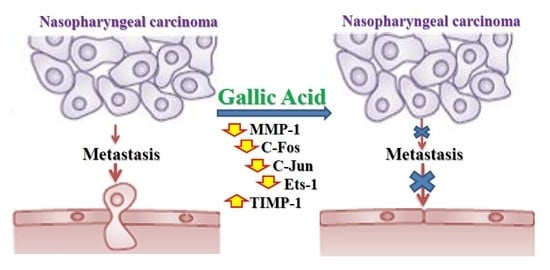
Gallic Acid Inhibited Matrix Invasion and AP-1/ETS-1-Mediated MMP-1 Transcription in Human Nasopharyngeal Carcinoma Cells
Abstract
1. Introduction
2. Results
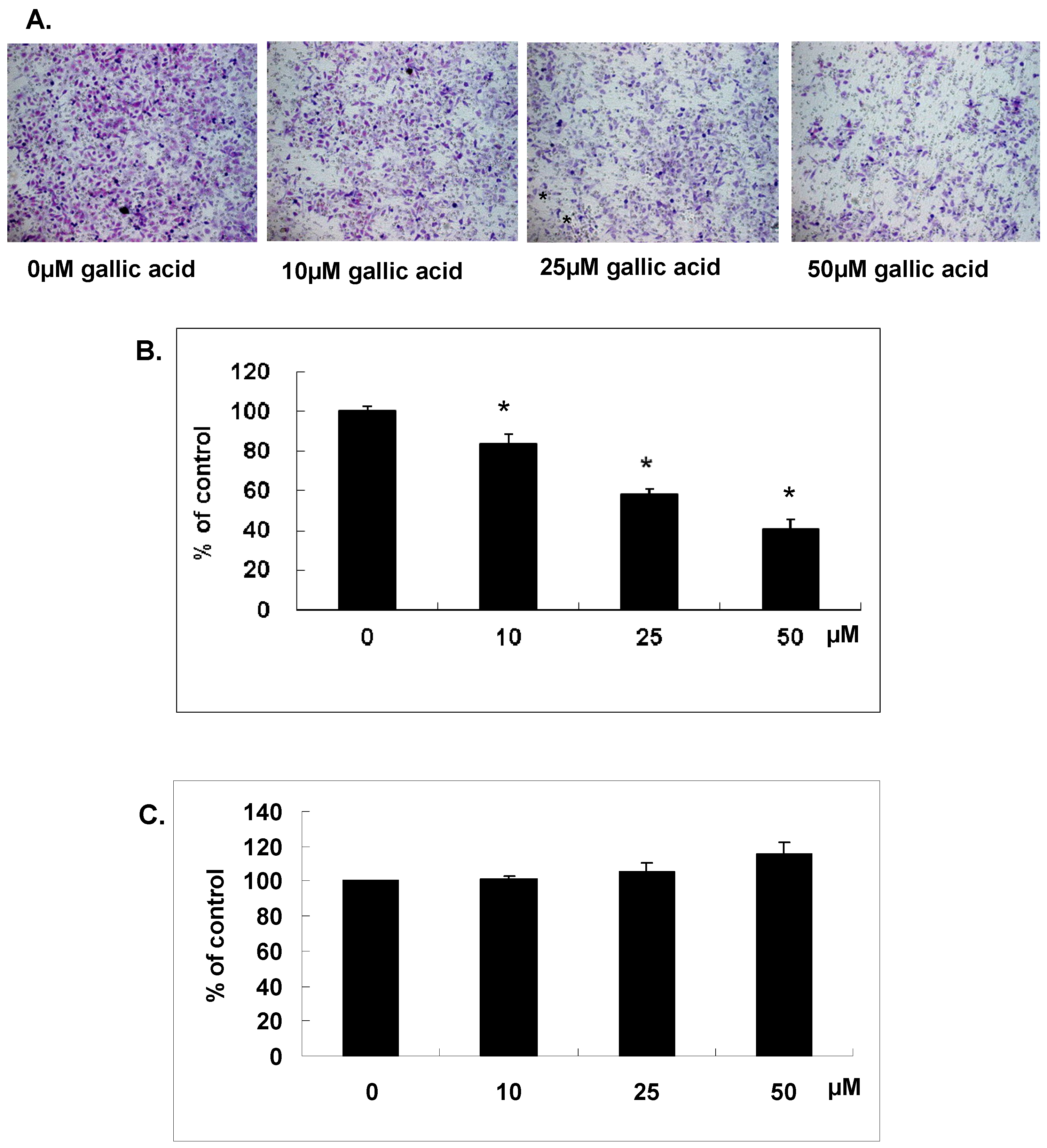
2.1. Gallic Acid Suppressed the In Vitro Matrix Invasion of NPC-BM1 Cells
2.2. Gallic Acid Reduced MMP-1 Expression in NPC-BM1 Cells
2.3. Gallic Acid Inhibited MMP-1 Promoter Activity
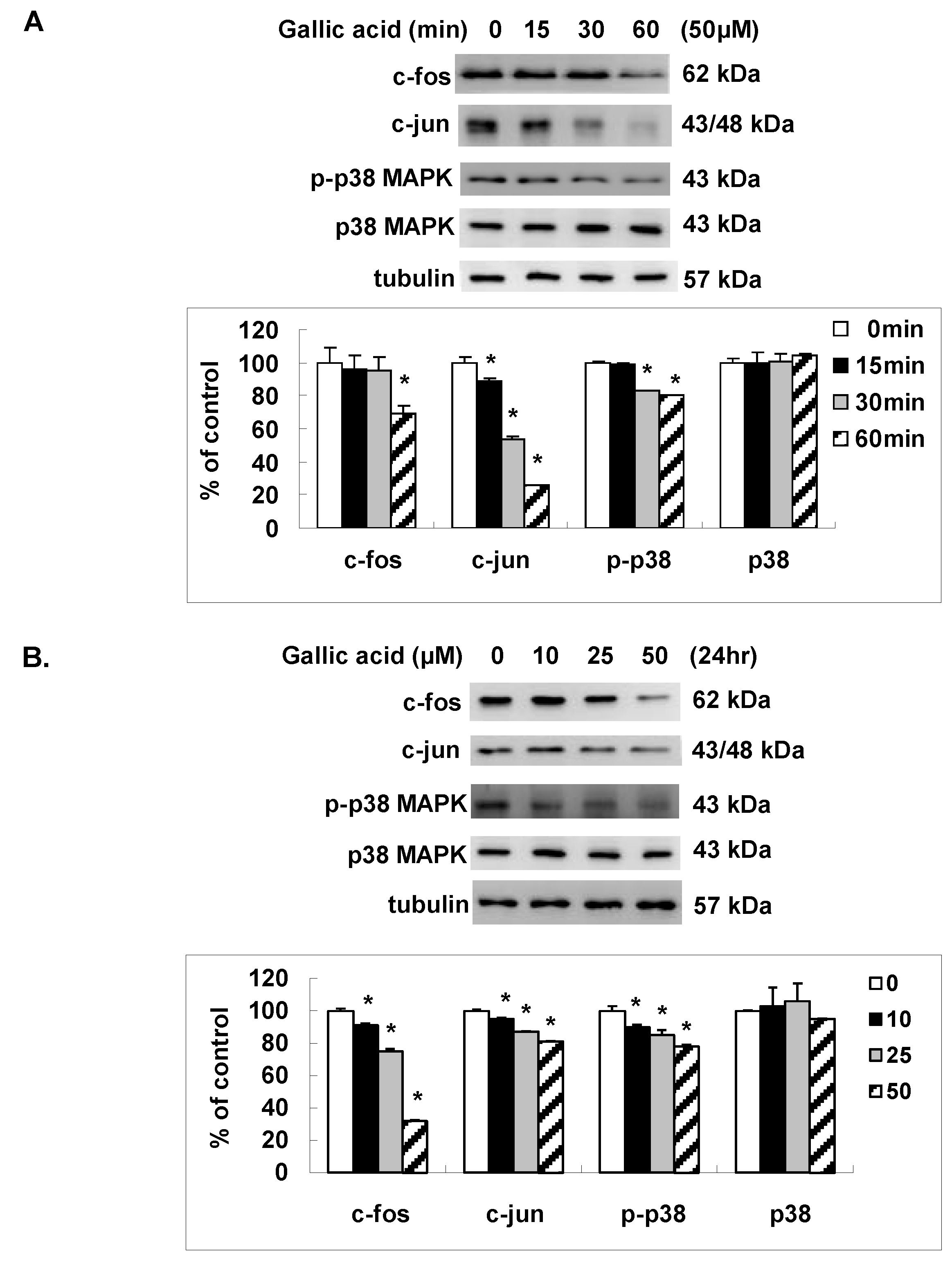
2.4. Gallic Acid Inhibited the AP-1 Expression and p38 MAPK Activation
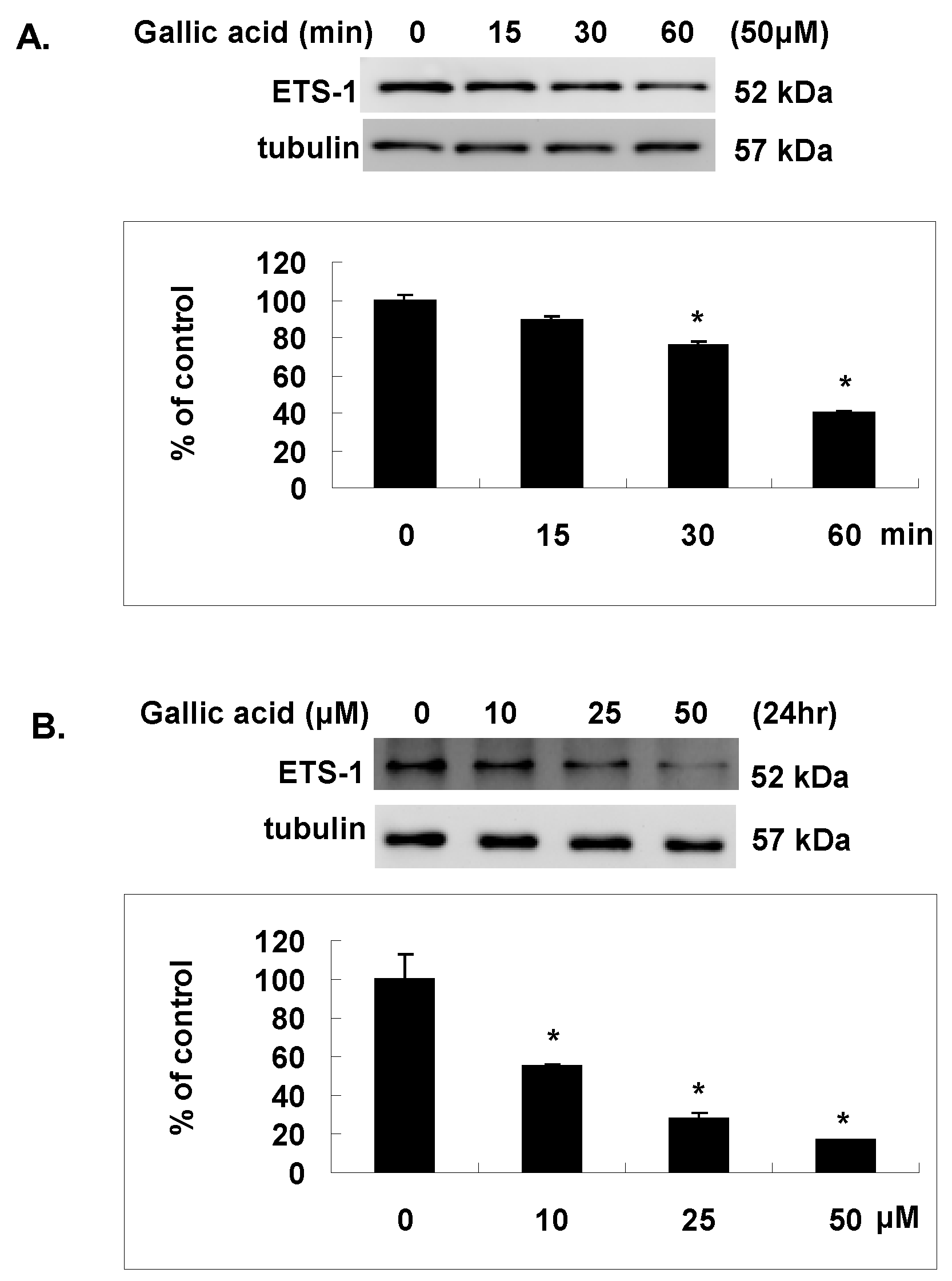
2.5. Gallic Acid Inhibited the Est-1 Expression in NPC-BM1 Cells
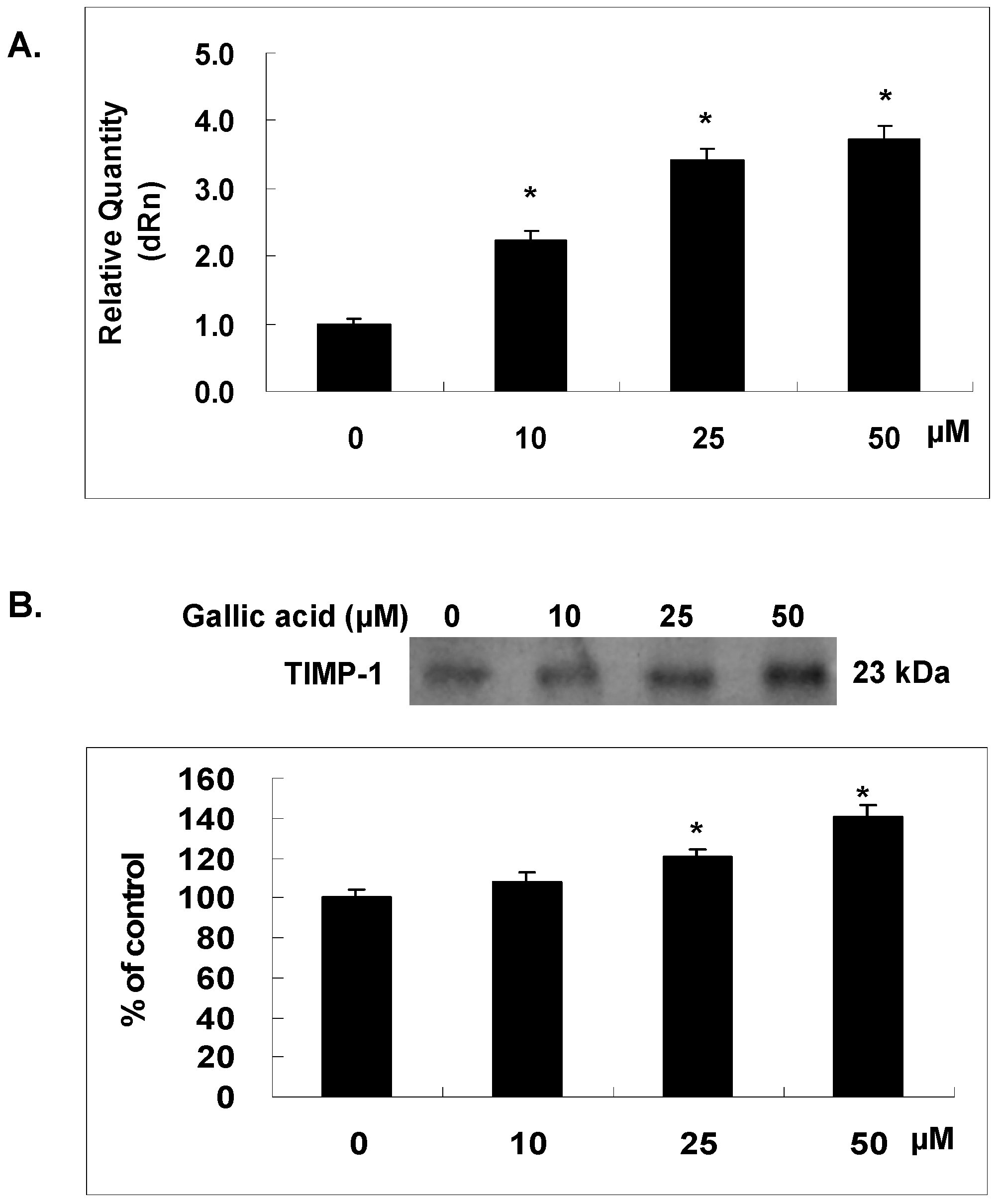
2.6. Gallic Acid Increased the TIMP-1 Expression in NPC-BM1 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Invasion Assay
4.4. Cytotoxicity Assay
4.5. Microarray Analysis
4.6. RNA Isolation
4.7. Real-Time RT-PCR
4.8. Western Blot Analysis
4.9. ELISA Assay
4.10. Plasmid Construction
4.11. Transient Transfection and Dual Luciferase Assay
4.12. Statistical Analysis
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
Abbreviations
| NPC | nasopharyngeal carcinoma |
| MMP | matrix metalloproteinase |
| TIMP | tissue inhibitor of matrix metalloproteinase |
| P. urinaria | Phyllanthus urinaria |
| GSH | glutathione |
| ROS | reactive oxygen species |
| EGFR | epidermal growth factor receptor |
| Ets-1 | proto-oncogene 1 |
| HPF | high-power microscopic field |
| LDH | lactate dehydrogenase |
| BSA | bovine serum albumin |
| AP-1 | activator protein 1 |
| MAPK | mitogen-activated protein kinase |
