Abstract
Oncolytic virotherapy has recently emerged as a promising strategy for inducing tumor-specific cell death. Adenoviruses are widely and frequently used in oncolytic virotherapy. The mechanism of oncolytic adenovirus-mediated tumor suppression involves virus-induced activation of the autophagic machinery in tumor cells. Autophagy is a cytoprotective process that produces energy via lysosomal degradation of intracellular components as a physiologic response to various stresses, including hypoxia, nutrient deprivation, and disruption of growth signaling. However, infection with oncolytic adenoviruses induces autophagy and subsequent death of tumor cells rather than enhancing their survival. In this review, we summarize the beneficial role of autophagy in oncolytic adenoviral therapy, including the roles of infection, replication, and cell lysis. Numerous factors are involved in the promotion and inhibition of oncolytic adenovirus-mediated autophagy. Furthermore, recent evidence has shown that oncolytic adenoviruses induce autophagy-related immunogenic cell death (ICD), which enhances the antitumor immune response by inducing the activation of danger signal molecules and thus represents a novel cancer immunotherapy. Understanding the precise role of oncolytic adenovirus-induced autophagy and ICD could enhance the therapeutic potential of oncolytic adenoviral therapy for treating various cancers.
1. Introduction
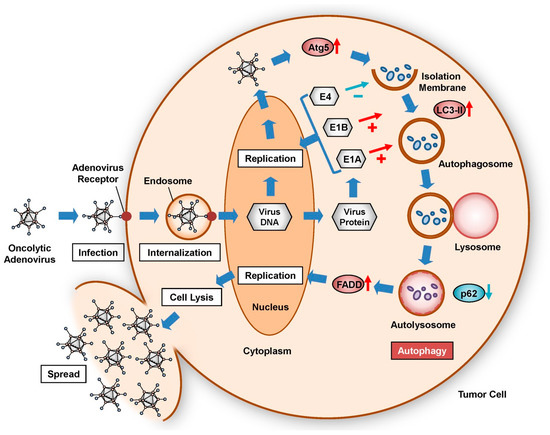
Oncolytic virotherapy has emerged as a novel antitumor strategy for inducing the lytic death of tumor cells as a result of rapid viral replication [1]. Although a number of different viruses are used in virotherapy, including adenoviruses, herpes simplex virus, measles virus, reovirus, and Newcastle disease virus, adenovirus serotype 5 (Ad5) is one of the most commonly used in oncolytic virotherapy [1]. Oncolytic adenoviruses are internalized by target cells via binding to the Coxsackie and adenovirus receptor (CAR) on the cell surface. As both normal and tumor cells express CAR within tumor-affected tissues, oncolytic adenoviruses are biogenetically modified to exhibit tumor-specific replication capability. Virus replication is regulated primarily by the induction of viral gene expression within the target cells. In adenovirus replication, induction of the early E1 gene is the most critical factor, as E1-deletion adenoviruses cannot replicate. Therefore, the wild-type E1 promoter has been biogenetically modified using several different tumor-specific promoters, including the human telomerase reverse transcriptase (hTERT) [2,3,4,5,6], midkine [7,8], cyclooxygenase-2 [9], and survivin [10] promoters. As hTERT is reportedly overexpressed in >80% of malignant tumor tissues [11], the hTERT promoter is a useful tool for tumor-specific regulation of viral gene expression [12]. Tumor-specific, promoter-regulated oncolytic adenoviruses are therefore ideal vectors for inducing tumor cell death through viral replication.
The mechanism of oncolytic adenovirus-mediated tumor suppression involves virus-induced activation of the autophagic machinery in tumor cells [13]. Autophagy produces energy through lysosomal degradation of cytoplasmic cellular components in autophagosomes [14]. Several physiologic conditions, including nutrient deprivation [15], hypoxia [16], and disruption of growth signaling [17], activate the autophagic machinery to enhance cell survival. However, some pathogenic viruses and bacteria also induce autophagy in infected cells [18,19]. Virus-induced autophagy plays both anti-viral defense and pro-viral replication roles in infected cells [18]. Several oncolytic adenoviruses are known to induce autophagy; in tumor cells, this process leads to cell death rather than survival [10,20,21]. For example, we generated a telomerase-specific, replication-competent oncolytic adenovirus, OBP-301, which drives the adenoviral E1A and E1B genes under control of the hTERT promoter for tumor-specific viral replication, and we found that OBP-301 induces lytic death of tumor cells with telomerase activity [6]. OBP-301-mediated autophagy induction is strongly associated with decreased viability of tumor cells [22,23]. Thus, induction of autophagy plays a crucial role in oncolytic adenovirus-mediated tumor suppression.
