4 A387P polymorphism with myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e183–e1844. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Randell, E.; Renouf, J.; Sun, G.; Green, R.; Han, F.Y.; Xie, Y.G. Thrombospondin-4 1186G>C (A387P) is a sex-dependent risk factor for myocardial infarction: A large replication study with increased sample size from the same population. Am. Heart J. 2006, 152, 543.e1–543.e5. [Google Scholar] [CrossRef] [PubMed]
- Wessel, J.; Topol, E.J.; Ji, M.; Meyer, J.; McCarthy, J.J. Replication of the association between the thrombospondin-4 A387P polymorphism and myocardial infarction. Am. Heart J. 2004, 147, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Peyvandi, F.; Palla, R.; Lombardi, R.; Canciani, M.T.; Cairo, A.; Ardissino, D.; Bernardinelli, L.; Bauer, K.A.; Lawler, J.; et al. The thrombospondin-1 N700S polymorphism is associated with early myocardial infarction without altering von Willebrand factor multimer size. Blood 2006, 108, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, J.P.; Ryan, D.; Moss, A.J.; McCarthy, J.; Goldenberg, I.; Zareba, W.; Sparks, C.E. Thrombospondin-4 polymorphism (A387P) predicts cardiovascular risk in postinfarction patients with high HDL cholesterol and C-reactive protein levels. Thromb. Haemost. 2011, 106, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Stenina, O.I.; Ustinov, V.; Krukovets, I.; Marinic, T.; Topol, E.J.; Plow, E.F. Polymorphisms A387P in thrombospondin-4 and N700S in thrombospondin-1 perturb calcium binding sites. FASEB J. 2005, 19, 1893–1895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Wei, C.Y.; Li, W.B.; Zhang, L.L.; Zhou, Y.; Wang, Z.H.; Tang, M.X.; Zhang, W.; Zhang, Y.; Zhong, M. Association between single nucleotide polymorphisms in thrombospondins genes and coronary artery disease: A meta-analysis. Thromb. Res. 2015, 136, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Adolph, K.W. Relative abundance of thrombospondin 2 and thrombospondin 3 mRNAs in human tissues. Biochem. Biophys. Res. Commun. 1999, 258, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Hu, Y.; Miller, G.G.; Mitchell, R.N.; Libby, P. Association of thrombospondin-1 and cardiac allograft vasculopathy in human cardiac allografts. Circulation 2001, 103, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Sezaki, S.; Hirohata, S.; Iwabu, A.; Nakamura, K.; Toeda, K.; Miyoshi, T.; Yamawaki, H.; Demircan, K.; Kusachi, S.; Shiratori, Y.; et al. Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp. Biol. Med. 2005, 230, 621–630. [Google Scholar]
- Mustonen, E.; Aro, J.; Puhakka, J.; Ilves, M.; Soini, Y.; Leskinen, H.; Ruskoaho, H.; Rysä, J. Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem. Biophys. Res. Commun. 2008, 373, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Stenina-Adognravi, O. Thrombospondins: Old players, new games. Curr. Opin. Lipidol. 2013, 24, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Robey, T.E.; Mignone, J.L.; Muskheli, V.; Bornstein, P.; Murry, C.E. Lack of thrombospondin-2 reduces fibrosis and increases vascularity around cardiac cell grafts. Cardiovasc. Pathol. 2013, 22, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, D.; van Almen, G.C.; Van Aelst, L.N.; Van Cleemput, J.; Droogné, W.; Jin, Y.; Van de Werf, F.; Carmeliet, P.; Vanhaecke, J.; Papageorgiou, A.P.; et al. Matricellular proteins and matrix metalloproteinases mark the inflammatory and fibrotic response in human cardiac allograft rejection. Eur. Heart J. 2013, 34, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, O.H.; Kirk, J.A.; Seo, K.; Koitabashi, N.; Lee, D.I.; Ramirez-Correa, G.; Bedja, D.; Barth, A.S.; Moens, A.L.; Kass, D.A. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ. Res. 2011, 109, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Frolova, E.G.; Sopko, N.; Blech, L.; Popovic, Z.B.; Li, J.; Vasanji, A.; Drumm, C.; Krukovets, I.; Jain, M.K.; Penn, M.S.; Plow, E.F.; et al. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J. 2012, 26, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Van Almen, G.C.; Swinnen, M.; Carai, P.; Verhesen, W.; Cleutjens, J.P.; D’hooge, J.; Verheyen, F.K.; Pinto, Y.M.; Schroen, B.; Carmeliet, P.; et al. Absence of thrombospondin-2 increases cardiomyocyte damage and matrix disruption in doxorubicin-induced cardiomyopathy. J. Mol. Cell Cardiol. 2011, 51, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Belmadani, S.; Bernal, J.; Wei, C.C.; Pallero, M.A.; Dell’italia, L.; Murphy-Ullrich, J.E.; Berecek, K.H. A thrombospondin-1 antagonist of transforming growth factor-β activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am. J. Pathol. 2007, 171, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quesada, C.; Cavalera, M.; Biernacka, A.; Kong, P.; Lee, D.W.; Saxena, A.; Frunza, O.; Dobaczewski, M.; Shinde, A.; Frangogiannis, N.G. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ. Res. 2013, 113, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, M.; Vanhoutte, D.; Van Almen, G.C.; Hamdani, N.; Schellings, M.W.; D’hooge, J.; Van der Velden, J.; Weaver, M.S.; Sage, E.H.; Bornstein, P.; et al. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation 2009, 120, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Ren, G.; Dewald, O.; Zymek, P.; Haudek, S.; Koerting, A.; Winkelmann, K.; Michael, L.H.; Lawler, J.; Entman, M.L. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation 2005, 111, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Chatila, K.; Ren, G.; Xia, Y.; Huebener, P.; Bujak, M.; Frangogiannis, N.G. The role of the thrombospondins in healing myocardial infarcts. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yuan, Z.; Fei, Q.; Zhao, J. Investigation of thrombospondin-1 and transforming growth factor-β expression in the heart of aging mice. Exp. Ther. Med. 2012, 3, 433–436. [Google Scholar] [PubMed]
