Erythropoietin and Its Angiogenic Activity
Abstract
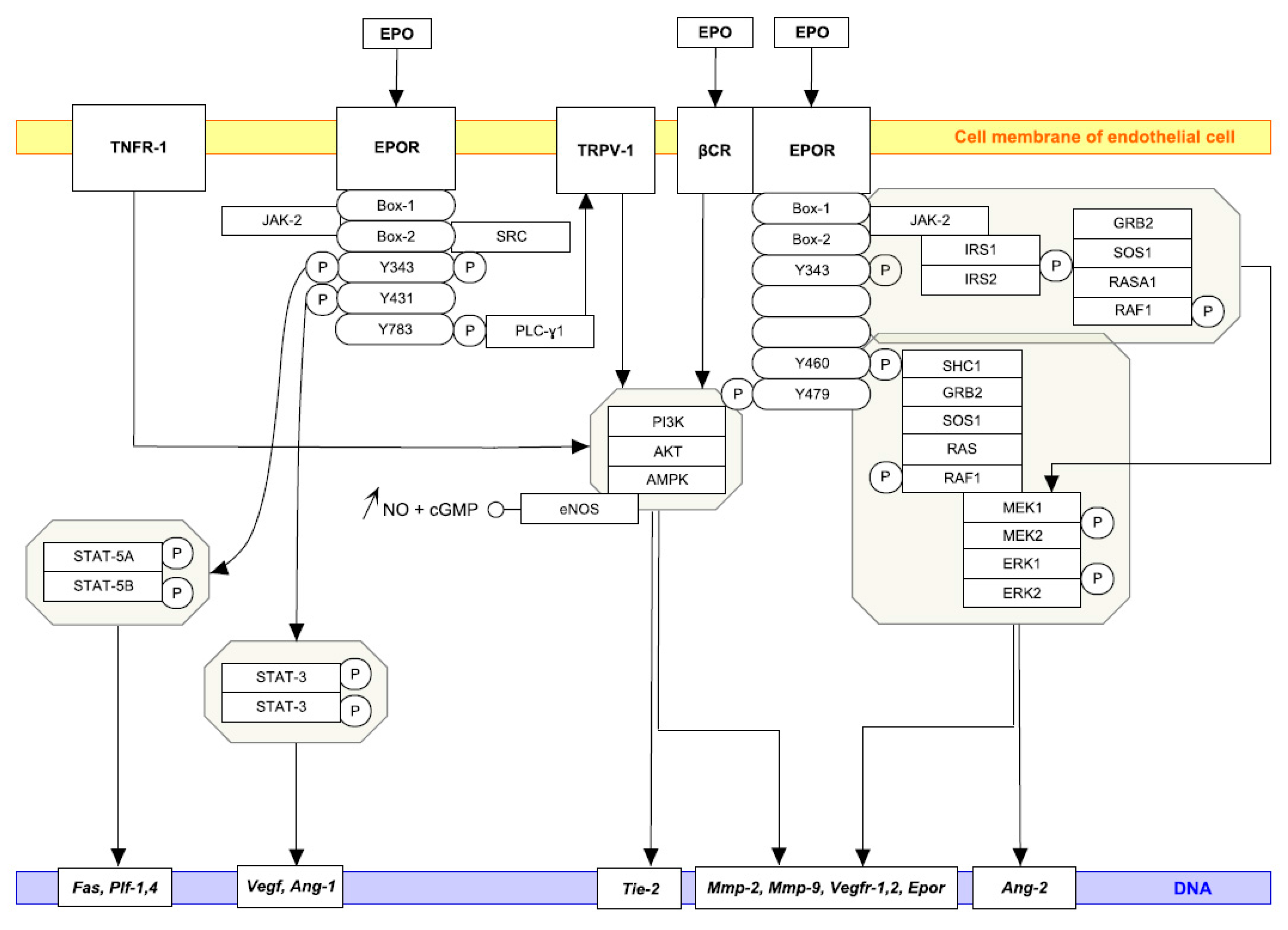
1. Introduction
1.1. Endothelial Cells
1.2. Bone Marrow
1.3. Adipose Tissue
1.4. Heart
1.5. Leg Ischemia
1.6. Retinopathy
1.7. Brain
1.8. Tumors
2. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jelkmann, W. Erythropoietin: Structure, control of production, and function. Physiol. Rev. 1992, 72, 449–489. [Google Scholar] [PubMed]
- Lin, C.S.; Lim, S.K.; D’Agati, V.; Costantini, F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996, 10, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Krantz, S.B. Erythropoietin. Blood 1991, 77, 419–434. [Google Scholar] [PubMed]
- Hardee, M.E.; Arcasoy, M.O.; Blackwell, K.L.; Kirkpatrick, J.P.; Dewhirst, M.W. Erythropoietin biology in cancer. Clin. Cancer Res. 2006, 12, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Watowich, S.S.; Hilton, D.J.; Lodish, H.F. Activation and inhibition of erythropoietin receptor function: Role of receptor dimerization. Mol. Cell. Biol. 1994, 14, 3535–3549. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Kampa, M.; Kafousi, M.; Dambaki, K.; Darivianaki, K.; Vrekoussis, T.; Sanidas, E.; Tsiftsis, D.D.; Stathopoulos, E.N.; Castanas, E. Erythropoietin and its receptor in breast cancer: Correlation with steroid receptors and outcome. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2016–2023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Darnell, J.E., Jr.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, M.L.; Heerkens, J.L.; de Bruin, A.M.; Klous, A.; de Waard, V.; de Vries, C.J. Erythropoietin accelerates smooth muscle cell-rich vascular lesion formation in mice through endothelial cell activation involving enhanced PDGF-BB release. Blood 2010, 115, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Christel, C.; Dannenberg, L.; Thiele, P.; Lindschau, C.; Luft, F.C. Signal transduction of erythropoietin in endothelial cells. Kidney Int. 1996, 50, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Carlini, R.G.; Dusso, A.S.; Obialo, C.I.; Alvarez, U.M.; Rothstein, M. Recombinant human erythropoietin (rHuEPO) increases endothelin-1 release by endothelial cells. Kidney Int. 1993, 43, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Carlini, R.G.; Reyes, A.A.; Rothstein, M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995, 47, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, A.; Lee, E.S.; Kessimian, N.; Levinson, R.; Steiner, M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc. Natl. Acad. Sci. USA 1990, 87, 5978–5982. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, A.; Liu, Z.; Steiner, M.; Chin, K.; Lee, E.S.; Kessimian, N.; Noguchi, C.T. Erythropoietin receptor mRNA expression in human endothelial cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, C.T.; Wang, L.; Rogers, H.M.; Teng, R.; Jia, Y. Survival and proliferative roles of erythropoietin beyond the erythroid lineage. Expert Rev. Mol. Med. 2008, 10, e36. [Google Scholar] [CrossRef] [PubMed]