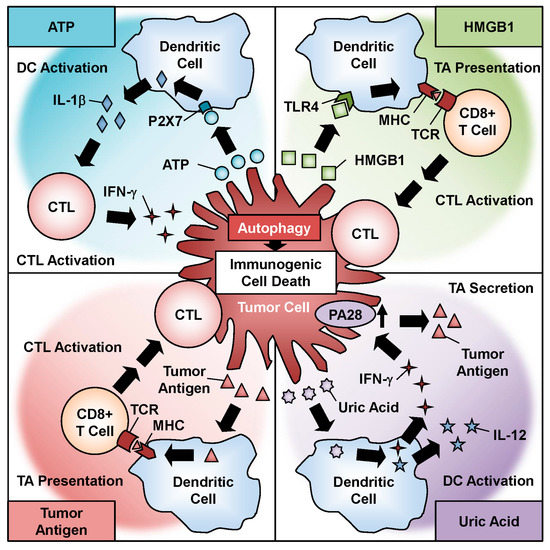
Recent evidence suggests that antitumor therapy-induced autophagy is associated with immunogenic cell death (ICD), which involves the induction of antitumor immune responses via the release of damage-associated molecular pattern (DAMP) molecules and tumor-associated antigens (TAAs) [24]. DAMP molecules include adenosine triphosphate (ATP), high-mobility group B1 (HMGB1), calreticulin (CRT), and uric acid [25]. A variety of ICD inducers are used in antitumor therapy, including chemotherapeutic agents, irradiation, and photodynamic treatment [26]. Oncolytic adenoviral therapy is also hypothesized to induce ICD via the induction of autophagy and the activation of DAMP molecules and TAAs [26,27]. Moreover, oncolytic virotherapy induces the release of pathogen-associated molecular pattern molecules, which function as another type of danger signal [27,28,29]. Therefore, oncolytic adenovirus-induced autophagy could function as both an antitumor effector and antitumor immune stimulator in oncolytic virotherapy.
In this review, we summarize the beneficial role of autophagy in oncolytic adenovirus-mediated tumor suppression processes, including virus infection, replication, and cell lysis. Factors that modulate autophagy are also described in the context of oncolytic adenoviral therapy. Moreover, the preclinical and clinical relevance of autophagy-mediated ICD in oncolytic adenoviral therapy are discussed in terms of antitumor immunotherapy.
3. Factors that Promote or Inhibit Oncolytic Adenovirus-Mediated Autophagy
A variety of factors can promote autophagy during the process of oncolytic adenoviral therapy, including infection, replication, and cell lysis (Figure 2). The efficacy of oncolytic adenoviruses in infecting target cells is one of the most important issues to consider for increasing the intracellular level of autophagy. Infection with adenoviruses depends primarily on the level of CAR expression on the surface of target cells. Upregulation of CAR expression enhances the infectivity of oncolytic adenoviruses, leading to extensive autophagy. For example, ionizing radiation increases the expression of CAR on the surface of tumor cells [39]. In addition, several histone deacetylase inhibitors, such as trichostatin A, sodium phenylbutyrate, FK228, and FR901228, increase the expression of CAR [40,41,42]. By contrast, modification of the fiber knob improves the infectivity of oncolytic adenoviruses independent of CAR expression. For example, incorporation of RGD peptide enhances binding to integrins αVβ3 and αVβ5. The RGD fiber-modified hTERT-driven OBP-301 variant (OBP-405) induces more profound autophagic death of malignant brain tumor cells than OBP-301, which encodes the wild-type fiber [43]. Fiber modification with the polylysine PK7 motif increases the affinity for heparin sulfate proteoglycans. PK7 fiber-modified survivin promoter-driven CRAd-S-pk7 induces autophagic death of malignant brain tumor cells in combination with temzolomide (TMZ) [44]. Moreover, a chimeric fiber knob composed of adenovirus serotype 3 (Ad3) and Ad5 fibers binds to CD46 on the cell surface. Ad5/3 fiber-modified human chorionic gonadotropin (hCG)-expressing Ad5/3∆24hCG, which lacks a 24-bp segment (919-943) in the E1A region, induces autophagic death of human cancer cells [45]. Enhanced infectivity contributes to the high uptake of oncolytic adenoviruses and induction of virus-mediated autophagy in tumor cells.
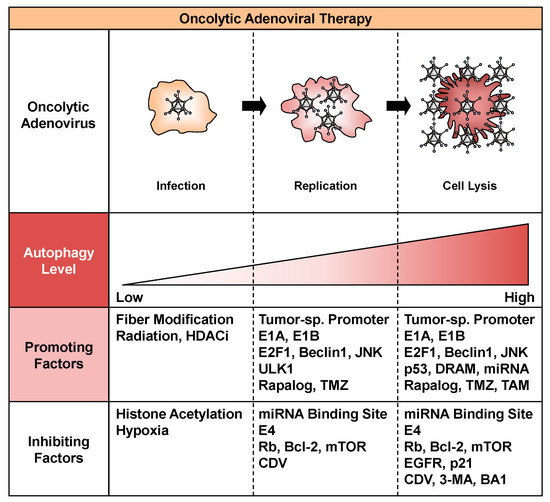
Figure 2.