- Sun, H.; Zhao, Y.; Bi, X.; Li, S.; Su, G.; Miao, Y.; Ma, X.; Zhang, Y.; Zhang, W.; Zhong, M. Valsartan blocks thrombospondin/transforming growth factor/Smads to inhibit aortic remodeling in diabetic rats. Diagn. Pathol. 2015, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Schroen, B.; Heymans, S.; Sharma, U.; Blankesteijn, W.M.; Pokharel, S.; Cleutjens, J.P.; Porter, J.G.; Evelo, C.T.; Duisters, R.; van Leeuwen, R.E.; Janssen, B.J.; et al. Thrombospondin-2 is essential for myocardial matrix integrity: Increased expression identifies failure-prone cardiac hypertrophy. Circ. Res. 2004, 95, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Elzie, C.A.; Kucik, D.F.; Murphy-Ullrich, J.E. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J. Cell Sci. 2003, 116, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Pallero, M.A.; Elzie, C.A.; Chen, J.; Mosher, D.F.; Murphy-Ullrich, J.E. Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis. FASEB J. 2008, 22, 3968–3979. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Murphy-Ullrich, J.E.; Song, Y. Structural insight into the role of thrombospondin-1 binding to calreticulin in calreticulin-induced focal adhesion disassembly. Biochemistry 2010, 49, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Maillet, M.; Vanhoutte, D.; Schloemer, A.; Sargent, M.A.; Blair, N.S.; Lynch, K.A.; Okada, T.; Aronow, B.J.; Osinska, H.; et al. A thrombospondin-dependent pathway for a protective ER stress response. Cell 2012, 149, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Frolova, E.G.; Drazba, J.; Krukovets, I.; Kostenko, V.; Blech, L.; Harry, C.; Vasanji, A.; Drumm, C.; Sul, P.; Jenniskens, G.J.; et al. Control of organization and function of muscle and tendon by thrombospondin-4. Matrix Biol. 2014, 37, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Pohjolainen, V.; Mustonen, E.; Taskinen, P.; Näpänkangas, J.; Leskinen, H.; Ohukainen, P.; Peltonen, T.; Aro, J.; Juvonen, T.; Satta, J.; et al. Increased thrombospondin-2 in human fibrosclerotic and stenotic aortic valves. Atherosclerosis 2012, 220, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Streit, M.; Riccardi, L.; Velasco, P.; Brown, L.F.; Hawighorst, T.; Bornstein, P.; Detmar, M. Thrombospondin-2: A potent endogenous inhibitor of tumor growth and angiogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 14888–14893. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell. Mol. Med. 2002, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.; Detmar, M. Tumor progression: The effects of thrombospondin-1 and -2. Int. J. Biochem. Cell Biol. 2004, 36, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lawler, J. Thrombospondin-based antiangiogenic therapy. Microvasc. Res. 2007, 74, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Almog, N.; Henke, V.; Flores, L.; Hlatky, L.; Kung, A.L.; Wright, R.D.; Berger, R.; Hutchinson, L.; Naumov, G.N.; Bender, E.; et al. Prolonged dormancy of human liposarcoma is associated with impaired tumor angiogenesis. FASEB J. 2006, 20, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Almog, N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010, 294, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Almog, N.; Ma, L.; Raychowdhury, R.; Schwager, C.; Erber, R.; Short, S.; Hlatky, L.; Vajkoczy, P.; Huber, P.E.; Folkman, J.; et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009, 69, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Sul, K.; Krukovets, I.; Nestor, C.; Li, J.; Adognravi, O.S. Novel tissue-specific mechanism of regulation of angiogenesis and cancer growth in response to hyperglycemia. J. Am. Heart Assoc. 2012, 1, e005967. [Google Scholar] [CrossRef] [PubMed]
- Krukovets, I.; Legerski, M.; Sul, P.; Stenina-Adognravi, O. Inhibition of hyperglycemia-induced angiogenesis and breast cancer tumor growth by systemic injection of microRNA-467 antagonist. FASEB J. 2015, 29, 3726–3736. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L.; Febbraio, M. CD36-TSP-HRGP interactions in the regulation of angiogenesis. Curr. Pharm. Des. 2007, 13, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Frieda, S.; Pearce, A.; Wu, J.; Silverstein, R.L. Rec mbinant GST/CD36 fusion proteins define a thrombospondin binding domain. Evidence for a single calcium-dependent binding site on CD36. J. Biol. Chem. 1995, 270, 2981–2986. [Google Scholar] [PubMed]
- Simantov, R.; Silverstein, R.L. CD36: A critical anti-angiogenic receptor. Front. Biosci. 2003, 8, s874–s882. [Google Scholar] [CrossRef] [PubMed]
- Simantov, R.; Febbraio, M.; Silverstein, R.L. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 2005, 24, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Martin-Manso, G.; Pendrak, M.L.; Garfield, S.H.; Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J. Biol. Chem. 2010, 285, 38923–38932. [Google Scholar] [CrossRef] [PubMed]
- Bazzazi, H.; Isenberg, J.S.; Popel, A.S. Inhibition of VEGFR2 activation and its downstream signaling to ERK1/2 and calcium by thrombospondin-1 (TSP1): In silico investigation. Front. Physiol 2017, in press. [Google Scholar]
- Isenberg, J.S.; Ridnour, L.A.; Dimitry, J.; Frazier, W.A.; Wink, D.A.; Roberts, D.D. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J. Biol. Chem. 2006, 281, 26069–26080. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Jia, Y.; Fukuyama, J.; Switzer, C.H.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J. Biol. Chem. 2007, 282, 15404–15415. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Hyodo, F.; Matsumoto, K.; Romeo, M.J.; Abu-Asab, M.; Tsokos, M.; Kuppusamy, P.; Wink, D.A.; Krishna, M.C.; Roberts, D.D. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood 2007, 109, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Annis, D.S.; Pendrak, M.L.; Ptaszynska, M.; Frazier, W.A.; Mosher, D.F.; Roberts, D.D. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J. Biol. Chem. 2009, 284, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Brandish, P.E.; Ballou, D.P.; Marletta, M.A. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA 1999, 96, 14753–14758. [Google Scholar] [CrossRef] [PubMed]
