The Implication of Substance P in the Development of Tendinopathy: A Case Control Study
Abstract
:1. Introduction
2. Results
2.1. Comparison between the Healthy Tenocyte and the Tendinopathy Tenocyte
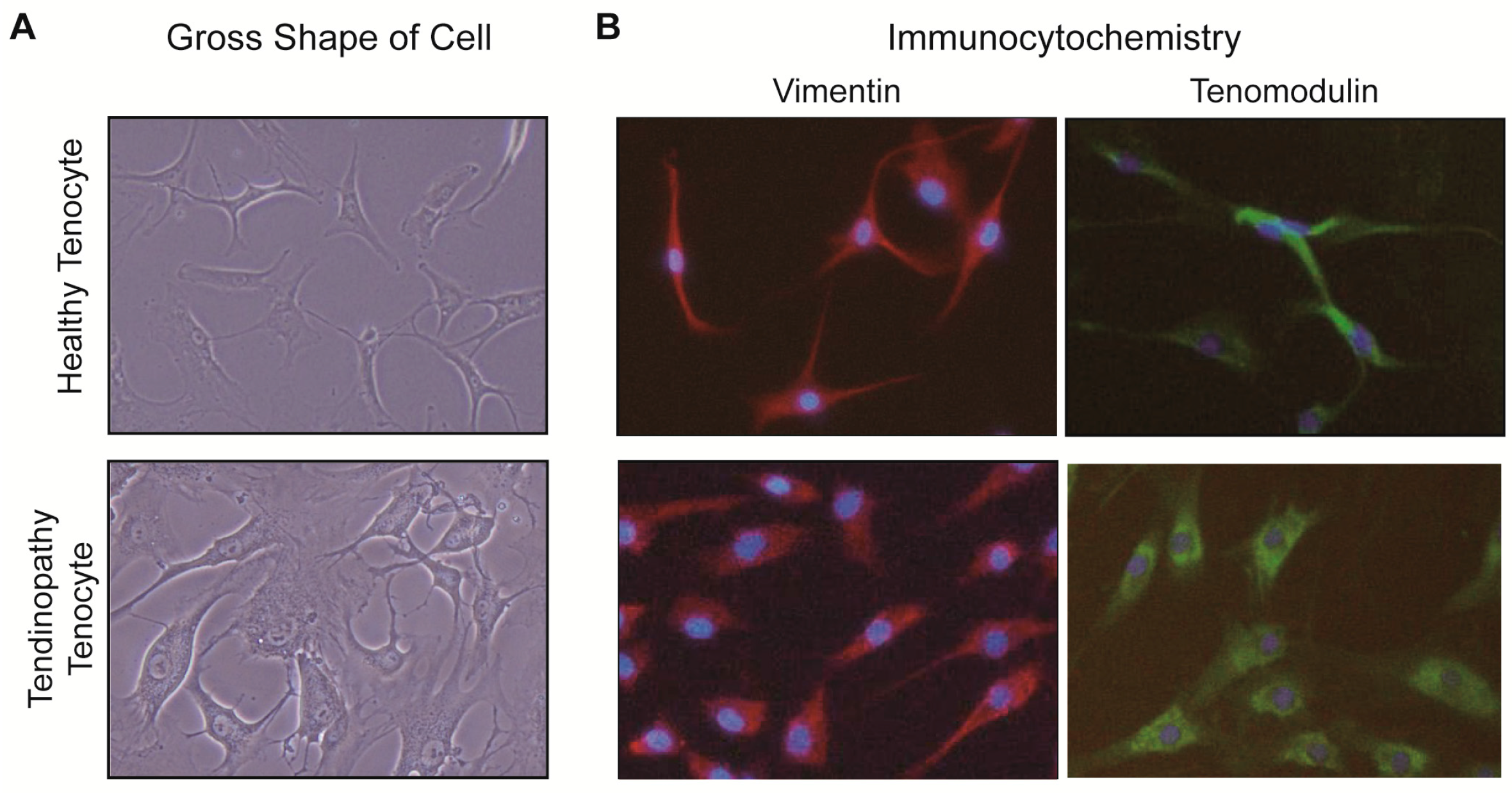
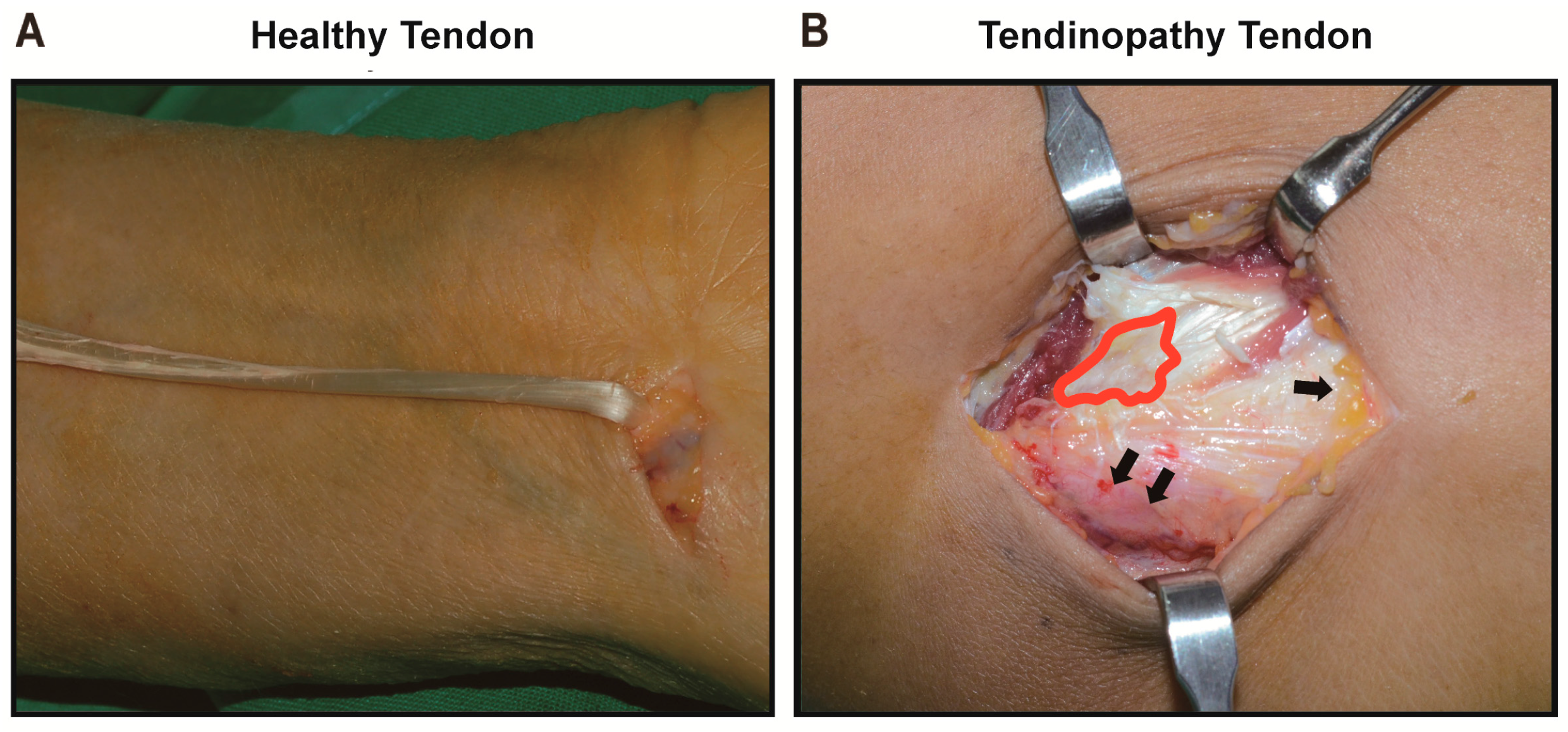
2.1.1. Gross Description of Cell Shape
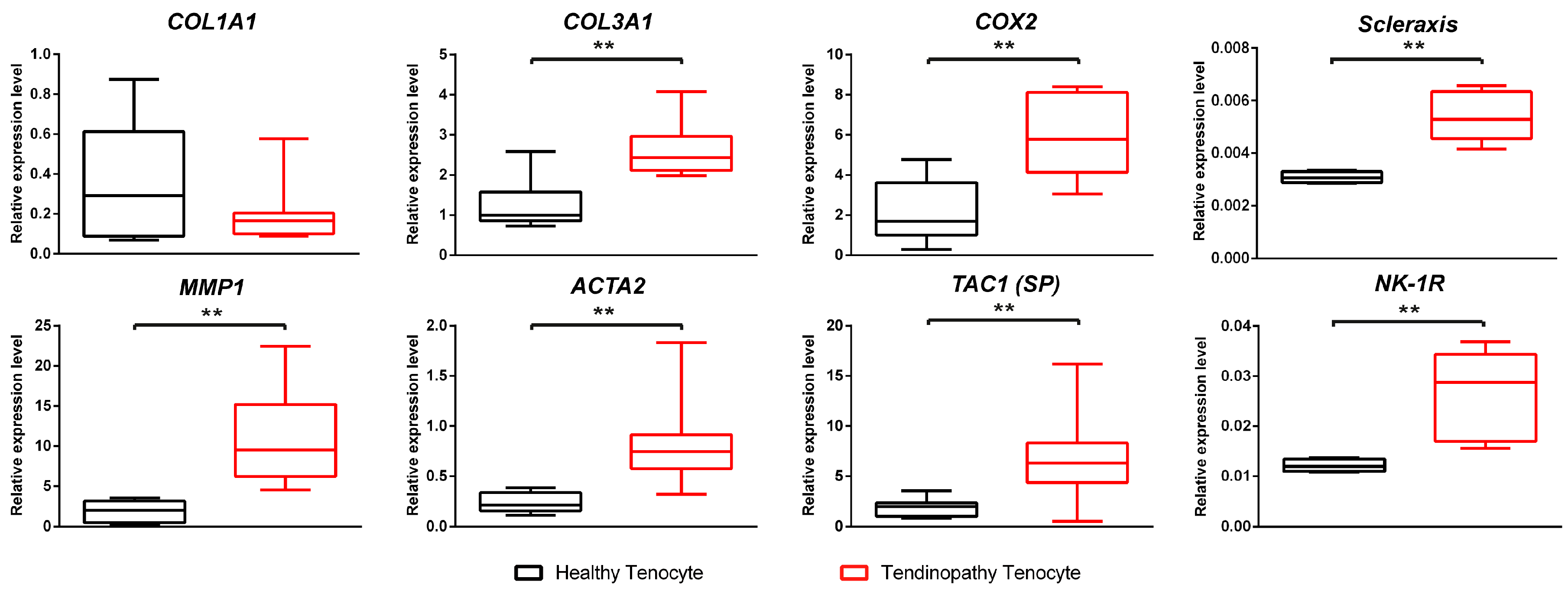
2.1.2. Differences of Expressions at the mRNA Level
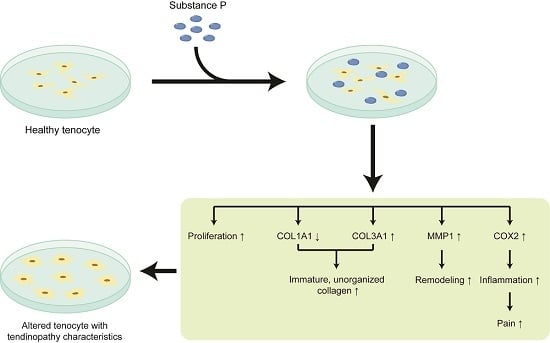
2.2. The Effect of Substance P (SP) on the Healthy Tenocyte in the Development of Tendinopathy
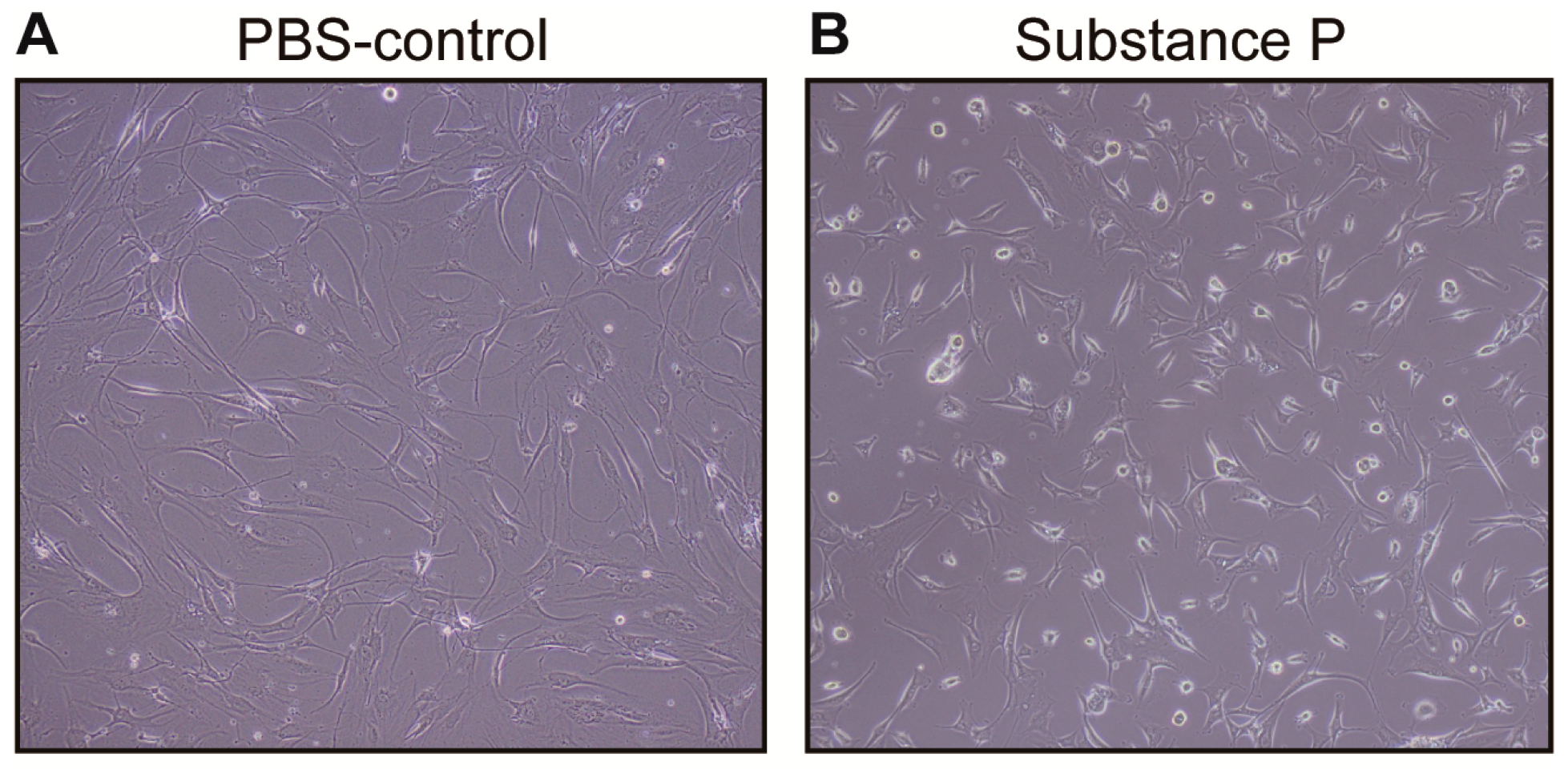
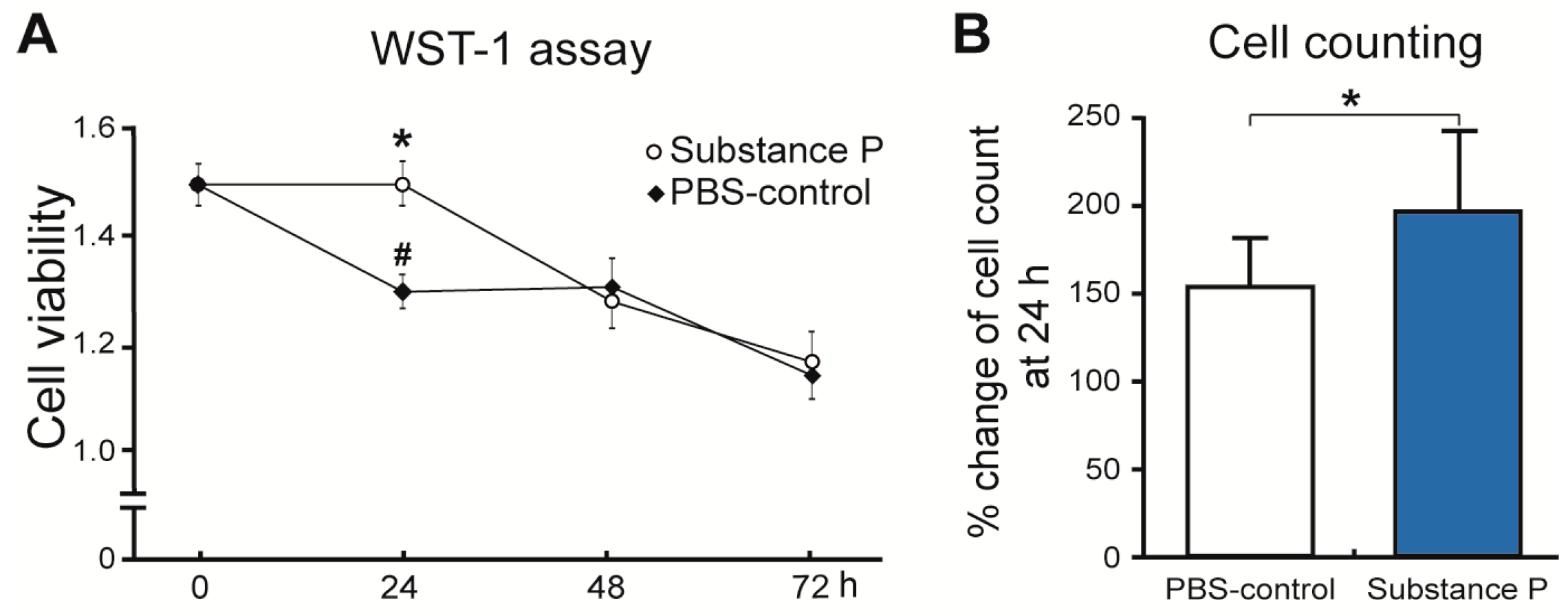
2.2.1. SP Increases Cellular Proliferation and Viability
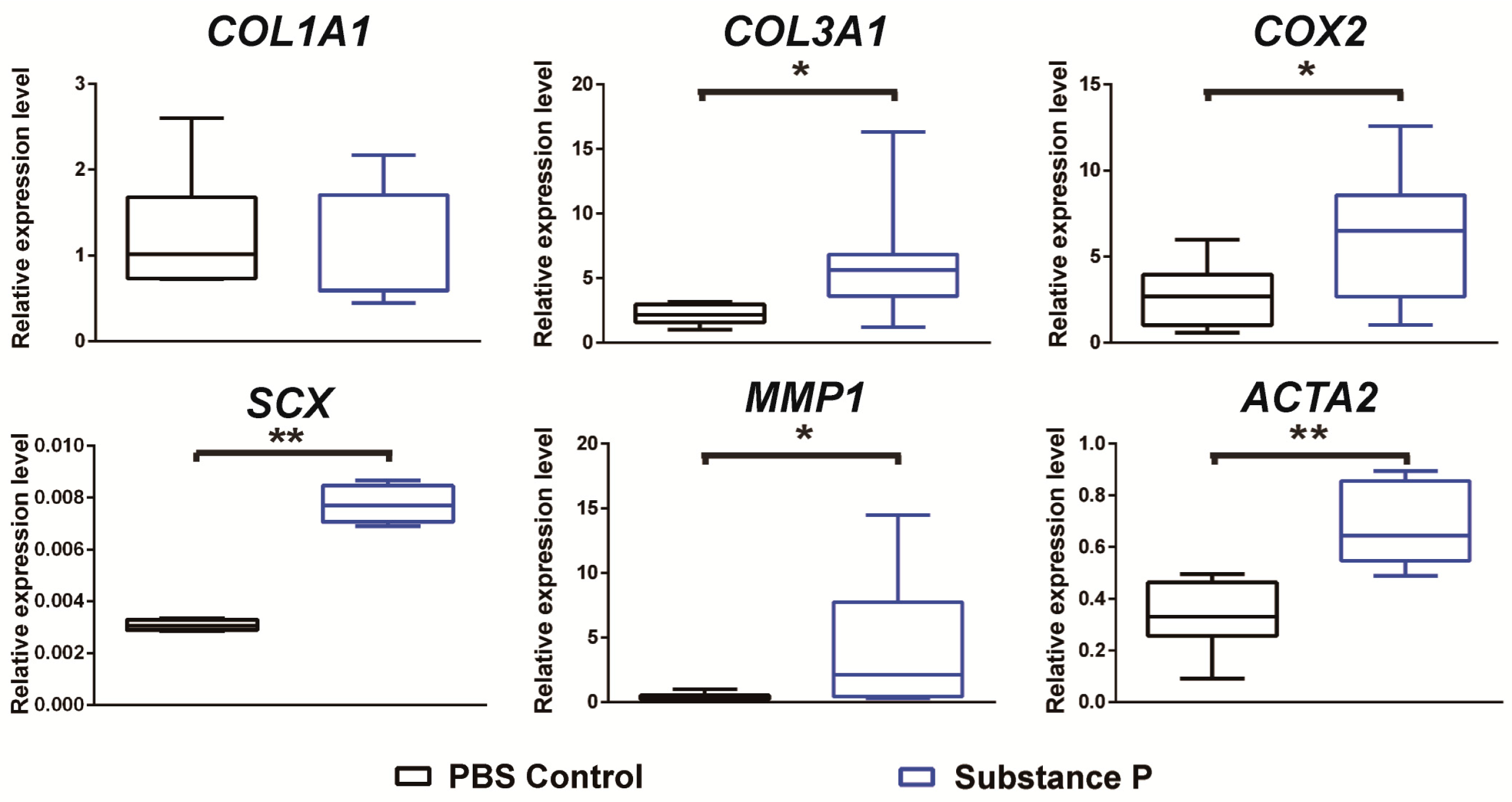
2.2.2. SP Up-Regulates the Relative mRNA Expressions Related to Tendinopathy Development in the Healthy Tenocyte
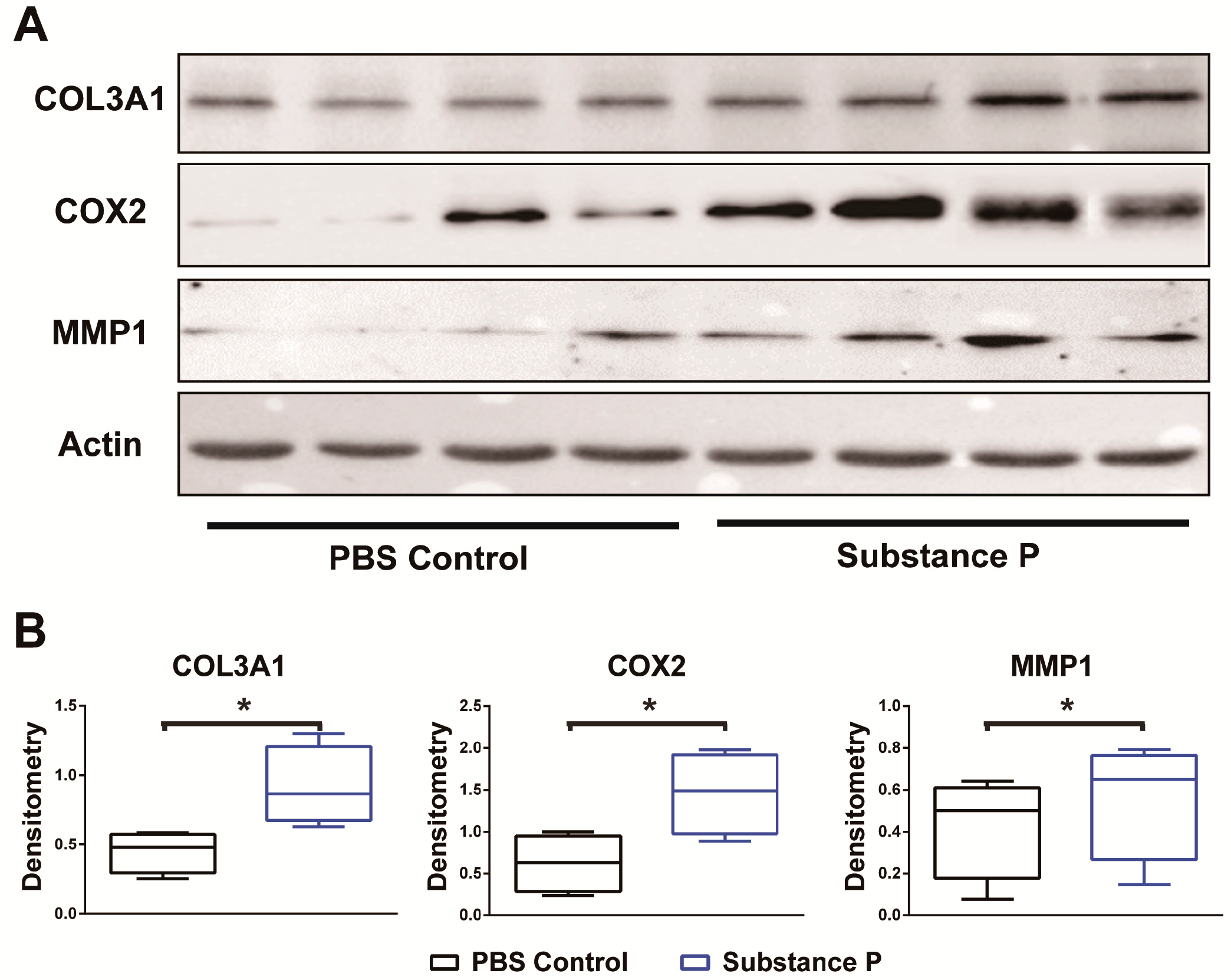
2.2.3. SP Increases the Protein Levels Related to Tendinopathy Development in the Healthy Tenocyte
3. Discussion
3.1. SP Is Closely Related to Tendon Healing
3.2. SP Has a Role in the Development of Tendinopathy
3.3. SP Contributed to the Development of Neurogenic Inflammation
3.4. Limitations
4. Materials and Methods
4.1. Experiment Overview
4.2. Tissue Sampling
4.3. Cell Culture
4.4. Cell Morphology
4.5. Immunocytochemistry (ICC)
4.6. Cell Counting Kit-8 (CCK-8) Assay
4.7. Cell Counting
4.8. RNA Isolation and qRT-PCR
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Maffulli, N.; Wong, J.; Almekinders, L.C. Types and epidemiology of tendinopathy. Clin. Sports Med. 2003, 22, 675–692. [Google Scholar] [CrossRef]
