Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention
Abstract
:1. Introduction
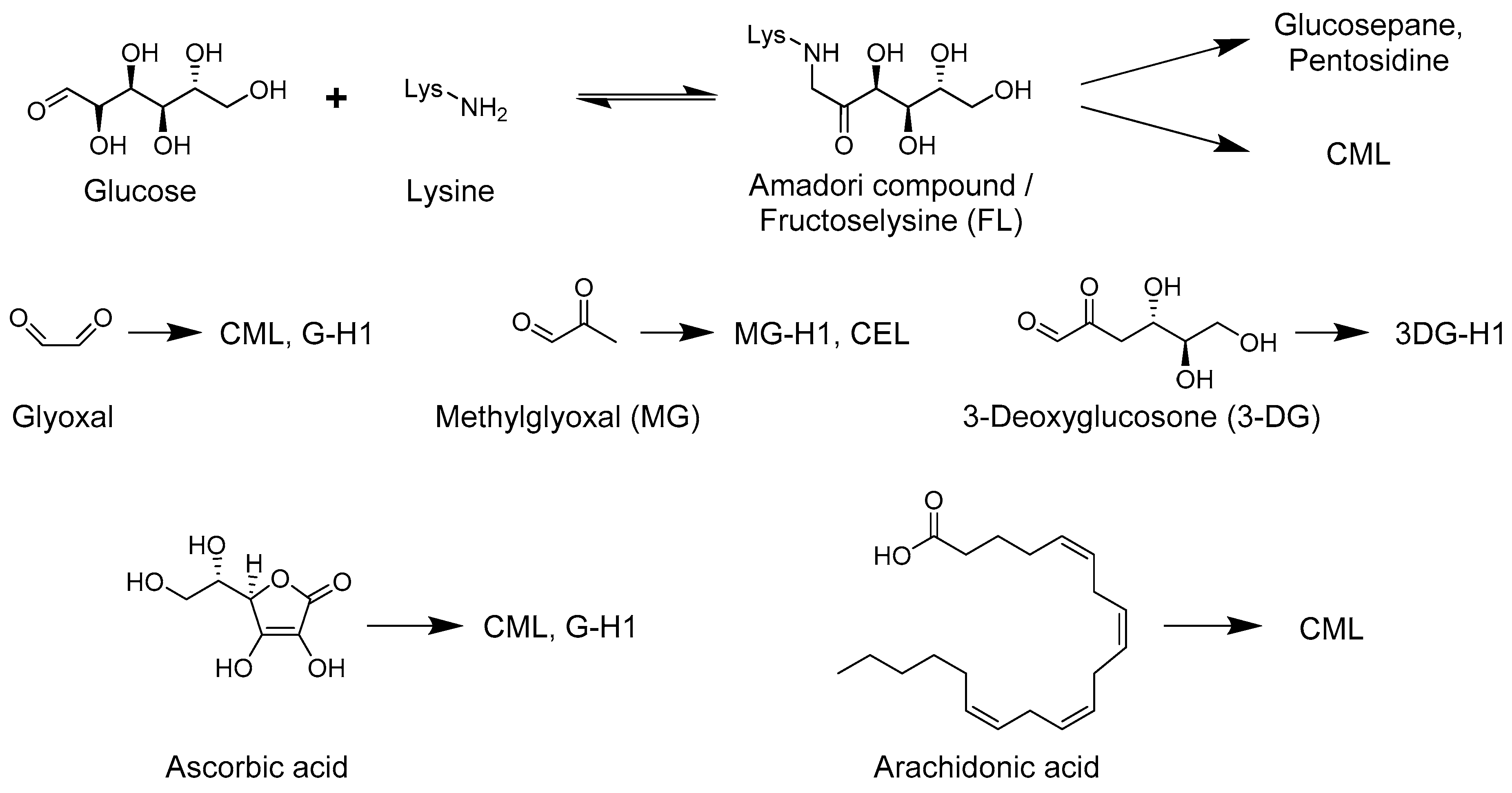
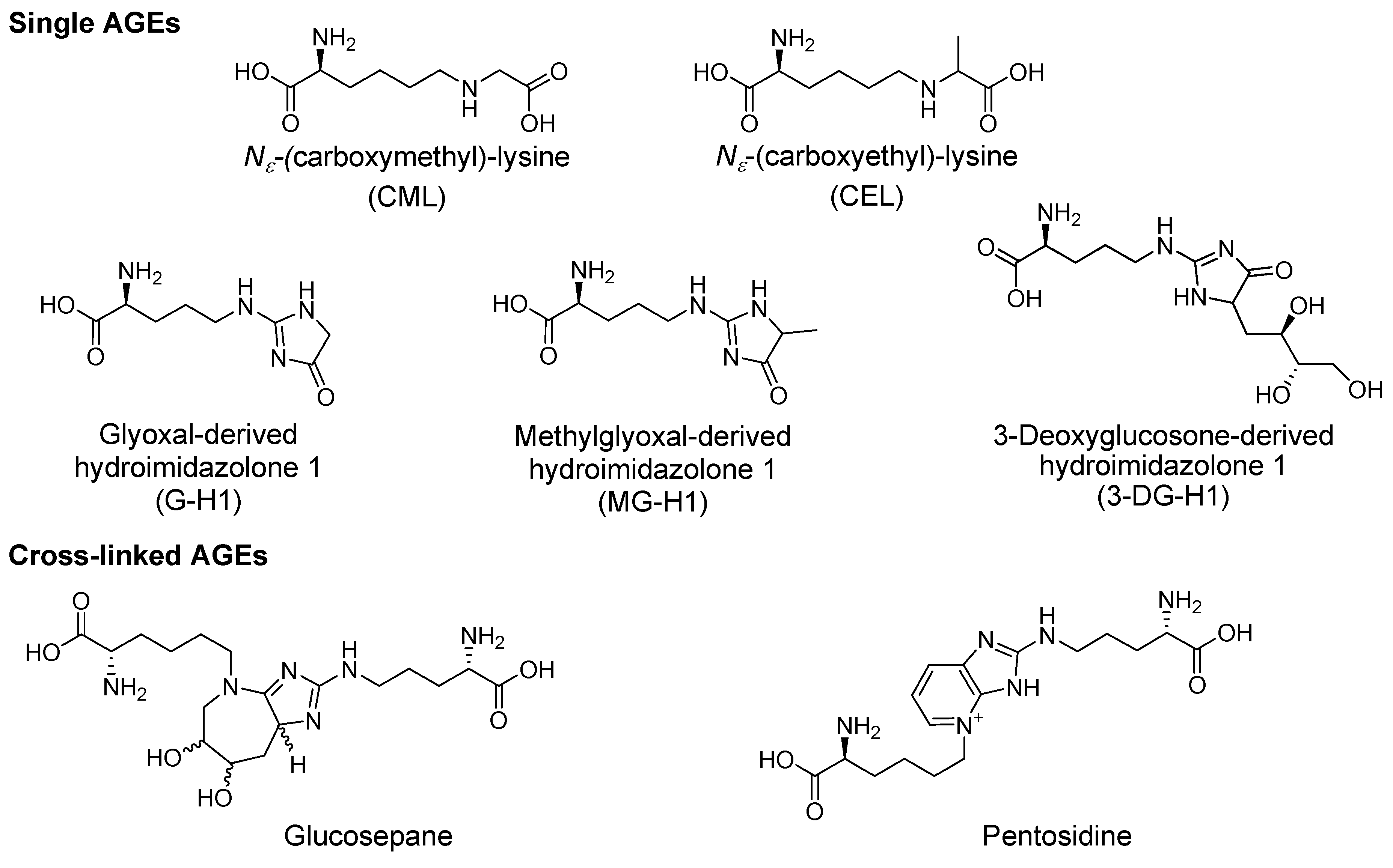
2. Glucose and Dicarbonyl Dependent Advanced Glycation End-Product Formation
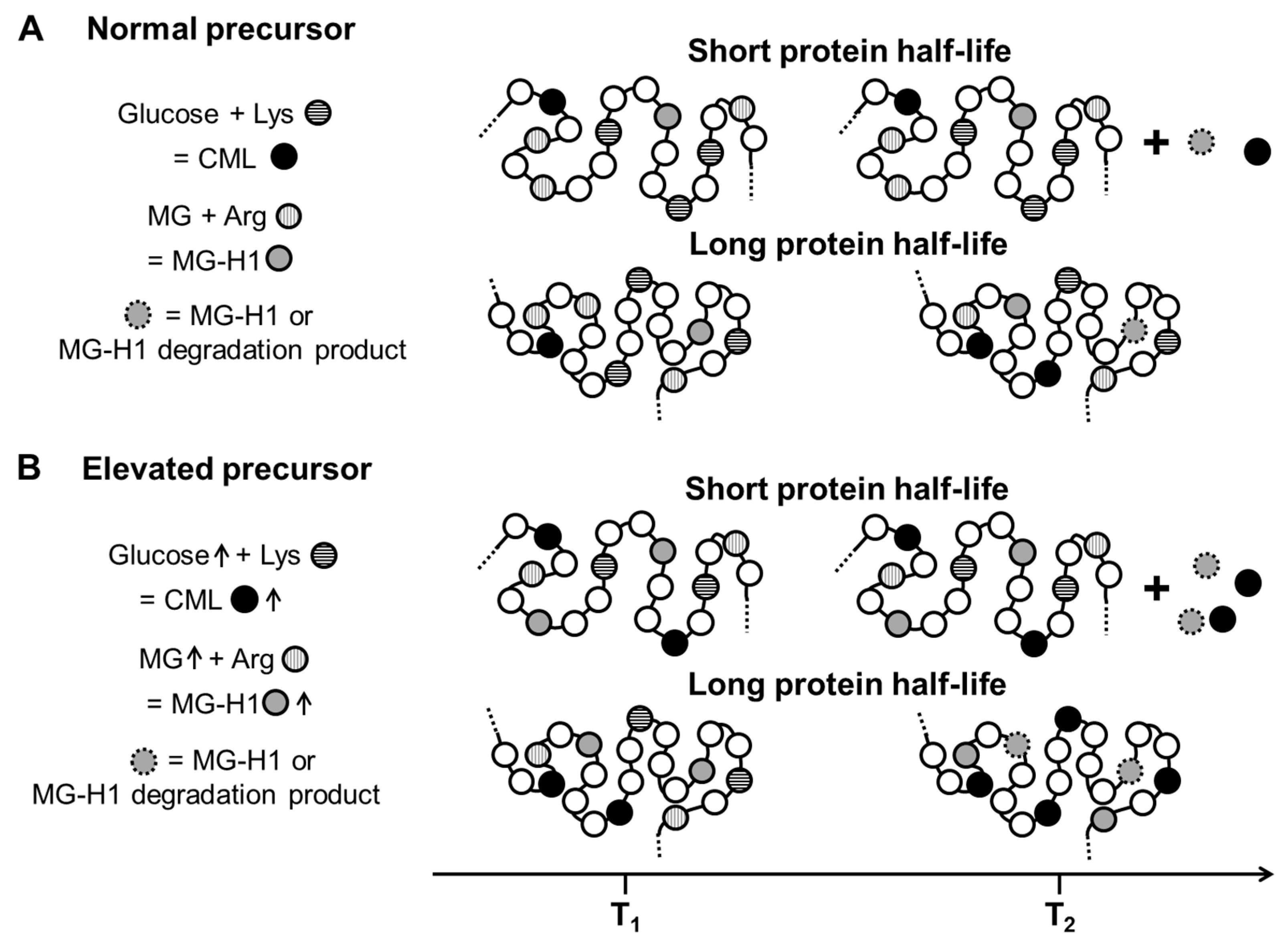
3. Interpretation of Advanced Glycation End-Product Levels In Vivo
4. Dicarbonyls in Diabetes and Relation to Diabetic Complications
4.1. Association of Methylglyoxal with Diabetic Complications
4.2. Association of 3-Deoxyclucosone and Glyoxal with Diabetic Complications
5. Quantification of Dicarbonyls and Advanced Glycation End-Products
6. Pathways of Dicarbonyl and Advanced Glycation End-Product Metabolism
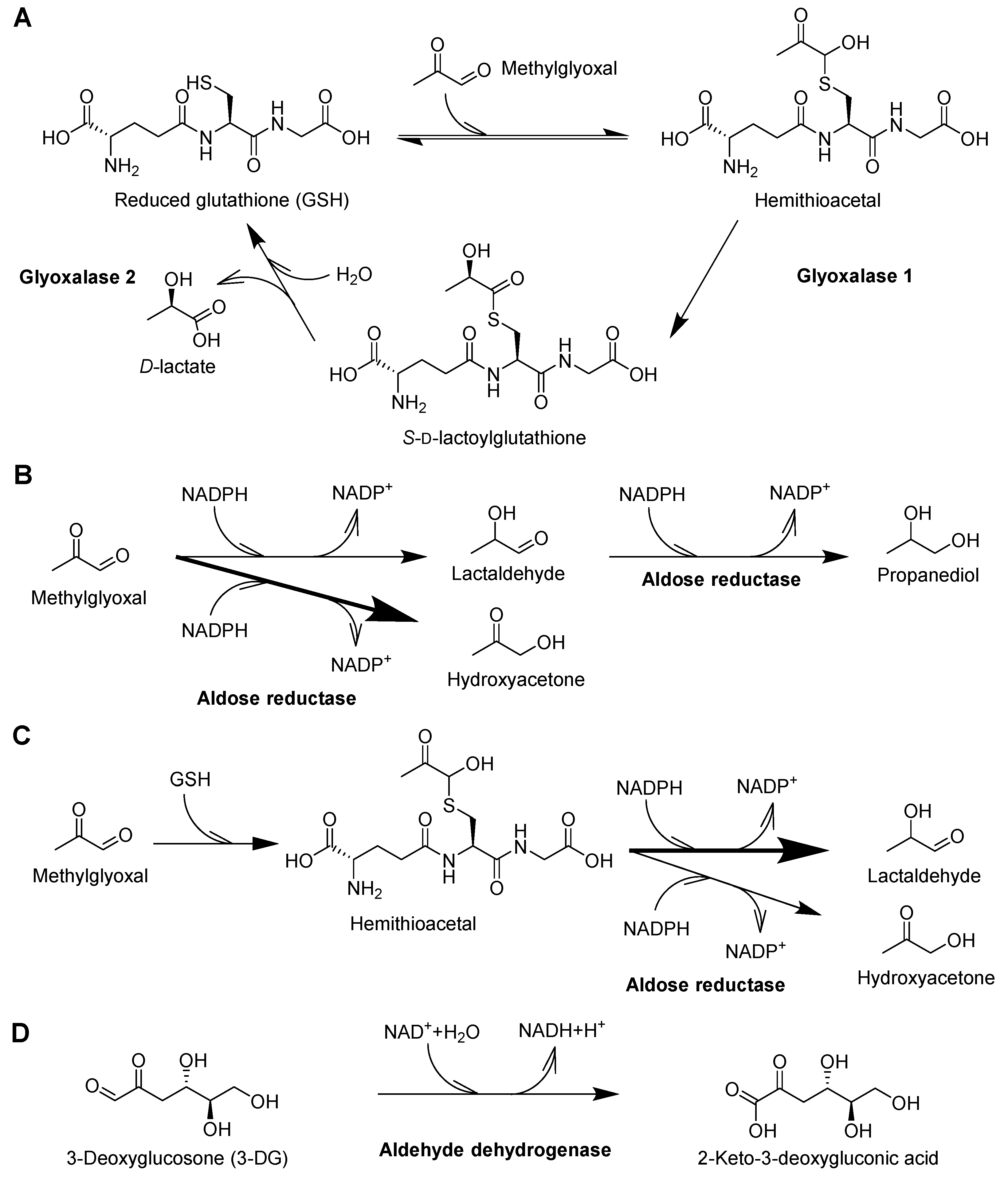
6.1. The Glyoxalase System
6.2. Aldose Reductase
6.3. Aldehyde Dehydrogenase
6.4. Fructosamine-3-kinase
6.5. The Proteolytic System
7. Therapeutic Targeting of Dicarbonyls
7.1. Dicarbonyl Scavengers
7.2. Alternative Advanced Glycation End-Product Lowering Strategies
7.3. Future Perspectives for Therapeutic Advanced Glycation End-Product Targeting
8. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACR | Albumin/creatinine ratio |
| AGE | Advanced glycation end-product |
| CEdA | N2-(1-carboxyethyl)-2′-deoxyadenosine |
| CEdG | N2-(1-carboxyethyl)-2′deoxyguanosine |
