miRNAs in Normal and Malignant Hematopoiesis
Abstract
:1. Introduction
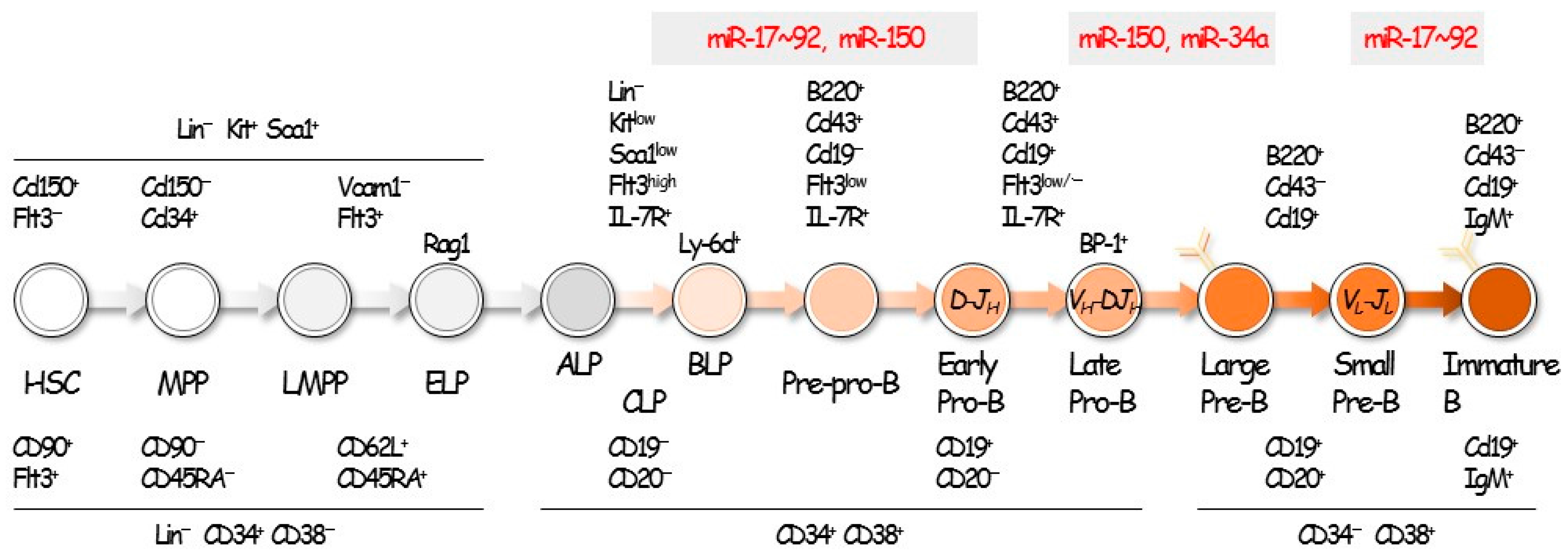
2. Hematopoiesis and B-Cell Development
3. miRNAs in B-Cell Differentiation
4. miRNAs in B Cell Malignancy
4.1. General View of miRNAs in Cancer
4.2. miR-21
4.3. miR-34a
4.4. miR-150
4.5. miR-155
4.6. miR-17‒92 Cluster
5. Application of miRNAs in Diagnosis and Therapy
6. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Mandel, E.M.; Grosschedl, R. Transcription control of early B cell differentiation. Curr. Opin. Immunol. 2010, 22, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Miyazaki, M.; Murre, C. The establishment of B versus T cell identity. Trends Immunol. 2014, 35, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, E.V. Transcriptional control of early T and B cell developmental choices. Annu. Rev. Immunol. 2014, 32, 283–321. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Ohmura, K.; Katsura, Y. Direct evidence for the commitment of hematopoietic stem cells to T, B and myeloid lineages in murine fetal liver. Int. Immunol. 1997, 9, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Ohmura, K.; Fujimoto, S.; Katsura, Y. Emergence of T cell progenitors without B cell or myeloid differentiation potential at the earliest stage of hematopoiesis in the murine fetal liver. J. Immunol. 1999, 162, 2725–2731. [Google Scholar] [PubMed]
- Kawamoto, H.; Ikawa, T.; Ohmura, K.; Fujimoto, S.; Katsura, Y. T cell progenitors emerge earlier than B cell progenitors in the murine fetal liver. Immunity 2000, 12, 441–450. [Google Scholar] [CrossRef]
- Kawamoto, H.; Ikawa, T.; Masuda, K.; Wada, H.; Katsura, Y. A map for lineage restriction of progenitors during hematopoiesis: The essence of the myeloid-based model. Immunol. Rev. 2010, 238, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, J.; Månsson, R.; Buza-Vidas, N.; Hultquist, A.; Liuba, K.; Jensen, C.T.; Bryder, D.; Yang, L.; Borge, O.J.; Thoren, L.A.; et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 2005, 121, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, E.C.; Serwold, T.; Kogan, S.; Weissman, I.L.; Passegué, E. New evidence supporting megakaryocyte-erythrocyte potential of Flk2/Flt3+ multipotent hematopoietic progenitors. Cell 2006, 126, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.W.; Chavez, A.; Xu, L.; Weber, B.N.; Shestova, O.; Schaffer, A.; Wertheim, G.; Pear, W.S.; Izon, D.; Bhandoola, A. Identification of Flt3⁺CD150− myeloid progenitors in adult mouse bone marrow that harbor t lymphoid developmental potential. Blood 2011, 118, 2723–2732. [Google Scholar] [CrossRef] [PubMed]
- Luc, S.; Luis, T.C.; Boukarabila, H.; Macaulay, I.C.; Buza-Vidas, N.; Bouriez-Jones, T.; Lutteropp, M.; Woll, P.S.; Loughran, S.J.; Mead, A.J.; et al. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nat. Immunol. 2012, 13, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, T.; Kawamoto, H.; Wright, L.Y.; Murre, C. Long-term cultured E2A-deficient hematopoietic progenitor cells are pluripotent. Immunity 2004, 20, 349–360. [Google Scholar] [CrossRef]