- Heeschen, C.; Aicher, A.; Lehmann, R.; Fichtlscherer, S.; Vasa, M.; Urbich, C.; Mildner-Rihm, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 2003, 102, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, A.V.; d’Uscio, L.V.; Peterson, T.E.; Katusic, Z.S. Activation of endothelial nitric oxide synthase is critical for erythropoietin-induced mobilization of progenitor cells. Peptides 2008, 29, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, B.D.; Lipsic, E.; van der Meer, P.; van der Harst, P.; Oeseburg, H.; Du Marchie Sarvaas, G.J.; Koster, J.; Voors, A.A.; van Veldhuisen, D.J.; van Gilst, W.H.; et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur. Heart J. 2007, 28, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Brines, M.; Cerami, A. Discovering erythropoietin’s extra-hematopoietic functions: Biology and clinical promise. Kidney Int. 2006, 70, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiao, Z.; Li, T.; Gu, X.; Fan, B. Erythropoietin promotes the growth of pituitary adenomas by enhancing angiogenesis. Int. J. Oncol. 2012, 40, 1230–1237. [Google Scholar] [PubMed]
- Kawachi, K.; Iso, Y.; Sato, T.; Wakabayashi, K.; Kobayashi, Y.; Takeyama, Y.; Suzuki, H. Effects of erythropoietin on angiogenesis after myocardial infarction in porcine. Heart Vessel. 2012, 27, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Min, S.K.; Min, S.I.; Suh, J.H.; Kim, S.J.; Ha, J. Early sustained injections of erythropoietin improve angiogenesis and restoration of perfusion in the ischemic mouse hindlimb. J. Korean Med. Sci. 2012, 27, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Presta, M.; Vacca, A.; Ria, R.; Giuliani, R.; Dell’Era, P.; Nico, B.; Roncali, L.; Dammacco, F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood 1999, 93, 2627–2636. [Google Scholar] [PubMed]
- Yamaji, R.; Okada, T.; Moriya, M.; Naito, M.; Tsuruo, T.; Miyatake, K.; Nakano, Y. Brain capillary endothelial cells express two forms of erythropoietin receptor mRNA. Eur. J. Biochem. 1996, 239, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.E.; Hiley, C.R.; Fan, T.P. Comparative studies of the angiogenic activity of vasoactive intestinal peptide, endothelins-1 and -3 and angiotensin II in a rat sponge model. Br. J. Pharmacol. 1996, 117, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Ashley, R.A.; Dubuque, S.H.; Dvorak, B.; Woodward, S.S.; Williams, S.K.; Kling, P.J. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr. Res. 2002, 51, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Arroyo, M.V.; Castilla, M.A.; Gonzalez Pacheco, F.R.; Tan, D.; Riesco, A.; Casado, S.; Caramelo, C. Role of vascular endothelial growth factor on erythropoietin-related endothelial cell proliferation. J. Am. Soc. Nephrol. 1998, 9, 1998–2004. [Google Scholar] [PubMed]
- Beleslin-Cokic, B.B.; Cokic, V.P.; Yu, X.; Weksler, B.B.; Schechter, A.N.; Noguchi, C.T. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 2004, 104, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Di Venosa, G.; Hasan, T.; Al, B. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Su, K.H.; Kou, Y.R.; Guo, B.C.; Lee, K.I.; Wei, J.; Lee, T.S. Role of transient receptor potential vanilloid 1 in regulating erythropoietin-induced activation of endothelial nitric oxide synthase. Acta Physiol. 2016, 219, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.W.; Li, L.H.; Hong, B.Z.; Xiao, J.Q.; Wei, D.M.; Jin, Z. Therapeutic effects and related mechanisms of erythropoietin sustained-release gelatin hydrogel microspheres on a murine model of hindlimb ischemia. Zhonghua Xin Xue Guan Bing Za Zhi 2016, 44, 524–529. [Google Scholar] [PubMed]