- Murad, F.; Rapoport, R.M.; Fiscus, R. Role of cyclic-GMP in relaxations of vascular smooth muscle. J. Cardiovasc. Pharmacol. 1985, 7, S111–S118. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Romeo, M.J.; Yu, C.; Yu, C.K.; Nghiem, K.; Monsale, J.; Rick, M.E.; Wink, D.A.; Frazier, W.A.; Roberts, D.D. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 2008, 111, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Koom, Y.K.; Kim, J.M.; Kim, S.Y.; Koo, J.Y.; Oh, D.; Park, S.; Yun-Choi, H.S. Elevated plasma concentration of NO and cGMP may be responsible for the decreased platelet aggregation and platelet leukocyte conjugation in platelets hypo-responsive to catecholamines. Platelets 2009, 20, 555–565. [Google Scholar]
- Miller, T.W.; Kaur, S.; Ivins-O’Keefe, K.; Roberts, D.D. Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol. 2013, 32, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Csányi, G.; Yao, M.; Rodríguez, A.I.; Al Ghouleh, I.; Sharifi-Sanjani, M.; Frazziano, G.; Huang, X.; Kelley, E.E.; Isenberg, J.S.; Pagano, P.J. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2966–2973. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Kleschyov, A.L.; Stessel, H.; Russwurm, M.; Münzel, T.; Koesling, D.; Schmidt, K. Inactivation of soluble guanylate cyclase by stoichiometric S-nitrosation. Mol. Pharmacol. 2009, 75, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.L.; Berka, V.; Sharina, I.; Martin, E. Dynamic ligand exchange in soluble guanylyl cyclase (sGC): Implications for sGC regulation and desensitization. J. Biol. Chem. 2011, 286, 43182–43192. [Google Scholar] [CrossRef] [PubMed]
- Muppala, S.; Frolova, E.; Xiao, R.; Krukovets, I.; Yoon, S.; Hoppe, G.; Vasanji, A.; Plow, E.; Stenina-Adognravi, O. Proangiogenic properties of thrombospondin-4. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Stenina, O.I.; Krukovets, I.; Wang, K.; Zhou, Z.; Forudi, F.; Penn, M.S.; Topol, E.J.; Plow, E.F. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation 2003, 107, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Asahara, T.; Krasinski, K.; Witzenbichler, B.; Yang, J.; Magner, M.; Kearney, M.; Frazier, W.A.; Isner, J.M.; Andrés, V. Antibody blockade of thrombospondin accelerates reendothelialization and reduces neointima formation in balloon-injured rat carotid artery. Circulation 1999, 100, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.; Tjwa, M.; Vandervoort, P.; Van Kerckhoven, S.; Holvoet, P.; Hoylaerts, M.F. Thrombospondin-1 deficiency accelerates atherosclerotic plaque maturation in ApoE−/− mice. Circ. Res. 2008, 103, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Stenina, O.I.; Plow, E.F. Counterbalancing forces: What is thrombospondin-1 doing in atherosclerotic lesions? Circ. Res. 2008, 103, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Siegel-Axel, D.I.; Runge, H.; Seipel, L.; Riessen, R. Effects of cerivastatin on human arterial smooth muscle cell growth and extracellular matrix expression at varying glucose and low-density lipoprotein levels. J. Cardiovasc. Pharmacol. 2003, 41, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.J.; Iruela-Arispe, L.; O’Brien, E.R.; Truong, T.; LaBell, T.; Bornstein, P.; Sage, E.H. Expression of thrombospondins by endothelial cells. Injury is correlated with TSP-1. Am. J. Pathol. 1995, 147, 1068–1080. [Google Scholar] [PubMed]
- Riessen, R.; Fenchel, M.; Chen, H.; Axel, D.I.; Karsch, K.R.; Lawler, J. Cartilage oligomeric matrix protein (thrombospondin-5) is expressed by human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Kong, W. ADAMTS-7, a novel proteolytic culprit in vascular remodeling. Sheng Li Xue Bao 2010, 62, 285–294. [Google Scholar] [PubMed]
- Wang, L.; Zheng, J.; Bai, X.; Liu, B.; Liu, C.J.; Xu, Q.; Zhu, Y.; Wang, N.; Kong, W.; Wang, X. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ. Res. 2009, 104, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.G.; Lindberg, F.P.; Finn, M.B.; Blystone, S.D.; Brown, E.J.; Frazier, W.A. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J. Biol. Chem. 1996, 271, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Roberts, D.D.; Isenberg, J.S. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol. Res. 2011, 63, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Frazier, W.A.; Krishna, M.C.; Wink, D.A.; Roberts, D.D. Enhancing cardiovascular dynamics by inhibition of thrombospondin-1/CD47 signaling. Curr. Drug Targets 2008, 9, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Qin, Y.; Maxhimer, J.B.; Sipes, J.M.; Despres, D.; Schnermann, J.; Frazier, W.A.; Roberts, D.D. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009, 28, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.M.; Qin, Y.; Miller, T.W.; Bandle, R.W.; Csanyi, G.; Pagano, P.J.; Bauer, P.M.; Schnermann, J.; Roberts, D.D.; Isenberg, J.S. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc. Res. 2010, 88, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Soto-Pantoja, D.R.; Kaur, S.; Roberts, D.D. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.D.; Miller, T.W.; Rogers, N.M.; Yao, M.; Isenberg, J.S. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012, 31, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Rogers, N.M.; Csányi, G.; Rodriguez, A.I.; Ross, M.A.; St Croix, C.; Knupp, H.; Novelli, E.M.; Thomson, A.W.; Pagano, P.J.; et al. Thrombospondin-1 activation of signal-regulatory protein-α stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J. Am. Soc. Nephrol. 2014, 25, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Khandelwal, A.R.; Qin, Z.; Wu, X.; Chen, L.; Ago, T.; Sadoshima, J.; Cohen, R.A. Role of smooth muscle Nox4-based NADPH oxidase in neointimal hyperplasia. J. Mol. Cell Cardiol. 2015, 89, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Khandelwal, A.R.; Wu, X.; Xu, Z.; Yu, W.; Chen, C.; Zhao, W.; Yang, J.; Qin, Z.; Weisbrod, R.M.; et al. Pro-atherogenic role of smooth muscle Nox4-based NADPH oxidase. J. Mol. Cell Cardiol. 2016, 92, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Csányi, G.; Feck, D.M.; Ghoshal, P.; Singla, B.; Lin, H.; Nagarajan, S.; Meijles, D.N.; Al Ghouleh, I.; Cantu-Medellin, N.; Kelley, E.E.; et al. CD47 and Nox1 mediate dynamic fluid-phase macropinocytosis of native LDL. Antioxid. Redox. Signal 2017, 26, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Tiyyagura, S.R.; Pinney, S.P. Left ventricular remodeling after myocardial infarction: Past, present, and future. Mt. Sinai J. Med. 2006, 73, 840–851. [Google Scholar] [PubMed]