| ECM | extracellular matrix |
| EBV | Epstein-Barr virus |
| LMP1 | latent membrane protein 1 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| VEGF | vascular endothelial growth factor |
References
- Faried, A.; Kurnia, D.; Faried, L.S.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from indonesian herbal medicine, Phaleria macrocarpa (scheff) boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Rajamanickam, S.; Deep, G.; Singh, M.; Agarwal, R.; Agarwal, C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in tramp mice. Mol. Cancer Ther. 2008, 7, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Wang, C.Y.; Yang, R.C.; Wu, H.T.; Yang, S.H.; Cheng, Y.C.; Pang, J.H. Ellagic acid, the active compound of Phyllanthus urinaria, exerts in vivo anti-angiogenic effect and inhibits MMP-2 activity. Evid. Based Complement. Alternat. Med. 2011, 2011, 215035. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Pang, J.H.; Yang, R.C. Anti-cancer effects of Phyllanthus urinaria and relevant mechanisms. Chang Gung Med. J. 2010, 33, 477–487. [Google Scholar]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Filippin-Monteiro, F.B.; Creczynski-Pasa, T.B. Alkyl esters of gallic acid as anticancer agents: A review. Eur. J. Med. Chem. 2013, 60, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Lin, T.K.; Kuo, H.M.; Huang, Y.L.; Liou, C.W.; Wang, P.W.; Chuang, J.H.; Huang, S.T. Phyllanthus urinaria induces apoptosis in human osteosarcoma 143B cells via activation of Fas/Fasl- and mitochondria-mediated pathways. Evid. Based Complement. Alternat. Med. 2012, 2012, 925824. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Wang, C.Y.; Yang, R.C.; Chu, C.J.; Wu, H.T.; Pang, J.H. Phyllanthus urinaria increases apoptosis and reduces telomerase activity in human nasopharyngeal carcinoma cells. Forsch Komplementmed 2009, 16, 34–40. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Park, W.H. Gallic acid-induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicol. In Vitro 2010, 24, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Kim, S.Z.; Kim, S.H.; Park, W.H. Gallic acid-induced lung cancer cell death is accompanied by ROS increase and glutathione depletion. Mol. Cell. Biochem. 2011, 357, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Lai, T.Y.; Yang, J.S.; Yang, J.H.; Ma, Y.S.; Weng, S.W.; Lin, H.Y.; Chen, H.Y.; Lin, J.G.; Chung, J.G. Gallic acid inhibits the migration and invasion of A375.S2 human melanoma cells through the inhibition of matrix metalloproteinase-2 and Ras. Melanoma Res. 2011, 21, 267–273. [Google Scholar] [PubMed]
- Liao, C.L.; Lai, K.C.; Huang, A.C.; Yang, J.S.; Lin, J.J.; Wu, S.H.; Gibson Wood, W.; Lin, J.G.; Chung, J.G. Gallic acid inhibits migration and invasion in human osteosarcoma U-2 OS cells through suppressing the matrix metalloproteinase-2/-9, protein kinase B (PKB) and PKC signaling pathways. Food Chem. Toxicol. 2012, 50, 1734–1740. [Google Scholar] [PubMed]
- Ho, H.H.; Chang, C.S.; Ho, W.C.; Liao, S.Y.; Wu, C.H.; Wang, C.J. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-κB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem. Toxicol. 2010, 48, 2508–2516. [Google Scholar] [PubMed]