Factors that promote or inhibit oncolytic adenovirus-mediated autophagy. Factors that promote or inhibit oncolytic adenovirus-mediated autophagy are shown. HDACi, histone deacetylase inhibitor; JNK, c-Jun N-terminal kinase; ULK1, unc-51-like autophagy-activating kinase 1; TMZ, temzolomide; miRNA, microRNA; mTOR, mammalian target of rapamycin; CDV, cidofovir; DRAM, damage-regulated autophagy modulator; TAM, tamoxifen; EGFR, epidermal growth factor receptor; 3-MA, 3-methyladenine; BA1, bafilomycin A1.
The replication of oncolytic adenoviruses also affects the intracellular level of autophagy. Modification of the wild-type E1 promoter is a promising strategy for improving the level of viral replication because the expression level of E1 is crucial for the replication of oncolytic adenoviruses. The tumor-specific promoter enhances the viral replication level only in tumor cells, without affecting normal cells. The hTERT promoter is one of the most useful tools for inducing tumor-specific cell death. For example, a conditionally replicating oncolytic adenovirus, hTERT-Ad, in which a 255-bp hTERT promoter fragment is inserted into the wild-type E1A promoter region, induces the autophagic death of malignant brain tumor cells [20]. OBP-301, which contains a 455-bp hTERT promoter, induces the autophagic death of a variety of tumor cells with telomerase activities [22,23]. Moreover, the hTERT promoter-driven oncolytic adenovirus OBP-301 can replicate more efficiently than wild-type Ad5, even in hypoxic tumor microenvironments [46].
The adenoviral proteins E1A and E1B play roles in the induction of pro-autophagic signaling pathways. Adenoviral E1A binds to the tumor suppressor Rb, resulting in release of the transcription factor E2F1 from the Rb-E2F1 complex [47]. E2F1 activation induces autophagy via the upregulation of autophagy-related proteins, including Atg5 and LC3, in transactivation-dependent and -independent manners [48,49]. By contrast, adenoviral E1B interacts with pro-autophagic Beclin1, resulting in dissociation of the Beclin1-Bcl-2 complex and the induction of Beclin1-dependent autophagy [50]. During replication of the oncolytic adenovirus Delta-24-RGD, activation of the c-Jun N-terminal kinase pathway is also involved in suppression of Beclin1-Bcl-2 complex formation via the phosphorylation of Bcl-2 [51]. Moreover, it has been shown that suppression of the mTOR signaling pathway by rapalogs such as rapamycin and everolimus enhances autophagic cell death in oncolytic adenoviral therapy [43,52]. TMZ has been shown to enhance oncolytic adenovirus-induced autophagic death of malignant tumor cells [44,53,54]. Tamoxifen enhances the antitumor effect of survivin-driven CRAd-S-5/3 in primary malignant brain tumor cells [55]. In addition, adenovirus infection has been shown to induce accumulation of the sphingolipid metabolite ceramide, which is associated with autophagy-related cell death [56,57]. As sphingolipid metabolism is proposed to be a key regulator in autophagy induction [58], ceramide-inducing reagents, such as anti-folate pemetrexed and the sphingosine-1-phosphate receptor modulator FTY720, may enhance oncolytic adenovirus-mediated autophagy [57]. Adenoviral E1 and autophagy-inducing reagents efficiently activate the autophagic machinery in oncolytic virotherapy.
The activation of therapeutic transgenes is another useful strategy for enhancing the antitumor effect of oncolytic adenoviruses through the induction of autophagy. Although there are many types of therapeutic transgenes, those that specifically induce autophagy are the best candidates for enhancing antitumor effects in oncolytic adenoviral therapy. For example, activation of pro-autophagic Beclin-1 expression by Beclin-1-armed oncolytic adenoviruses enhances the autophagic death of malignant tumor cells [59]. In addition, the tumor suppressor gene p53 is a multifunctional transcription factor that regulates diverse cellular processes, including autophagy, for tumor suppression [60]. We generated a hTERT promoter-driven OBP-301 variant (OBP-702) that expresses p53 [61,62]. OBP-702 exhibited a more profound autophagy-associated antitumor effect than OBP-301 [63]. OBP-702 induced significant autophagy by inducing expression of the pro-autophagic protein damage-regulated autophagy modulator and suppressing expression of the anti-autophagic factor p21 [63]. We demonstrated the involvement of E2F1-regulated microRNAs (miRNAs) in oncolytic adenovirus-induced autophagic cell death [23,63]. E2F1-mediated activation of miR-7 is involved in OBP-301-mediated autophagic death of human lung cancer cells through suppression of the anti-autophagic factor, epidermal growth factor receptor [23]. E2F1-mediated activation of miR-93 and miR-106 suppresses the expression of anti-autophagic p21 in OBP-702-mediated autophagy in human osteosarcoma cells [61,63]. These findings suggest that p53 and E2F1-regulated miRNAs are crucial factors that must be considered for fine-tuning oncolytic adenovirus-induced autophagy.