- Buja, L.M.; Vela, D. Cardiomyocyte death and renewal in the normal and diseased heart. Cardiovasc Pathol. 2008, 17, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Jugdutt, B.I. Ventricular remodeling after infarction and the extracellular collagen matrix: When is enough enough? Circulation 2003, 108, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. The role of cardiac fibroblasts in post-myocardial heart tissue repair. Exp. Mol. Pathol. 2016, 101, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.W.; Borg, T.K.; Covell, J.W. Structure and mechanics of healing myocardial infarcts. Annu. Rev. Biomed. Eng. 2005, 7, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Lamy, L.; Ticchioni, M.; Rouquette-Jazdanian, A.K.; Samson, M.; Deckert, M.; Greenberg, A.H.; Bernard, A. CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J. Biol. Chem. 2003, 278, 23915–23921. [Google Scholar] [CrossRef] [PubMed]
- Lamy, L.; Foussat, A.; Brown, E.J.; Bornstein, P.; Ticchioni, M.; Bernard, A. Interactions between CD47 and thrombospondin reduce inflammation. J. Immunol. 2007, 178, 5930–5939. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Roberts, D.D.; Frazier, W.A. CD47: A new target in cardiovascular therapy. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Hyodo, F.; Pappan, L.K.; Abu-Asab, M.; Tsokos, M.; Krishna, M.C.; Frazier, W.A.; Roberts, D.D. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Mirochnik, Y.; Kwiatek, A.; Volpert, O.V. Thrombospondin and apoptosis: Molecular mechanisms and use for design of complementation treatments. Curr. Drug Targets 2008, 9, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Krady, M.M.; Zeng, J.; Yu, J.; MacLauchlan, S.; Skokos, E.A.; Tian, W.; Bornstein, P.; Sessa, W.C.; Kyriakides, T.R. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am. J. Pathol. 2008, 173, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Maclauchlan, S.; Skokos, E.A.; Agah, A.; Zeng, J.; Tian, W.; Davidson, J.M.; Bornstein, P.; Kyriakides, T.R. Enhanced angiogenesis and reduced contraction in thrombospondin-2-null wounds is associated with increased levels of matrix metalloproteinases-2 and -9, and soluble VEGF. J. Histochem. Cytochem. 2009, 57, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Zhu, Y.H.; Smith, L.T.; Bain, S.D.; Yang, Z.; Lin, M.T.; Danielson, K.G.; Iozzo, R.V.; LaMarca, M.; McKinney, C.E.; et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J. Cell Biol. 1998, 140, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Leach, K.J.; Hoffman, A.S.; Ratner, B.D.; Bornstein, P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc. Natl. Acad. Sci. USA 1999, 96, 4449–4454. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P.; Kyriakides, T.R.; Yang, Z.; Armstrong, L.C.; Birk, D.E. Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J. Investig. Dermatol. Symp. Proc. 2000, 5, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Van Oorschot, A.A.; Smits, A.M.; Pardali, E.; Doevendans, P.A.; Goumans, M.J. Low oxygen tension positively influences cardiomyocyte progenitor cell function. J. Cell Mol. Med. 2011, 15, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, E.; Leskinen, H.; Aro, J.; Luodonpää, M.; Vuolteenaho, O.; Ruskoaho, H.; Rysä, J. Metoprolol treatment lowers thrombospondin-4 expression in rats with myocardial infarction and left ventricular hypertrophy. Basic Clin. Pharmacol. Toxicol. 2010, 107, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Brody, M.J.; Schips, T.G.; Vanhoutte, D.; Kanisicak, O.; Karch, J.; Maliken, B.D.; Blair, N.S.; Sargent, M.A.; Prasad, V.; Molkentin, J.D. Dissection of thrombospondin-4 domains involved in intracellular adaptive endoplasmic reticulum stress-responsive signaling. Mol. Cell Biol. 2015, 36, 2–12. [Google Scholar] [PubMed]
- Jin, J.K.; Blackwood, E.A.; Azizi, K.; Thuerauf, D.J.; Fahem, A.G.; Hofmann, C.; Kaufman, R.J.; Doroudgar, S.; Glembotski, C.C. ATF6 decreases myocardial ischemia/reperfusion damage and links ER stress and oxidative stress signaling pathways in the heart. Circ. Res. 2017, 120, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; Creighton, W.; Tavora, F.; Li, L.; Fowler, D. Decreased frequency of the 3′UTR T>G single nucleotide polymorphism of thrombospondin-2 gene in sudden death due to plaque erosion. Cardiovasc. Pathol. 2010, 19, e45–e49. [Google Scholar] [CrossRef] [PubMed]