- Yang, R.; Xu, Y.; Li, P.; Zhang, X.; Wang, J.; Gu, D.; Wang, Y. Combined upregulation of matrix metalloproteinase-1 and proteinase-activated receptor-1 predicts unfavorable prognosis in human nasopharyngeal carcinoma. Onco. Targets Ther. 2013, 6, 1139–1146. [Google Scholar] [PubMed]
- Li, A.C.; Xiao, W.W.; Shen, G.Z.; Wang, L.; Xu, A.A.; Cao, Y.Q.; Huang, S.M.; Lin, C.G.; Han, F.; Deng, X.W.; et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of imrt: Long-term results and benefits of chemotherapy. Oncotarget 2015, 6, 24511–24521. [Google Scholar] [PubMed]
- Nelson, A.R.; Fingleton, B.; Rothenberg, M.L.; Matrisian, L.M. Matrix metalloproteinases: Biologic activity and clinical implications. J. Clin. Oncol. 2000, 18, 1135–1149. [Google Scholar] [PubMed]
- Davies, K.J. The complex interaction of matrix metalloproteinases in the migration of cancer cells through breast tissue stroma. Int. J. Breast Cancer 2014, 2014, 839094. [Google Scholar] [PubMed]
- Wong, T.S.; Kwong, D.L.; Sham, J.S.; Wei, W.I.; Kwong, Y.L.; Yuen, A.P. Clinicopathologic significance of plasma matrix metalloproteinase-2 and -9 levels in patients with undifferentiated nasopharyngeal carcinoma. Eur. J. Surg. Oncol. 2004, 30, 560–564. [Google Scholar] [PubMed]
- Ben Nasr, H.; Chahed, K.; Remadi, S.; Zakhama, A.; Chouchane, L. Expression and clinical significance of latent membrane protein-1, matrix metalloproteinase-1 and Ets-1 transcription factor in tunisian nasopharyngeal carcinoma patients. Arch. Med. Res. 2009, 40, 196–203. [Google Scholar] [PubMed]