- Su, K.H.; Shyue, S.K.; Kou, Y.R.; Ching, L.C.; Chiang, A.N.; Yu, Y.B.; Chen, C.Y.; Pan, C.C.; Lee, T.S. β Common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J. Cell. Physiol. 2011, 226, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Lamanuzzi, A.; Saltarella, I.; Ferrucci, A.; Ria, R.; Ruggieri, S.; Racanelli, V.; Rao, L.; Annese, T.; Nico, B.; Vacca, A.; Ribatti, D. Role of erythropoietin in the angiogenic activity of bone marrow endothelial cells of MGUS and multiple myeloma patients. Oncotarget 2016, 7, 14510–14521. [Google Scholar] [PubMed]
- De Luisi, A.; Binetti, L.; Ria, R.; Ruggieri, S.; Berardi, S.; Catacchio, I.; Racanelli, V.; Pavone, V.; Rossini, B.; Vacca, A.; Ribatti, D. Erythropoietin is involved in the angiogenic potential of bone marrow macrophages in multiple myeloma. Angiogenesis 2013, 16, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, Z.; Cui, S.; Ji, L.; Geng, H.; Chai, K.; Ma, X.; Bai, Z.; Yang, Y.; Wuren, T.; Ge, R.L.; Rondina, M.T. The local HIF-2α/EPO pathway in the bone marrow is associated with excessive erythrocytosis and the increase in bone marrow microvessel density in chronic mountain sickness. High Alt. Med. Biol. 2015, 16, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhang, F.; He, Q.; Tsang, W.P.; Lu, L.; Li, Q.; Wu, Z.; Qiu, G.; Zhou, G.; Wan, C. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis. PLoS ONE 2014, 9, e102010. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.H.; Orth, M.; Scheuer, C.; Tami, A.; Becker, S.C.; Garcia, P.; Histing, T.; Morsdorf, P.; Klein, M.; Pohlemann, T.; Menger, M.D. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone 2011, 49, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Luk, C.T.; Shi, S.Y.; Choi, D.; Cai, E.P.; Schroer, S.A.; Woo, M. In vivo knockdown of adipocyte erythropoietin receptor does not alter glucose or energy homeostasis. Endocrinology 2013, 154, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Mikolas, E.; Cseh, J.; Pap, M.; Szijarto, I.A.; Balogh, A.; Laczy, B.; Beko, V.; Fisi, V.; Molnar, G.A.; Merei, A.; et al. Effects of erythropoietin on glucose metabolism. Horm Metab. Res. 2012, 44, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Egozi, D.; Kruchevsky, D.; Teot, L.; Gilhar, A.; Ullmann, Y. Erythropoietin improves the survival of fat tissue after its transplantation in nude mice. PLoS ONE 2010, 5, e13986. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, A.; Hogger, D.C.; Borozadi, M.K.; Schmidt, C.A.; Plock, J.; Largo, R.D.; Lindenblatt, N.; Giovanoli, P.; Contaldo, C. EPO reverses defective wound repair in hypercholesterolaemic mice by increasing functional angiogenesis. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.; Gavrilova, O.; Suzuki, N.; Chanturiya, T.; Schimel, D.; Hugendubler, L.; Mammen, S.; Yver, D.R.; Cushman, S.W.; Mueller, E.; et al. Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat. Commun. 