- Pongubala, J.M.; Northrup, D.L.; Lancki, D.W.; Medina, K.L.; Treiber, T.; Bertolino, E.; Thomas, M.; Grosschedl, R.; Allman, D.; Singh, H. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat. Immunol. 2008, 9, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Györy, I.; Boller, S.; Nechanitzky, R.; Mandel, E.; Pott, S.; Liu, E.; Grosschedl, R. Transcription factor Ebf1 regulates differentiation stage-specific signaling, proliferation, and survival of B cells. Genes Dev. 2012, 26, 668–682. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Jhunjhunwala, S.; Benner, C.; Heinz, S.; Welinder, E.; Mansson, R.; Sigvardsson, M.; Hagman, J.; Espinoza, C.A.; Dutkowski, J.; et al. A global network of transcription factors, involving E2A, Ebf1 and FOXO1, that orchestrates B cell fate. Nat. Immunol. 2010, 11, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Bagga, S.; Bracht, J.; Hunter, S.; Massirer, K.; Holtz, J.; Eachus, R.; Pasquinelli, A.E. Regulation by let-7 and lin-4 miRNAs results in target mrna degradation. Cell 2005, 122, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Yekta, S.; Shih, I.; Bartel, D. MicroRNA-directed cleavage of hoxb8 mRNA. Science 2004, 304, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Kotani, A.; Ha, D.; Hsieh, J.; Rao, P.K.; Schotte, D.; den Boer, M.L.; Armstrong, S.A.; Lodish, H.F. miR-128b is a potent glucocorticoid sensitizer in MLL-AF4 acute lymphocytic leukemia cells and exerts cooperative effects with miR-221. Blood 2009, 114, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, D.; Mecklenbrauker, I.; Das, P.; Santana, A.; Koenig, U.; Enright, A.; Miska, E.; Tarakhovsky, A. A slicer-independent role for argonaute 2 in hematopoiesis and the microrna pathway. Genes Dev. 2007, 21, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Koralov, S.; Muljo, S.; Galler, G.; Krek, A.; Chakraborty, T.; Kanellopoulou, C.; Jensen, K.; Cobb, B.; Merkenschlager, M.; Rajewsky, N.; et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 2008, 132, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Ademokun, A.; Turner, M. Regulation of B-cell differentiation by microRNAs and RNA-binding proteins. Biochem. Soc. Trans. 2008, 36, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.S.; O’Connell, R.M.; Chaudhuri, A.A.; Garcia-Flores, Y.; Geiger, T.L.; Baltimore, D. MicroRNA-34a perturbs b lymphocyte development by repressing the forkhead box transcription factor FOXP1. Immunity 2010, 33, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, M.; Haga, C.L.; Das, S.; Leu, C.M.; Hodson, D.; Josson, S.; Turner, M.; Cooper, M.D. MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int. Immunol. 2010, 22, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.A.; So, A.Y.; Mehta, A.; Minisandram, A.; Sinha, N.; Jonsson, V.D.; Rao, D.S.; O’Connell, R.M.; Baltimore, D. Oncomir miR-125b regulates hematopoiesis by targeting the gene lin28a. Proc. Natl. Acad. Sci. USA 2012, 109, 4233–4238. [Google Scholar] [CrossRef] [PubMed]
- Puissegur, M.P.; Eichner, R.; Quelen, C.; Coyaud, E.; Mari, B.; Lebrigand, K.; Broccardo, C.; Nguyen-Khac, F.; Bousquet, M.; Brousset, P. B-cell regulator of immunoglobulin heavy-chain transcription (bright)/arid3a is a direct target of the oncomir microRNA-125b in progenitor B-cells. Leukemia 2012, 26, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Calado, D.; Galler, G.; Thai, T.; Patterson, H.; Wang, J.; Rajewsky, N.; Bender, T.; Rajewsky, K. miR-150 controls B cell differentiation by targeting the transcription factor c-myb. Cell 2007, 131, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Mann, M.; Zhao, J.L.; Marinov, G.K.; Majumdar, D.; Garcia-Flores, Y.; Du, X.; Erikci, E.; Chowdhury, K.; Baltimore, D. The microRNA-212/132 cluster regulates B cell development by targeting SOX4. J. Exp. Med. 2015, 212, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.P.; Wang, M.; Robertus, J.L.; Schakel, R.N.; Gibcus, J.H.; Diepstra, A.; Harms, G.; Peh, S.C.; Reijmers, R.M.; Pals, S.T.; et al. Mirna profiling of B-cell subsets: Specific miRNA profile for germinal center B cells with variation between centroblasts and centrocytes. Lab. Investig. 