- Lysholm, J.; Wiklander, J. Injuries in runners. Am. J. Sports Med. 1987, 15, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Jozsa, L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J. Bone Jt. Surg. Am. 1991, 73, 1507–1525. [Google Scholar] [CrossRef]
- Kvist, M.; Jozsa, L.; Jarvinen, M.J.; Kvist, H. Chronic Achilles paratenonitis in athletes: A histological and histochemical study. Pathology 1987, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Uchio, Y.; Ochi, M.; Ryoke, K.; Sakai, Y.; Ito, Y.; Kuwata, S. Expression of neuropeptides and cytokines at the extensor carpi radialis brevis muscle origin. J. Shoulder Elbow Surg. 2002, 11, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Fong, G.; Backman, L.J.; Hart, D.A.; Danielson, P.; McCormack, B.; Scott, A. Substance P enhances collagen remodeling and MMP-3 expression by human tenocytes. J. Orthop. Res. 2013, 31, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Backman, L.J.; Fong, G.; Andersson, G.; Scott, A.; Danielson, P. Substance P is a mechanoresponsive, autocrine regulator of human tenocyte proliferation. PLoS ONE 2011, 6, e27209. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, P.W. Neuronal regulation of tendon homoeostasis. Int. J. Exp. Pathol. 2013, 94, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Lian, O.; Dahl, J.; Ackermann, P.W.; Frihagen, F.; Engebretsen, L.; Bahr, R. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am. J. Sports Med. 2006, 34, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Alfonso, V.; Rosello-Sastre, E.; Subias-Lopez, A. Neuroanatomic basis for pain in patellar tendinosis (“jumper’s knee”): A neuroimmunohistochemical study. Am. J. Knee Surg. 2001, 14, 174–177. [Google Scholar] [PubMed]