| CEL | Nε-(carboxyethyl)lysine |
| CML | Nε-(carboxymethyl)-lysine |
| FL | Fructoselysine |
| FN3K | Fructoseamine-3-kinase |
| GdG | 3-(2′-Deoxyribosyl)-6,7-dihydro-6,7-dihydroxyimidazo-[2,3-b]purine-9(8)one |
| G-H1 | Glyoxal-derived hydroimidazolone 1 |
| Glucosepane | 6-[2-{[(4S)-4-Ammonio-5-oxido-5-oxopentyl]amino}-6,7-dihydroxy-6,7,8,8a-tetrahydroimidazo[4,5-b]-azepin-4(5H)-yl]-l-norleucinate |
| GLO1 | Glyoxalase 1 |
| GLO2 | Glyoxalase 2 |
| GSH | Glutathione |
| HSA | Human serum albumin |
| MG | Methylglyoxal |
| MG-H1 | Methylglyoxal-derived hydroimidazolone 1 |
| MGdG | 3-(2′-Deoxyribosyl)6,7-dihydro-6,7-dihydroxy-6/7-methylimidazo-[2,3-b]purine-9(8)one |
| Pentosidine | (2S)-2-Amino-6-[2-[[(4S)-4-amino-4-carboxybutyl]amino]imidazo[4,5-b]pyridin-4-yl]hexanoic acid |
| STZ | Streptozotocin |
| 3-DG | 3-Deoxyglucosone |
| 3-DG-H1 | 3-Deoxyglucosone-derived hydroimidazolone 1 |
References
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.; Sun, W.; Cleary, P.; Gao, X.; Sell, D.R.; Lachin, J.; Group, D.E.R.; Monnier, V.M. Skin Advanced Glycation End Products Glucosepane and Methylglyoxal Hydroimidazolone Are Independently Associated with Long-Term Microvascular Complication Progression of Type 1 Diabetes. Diabetes 2015, 64, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.; Sun, W.; Cleary, P.; Sell, D.R.; Dahms, W.; Malone, J.; Sivitz, W.; Monnier, V.M. Glycation