- Farb, A.; Burke, A.P.; Tang, A.L.; Liang, T.Y.; Mannan, P.; Smialek, J.; Virmani, R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996, 93, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Hoppmann, P.; de Waha, A.; Schömig, A.; Kastrati, A. Polymorphisms in thrombospondin genes and myocardial infarction: A case-control study and a meta-analysis of available evidence. Hum. Mol. Genet. 2008, 17, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M.; Anbarasan, C.; Saibabu, R.; Kuram, S.; Raman, S.C.; Cherian, K.M. An association study of thrombospondin 1 and 2 SNPs with coronary artery disease and myocardial infarction among South Indians. Thromb. Res. 2011, 128, e49–e53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, J.; Chen, J.; Zhao, J.; Yang, W.; Wang, X.; Gu, D. Thrombospondin-4 A387P polymorphism is not associated with coronary artery disease and myocardial infarction in the Chinese Han population. Clin. Sci. 2004, 106, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, J.; Chen, J.; Zhao, J.; Ge, D.; Yang, W.; Gu, D. Genetic association analysis of myocardial infarction with thrombospondin-1 N700S variant in a Chinese population. Thromb. Res. 2004, 113, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Burlew, B.S.; Weber, K.T. Connective tissue and the heart. Functional significance and regulatory mechanisms. Cardiol. Clin. 2000, 18, 435–442. [Google Scholar] [CrossRef]
- Fomovsky, G.M.; Thomopoulos, S.; Holmes, J.W. Contribution of extracellular matrix to the mechanical properties of the heart. J. Mol. Cell Cardiol. 2010, 48, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circ. Res. 2002, 90, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd. Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc. Res. 2009, 81, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lee, K.; Li, N.; Corbett, D.; Mendoza, L.; Frangogiannis, N.G. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem. Cell Biol. 2009, 131, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Dobaczewski, M.; Gonzalez-Quesada, C.; Chen, W.; Biernacka, A.; Li, N.; Lee, D.W.; Frangogiannis, N.G. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 2011, 58, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Rohini, A.; Agrawal, N.; Koyani, C.N.; Singh, R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol. Res. 2010, 61, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.H.; Olfert, I.M. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp. Physiol. 2009, 94, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Moens, A.L.; Cingolani, O.; Arkenbout, E.; Kass, D.A. Exacerbated cardiac remodeling to pressure-overload in mice lacking thrombospondin-4. Eur. J. Heart Fail. 2008, 7, 149–150. [Google Scholar] [CrossRef]
- Bronzwaer, J.G.; Paulus, W.J. Diastolic and systolic heart failure: Different stages or distinct phenotypes of the heart failure syndrome? Curr. Heart Fail. Rep. 2009, 6, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Massie, B. Systolic and diastolic heart failure: Differences and similarities. J. Card. Fail. 2007, 13, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Diwan, A.; Dorn, G.W., 2nd. Decompensation of cardiac hypertrophy: Cellular mechanisms and novel therapeutic targets. Physiology 2007, 22, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Otsu, K. Cell death in heart failure. Circ. J. 2008, 72, A17–A21. [Google Scholar] [CrossRef]
- Shan, K.; Kurrelmeyer, K.; Seta, Y.; Wang, F.; Dibbs, Z.; Deswal, A.; Lee-Jackson, D.; Mann, D.L. The role of cytokines in disease progression in heart failure. Curr. Opin. Cardiol. 1997, 12, 218–223. [Google Scholar] [CrossRef]
- Batlle, M.; Pérez-Villa, F.; Lázaro, A.; García-Pras, E.; Vallejos, I.; Sionis, A.; Castel, M.A.; Roig, E. Decreased expression of thrombospondin-1 in failing hearts may favor ventricular remodeling. Transplant. Proc. 2009, 41, 2231–2233. [Google Scholar] [CrossRef]
- Muñoz-Pacheco, P.; Ortega-Hernández, A.; Caro-Vadillo, A.; Casanueva-Eliceiry, S.; Aragoncillo, P.; Egido, J.; Fernández-Cruz, A.; Gómez-Garre, D. Eplerenone enhances cardioprotective effects of standard heart failure therapy through matricellular proteins in hypertensive heart failure. J. Hypertens. 2013, 31, 2309–2319. [Google Scholar] [CrossRef]
- Sharifi-Sanjani, M.; Shoushtari, A.H.; Quiroz, M.; Baust, J.; Sestito, S.F.; Mosher, M.; Ross, M.; McTiernan, C.F.; St Croix, C.M.; Bilonick, R.A.; et al. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J. Am. Heart Assoc. 2014, 3, e000670. [Google Scholar] [CrossRef]
- Van Almen, G.C.; Verhesen, W.; Van Leeuwen, R.E.; Van de Vrie, M.; Eurlings, C.; Schellings, M.W.; Swinnen, M.; Cleutjens, J.P.; Van Zandvoort, M.A.; Heymans, S.; et al. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell 2011, 10, 769–779. [Google Scholar] [CrossRef]
- Papageorgiou, A.P.; Swinnen, M.; Vanhoutte, D.; VandenDriessche, T.; Chuah, M.; Lindner, D.; Verhesen, W.; De Vries, B.; D’hooge, J.; Lutgens, E.; et al. Thrombospondin-2 prevents cardiac injury and dysfunction in viral myocarditis through the activation of regulatory T-cells. Cardiovasc. Res. 2012, 94, 115–124. [Google Scholar] [CrossRef]
- Rysä, J.; Leskinen, H.; Ilves, M.; Ruskoaho, H. Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension 2005, 45, 927–933. [Google Scholar] [CrossRef]
- Melenovsky, V.; Benes, J.; Skaroupkova, P.; Sedmera, D.; Strnad, H.; Kolar, M.; Vlcek, C.; Petrak, J.; Benes, J., Jr.; Papousek, F.; et al. Metabolic characterization of volume overload heart failure due to aorto-caval fistula in rats. Mol. Cell Biochem. 2011, 354, 83–96. [Google Scholar] [CrossRef]
- Yetkin, E.; Waltenberger, J. Molecular and cellular mechanisms of aortic stenosis. Int. J. Cardiol. 2009, 135, 4–13. [Google Scholar] [CrossRef]
- Rawat, D.K.; Alzoubi, A.; Gupte, R.; Chettimada, S.; Watanabe, M.; Kahn, A.G.; Okada, T.; McMurtry, I.F.; Gupte, S.A. Increased reactive oxygen species, metabolic maladaptation, and autophagy contribute to pulmonary arterial hypertension-induced ventricular hypertrophy and diastolic heart failure. Hypertension 2014, 64, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Sage, E.; Mercier, O.; Van den Eyden, F.; De Perrot, M.; Barlier-Mur, A.M.; Dartevelle, P.; Eddahibi, S.; Herve, P.; Fadel, E. Endothelial cell apoptosis in chronically obstructed and reperfused pulmonary artery. Respir. Res. 2008, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Imoto, K.; Okada, M.; Yamawaki, H. Expression profile of matricellular proteins in hypertrophied right ventricle of monocrotaline-induced pulmonary hypertensive rats. J. Vet. Med. Sci. 2017, 79, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Frantz, C.; Bals, R.; Wilkens, H. The role of circulating thrombospondin-1 in patients with precapillary pulmonary hypertension. Respir. Res. 2016, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, C.D.; Yu, L.; Al-Ansari, E.; Hales, C.A.; Quinn, D.A. Thrombospondin-1 null mice are resistant to hypoxia-induced pulmonary hypertension. J. Cardiothorac. Surg. 2010, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mickael, C.; Kassa, B.; Gebreab, L.; Robinson, J.C.; Koyanagi, D.E.; Sanders, L.; Barthel, L.; Meadows, C.; Fox, D.; et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat. Commun. 2017, 8, 15494. [Google Scholar] [CrossRef] [PubMed]