- Schubert, T.E.; Weidler, C.; Lerch, K.; Hofstadter, F.; Straub, R.H. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann. Rheum. Dis. 2005, 64, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Alpantaki, K.; McLaughlin, D.; Karagogeos, D.; Hadjipavlou, A.; Kontakis, G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J. Bone Jt. Surg. Am. 2005, 87, 1580–1583. [Google Scholar] [CrossRef]
- Fedorczyk, J.M.; Barr, A.E.; Rani, S.; Gao, H.G.; Amin, M.; Amin, S.; Litvin, J.; Barbe, M.F. Exposure-dependent increases in IL-1β, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J. Orthop. Res. 2010, 28, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Singaraju, V.M.; Kang, R.W.; Yanke, A.B.; McNickle, A.G.; Lewis, P.B.; Wang, V.M.; Williams, J.M.; Chubinskaya, S.; Romeo, A.A.; Cole, B.J. Biceps tendinitis in chronic rotator cuff tears: A histologic perspective. J. Shoulder Elbow Surg. 2008, 17, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.; Chan, L.S.; Fu, S.C.; Chan, K.M. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am. J. Sports Med. 2010, 38, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, P.W.; Li, J.; Lundeberg, T.; Kreicbergs, A. Neuronal plasticity in relation to nociception and healing of rat Achilles tendon. J. Orthop. Res. 2003, 21, 432–441. [Google Scholar] [CrossRef]
- Burssens, P.; Steyaert, A.; Forsyth, R.; van Ovost, E.J.; Depaepe, Y.; Verdonk, R. Exogenously administered substance P and neutral endopeptidase inhibitors stimulate fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing. Foot Ankle Int. 2005, 26, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Bring, D.K.; Paulson, K.; Renstrom, P.; Salo, P.; Hart, D.A.; Ackermann, P.W. Residual substance P levels after capsaicin treatment correlate with tendon repair. Wound Repair Regen. 