2009, 89, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jima, D.; Jacobs, C.; Fischer, R.; Gottwein, E.; Huang, G.; Lugar, P.; Lagoo, A.; Rizzieri, D.; Friedman, D.; et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood 2009, 113, 4586–4594. [Google Scholar] [CrossRef] [PubMed]
- Malpeli, G.; Barbi, S.; Zupo, S.; Tosadori, G.; Scardoni, G.; Bertolaso, A.; Sartoris, S.; Ugel, S.; Vicentini, C.; Fassan, M.; et al. Identification of microRNAs implicated in the late differentiation stages of normal B cells suggests a central role for miRNA targets zeb1 and tp53. Oncotarget 2017, 8, 11809–11826. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Grosshans, H.; Shingara, J.; Byrom, M.; Jarvis, R.; Cheng, A.; Labourier, E.; Reinert, K.L.; Brown, D.; Slack, F.J. Ras is regulated by the let-7 microrna family. Cell 2005, 120, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, J.; Konishi, H.; Yanagisawa, K.; Tomida, S.; Osada, H.; Endoh, H.; Harano, T.; Yatabe, Y.; Nagino, M.; Nimura, Y.; et al. Reduced expression of the let-7 micrornas in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004, 64, 3753–3756. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.M.; Hiebert, S.W.; Eischen, C.M. Myc induces miRNA-mediated apoptosis in response to HDAC inhibition in hematologic malignancies. Cancer Res. 2016, 76, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Schotte, D.; De Menezes, R.X.; Akbari Moqadam, F.; Khankahdani, L.M.; Lange-Turenhout, E.; Chen, C.; Pieters, R.; Den Boer, M.L. Microrna characterize genetic diversity and drug resistance in pediatric acute lymphoblastic leukemia. Haematologica 2011, 96, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting zeb1 and sip1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors zeb1 and zeb2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Kuo, S.H.; Cheng, A.L.; Lin, C.W. Inhibition of zeb1 by miR-200 characterizes helicobacter pylori-positive gastric diffuse large B-cell lymphoma with a less aggressive behavior. Mod. Pathol. 2014, 27, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Wilda, M.; Busch, K.; Viehmann, S.; Borkhardt, A. High expression of precursor microRNA-155/bic RNA in children with burkitt lymphoma. Genes Chromosomes Cancer 2004, 39, 167–169. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Thomson, J.; Hemann, M.; Hernando-Monge, E.; Mu, D.; Goodson, S.; Powers, S.; Cordon-Cardo, C.; Lowe, S.; Hannon, G.; et al. A microRNA polycistron as a potential human oncogene. Nature 2005, 435, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Costinean, S.; Zanesi, N.; Pekarsky, Y.; Tili, E.; Volinia, S.; Heerema, N.; Croce, C.M. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in Eμ-miR155 transgenic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. miRNAs and cancer: An epigenetics view. Mol. Asp. Med. 2013, 34, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.; Soneji, S.; Marafioti, T.; Cooper, C.; Palazzo, S.; Paterson, J.; Cattan, H.; Enver, T.; Mager, R.; Boultwood, J.; et al. MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int. J. Cancer 2007, 121, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Shimizu, M.; Barbarotto, E.; Nicoloso, M.S.; Dimitri, F.; Sampath, D.; Fabbri, M.; Lerner, S.; Barron, L.L.; Rassenti, L.Z.; et al. MicroRNA fingerprinting of CLL patients with chromosome 17p deletion identify a miR-21 score that stratifies early survival. Blood 2010, 116, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Rosato, P.; Anastasiadou, E.; Garg, N.; Lenze, D.; Boccellato, F.; Vincenti, S.; Severa, M.; Coccia, E.M.; Bigi, R.; Cirone, M.; et al. Differential regulation of miR-21 and miR-146a by epstein-barr virus-encoded EBNA2. Leukemia 2012, 26, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wei, J.; Deng, C.; Yang, X.; Wang, C.; Xu, R. MicroRNA-21 regulates the sensitivity of diffuse large B-cell lymphoma cells to the CHOP chemotherapy regimen. Int. J. Hematol. 2013, 97, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Medina, P.P.; Nolde, M.; Slack, F.J. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature 2010, 467, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Shi, Y.; Tan, G.; Yang, C.H.; Fan, M.; Pfeffer, L.M.; Wu, Z.H. DNA damage induces NF-κB-dependent microRNA-21 up-regulation and promotes breast cancer cell invasion. J. Biol. Chem. 2012, 287, 21783–21795. [Google Scholar] [CrossRef] [PubMed]
- Koti, M.; Gooding, R.J.; Nuin, P.; Haslehurst, A.; Crane, C.; Weberpals, J.; Childs, T.; Bryson, P.; Dharsee, M.; Evans, K.; et al. Identification of the IGF1/PI3K/NF κB/ERK gene signalling networks associated with chemotherapy resistance and treatment response in high-grade serous epithelial ovarian cancer. BMC Cancer 2013, 13, 549. [Google Scholar] [CrossRef] [PubMed]
- Boysen, J.; Sinha, S.; Price-Troska, T.; Warner, S.L.; Bearss, D.J.; Viswanatha, D.; Shanafelt, T.D.; Kay, N.E.; Ghosh, A.K. The tumor suppressor axis p53/miR-34a regulates axl expression in B-cell chronic lymphocytic leukemia: Implications for therapy in p53-defective CLL patients. Leukemia 2014, 28, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Cogliatti, S.B.; Imig, J.; Renner, C.; Neuenschwander, S.; Rehrauer, H.; Schlapbach, R.; Dirnhofer, S.; Tzankov, A.; Müller, A. Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FOXP1. Blood 2011, 117, 6227–6236. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Z.; Dai, X.; Pan, J.; Ge, J.; Han, X.; Wu, Z.; Zhou, X.; Zhao, T. Re-expression of microRNA-150 induces EBV-positive burkitt lymphoma differentiation by modulating c-myb in vitro. Cancer Sci. 2013, 104, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Mraz, M.; Chen, L.; Rassenti, L.Z.; Ghia, E.M.; Li, H.; Jepsen, K.; Smith, E.N.; Messer, K.; Frazer, K.A.; Kipps, T.J. miR-150 influences B-cell receptor signaling in chronic lymphocytic leukemia by regulating expression of GAB1 and FOXP1. Blood 2014, 124, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Tano, N.; Kim, H.W.; Ashraf, M. MicroRNA-150 regulates mobilization and migration of bone marrow-derived mononuclear cells by targeting CXCR4. PLoS ONE 2011, 6, e23114. [Google Scholar] [CrossRef] [PubMed]