- Green, D.E.; Kang, B.Y.; Murphy, T.C.; Hart, C.M. Peroxisome proliferator-activated receptor gamma (PPARγ) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation. Pulm. Circ. 2012, 2, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Cucoranu, I.; Clempus, R.; Dikalova, A.; Phelan, P.J.; Ariyan, S.; Dikalov, S.; Sorescu, D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005, 97, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Sturrock, A.; Cahill, B.; Norman, K.; Huecksteadt, T.P.; Hill, K.; Sanders, K.; Karwande, S.V.; Stringham, J.C.; Bull, D.A.; Gleich, M.; Kennedy, T.P.; Hoidal, J.R. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L661–L673. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Sturrock, A.; Wu, P.; Cahill, B.; Norman, K.; Huecksteadt, T.; Sanders, K.; Kennedy, T.; Hoidal, J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: The role of autocrine production of transforming growth factor-β-1 and insulin-like growth factor binding protein-3. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L489–L499. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Murphy, T.C.; Nanes, M.S.; Hart, C.M. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L559–L566. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Bijli, K.M.; Ramirez, A.; Murphy, T.C.; Kleinhenz, J.; Hart, C.M. Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radic. Biol. Med. 2013, 63, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.N.; Kim, G.M.; Chen, J.J.; Cheung, W.M.; He, Y.Y.; Hsu, C.Y. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke 2003, 34, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Yang, J.; Beltran, C.; Cho, S. Cell surface CD36 protein in monocyte/macrophage contributes to phagocytosis during the resolution phase of ischemic stroke in mice. J. Biol. Chem. 2016, 291, 23654–23661. [Google Scholar] [CrossRef] [PubMed]
- Liauw, J.; Hoang, S.; Choi, M.; Eroglu, C.; Choi, M.; Sun, G.H.; Percy, M.; Wildman-Tobriner, B.; Bliss, T.; Guzman, R.G.; Barres, B.A.; Steinberg, G.K. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J. Cereb. Blood Flow Metab. 2008, 28, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Jurk, K.; Jahn, U.R.; Van Aken, H.; Schriek, C.; Droste, D.W.; Ritter, M.A.; Bernd Ringelstein, E.; Kehrel, B.E. Platelets in patients with acute ischemic stroke are exhausted and refractory to thrombin, due to cleavage of the seven-transmembrane thrombin receptor (PAR-1). Thromb. Haemost. 2004, 91, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Yesner, L.M.; Huh, H.Y.; Pearce, S.F.; Silverstein, R.L. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Buée, L.; Hof, P.R.; Roberts, D.D.; Delacourte, A.; Morrison, J.H.; Fillit, H.M. Immunohistochemical identification of thrombospondin in normal human brain and in Alzheimer’s disease. Am. J. Pathol. 1992, 141, 783–788. [Google Scholar] [PubMed]
- Son, S.M.; Nam, D.W.; Cha, M.Y.; Kim, K.H.; Byun, J.; Ryu, H.; Mook-Jung, I. Thrombospondin-1 prevents amyloid beta-mediated synaptic pathology in Alzheimer’s disease. Neurobiol. Aging 2015, 36, 3214–3227. [Google Scholar] [CrossRef] [PubMed]
- Rama Rao, K.V.; Curtis, K.M.; Johnstone, J.T.; Norenberg, M.D. Amyloid-β inhibits thrombospondin 1 release from cultured astrocytes: Effects on synaptic protein expression. J. Neuropathol. Exp. Neurol. 2013, 72, 735–744. [Google Scholar] [PubMed]
- Garcia, O.; Torres, M.; Helguera, P.; Coskun, P.; Busciglio, J. A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down‘s syndrome. PLoS ONE 2010, 5, e14200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Lau, S.K.; Doering, L.C. Astrocyte-secreted thrombospondin-1 modulates synapse and spine defects in the fragile X mouse model. Mol. Brain 2016, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Chu, Y.Y.; Narumiya, S.; Chi, J.Y.; Furuyashiki, T.; Aoki, T.; Wang, S.M.; Chang, W.C.; Wang, J.M. CCAAT/enhancer-binding protein δ/miR135a/thrombospondin 1 axis mediates PGE2-induced angiogenesis in Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Liu, S.; Zeng, X.; Yang, X.; Huang, H.; Zhang, Y.; Chen, J.; Xu, Y.; Huang, D.; Qiu, X. Population study confirms serum proteins’ change and reveals diagnostic values in congenital ventricular septal defect. Pediatr. Cardiol 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nagy, A.; Larsson, J.; Dudas, M.; Sucov, H.M.; Kaartinen, V. Defective ALK5 signaling in the neural crest leads to increased postmigratory neural crest cell apoptosis and severe outflow tract defects. BMC Dev. Biol. 2006, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Kim, G.H.; Mackinnon, A.C.; Flagg, A.E.; Bassett, B.; Earley, J.U.; Svensson, E.C. Ets1 is required for proper migration and differentiation of the cardiac neural crest. Development 2010, 137, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Fouladkou, F.; Lu, C.; Jiang, C.; Zhou, L.; She, Y.; Walls, J.R.; Kawabe, H.; Brose, N.; Henkelman, R.M.; Huang, A.; Bruneau, B.G.; Rotin, D. The ubiquitin ligase Nedd4–1 is required for heart development and is a suppressor of thrombospondin-1. J. Biol. Chem. 2010, 285, 6770–6780. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kumar, S. Nedd4 and Nedd4–2: Closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 2010, 17, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, J.; Treins, C.; Monthouël-Kartmann, M.N.; Pontier-Bres, R.; Kumar, S.; Van Obberghen, E.; Giorgetti-Peraldi, S. Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J. Biol. Chem. 2004, 279, 26754–26761. [Google Scholar] [CrossRef] [PubMed]