2012, 20, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, O.; Schizas, N.; Li, J.; Ackermann, P.W. Substance P injections enhance tissue proliferation and regulate sensory nerve ingrowth in rat tendon repair. Scand. J. Med. Sci. Sports 2011, 21, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.S.; Lee, J.; Lee, E.; Kwon, Y.S.; Lee, E.; Ahn, W.; Jiang, M.H.; Kim, J.C.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29+ stromal-like cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Z.; Helme, R. Sensory peptides as neuromodulators of wound healing in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 1996, 51, B354–B361. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; von Euler, A.M.; Dalsgaard, C.J. Stimulation of connective tissue cell growth by substance P and substance K. Nature 1985, 315, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Steyaert, A.E.; Burssens, P.J.; Vercruysse, C.W.; Vanderstraeten, G.G.; Verbeeck, R.M. The effects of substance P on the biomechanic properties of ruptured rat Achilles’ tendon. Arch. Phys. Med. Rehabil. 2006, 87, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Morbidelli, L.; Pacini, M.; Geppetti, P.; Alessandri, G.; Maggi, C.A. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc. Res. 1990, 40, 264–278. [Google Scholar] [CrossRef]
- Lotz, M.; Vaughan, J.H.; Carson, D.A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988, 241, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.N.; Wang, Z.G.; Zhu, J.M.; Wang, L.L. Effect of substance P on gene expression of transforming growth factor β-1 and its receptors in rat’s fibroblasts. Chin. J. Traumatol. 2003, 6, 350–354. [Google Scholar] [PubMed]