A number of factors can also inhibit autophagy by suppressing the infection, replication, or autophagy-inducing activity of oncolytic adenoviruses (Figure 2). Low infectivity of oncolytic adenoviruses results in poor induction of autophagy. CAR-negative tumor cells are highly resistant to oncolytic adenovirus-mediated antitumor effects. Downregulation of CAR expression by histone acetylation in the CAR gene promoter attenuates the infectivity of oncolytic adenoviruses for tumor cells [64]. The presence of a hypoxic tumor microenvironment also suppresses the expression of CAR, resulting in low infectivity [65]. In addition, a low rate of oncolytic adenovirus replication can suppress the induction of autophagy. The insertion of a miRNA binding site was recently reported as a means of suppressing the replication and cytotoxic effects of oncolytic adenoviruses in normal tissue. For example, insertion of the miR-122 binding site into the 3’-untranslated region of the gene encoding E1 effectively attenuates the replication of oncolytic adenoviruses in liver tissue, in which miR-122 is highly expressed [66,67,68]. This strategy is very useful for circumventing the hepatotoxicity of oncolytic adenoviruses. By contrast, we previously reported that cidofovir, an antiviral compound approved for the clinical treatment of adenovirus infection [69], inhibits the antitumor effect of hTERT-driven OBP-301 by suppressing viral replication [70]. Adenoviral E4 suppresses autophagy through activation of the mTOR signaling pathway [31] and subsequent inhibition of ULK1 activity [32] during viral replication. Oncolytic adenovirus-mediated autophagy can also be inhibited by directly suppressing the autophagic machinery. For example, 3-methyladenine, an inhibitor of pro-autophagic phosphatidylinositol 3-kinase class III, inhibits the induction of autophagy associated with oncolytic adenoviruses through suppression of both the autophagy signaling pathway and viral replication [30]. Bafilomycin A1, an inhibitor of lysosomal function, inhibits the induction of autophagy and antitumor effects of oncolytic adenoviruses by preventing fusion of the autophagosome and lysosome [37]. Thus, the life cycle of oncolytic adenoviruses is a key target that can be exploited to suppress oncolytic adenovirus-mediated autophagy.
5. Conclusions
Oncolytic adenoviral therapy is a promising strategy for inducing tumor-specific cell death via the activation of autophagy. Recent reports have demonstrated the beneficial role of autophagy in oncolytic adenoviral therapy. Persistent and extensive autophagy induced by oncolytic adenoviruses plays a crucial role in the death of tumor cells. Stimulation of CAR expression, fiber modification, insertion of tumor-specific promoters, induction of therapeutic transgenes, and the use of autophagy-inducing reagents are useful strategies for inducing extensive autophagy through enhancement of viral infection, replication, and cell lysis. However, the precise roles of oncolytic adenovirus-induced autophagy and ICD in antitumor immunity remain to be elucidated. Therefore, exploring the functional role of oncolytic adenovirus-induced autophagy and ICD could improve the therapeutic potential of oncolytic adenoviral anticancer therapy.
Acknowledgments
The writing of this review was supported in part by grants from the Ministry of Health, Labor, and Welfare of Japan and from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Ad5 | Adenovirus serotype 5 |
| CAR | Coxsackie and adenovirus receptor |
| hTERT | Human telomerase reverse transcriptase |
| ICD | Immunogenic cell death |
| DAMP | Damage-associated molecular pattern |
| TAA | Tumor-associated antigen |
| ATP | Adenosine triphosphate |
| HMGB1 | High-mobility group protein B1 |
| CRT | Calreticulin |
| mTOR | Mammalian target of rapamycin |
| ULK1 | unc-51-like kinase 1 |
| Atg5 | Autophagy-related 5 |
| LC3 | Microtubule-associated protein 1 light chain 3 |
| AVO | Acidic vesicular organelle |
| FADD | Fas-associated via death domain |
| TMZ | Temzolomide |
| Ad3 | Adenovirus serotype 3 |
| hCG | Human chorionic gonadotropin |
| miRNA | microRNA |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| DC | Dendritic cell |
| CTL | Cytotoxic T lymphocyte |
| ER | Endoplasmic reticulum |
References
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Zender, L.; Schulte, B.; Mundt, B.; Plentz, R.; Rudolph, K.L.; Manns, M.; Kubicka, S.; Kuhnel, F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003, 63, 3181–3188. [Google Scholar] [PubMed]