- Lu, J.; Chua, H.H.; Chen, S.Y.; Chen, J.Y.; Tsai, C.H. Regulation of matrix metalloproteinase-1 by epstein-barr virus proteins. Cancer Res. 2003, 63, 256–262. [Google Scholar] [PubMed]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Nasr, H.B.; Mestiri, S.; Chahed, K.; Bouaouina, N.; Gabbouj, S.; Jalbout, M.; Chouchane, L. Matrix metalloproteinase-1 (-1607) 1G/2G and -9 (-1562) C/T promoter polymorphisms: Susceptibility and prognostic implications in nasopharyngeal carcinomas. Clin. Chim. Acta. 2007, 384, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.S.; Tymms, M.J.; McKinlay, L.H.; Shannon, M.F.; Seth, A.; Kola, I. ETS1, NF-κB and AP1 synergistically transactivate the human GM-CSF promoter. Oncogene 1997, 14, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, P.; Parikh, N.; Garrett-Sinha, L.A.; Sinha, S. Ets1 induces dysplastic changes when expressed in terminally-differentiating squamous epidermal cells. PLoS ONE 2009, 4, e4179. [Google Scholar] [CrossRef] [PubMed]
- Flint, P.W.; Cummings, C.W. Cummings Otolaryngology—Head & Neck Surgery, 5th ed.; Mosby/Elsevier: Philadelphia, PA, USA, 2010; Volume 3, p. 1344. [Google Scholar]
- Ai, J.; Li, W.; Zeng, R.; Xie, Z.; Liu, H.; Hou, M.; Tan, G. Blockage of SSRP1/Ets-1/Pim-3 signalling enhances chemosensitivity of nasopharyngeal carcinoma to docetaxel in vitro. Biomed. Pharmacother 2016, 83, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Kida, Y.; Mai, M.; Endo, Y.; Sasaki, T.; Tanaka, J.; Seiki, M. Expression of genes encoding type IV collagen-degrading metalloproteinases and tissue inhibitors of metalloproteinases in various human tumor cells. Oncogene 1992, 7, 77–83. [Google Scholar] [PubMed]