2001, 2, 520. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, B.D.; Oeseburg, H.; Kleijn, L.; van der Harst, P.; Belonje, A.M.; Voors, A.A.; Schoemaker, R.G.; de Boer, R.A.; van Veldhuisen, D.J.; van Gilst, W.H. Erythropoietin stimulates normal endothelial progenitor cell-mediated endothelial turnover, but attributes to neovascularization only in the presence of local ischemia. Cardiovasc. Drugs Ther. 2008, 22, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Imamura, R.; Moriyama, T.; Isaka, Y.; Namba, Y.; Ichimaru, N.; Takahara, S.; Okuyama, A. Erythropoietin protects the kidneys against ischemia reperfusion injury by activating hypoxia inducible factor-1α. Transplantation 2007, 83, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Wang, Y.; Zhang, R.; Chopp, M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 2004, 35, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Satoh, K.; Fukumoto, Y.; Ito, Y.; Kagaya, Y.; Ishii, N.; Sugamura, K.; Shimokawa, H. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ. Res. 2007, 100, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Zentilin, L.; Tafuro, S.; Zacchigna, S.; Arsic, N.; Pattarini, L.; Sinigaglia, M.; Giacca, M. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood 2006, 107, 3546–3554. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Fukumoto, Y.; Nakano, M.; Kagaya, Y.; Shimokawa, H. Emergence of the erythropoietin/erythropoietin receptor system as a novel cardiovascular therapeutic target. J. Cardiovasc. Pharmacol. 2011, 58, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Garcia-Gimenez, J.L.; Pareja-Galeano, H.; Romagnoli, M.; Perez-Quilis, C.; Lippi, G. Erythropoietin and the heart: Physiological effects and the therapeutic perspective. Int. J. Cardiol. 2014, 171, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Miura, T.; Ishida, H.; Miki, T.; Tanno, M.; Yano, T.; Sato, T.; Hotta, H.; Shimamoto, K. Limitation of infarct size by erythropoietin is associated with translocation of AKT to the mitochondria after reperfusion. Clin. Exp. Pharmacol. Physiol. 2008, 35, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.A.; Wei, L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yao, Y.Y.; Dai, Q.M.; Ma, G.S.; Zhang, S.F.; Cao, L.; Ren, L.Q.; Liu, N.F. Erythropoietin attenuates cardiac dysfunction by increasing myocardial angiogenesis and inhibiting interstitial fibrosis in diabetic rats. Cardiovasc. Diabetol. 2012, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Tsui, J.; Ho, T.K.; Selvakumar, S.; Abraham, D.J.; Baker, D.M. Review of the role of erythropoietin in critical leg ischemia. Angiology 2010, 61, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Bennis, Y.; Sarlon-Bartoli, G.; Guillet, B.; Lucas, L.; Pellegrini, L.; Velly, L.; Blot-Chabaud, M.; Dignat-Georges, F.; Sabatier, F.; Pisano, P. Priming of late endothelial progenitor cells with erythropoietin before transplantation requires the CD131 receptor subunit and enhances their angiogenic potential. J. Thromb. Haemost. 2012, 10, 1914–1928. [Google Scholar] [CrossRef] [PubMed]