- Kim, E.; Kim, J.H.; Shin, H.Y.; Lee, H.S.; Sohn, J.H.; Yang, J.M.; Kim, J.; Yun, C.O. Development of a conditional replication competent adenovirus, controlled by the human telomerase promoter (htert). Cancer Res. Treat. 2003, 35, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Lanson, N.A., Jr.; Friedlander, P.L.; Schwarzenberger, P.; Kolls, J.K.; Wang, G. Replication of an adenoviral vector controlled by the human telomerase reverse transcriptase promoter causes tumor-selective tumor lysis. Cancer Res. 2003, 63, 7936–7941. [Google Scholar] [PubMed]
- Zou, W.; Luo, C.; Zhang, Z.; Liu, J.; Gu, J.; Pei, Z.; Qian, C.; Liu, X. A novel oncolytic adenovirus targeting to telomerase activity in tumor cells with potent. Oncogene 2004, 23, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Kagawa, S.; Kobayashi, N.; Shirakiya, Y.; Umeoka, T.; Teraishi, F.; Taki, M.; Kyo, S.; Tanaka, N.; Fujiwara, T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004, 10, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Nakagawa, K.; Hamada, K.; Harada, H.; Yamasaki, K.; Hashimoto, K.; Tagawa, M.; Nagato, S.; Furukawa, K.; Ohnishi, T. Midkine promoter-based conditionally replicative adenovirus for malignant glioma therapy. Oncol. Rep. 2004, 12, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Kawasaki, Y.; Yamaoka, N.; Tagawa, M.; Kasahara, N.; Terada, N.; Okamura, H. Complete regression of human malignant mesothelioma xenografts following local injection of midkine promoter-driven oncolytic adenovirus. J. Gene Med. 2010, 12, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.A.; Davydova, J.G.; Adachi, Y.; Takayama, K.; Barker, S.D.; Reynolds, P.N.; Krasnykh, V.N.; Kunisaki, C.; Shimada, H.; Curiel, D.T.; et al. Promoter-controlled infectivity-enhanced conditionally replicative adenoviral vectors for the treatment of gastric cancer. J. Gastroenterol. 2005, 40, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ulasov, I.V.; Tyler, M.A.; Zhu, Z.B.; Han, Y.; He, T.C.; Lesniak, M.S. Oncolytic adenoviral vectors which employ the survivin promoter induce glioma oncolysis via a process of beclin-dependent autophagy. Int. J. Oncol. 2009, 34, 729–742. [Google Scholar] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Kyo, S.; Takakura, M.; Fujiwara, T.; Inoue, M. Understanding and exploiting htert promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008, 99, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, H.; Kagawa, S.; Fujiwara, T. Oncolytic adenovirus-induced autophagy: Tumor-suppressive effect and molecular basis. Acta Med. Okayama 2013, 67, 333–342. [Google Scholar] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Tsuchihara, K.; Fujii, S.; Sugiyama, M.; Goya, T.; Atomi, Y.; Ueno, T.; Ochiai, A.; Esumi, H. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007, 67, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; DeLay, M.; Jahangiri, A.; Molinaro, A.M.; Rose, S.D.; Carbonell, W.S.; Aghi, M.K. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012, 72, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Lum, J.J.; Bauer, D.E.; Kong, M.; Harris, M.H.; Li, C.; Lindsten, T.; Thompson, C.B. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005, 120, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kudchodkar, S.B.; Levine, B. Viruses and autophagy. Rev. Med. Virol. 2009, 19, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Lee, H.K. Modulation of pathogen recognition by autophagy. Front. Immunol. 2012, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Aoki, H.; Kuhnel, F.; Kondo, Y.; Kubicka, S.; Wirth, T.; Iwado, E.; Iwamaru, A.; Fujiwara, K.; Hess, K.R.; et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J. Natl. Cancer Inst. 2006, 98, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gomez-Manzano, C.; Aoki, H.; Alonso, M.M.; Kondo, S.; McCormick, F.; Xu, J.; Kondo, Y.; Bekele, B.N.; Colman, H.; et al. Examination of the therapeutic potential of delta-24-RGD in brain tumor stem cells: Role of autophagic cell death. J. Natl. Cancer Inst. 2007, 99, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Sakai, R.; Ouchi, M.; Onimatsu, H.; Hioki, M.; Kagawa, S.; Uno, F.; Watanabe, Y.; Urata, Y.; Tanaka, N.; et al. Virus-mediated oncolysis induces danger signal and stimulates cytotoxic T-lymphocyte activity via proteasome activator upregulation. Oncogene 2008, 27, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, H.; Yano, S.; Yoshida, R.; Yamasaki, Y.; Sasaki, T.; Hashimoto, Y.; Kuroda, S.; Ouchi, M.; Onishi, T.; Uno, F.; et al. Genetically engineered oncolytic adenovirus induces autophagic cell death through an e2f1-microRNA-7-epidermal growth factor receptor axis. Int. J. Cancer 2012, 131, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zhang, Q.; Yan, Z.; Chen, R.; Zeh Iii, H.J.; Kang, R.; Lotze, M.T.; Tang, D. Strange attractors: Damps and autophagy link tumor cell death and immunity. Cell Death Dis. 2013, 4, e966. [Google Scholar] [CrossRef] [PubMed]