- Lee, W.; Mitchell, P.; Tjian, R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell 1987, 49, 741–752. [Google Scholar] [CrossRef]
- Vincenti, M.P.; White, L.A.; Schroen, D.J.; Benbow, U.; Brinckerhoff, C.E. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): Mechanisms that control enzyme activity, transcription, and mRNA stability. Crit. Rev. Eukaryot Gene Expr. 1996, 6, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Milde-Langosch, K. The fos family of transcription factors and their role in tumourigenesis. Eur. J. Cancer 2005, 41, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, A.M.; Methner, C.; Lisboa, B.W.; Stadtler, C.; Schulte, H.M.; Loning, T.; Milde-Langosch, K. Expression pattern of the AP-1 family in breast cancer: Association of fosB expression with a well-differentiated, receptor-positive tumor phenotype. Int. J. Cancer 1999, 84, 533–538. [Google Scholar] [CrossRef]
- Ozanne, B.W.; Spence, H.J.; McGarry, L.C.; Hennigan, R.F. Transcription factors control invasion: Ap-1 the first among equals. Oncogene 2007, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Maishi, N.; Torii, C.; Hida, Y. Tumor angiogenesis-characteristics of tumor endothelial cells. Int. J. Clin. Oncol. 2016, 21, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lu, R.N.; Li, J. Angiogenic factors in chronic lymphocytic leukemia. Leuk. Res. 2012, 36, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, A.Y.; Rojanasakul, Y.; Rankin, G.O.; Chen, Y.C. Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1α/VEGF signaling pathway in ovarian cancer cells. Oncol. Rep. 2016, 35, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.; Suliburska, J.; Ferreira, I.M. New insights into the antiangiogenic and proangiogenic properties of dietary polyphenols. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]


| Gene Description | UniGene Symbol | Fold Decrease |
|---|---|---|
| matrix metallopeptidase 1 (interstitial collagenase) | MMP1 | 2.9 |
| interferon, α-inducible protein 27 | IFI27 | 2.8 |
| tripartite motif-containing 22 | TRIM22 | 2.4 |
| 2′-5′-oligoadenylate synthetase 2, 69/71kDa | OAS2 | 2.4 |
| 2′,5′-oligoadenylate synthetase 1, 40/46kDa | OAS1 | 2.3 |
| interferon-induced protein with tetratricopeptide repeats 1 | IFIT1 | 2.2 |
| keratin 13 | KRT13 | 2.2 |
| small proline-rich protein 1B (cornifin) | SPRR1B | 2.2 |
| lectin, galactoside-binding, soluble, 7 (galectin 7) | LGALS7 | 2.1 |
| chemokine (C-C motif) ligand 5 | CCL5 | 2.1 |
| interferon-induced protein 44-like | IFI44L | 2.0 |
| Gene Description | UniGene Symbol | Fold Increase |
|---|---|---|
| hemoglobin, α 2 | HBA2 | 0.26 |
| hemoglobin, α 1 | HBA1 | 0.27 |
| aldo-keto reductase family 1 | AKR1C2 | 0.28 |
| chromosome 6 open reading frame 48 | C6orf48 | 0.32 |
| hemoglobin, β | HBB | 0.35 |
| cytochrome P450, family 1, subfamily A, polypeptide 1 | CYP1A1 | 0.37 |
| lipocalin 2 (oncogene 24p3) | LCN2 | 0.40 |
| N-myc downstream regulated gene 1 | NDRG1 | 0.44 |
| thioredoxin interacting protein | TXNIP | 0.44 |
| laminin, β 3 | LAMB3 | 0.44 |