- Van Bemmelen, M.X.; Rougier, J.S.; Gavillet, B.; Apothéloz, F.; Daidié, D.; Tateyama, M.; Rivolta, I.; Thomas, M.A.; Kass, R.S.; Staub, O.; et al. Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4–2 mediated ubiquitination. Circ. Res. 2004, 95, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Fotia, A.B.; Ekberg, J.; Adams, D.J.; Cook, D.I.; Poronnik, P.; Kumar, S. Regulation of neuronal voltage-gated sodium channels by the ubiquitin-protein ligases Nedd4 and Nedd4–2. J. Biol. Chem. 2004, 279, 28930–28935. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, I.P.; Pizard, A.; Patel, V.V.; Bruneau, B.G.; Kim, J.B.; Kupershmidt, S.; Roden, D.; Berul, C.I.; Seidman, C.E.; Seidman, J.G. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development 2004, 131, 4107–4116. [Google Scholar] [CrossRef] [PubMed]
- Murhy-Ullrich, J.E.; Sage, E.H. Revisiting the matricellular concept. Matrix Biol. 2014, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marganski, W.A.; Gangopadhyay, S.S.; Je, H.D.; Gallant, C.; Morgan, K.G. Targeting of a novel Ca+2/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ. Res. 2005, 97, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Z.; Wu, H.E.; Luo, Z.D.; Hogan, Q.H.; Pan, B. Increased thrombospondin-4 after nerve injury mediates disruption of intracellular calcium signaling in primary sensory neurons. Neuropharmacology 2017, 117, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Sandell, L.J. Antiangiogenic and anticancer molecules in cartilage. Expert Rev. Mol. Med. 2012, 14, e10. [Google Scholar] [CrossRef] [PubMed]
- Rusnati, M.; Urbinati, C.; Bonifacio, S.; Presta, M.; Taraboletti, G. Thrombospondin-1 as a paradigm for the development of antiangiogenic agents endowed with multiple mechanisms of action. Pharmaceuticals 2010, 3, 1241–1278. [Google Scholar] [CrossRef] [PubMed]
- Henkin, J.; Volpert, O.V. Therapies using anti-angiogenic peptide mimetics of thrombospondin-1. Expert Opin. Ther. Targets 2011, 15, 1369–1386. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.W.; Volpert, O.V.; Pearce, S.F.; Schneider, A.J.; Silverstein, R.L.; Henkin, J.; Bouck, N.P. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol. Pharmacol. 1999, 55, 332–338. [Google Scholar] [PubMed]
- Westphal, J.R. Technology evaluation: ABT-510, Abbott. Curr. Opin. Mol. Ther. 2004, 6, 451–457. [Google Scholar] [PubMed]
- Amato, R.J. Renal cell carcinoma: Review of novel single-agent therapeutics and combination regimens. Ann. Oncol. 2005, 16, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, R.; de Vos, F.Y.; Eskens, F.A.; Gietema, J.A.; Van der Gaast, A.; Groen, H.J.; Knight, R.A.; Carr, R.A.; Humerickhouse, R.A.; Verweij, J.; et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced cancer. J. Clin. Oncol. 2005, 23, 5188–5197. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, R.; de Vos, F.Y.; Eskens, F.A.; de Vries, E.G.; Uges, D.R.; Knight, R.; Carr, R.A.; Humerickhouse, R.; Verweij, J.; Gietema, J.A. Phase I study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with 5-fluorouracil and leucovorin: A safe combination. Eur. J. Cancer 2006, 42, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Gietema, J.A.; Hoekstra, R.; De Vos, F.Y.; Uges, D.R.; Van der Gaast, A.; Groen, H.J.; Loos, W.J.; Knight, R.A.; Carr, R.A.; Humerickhouse, R.A.; et al. A phase I study assessing the safety and pharmacokinetics of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with gemcitabine and cisplatin in patients with solid tumors. Ann. Oncol. 2006, 17, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.S.; Mendelson, D.; Carr, R.; Knight, R.A.; Humerickhouse, R.A.; Iannone, M.; Stopeck, A.T. A phase 1 trial of 2 dose schedules of ABT-510, an antiangiogenic, thrombospondin-1-mimetic peptide, in patients with advanced cancer. Cancer 2008, 113, 3420–3429. [Google Scholar] [CrossRef] [PubMed]
- Nabors, L.B.; Fiveash, J.B.; Markert, J.M.; Kekan, M.S.; Gillespie, G.Y.; Huang, Z.; Johnson, M.J.; Meleth, S.; Kuo, H.; Gladson, C.L.; et al. A phase 1 trial of ABT-510 concurrent with standard chemoradiation for patients with newly diagnosed glioblastoma. Arch. Neurol. 2010, 67, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Uronis, H.E.; Cushman, S.M.; Bendell, J.C.; Blobe, G.C.; Morse, M.A.; Nixon, A.B.; Dellinger, A.; Starr, M.D.; Li, H.; Meadows, K.; et al. A phase I study of ABT-510 plus bevacizumab in advanced solid tumors. Cancer Med. 2013, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.N.; Suman, V.J.; Rao, R.A.; Ingle, J.N.; Kaur, J.S.; Erickson, L.A.; Pitot, H.C.; Croghan, G.A.; McWilliams, R.R.; Merchan, J.; et al. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am. J. Clin. Oncol. 2007, 30, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, S.; Hussain, M.; Tannir, N.; Gordon, M.; Desai, A.A.; Knight, R.A.; Humerickhouse, R.A.; Qian, J.; Gordon, G.B.; Figlin, R. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin. Cancer Res. 2007, 13, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.H.; Rowinsky, E.K.; Mendelson, D.; Humerickhouse, R.A.; Knight, R.A.; Qian, J.; Carr, R.A.; Gordon, G.B.; Demetri, G.D. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J. Clin. Oncol. 2008, 26, 5583–5588. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Romeo, M.; Abu-Asab, M.; Tsokos, M.; Oldenborg, A.; Pappan, L.; Wink, D.A.; Frazier, W.A.; Roberts, D.D. Increasing survival of ischemic tissue by targeting CD47. Circ. Res. 2007, 100, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS ONE 2015, 10, e0137345. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C. Monoclonal antibodies against human cancer stem cells. Immunotherapy 2014, 6, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.M.; Poczatek, M.; Schultz-Cherry, S.; Villain, M.; Murphy-Ullrich, J.E. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J. Biol. Chem. 1999, 274, 13586–13593. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Ullrich, J.E.; Poczatek, M. Activation of latent TGF-β by thrombospondin-1: Mechanisms and physiology. Cytokine Growth Factor Rev. 2000, 11, 59–69. [Google Scholar] [CrossRef]