- Zelenay, S.; Reis e Sousa, C. Adaptive immunity after cell death. Trends Immunol. 2013, 34, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Tani, K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014, 21, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fueyo, J. Healing after death: Antitumor immunity induced by oncolytic adenoviral therapy. Oncoimmunology 2014, 3, e947872. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.L.; Liu, Z.; Sathaiah, M.; Ravindranathan, R.; Guo, Z.; He, Y.; Guo, Z.S. Oncolytic viruses as therapeutic cancer vaccines. Mol. Cancer 2013, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Liu, Z.; Bartlett, D.L. Oncolytic immunotherapy: Dying the right way is a key to eliciting potent antitumor immunity. Front. Oncol. 2014, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rocha, H.; Gomez-Gutierrez, J.G.; Garcia-Garcia, A.; Rao, X.M.; Chen, L.; McMasters, K.M.; Zhou, H.S. Adenoviruses induce autophagy to promote virus replication and oncolysis. Virology 2011, 416, 9–15. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, C.; Klupsch, K.; Choi, S.; Bagus, B.; Soria, C.; Shen, J.; McCormick, F.; Stokoe, D. Adenoviral proteins mimic nutrient/growth signals to activate the mtor pathway for viral replication. EMBO J. 2005, 24, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. Ampk and mtor regulate autophagy through direct phosphorylation of ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Broughton, R.S.; Goodrum, F.D.; Ornelles, D.A. E4orf1 limits the oncolytic potential of the e1b-55k deletion mutant adenovirus. J. Virol. 2009, 83, 2406–2416. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Noda, T.; Yoshimori, T.; Tanaka, Y.; Ishii, T.; George, M.D.; Klionsky, D.J.; Ohsumi, M.; Ohsumi, Y. A protein conjugation system essential for autophagy. Nature 1998, 395, 395–398. [Google Scholar] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Bjorkoy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Overvatn, A.; Stenmark, H.; Johansen, T. P62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005, 171, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; White, E.J.; Rios-Vicil, C.I.; Xu, J.; Gomez-Manzano, C.; Fueyo, J. Human adenovirus type 5 induces cell lysis through autophagy and autophagy-triggered caspase activity. J. Virol. 2011, 85, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.O.; Jang, M.H.; Kwon, Y.K.; Lee, H.J.; Jun, J.I.; Woo, H.N.; Cho, D.H.; Choi, B.; Lee, H.; Kim, J.H.; et al. Essential roles of atg5 and fadd in autophagic cell death: Dissection of autophagic cell death into vacuole formation and cell death. J. Biol. Chem. 2005, 280, 20722–20729. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Fujiwara, T.; Shirakawa, Y.; Yamasaki, Y.; Yano, S.; Uno, F.; Tazawa, H.; Hashimoto, Y.; Watanabe, Y.; Noma, K.; et al. Telomerase-dependent oncolytic adenovirus sensitizes human cancer cells to ionizing radiation via inhibition of DNA repair machinery. Cancer Res. 2010, 70, 9339–9348. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.D.; Ramamurthy, M.; Poel, H.; Wickham, T.J.; Lamfers, M.; Gerritsen, W.; Chowdhury, W.; Li, Y.; Schoenberg, M.P.; Rodriguez, R. Histone deacetylase inhibitors upregulate expression of the coxsackie adenovirus receptor (CAR) preferentially in bladder cancer cells. Cancer Gene Ther. 2004, 11, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Pong, R.C.; Roark, R.; Ou, J.Y.; Fan, J.; Stanfield, J.; Frenkel, E.; Sagalowsky, A.; Hsieh, J.T. Mechanism of increased coxsackie and adenovirus receptor gene expression and adenovirus uptake by phytoestrogen and histone deacetylase inhibitor in human bladder cancer cells and the potential clinical application. Cancer Res. 2006, 66, 8822–8828. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hioki, M.; Fujiwara, T.; Nishizaki, M.; Kagawa, S.; Taki, M.; Kishimoto, H.; Endo, Y.; Urata, Y.; Tanaka, N. Histone deacetylase inhibitor fr901228 enhances the antitumor effect of telomerase-specific replication-selective adenoviral agent obp-301 in human lung cancer cells. Exp. Cell Res. 2006, 312, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Iwado, E.; Kondo, Y.; Aoki, H.; Hayashi, Y.; Georgescu, M.M.; Sawaya, R.; Hess, K.R.; Mills, G.B.; Kawamura, H.; et al. Autophagy-inducing agents augment the antitumor effect of telerase-selve oncolytic adenovirus obp-405 on glioblastoma cells. Gene Ther. 2008, 15, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Ulasov, I.V.; Sonabend, A.M.; Nandi, S.; Khramtsov, A.; Han, Y.; Lesniak, M.S. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagic and apoptotic cell death in vivo. Br. J. Cancer 2009, 100, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Rajecki, M.; Hallstrom, T.A.; Hakkarainen, T.; Nokisalmi, P.; Hautaniemi, S.; Nieminen, A.I.; Tenhunen, M.; Rantanen, V.; Desmond, R.A.; Chen, D.T.; et al. Mre11 inhibition by oncolytic adenovirus associates with autophagy and underlies synergy with ionizing radiation. Int. J. Cancer 2009, 125, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Tazawa, H.; Teraishi, F.; Kojima, T.; Watanabe, Y.; Uno, F.; Yano, S.; Urata, Y.; Kagawa, S.; Fujiwara, T. The htert promoter enhances the antitumor activity of an oncolytic adenovirus under a hypoxic microenvironment. PLoS ONE 2012, 7, e39292. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Raychaudhuri, P.; Nevins, J.R. Adenovirus e1a proteins can dissociate heteromeric complexes involving the E2f transcription factor: A novel mechanism for E1a trans-activation. Cell 1990, 62, 659–669. [Google Scholar] [CrossRef]
- Polager, S.; Ofir, M.; Ginsberg, D. E2f1 regulates autophagy and the transcription of autophagy genes. Oncogene 2008, 27, 4860–4864. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, A.; Rodriguez-Rocha, H.; Tseng, M.T.; Montes de Oca-Luna, R.; Zhou, H.S.; McMasters, K.M.; Gomez-Gutierrez, J.G. E2f-1 lacking the transcriptional activity domain induces autophagy. Cancer Biol. Ther. 2012, 13, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Piya, S.; White, E.J.; Klein, S.R.; Jiang, H.; McDonnell, T.J.; Gomez-Manzano, C.; Fueyo, J. The e1b19k oncoprotein complexes with beclin 1 to regulate autophagy in adenovirus-infected cells. PLoS ONE 2011, 6, e29467. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.R.; Piya, S.; Lu, Z.; Xia, Y.; Alonso, M.M.; White, E.J.; Wei, J.; Gomez-Manzano, C.; Jiang, H.; Fueyo, J. C-jun N-terminal kinases are required for oncolytic adenovirus-mediated autophagy. Oncogene 2015, 34, 5295–5301. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.M.; Jiang, H.; Yokoyama, T.; Xu, J.; Bekele, N.B.; Lang, F.F.; Kondo, S.; Gomez-Manzano, C.; Fueyo, J. Delta-24-RGD in combination with rad001 induces enhanced anti-glioma effect via autophagic cell death. Mol. Ther. 2008, 16, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Liikanen, I.; Ahtiainen, L.; Hirvinen, M.L.; Bramante, S.; Cerullo, V.; Nokisalmi, P.; Hemminki, O.; Diaconu, I.; Pesonen, S.; Koski, A.; et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol. Ther. 2013, 21, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gutierrez, J.G.; Nitz, J.; Sharma, R.; Wechman, S.L.; Riedinger, E.; Martinez-Jaramillo, E.; Sam Zhou, H.; McMasters, K.M. Combined therapy of oncolytic adenovirus and temozolomide enhances lung cancer virotherapy in vitro and in vivo. Virology 2016, 487, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Ulasov, I.V.; Shah, N.; Kaverina, N.V.; Lee, H.; Lin, B.; Lieber, A.; Kadagidze, Z.G.; Yoon, J.G.; Schroeder, B.; Hothi, P.; et al. Tamoxifen improves cytopathic effect of oncolytic adenovirus in primary glioblastoma cells mediated through autophagy. Oncotarget 2015, 6, 3977–3987. [Google Scholar] [CrossRef] [PubMed]
- Kanj, S.S.; Dandashi, N.; El-Hed, A.; Harik, H.; Maalouf, M.; Kozhaya, L.; Mousallem, T.; Tollefson, A.E.; Wold, W.S.; Chalfant, C.E.; et al. Ceramide regulates SR protein phosphorylation during adenoviral infection. Virology 2006, 345, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Cruickshanks, N.; Roberts, J.L.; Bareford, M.D.; Tavallai, M.; Poklepovic, A.; Booth, L.; Spiegel, S.; Dent, P. Differential regulation of autophagy and cell viability by ceramide species. Cancer Biol. Ther. 2015, 16, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Harvald, E.B.; Olsen, A.S.B.; Faergeman, N.J. Autophagy in the light of sphingolipid metabolism. Apoptosis 2015, 20, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; You, L.; Liu, H.; Li, L.; Meng, H.; Qian, Q.; Qian, W. Potent antitumor activity of oncolytic adenovirus expressing beclin-1 via induction of autophagic cell death in leukemia. Oncotarget 2013, 4, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the light: The growing complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Tazawa, H.; Hashimoto, Y.; Kojima, T.; Kuroda, S.; Yano, S.; Yoshida, R.; Uno, F.; Mizuguchi, H.; Ohtsuru, A.; et al. A novel apoptotic mechanism of genetically engineered adenovirus-mediated tumour-specific p53 overexpression through E1a-dependent p21 and mdm2 suppression. Eur. J. Cancer 2012, 48, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, H.; Kagawa, S.; Fujiwara, T. Advances in adenovirus-mediated p53 cancer gene therapy. Expert Opin. Biol. Ther. 2013, 13, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Hasei, J.; Sasaki, T.; Tazawa, H.; Osaki, S.; Yamakawa, Y.; Kunisada, T.; Yoshida, A.; Hashimoto, Y.; Onishi, T.; Uno, F.; et al. Dual programmed cell death pathways induced by p53 transactivation overcome resistance to oncolytic adenovirus in human osteosarcoma cells. Mol. Cancer Ther. 2013, 12, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Pong, R.C.; Lai, Y.J.; Chen, H.; Okegawa, T.; Frenkel, E.; Sagalowsky, A.; Hsieh, J.T. Epigenetic regulation of coxsackie and adenovirus receptor (car) gene promoter in urogenital cancer cells. Cancer Res. 2003, 63, 8680–8686. [Google Scholar] [PubMed]
- Kuster, K.; Koschel, A.; Rohwer, N.; Fischer, A.; Wiedenmann, B.; Anders, M. Downregulation of the coxsackie and adenovirus receptor in cancer cells by hypoxia depends on hif-1α. Cancer Gene Ther. 2010, 17, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Ylosmaki, E.; Hakkarainen, T.; Hemminki, A.; Visakorpi, T.; Andino, R.; Saksela, K. Generation of a conditionally replicating adenovirus based on targeted destruction of E1a mrna by a cell type-specific microrna. J. Virol. 2008, 82, 11009–11015. [Google Scholar] [CrossRef] [PubMed]
- Sugio, K.; Sakurai, F.; Katayama, K.; Tashiro, K.; Matsui, H.; Kawabata, K.; Kawase, A.; Iwaki, M.; Hayakawa, T.; Fujiwara, T.; et al. Enhanced safety profiles of the telomerase-specific replication-competent adenovirus by incorporation of normal cell-specific microrna-targeted sequences. Clin. Cancer Res. 2011, 17, 2807–2818. [Google Scholar] [CrossRef] [PubMed]
- Ylosmaki, E.; Lavilla-Alonso, S.; Jaamaa, S.; Vaha-Koskela, M.; Hallstrom, T.A.; Hemminki, A.; Arola, J.; Makisalo, H.; Saksela, K. Microrna-mediated suppression of oncolytic adenovirus replication in human liver. PLoS ONE 2013, 8, e54506. [Google Scholar] [CrossRef] [PubMed]
- Ganapathi, L.; Arnold, A.; Jones, S.; Patterson, A.; Graham, D.; Harper, M.; Levy, O. Use of cidofovir in pediatric patients with adenovirus infection. F1000Res 2016, 5, 758. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, M.; Kawamura, H.; Urata, Y.; Fujiwara, T. Antiviral activity of cidofovir against telomerase-specific replication-selective oncolytic adenovirus, obp-301 (telomelysin). Investig. New Drug. 2009, 27, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Munz, C.; Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015, 16, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Martins, I.; Ma, Y.; Kepp, O.; Galluzzi, L.; Kroemer, G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 2013, 9, 1624–1625. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.T.; Pellegatti, P.; Shen, S.S.; Kepp, O.; Scoazec, M.; Mignot, G.; et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Gump, J.M.; Staskiewicz, L.; Morgan, M.J.; Bamberg, A.; Riches, D.W.H.; Thorburn, A. Autophagy variation within a cell population determines cell fate through selective degradation of Fap-1. Nat. Cell Biol. 2014, 16, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, J.; Horita, H.; Redzic, J.; Hansen, K.; Frankel, A.E.; Thorburn, A. Autophagy regulates selective hmgb1 release in tumor cells that are destined to die. Cell Death Differ. 2009, 16, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Liikanen, I.; Koski, A.; Merisalo-Soikkeli, M.; Hemminki, O.; Oksanen, M.; Kairemo, K.; Joensuu, T.; Kanerva, A.; Hemminki, A. Serum hmgb1 is a predictive and prognostic biomarker for oncolytic immunotherapy. Oncoimmunology 2015, 4, e989771. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.R.; Jiang, H.; Hossain, M.B.; Fan, X.J.; Gumin, J.; Dong, A.; Alonso, M.M.; Gomez-Manzano, C.; Fueyo, J. Critical role of autophagy in the processing of adenovirus capsid-incorporated cancer-specific antigens. PLoS ONE 2016, 11, e0153814. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lei, Z.; Lichty, B.D.; Li, D.; Zhang, G.M.; Feng, Z.H.; Wan, Y.H.; Huang, B. Autophagy facilitates major histocompatibility complex class I expression induced by IFN-γ in b16 melanoma cells. Cancer Immunol. Immun. 2010, 59, 313–321. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).