- Eis, P.; Tam, W.; Sun, L.; Chadburn, A.; Li, Z.; Gomez, M.; Lund, E.; Dahlberg, J. Accumulation of miR-155 and bic RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. USA 2005, 102, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Kluiver, J.; Poppema, S.; de Jong, D.; Blokzijl, T.; Harms, G.; Jacobs, S.; Kroesen, B.; van den Berg, A. Bic and miR-155 are highly expressed in hodgkin, primary mediastinal and diffuse large B cell lymphomas. J. Pathol. 2005, 207, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.; Dahlberg, J. miR-155/bic as an oncogenic microRNA. Genes Chromosomes Cancer 2006, 45, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.; Harris, A.; Martelli, F.; Kulshreshtha, R. Hypoxia response and micrornas: No longer two separate worlds. J. Cell. Mol. Med. 2008, 12, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, L.; Dijkstra, M.; van Lom, K.; Robertus, J.; Harms, G.; Blokzijl, T.; Kooistra, K.; van T’veer, M.; Rosati, S.; et al. miRNA analysis in B-cell chronic lymphocytic leukaemia: Proliferation centres characterized by low miR-150 and high bic/miR-155 expression. J. Pathol. 2008, 215, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, Q.; He, L. Role of TLR4 and miR-155 in peripheral blood mononuclear cell-mediated inflammatory reaction in coronary slow flow and coronary arteriosclerosis patients. J. Clin. Lab. Anal. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Liu, F.; Ding, L.; You, M.; Yue, H.; Zhou, Y.; Hou, Y. Anti-inflammatory effects of curcumin are associated with down regulating microRNA-155 in LPS-treated macrophages and mice. Pharm. Biol. 2017, 55, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Louafi, F.; Martinez-Nunez, R.T.; Sanchez-Elsner, T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-β. J. Biol. Chem. 2010, 285, 41328–41336. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, Y.; Jiang, F.; Wang, X.; Zhang, J.; Shen, J.; Yang, X. Inhibition of transforming growth factor β/SMAD signal by miR-155 is involved in arsenic trioxide-induced anti-angiogenesis in prostate cancer. Cancer Sci. 2014, 105, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Cazac, B.B.; Roes, J. TGF-β receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 2000, 13, 443–451. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, Y.; Li, B.; Luo, X.; Tang, Y. miR-155 regulates high glucose-induced cardiac fibrosis via the TGF-β signaling pathway. Mol. Biosyst. 2016, 13, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Dagan, L.N.; Jiang, X.; Bhatt, S.; Cubedo, E.; Rajewsky, K.; Lossos, I.S. miR-155 regulates HGAL expression and increases lymphoma cell motility. Blood 2012, 119, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Shen, Y.; Huang, X.; Liu, Y.; Wake, L.; Liu, C.; Deffenbacher, K.; Lachel, C.M.; Wang, C.; Rohr, J.; et al. Global microRNA expression profiling uncovers molecular markers for classification and prognosis in aggressive B-cell lymphoma. Blood 2015, 125, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, L.E. Knockdown of LMP1-induced miR-155 sensitizes nasopharyngeal carcinoma cells to radiotherapy in vitro. Oncol. Lett. 2016, 11, 3451–3456. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Srinivasan, L.; Calado, D.; Patterson, H.; Zhang, B.; Wang, J.; Henderson, J.; Kutok, J.; Rajewsky, K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17–92 expression in lymphocytes. Nat. Immunol. 2008, 9, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.; Thomson, J.; Hammond, S. Direct regulation of an oncogenic micro-rna cluster by E2F transcription factors. J. Biol. Chem. 2007, 282, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Olive, V.; Sabio, E.; Bennett, M.J.; De Jong, C.S.; Biton, A.; McGann, J.C.; Greaney, S.K.; Sodir, N.M.; Zhou, A.Y.; Balakrishnan, A.; et al. A component of the miR-17–92 polycistronic oncomir promotes oncogene-dependent apoptosis. Elife 2013, 2, e00822. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, W.; Xie, C.; Huang, C.; Zhu, J.; Liang, Z.; Deng, F.; Zhu, M.; Zhu, W.; Wu, R.; et al. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol a-induced MCF-7 breast cancer cell proliferation. Phytother. Res. 2014, 28, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Karanti, S.; Jung, I.; Dahia, P.; Aguiar, R. Coordinated expression of microRNA-155 and predicted target genes in diffuse large B-cell lymphoma. Cancer Genet. Cytogenet. 2008, 181, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Song, G.; Chen, L.; Nie, Z.; He, B.; Pan, Y.; Xu, Y.; Li, R.; Gao, T.; Cho, W.C.; et al. Inhibition of miR-21 induces biological and behavioral alterations in diffuse large B-cell lymphoma. Acta. Haematol. 2013, 130, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Jang, J.Y.; Kim, P.J.; Kim, Y.G.; Nam, S.J.; Paik, J.H.; Kim, T.M.; Heo, D.S.; Kim, C.W.; Jeon, Y.K. MicroRNA-21 plays an oncogenic role by targeting FOXO1 and activating the PI3K/AKT pathway in diffuse large B-cell lymphoma. Oncotarget 2015, 6, 15035–15049. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, R.; Yang, L.; Tu, W. miR-21 expression predicts prognosis in diffuse large B-cell lymphoma. Int. J. Clin. Exp. Pathol. 2015, 8, 15019–15024. [Google Scholar] [PubMed]
- Caivano, A.; Laurenzana, I.; De Luca, L.; La Rocca, F.; Simeon, V.; Trino, S.; D’Auria, F.; Traficante, A.; Maietti, M.; Izzo, T.; et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumour Biol. 2015, 36, 9739–9752. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, S.; Sekizuka, T.; Kataoka, M.; Hasegawa, H.; Hamada, H.; Kuroda, M.; Katano, H. Profile of exosomal and intracellular microrna in γ-herpesvirus-infected lymphoma cell lines. PLoS ONE 2016, 11, e0162574. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Lotvall, J.; Valadi, H. Cell to cell signalling via exosomes through esRNA. Cell Adhes. Migr. 2007, 1, 156–158. [Google Scholar] [CrossRef]
- Lanford, R.E.; Hildebrandt-Eriksen, E.S.; Petri, A.; Persson, R.; Lindow, M.; Munk, M.E.; Kauppinen, S.; Ørum, H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 2010, 327, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Bahal, R.; Babar, I.A.; Pincus, Z.; Barrera, F.; Liu, C.; Svoronos, A.; Braddock, D.T.; Glazer, P.M.; Engelman, D.M.; et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015, 518, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G. miR-34—A microRNA replacement therapy is headed to the clinic. Front. Genet. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotaki, R.; Koyama-Nasu, R.; Yamakawa, N.; Kotani, A. miRNAs in Normal and Malignant Hematopoiesis. Int. J. Mol. Sci. 2017, 18, 1495. https://doi.org/10.3390/ijms18071495
Kotaki R, Koyama-Nasu R, Yamakawa N, Kotani A. miRNAs in Normal and Malignant Hematopoiesis. International Journal of Molecular Sciences. 2017; 18(7):1495. https://doi.org/10.3390/ijms18071495
Chicago/Turabian StyleKotaki, Ryutaro, Ryo Koyama-Nasu, Natsuko Yamakawa, and Ai Kotani. 2017. "miRNAs in Normal and Malignant Hematopoiesis" International Journal of Molecular Sciences 18, no. 7: 1495. https://doi.org/10.3390/ijms18071495
APA StyleKotaki, R., Koyama-Nasu, R., Yamakawa, N., & Kotani, A. (2017). miRNAs in Normal and Malignant Hematopoiesis. International Journal of Molecular Sciences, 18(7), 1495. https://doi.org/10.3390/ijms18071495