- Li, H.G.; Li, J.S.; Chen, W.L.; Wang, L.; Wu, D.H.; Lin, Z.Y. Prognostic significance of erythropoietin and erythropoietin receptor in tongue squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 2009, 47, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; deMuinck, E.D.; Zhuang, Z.; Drinane, M.; Kauser, K.; Rubanyi, G.M.; Qian, H.S.; Murata, T.; Escalante, B.; Sessa, W.C. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc. Natl. Acad. Sci. USA 2005, 102, 10999–11004. [Google Scholar] [CrossRef] [PubMed]
- Kupatt, C.; Hinkel, R.; von Bruhl, M.L.; Pohl, T.; Horstkotte, J.; Raake, P.; El Aouni, C.; Thein, E.; Dimmeler, S.; Feron, O.; et al. Endothelial nitric oxide synthase overexpression provides a functionally relevant angiogenic switch in hibernating pig myocardium. J. Am. Coll. Cardiol. 2007, 49, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Amano, H.; Ito, Y.; Eshima, K.; Aoyama, N.; Tamaki, H.; Sakagami, H.; Satoh, Y.; Izumi, T.; Majima, M. Effect of erythropoietin on angiogenesis with the increased adhesion of platelets to the microvessels in the hind-limb ischemia model in mice. J. Pharmacol. Sci. 2010, 112, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Connor, K.M.; Aderman, C.M.; Smith, L.E. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Investig. 2008, 118, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.B.; May, W.S.; Caballero, S.; Brown, G.A.; Guthrie, S.M.; Mames, R.N.; Byrne, B.J.; Vaught, T.; Spoerri, P.E.; Peck, A.B.; et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat. Med. 2002, 8, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Otani, A.; Kinder, K.; Ewalt, K.; Otero, F.J.; Schimmel, P.; Friedlander, M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat. Med. 2002, 8, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.R.; Banin, E.; Moreno, S.K.; Aguilar, E.; Dorrell, M.I.; Friedlander, M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J. Clin. Investig. 2006, 116, 3266–3276. [Google Scholar] [CrossRef] [PubMed]
- Checchin, D.; Sennlaub, F.; Levavasseur, E.; Leduc, M.; Chemtob, S. Potential role of microglia in retinal blood vessel formation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Suzuma, K.; Matsui, S.; Kurimoto, M.; Kiryu, J.; Kita, M.; Suzuma, I.; Ohashi, H.; Ojima, T.; Murakami, T.; et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N. Engl. J. Med. 2005, 353, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Connor, K.M.; Aderman, C.M.; Willett, K.L.; Aspegren, O.P.; Smith, L.E. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Eldweik, L.; Mantagos, I.S. Role of VEGF inhibition in the treatment of retinopathy of prematurity. Semin. Ophthalmol. 2016, 31, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Van Wijngaarden, P.; Brereton, H.M.; Gibbins, I.L.; Coster, D.J.; Williams, K.A. Kinetics of strain-dependent differential gene expression in oxygen-induced retinopathy in the rat. Exp. Eye Res. 2007, 85, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Caprara, C.; Britschgi, C.; Samardzija, M.; Grimm, C. The erythropoietin receptor is not required for the development, function, and aging of rods and cells in the retinal periphery. Mol. Vis. 2014, 20, 307–324. [Google Scholar] [PubMed]