- Cuesta, M.C.; Quintero, L.; Pons, H.; Suarez-Roca, H. Substance P and calcitonin gene-related peptide increase IL-1β, IL-6 and TNFα secretion from human peripheral blood mononuclear cells. Neurochem. Int. 2002, 40, 301–306. [Google Scholar] [CrossRef]
- Saria, A.; Lundberg, J.M.; Skofitsch, G.; Lembeck, F. Vascular protein linkage in various tissue induced by substance P, capsaicin, bradykinin, serotonin, histamine and by antigen challenge. Naunyn Schmiedebergs Arch. Pharmacol. 1983, 324, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Li, J.; Abdul, A.M.; Ahmed, M.; Ostenson, C.G.; Salo, P.T.; Hewitt, C.; Hart, D.A.; Ackermann, P.W. Compromised neurotrophic and angiogenic regenerative capability during tendon healing in a rat model of type-II diabetes. PLoS ONE 2017, 12, e0170748. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, A.E.; Bragdon, G.A.; Jacobson, J.A.; Hasslund, S.; Cortes, Z.E.; Schwarz, E.M.; Mitten, D.J.; Awad, H.A.; O’Keefe, R.J. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J. Orthop. Res. 2009, 27, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.; Chan, L.S.; Lee, Y.W.; Fu, S.C.; Chan, K.M. Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatol. 2010, 49, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.P.; Harrall, R.L.; Constant, C.R.; Chard, M.D.; Cawston, T.E.; Hazleman, B.L. Tendon degeneration and chronic shoulder pain: Changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 1994, 53, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef]