and Carboxymethyllysine Levels in Skin Collagen Predict the Risk of Future 10-Year Progression of Diabetic Retinopathy and Nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications Participants with Type 1 Diabetes. Diabetes 2005, 54, 3103–3111. [Google Scholar] [PubMed]
- Waris, S.; Winklhofer-Roob, B.M.; Roob, J.M.; Fuchs, S.; Sourij, H.; Rabbani, N.; Thornalley, P.J. Increased DNA Dicarbonyl Glycation and Oxidation Markers in Patients with Type 2 Diabetes and Link to Diabetic Nephropathy. J. Diabetes Res. 2015, 2015, 915486. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Thoss, G.; Hubner-Parajsz, C.; Kientsch-Engel, R.; Stahl, P.; Pischetsrieder, M. Determination of Glycated Nucleobases in Human Urine by a New Monoclonal Antibody Specific for N2-Carboxyethyl-2′-Deoxyguanosine. Chem. Res. Toxicol. 2004, 17, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Henning, C.; Glomb, M.A. Pathways of the Maillard Reaction Under Physiological Conditions. Glycoconj. J. 2016, 33, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced Glycoxidation and Lipoxidation End Products (Ages and Ales): An Overview of Their Mechanisms of Formation. Free Radic. Res. 2013, 47 (Suppl. S1), 3–27. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Ramdas, M.; Goodman, S.; Pyzik, R.; Chen, X.; Zhu, L.; Striker, G.E.; Vlassara, H. Restriction of Advanced Glycation End Products Improves Insulin Resistance in Human Type 2 Diabetes: Potential Role of Ager1 and Sirt1. Diabetes Care 2011, 34, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ramdas, M.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. Oral Advanced Glycation Endproducts (AGEs) Promote Insulin Resistance and Diabetes by Depleting the Antioxidant Defenses Age Receptor-1 and Sirtuin 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15888–15893. [Google Scholar] [CrossRef] [PubMed]
- Maillard, L.C. Action Des Acides Aminés Sur Les Sucres; Formation Des Mélanoïdines Par Voie Méthodique. C. R. Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Dunn, J.A.; Ahmed, M.U.; Murtiashaw, M.H.; Richardson, J.M.; Walla, M.D.; Thorpe, S.R.; Baynes, J.W. Reaction of Ascorbate with Lysine and Protein Under Autoxidizing Conditions: Formation of Nε-(Carboxymethyl)lysine by Reaction between Lysine and Products of Autoxidation of Ascorbate. Biochemistry 1990, 29, 10964–10970. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The Advanced Glycation End Product, Nε-(Carboxymethyl)lysine, Is A Product of Both Lipid Peroxidation and Glycoxidation Reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [PubMed]