- Yang, Z.; Wang, H.; Jiang, Y.; Hartnett, M.E. VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. Am. J. Pathol. 2014, 184, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Filippi, L.; Bagnoli, P.; La Marca, G.; Cristofori, G.; Raffaeli, G.; Padrini, L.; Araimo, G.; Fumagalli, M.; Groppo, M.; et al. The pathophysiology of retinopathy of prematurity: An update of previous and recent knowledge. Acta Ophthalmol. 2014, 92, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Keogh, C.L.; Yu, S.P.; Wei, L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J. Cereb. Blood Flow Metab. 2006, 27, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.Y.; Ogle, M.; Wei, L. Erythropoietin prevents blood brain barrier damage induced by focal cerebral ischemia in mice. Neurochem. Res. 2007, 32, 2132–2141. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, M.; Meng, Y.; Li, H.; Yu, L.; Fu, X.; Tang, Y.; Jiang, C. Erythropoietin improves hypoxic-ischemic encephalopathy in neonatal rats after short-term anoxia by enhancing angiogenesis. Brain Res. 2016, 1651, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, Y.; Mahmood, A.; Meng, Y.; Qu, C.; Chopp, M. Erythropoietin mediates neurobehavioral recovery and neurovascular remodeling following traumatic brain injury in rats by increasing expression of vascular endothelial growth factor. Transl. Stroke Res. 2011, 2, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chopp, M.; Teng, H.; Bolz, M.; Francisco, M.A.; Aluigi, D.M.; Wang, X.L.; Zhang, R.L.; Chrsitensen, S.; Sager, T.N.; et al. Tumor necrosis factor α primes cerebral endothelial cells for erythropoietin-induced angiogenesis. J. Cereb. Blood Flow Metab. 2011, 31, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Goodison, S.; Lawton, A.; Zhang, G.; Gomes-Giacoia, E.; Rosser, C.J. Erythropoietin is a JAK2 and ERK1/2 effector that can promote renal tumor cell proliferation under hypoxic conditions. J. Hematol. Oncol. 2013, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- El Hasnaoui-Saadani, R.; Pichon, A.; Marchant, D.; Olivier, P.; Launay, T.; Quidu, P.; Beaudry, M.; Duvallet, A.; Richalet, J.P.; Favret, F. Cerebral adaptations to chronic anemia in a model of erythropoietin-deficient mice exposed to hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R801–R811. [Google Scholar] [CrossRef] [PubMed]
- Pichon, A.; Jeton, F.; El Hasnaoui-Saadani, R.; Hagstrom, L.; Launay, T.; Beaudry, M.; Marchant, D.; Quidu, P.; Macarlupu, J.L.; Favret, F.; et al. Erythropoietin and the use of a transgenic model of erythropoietin-deficient mice. Hypoxia 2016, 4, 29–39. [Google Scholar] [PubMed]
- Lee, S.T.; Chu, K.; Park, J.E.; Jung, K.H.; Jeon, D.; Lim, J.Y.; Lee, S.K.; Kim, M.; Roh, J.K. Erythropoietin improves memory function with reducing endothelial dysfunction and amyloid-β burden in Alzheimer’s disease models. J. Neurochem. 2012, 120, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, V.; Juul, S.E. Erythropoietin: Emerging role of erythropoietin in neonatal neuroprotection. Pediatr. Neurol. 2014, 51, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Fujita, Y.; Musha, T.; Tanaka, H.; Shiokawa, S.; Nakamatsu, K.; Mori, S.; Matsuo, T.; Nakamura, Y. Expression of erythropoietin in human female reproductive organs. Ital. J. Anat. Embryol. 2001, 106, 215–222. [Google Scholar] [PubMed]