| thioredoxin reductase 1 | TXNRD1 | 0.44 |
| hypothetical protein DJ328E19.C1.1 | DJ328E19.C1.1 | 0.45 |
| sialidase 1 (lysosomal sialidase) | NEU1 | 0.48 |
| uridine phosphorylase 1 | UPP1 | 0.53 |
| calbindin 1, 28kDa | CALB1 | 0.54 |
| aldolase C, fructose-bisphosphate | ALDOC | 0.55 |
| hypothetical protein MAC30 | MAC30 | 0.57 |
| hypothetical protein MGC14376 | MGC14376 | 0.58 |
| lipin 1 | LPIN1 | 0.58 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, J.-H.S.; Yen, J.-H.; Wu, H.-T.; Huang, S.-T. Gallic Acid Inhibited Matrix Invasion and AP-1/ETS-1-Mediated MMP-1 Transcription in Human Nasopharyngeal Carcinoma Cells. Int. J. Mol. Sci. 2017, 18, 1354. https://doi.org/10.3390/ijms18071354
Pang J-HS, Yen J-H, Wu H-T, Huang S-T. Gallic Acid Inhibited Matrix Invasion and AP-1/ETS-1-Mediated MMP-1 Transcription in Human Nasopharyngeal Carcinoma Cells. International Journal of Molecular Sciences. 2017; 18(7):1354. https://doi.org/10.3390/ijms18071354
Chicago/Turabian StylePang, Jong-Hwei S., Jia-Hau Yen, Hsiao-Ting Wu, and Sheng-Teng Huang. 2017. "Gallic Acid Inhibited Matrix Invasion and AP-1/ETS-1-Mediated MMP-1 Transcription in Human Nasopharyngeal Carcinoma Cells" International Journal of Molecular Sciences 18, no. 7: 1354. https://doi.org/10.3390/ijms18071354
APA StylePang, J.-H. S., Yen, J.-H., Wu, H.-T., & Huang, S.-T. (2017). Gallic Acid Inhibited Matrix Invasion and AP-1/ETS-1-Mediated MMP-1 Transcription in Human Nasopharyngeal Carcinoma Cells. International Journal of Molecular Sciences, 18(7), 1354. https://doi.org/10.3390/ijms18071354