- Cury, P.R.; Canavez, F.; de Araujo, V.C.; Furuse, C.; de Araujo, N.S. Substance P regulates the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in cultured human gingival fibroblasts. J. Periodontal Res. 2008, 43, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A.; Kydd, A.; Reno, C. Gender and pregnancy affect neuropeptide responses of the rabbit Achilles tendon. Clin. Orthop. Relat. Res. 1999, 365, 237–246. [Google Scholar] [CrossRef]
- Hart, D.A.; Reno, C. Pregnancy alters the in vitro responsiveness of the rabbit medial collateral ligament to neuropeptides: Effect on mRNA levels for growth factors, cytokines, iNOS, COX-2, metalloproteinases and TIMPs. Biochim. Biophys. Acta 1998, 1408, 35–43. [Google Scholar] [CrossRef]
- Gabbiani, G.; Ryan, G.B.; Majne, G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994, 124, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Schurch, W.; Seemayer, T.A.; Gabbiani, G. The myofibroblast: A quarter century after its discovery. Am. J. Surg. Pathol. 1998, 22, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.D.; McCulloch, C.A. Dependence of collagen remodelling on α-smooth muscle actin expression by fibroblasts. J. Cell. Physiol. 1994, 159, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, G. The myofibroblast: A key cell for wound healing and fibrocontractive diseases. Prog. Clin. Biol. Res. 1981, 54, 183–194. [Google Scholar] [PubMed]
- Khan, K.M.; Cook, J.L.; Bonar, F.; Harcourt, P.; Astrom, M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999, 27, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Hamada, K.; Yamakawa, H.; Inoue, A.; Fukuda, H. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J. Orthop. Res. 1998, 16, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; An, H.J.; Song, J.Y.; Shin, D.E.; Kwon, Y.D.; Shim, J.S.; Lee, S.C. Effects of corticosteroid on the expressions of neuropeptide and cytokine mRNA and on tenocyte viability in lateral epicondylitis. J. Inflamm. 