- Yasuda, Y.; Fujita, Y.; Masuda, S.; Musha, T.; Ueda, K.; Tanaka, H.; Fujita, H.; Matsuo, T.; Nagao, M.; Sasaki, R.; et al. Erythropoietin is involved in growth and angiogenesis in malignant tumours of female reproductive organs. Carcinogenesis 2002, 23, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Hardee, M.E.; Cao, Y.; Fu, P.; Jiang, X.; Zhao, Y.; Rabbani, Z.N.; Vujaskovic, Z.; Dewhirst, M.W.; Arcasoy, M.O. Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS ONE 2007, 2, e549. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Fujita, Y.; Matsuo, T.; Koinuma, S.; Hara, S.; Tazaki, A.; Onozaki, M.; Hashimoto, M.; Musha, T.; Ogawa, K.; et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis 2003, 24, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Nakamatsu, K.; Nishimura, Y.; Suzuki, M.; Kanamori, S.; Maenishi, O.; Yasuda, Y. Erythropoietin/erythropoietin-receptor system as an angiogenic factor in chemically induced murine hepatic tumors. Int. J. Clin. Oncol. 2004, 9, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Ebihara, S.; Asada, M.; Yamanda, S.; Niu, K.; Arai, H. Erythropoietin promotes the growth of tumors lacking its receptor and decreases survival of tumor-bearing mice by enhancing angiogenesis. Neoplasia 2008, 10, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Rupertus, K.; Senger, S.; Menger, M.D.; Schilling, M.K.; Kollmar, O. Darbepoetin-α promotes neovascularization and cell proliferation in established colorectal liver metastases. J. Surg. Res. 2012, 176, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Nico, B.; Annese, T.; Guidolin, D.; Finato, N.; Crivellato, E.; Ribatti, D. EPO is involved in angiogenesis in human glioma. J. Neurooncol. 2011, 102, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Bohle, B.; Alonso, S.; Mayol, X.; Salvans, S.; Grande, L.; Pera, M. Preoperative administration of erythropoietin stimulates tumor recurrence after surgical excision of colon cancer in mice by a vascular endothelial growth factor-independent mechanism. J. Surg. Res. 2013, 183, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Marzullo, A.; Gentile, A.; Longo, V.; Nico, B.; Vacca, A.; Dammacco, F. Erythropoietin/erythropoietin-receptor system is involved in angiogenesis in human hepatocellular carcinoma. Histopathology 2007, 50, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Poliani, P.L.; Longo, V.; Mangieri, D.; Nico, B.; Vacca, A. Erythropoietin/erythropoietin receptor system is involved in angiogenesis in human neuroblastoma. Histopathology 2007, 50, 636–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribatti, D. Erythropoietin and tumor angiogenesis. Stem Cells Dev. 2010, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Perra, M.T.; Longo, V.; Maxia, C.; Annese, T.; Piras, F.; Murtas, D.; Sirigu, P. Erythropoietin is involved in angiogenesis in human primary melanoma. Int. J. Exp. Pathol. 2010, 91, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.G.; Xia, Z.S.; Wen, J.M.; Lv, J. Prognostic significance of erythropoietin and erythropoietin receptor in gastric adenocarcinoma. World J. Gastroenterol. 2011, 17, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.G.; Yu, T.T.; Shan, L. Expression of erythropoietin and erythropoietin receptor in non-small cell lung cancer and its correlation with microvessel density. Zhonghua Zhong Liu Za Zhi 2012, 34, 605–608. [Google Scholar] [PubMed]