- Kondou, H.; Mushiake, S.; Etani, Y.; Miyoshi, Y.; Michigami, T.; Ozono, K. A blocking peptide for transforming growth factor-β1 activation prevents hepatic fibrosis in vivo. J. Hepatol. 2003, 39, 742–748. [Google Scholar] [CrossRef]
- Xie, X.S.; Li, F.Y.; Liu, H.C.; Deng, Y.; Li, Z.; Fan, J.M. LSKL, a peptide antagonist of thrombospondin-1, attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Arch. Pharm. Res. 2010, 33, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Miao, M.; Schoeb, T.R.; Agarwal, A.; Murphy-Ullrich, J.E. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am. J. Pathol. 2011, 178, 2573–2586. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Li, G.; Yuan, W.; Chen, Y.; Zuo, Y.; Rashid, K.; Zhang, J.H.; Feng, H.; Liu, F. LSKL peptide alleviates subarachnoid fibrosis and hydrocephalus by inhibiting TSP1-mediated TGF-β1 signaling activity following subarachnoid hemorrhage in rats. Exp. Ther. Med. 2016, 12, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Seto, S.W.; Jose, R.J.; Biros, E.; Moran, C.S.; Wang, Y.; Clancy, P.; Golledge, J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Gao, J.; Pawlyk, B.; Starcher, B.; Spencer, J.A.; Yanagisawa, H.; Zuo, J.; Li, T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004, 36, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Wang, R.; Wang, W. Cell therapies in cardiomyopathy: Current status of clinical trials. Anal. Cell Pathol. 2017, 2017, 9404057. [Google Scholar] [CrossRef] [PubMed]
- Cointe, S.; Rhéaume, É.; Martel, C.; Blanc-Brude, O.; Dubé, E.; Sabatier, F.; Dignat-George, F.; Tardif, J.C.; Bonnefoy, A. Thrombospondin-1-derived peptide RFYVVMWK improves the adhesive phenotype of CD34+ cells from atherosclerotic patients with type 2 diabetes. Cell Transplant. 2017, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Efimenko, A.; Starostina, E.; Kalinina, N.; Stolzing, A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J. Transl. Med. 2011, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Noiseux, N.; Borie, M.; Desnoyers, A.; Menaouar, A.; Stevens, L.M.; Mansour, S.; Danalache, B.A.; Roy, D.C.; Jankowski, M.; Gutkowska, J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology 2012, 153, 5361–5372. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Iyer, R.P.; Zamilpa, R.; Yabluchanskiy, A.; DeLeon-Pennell, K.Y.; Hall, M.E.; Kaplan, A.; Zouein, F.A.; Bratton, D.; Flynn, E.R.; Cannon, P.L.; et al. A novel collagen matricryptin reduces left ventricular dilation post-myocardial infarction by promoting scar formation and angiogenesis. J. Am. Coll. Cardiol. 2015, 66, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Bein, K.; Simons, M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J. Biol. Chem. 2000, 275, 32167–32173. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | TSP1 | TSP2 | TSP3 | TSP4 | TSP5 |
|---|---|---|---|---|---|
| Expression in the vascular wall | Yes | Yes | Yes | Yes | Yes |
| Expression in the atherosclerotic plaque | Yes | Yes | Yes | Yes | Yes |
| Cardiac expression | Yes | Yes | No? | Yes | Unknown |
| Angiogenesis in the myocardium | Inhibition | Inhibition | Unknown | Activation | Unknown |
| Up-regulated expression in cardiac remodeling | Yes | Yes | Yes | Yes | Yes |
| Inhibition of MMP-2/3/9 | Yes | Yes | No | No | No |
| Cardiac fibrosis | Activation/Inhibition | Inhibition | Unknown | Inhibition | Unknown |
| VSMC proliferation/hyperplasia | Activation | Activation | Unknown | No effect | Inhibition |
| Blood pressure | Vasoconstriction | Vasoconstriction | Unknown | Unknown | Unknown |
| Inflammation | Activation/Inhibition | Inhibition | Unknown | Activation (moderate) | Unknown |
| Effects on macrophages | Stimulation of phagocytosis Foam cell formation | Unknown | Unknown | Recruitment to the plaque | Unknown |
| Plaque progression | Activation | Unknown | Unknown | Activation | Unknown |
| Oxidative stress | Activation | Unknown | Unknown | Unknown | Unknown |
| Cardiomyocyte apoptosis | Inhibition | Inhibition | Unknown | Unknown | Unknown |
| Cardiac contractility | No effect | Unknown | Unknown | Activation | Unknown |
| Cardiac hypertrophy | Inhibition | Inhibition | Unknown | Inhibition | Unknown |
| Heart failure | Inhibition? | Inhibition | Unknown | Inhibition | Unknown |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Thrombospondins: A Role in Cardiovascular Disease. Int. J. Mol. Sci. 2017, 18, 1540. https://doi.org/10.3390/ijms18071540
Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN. Thrombospondins: A Role in Cardiovascular Disease. International Journal of Molecular Sciences. 2017; 18(7):1540. https://doi.org/10.3390/ijms18071540
Chicago/Turabian StyleChistiakov, Dimitry A., Alexandra A. Melnichenko, Veronika A. Myasoedova, Andrey V. Grechko, and Alexander N. Orekhov. 2017. "Thrombospondins: A Role in Cardiovascular Disease" International Journal of Molecular Sciences 18, no. 7: 1540. https://doi.org/10.3390/ijms18071540
APA StyleChistiakov, D. A., Melnichenko, A. A., Myasoedova, V. A., Grechko, A. V., & Orekhov, A. N. (2017). Thrombospondins: A Role in Cardiovascular Disease. International Journal of Molecular Sciences, 18(7), 1540. https://doi.org/10.3390/ijms18071540