2012, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Ljung, B.O.; Alfredson, H.; Forsgren, S. Neurokinin 1-receptors and sensory neuropeptides in tendon insertions at the medial and lateral epicondyles of the humerus. Studies on tennis elbow and medial epicondylalgia. J. Orthop. Res. 2004, 22, 321–327. [Google Scholar] [CrossRef]
- Fearon, A.M.; Twin, J.; Dahlstrom, J.E.; Cook, J.L.; Cormick, W.; Smith, P.N.; Scott, A. Increased substance P expression in the trochanteric bursa of patients with greater trochanteric pain syndrome. Rheumatol. Int. 2014, 34, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Noorbaloochi, S.; Knutson, K.L. Cytokine and neuropeptide levels are associated with pain relief in patients with chronically painful total knee arthroplasty: A pilot study. BMC Musculoskelet. Disord. 2017, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Warner, S.C.; Walsh, D.A.; Laslett, L.L.; Maciewicz, R.A.; Soni, A.; Hart, D.J.; Zhang, W.; Muir, K.R.; Dennison, E.M.; Leaverton, P.; et al. Pain in knee osteoarthritis is associated with variation in the neurokinin 1/substance P receptor (TACR1) gene. Eur. J. Pain 2017. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Santana, A.; Marrero-Hernandez, S.; Dorta, I.; Hernandez, M.; Pinto, F.M.; Baez, D.; Bello, A.R.; Candenas, L.; Almeida, T.A. Altered expression of the tachykinins substance P/neurokinin A/hemokinin-1 and their preferred neurokinin 1/neurokinin 2 receptors in uterine leiomyomata. Fertil. Steril. 