- Diensthuber, M.; Potinius, M.; Rodt, T.; Stan, A.C.; Welkoborsky, H.J.; Samii, M.; Schreyogg, J.; Lenarz, T.; Stover, T. Expression of Bcl-2 is associated with microvessel density in olfactory neuroblastoma. J. Neurooncol. 2008, 89, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kase, S.; Osaki, M.; Jin, X.H.; Ohgami, K.; Yoshida, K.; Saito, W.; Takahashi, S.; Nakanishi, K.; Ito, H.; Ohno, S. Increased expression of erythropoietin receptor in human pterygial tissues. Int. J. Mol. Med. 2007, 20, 699–702. [Google Scholar] [PubMed]
- Ribatti, D.; Marzullo, A.; Nico, B.; Crivellato, E.; Ria, R.; Vacca, A. Erythropoietin as an angiogenic factor in gastric carcinoma. Histopathology 2003, 42, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Tankiewicz-Kwedlo, A.; Hermanowicz, J.; Surazynski, A.; Rozkiewicz, D.; Pryczynicz, A.; Domaniewski, T.; Pawlak, K.; Kemona, A.; Pawlak, D. Erythropoietin accelerates tumor growth through increase of erythropoietin receptor (EPOR) as well as by the stimulation of angiogenesis in DLD-1 and Ht-29 xenografts. Mol. Cell. Biochem. 2016, 421, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Jiang, Y.; Xu, M.; Lu, M.Z.; Zhou, B.; Ding, Y. Correlation of adrenomedullin with the erythropoietin receptor and microvessel density in hepatocellular carcinoma. Arch. Med. Sci. 2015, 11, 978–981. [Google Scholar] [PubMed]
- Tovari, J.; Gilly, R.; Raso, E.; Paku, S.; Bereczky, B.; Varga, N.; Vago, A.; Timar, J. Recombinant human erythropoietin α targets intratumoral blood vessels, improving chemotherapy in human xenograft models. Cancer Res. 2005, 65, 7186–7193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, A.S.; Kim, D.H.; Lee, J.E.; Jung, Y.J.; Kang, K.P.; Lee, S.; Park, S.K.; Kwak, J.Y.; Lee, S.Y.; Lim, S.T.; et al. Erythropoietin induces lymph node lymphangiogenesis and lymph node tumor metastasis. Cancer Res. 2011, 71, 4506–4517. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimáková, P.; Solár, P.; Solárová, Z.; Komel, R.; Debeljak, N. Erythropoietin and Its Angiogenic Activity. Int. J. Mol. Sci. 2017, 18, 1519. https://doi.org/10.3390/ijms18071519
Kimáková P, Solár P, Solárová Z, Komel R, Debeljak N. Erythropoietin and Its Angiogenic Activity. International Journal of Molecular Sciences. 2017; 18(7):1519. https://doi.org/10.3390/ijms18071519
Chicago/Turabian StyleKimáková, Patrícia, Peter Solár, Zuzana Solárová, Radovan Komel, and Nataša Debeljak. 2017. "Erythropoietin and Its Angiogenic Activity" International Journal of Molecular Sciences 18, no. 7: 1519. https://doi.org/10.3390/ijms18071519
APA StyleKimáková, P., Solár, P., Solárová, Z., Komel, R., & Debeljak, N. (2017). Erythropoietin and Its Angiogenic Activity. International Journal of Molecular Sciences, 18(7), 1519. https://doi.org/10.3390/ijms18071519