2016, 106, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Mousavizadeh, R.; Backman, L.; McCormack, R.G.; Scott, A. Dexamethasone decreases substance P expression in human tendon cells: An in vitro study. Rheumatol. 2015, 54, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Meng, F.; Wu, N.; Zhou, T.; Venter, J.; Francis, H.; Kennedy, L.; Glaser, T.; Bernuzzi, F.; Invernizzi, P.; et al. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology 2017. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.-H.; Choi, W.; Song, J.; Kim, J.; Lee, S.; Choi, Y.; Byun, S.-E.; Ahn, T.; Ahn, H.; Ding, C.; et al. The Implication of Substance P in the Development of Tendinopathy: A Case Control Study. Int. J. Mol. Sci. 2017, 18, 1241. https://doi.org/10.3390/ijms18061241
Han S-H, Choi W, Song J, Kim J, Lee S, Choi Y, Byun S-E, Ahn T, Ahn H, Ding C, et al. The Implication of Substance P in the Development of Tendinopathy: A Case Control Study. International Journal of Molecular Sciences. 2017; 18(6):1241. https://doi.org/10.3390/ijms18061241
Chicago/Turabian StyleHan, Soo-Hong, Wonchul Choi, Jiye Song, Jaehee Kim, Seungyong Lee, Youngrak Choi, Seong-Eun Byun, Taekeun Ahn, Heejung Ahn, Catherine Ding, and et al. 2017. "The Implication of Substance P in the Development of Tendinopathy: A Case Control Study" International Journal of Molecular Sciences 18, no. 6: 1241. https://doi.org/10.3390/ijms18061241
APA StyleHan, S.-H., Choi, W., Song, J., Kim, J., Lee, S., Choi, Y., Byun, S.-E., Ahn, T., Ahn, H., Ding, C., Baik, L., Ward, S., Ting, K., & Lee, S. (2017). The Implication of Substance P in the Development of Tendinopathy: A Case Control Study. International Journal of Molecular Sciences, 18(6), 1241. https://doi.org/10.3390/ijms18061241