- Ahmed, M.U.; Thorpe, S.R.; Baynes, J.W. Identification of N Epsilon-Carboxymethyllysine As A Degradation Product of Fructoselysine in Glycated Protein. J. Biol. Chem. 1986, 261, 4889–4894. [Google Scholar] [PubMed]
- Hunt, J.V.; Bottoms, M.A.; Mitchinson, M.J. Oxidative Alterations in the Experimental Glycation Model of Diabetes Mellitus Are Due to Protein-Glucose Adduct Oxidation. Some Fundamental Differences in Proposed Mechanisms of Glucose Oxidation and Oxidant Production. Biochem. J. 1993, 291, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Namiki, M. Formation of Two-Carbon Sugar Fragment At An Early Stage of the Browning Reaction of Sugar with Amine. Agric. Biol. Chem. 1980, 44, 2575–2580. [Google Scholar]
- Valencia, J.V.; Weldon, S.C.; Quinn, D.; Kiers, G.H.; Degroot, J.; Tekoppele, J.M.; Hughes, T.E. Advanced Glycation End Product Ligands for the Receptor for Advanced Glycation End Products: Biochemical Characterization and Formation Kinetics. Anal. Biochem. 2004, 324, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Duran-Jimenez, B.; Dobler, D.; Moffatt, S.; Rabbani, N.; Streuli, C.H.; Thornalley, P.J.; Tomlinson, D.R.; Gardiner, N.J. Advanced Glycation End Products in Extracellular Matrix Proteins Contribute to the Failure of Sensory Nerve Regeneration in Diabetes. Diabetes 2009, 58, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Wadman, S.K.; De Bree, P.K.; Van Sprang, F.J.; Kamerling, J.P.; Haverkamp, J.; Vliegenthart, J.F.G. Nε-(Carboxymethyl)Lysine, A Constituent of Human Urine. Clin. Chim. Acta 1975, 59, 313–320. [Google Scholar] [CrossRef]
- Thorpe, S.R.; Baynes, J.W. Cml: A Brief History. Int. Congr. Ser. 2002, 1245, 91–99. [Google Scholar] [CrossRef]
- Biemel, K.M.; Reihl, O.; Conrad, J.; Lederer, M.O. Formation Pathways for Lysine-Arginine Cross-Links Derived From Hexoses and Pentoses by Maillard Processes: Unraveling the Structure of A Pentosidine Precursor. J. Biol. Chem. 2001, 276, 23405–23412. [Google Scholar] [CrossRef] [PubMed]
- Biemel, K.M.; Friedl, D.A.; Lederer, M.O. Identification and Quantification of Major Maillard Cross-Links in Human Serum Albumin and Lens Protein. Evidence for Glucosepane As the Dominant Compound. J. Biol. Chem. 2002, 277, 24907–24915. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Yurek-George, A.; Argirov, O.K. Kinetics and Mechanism of the Reaction of Aminoguanidine with the α-Oxoaldehydes Glyoxal, Methylglyoxal, and 3-Deoxyglucosone Under Physiological Conditions. Biochem. Pharmacol. 2000, 60, 55–65. [Google Scholar] [CrossRef]
- Phillips, S.A.; Thornalley, P.J. The Formation of Methylglyoxal From Triose Phosphates. Investigation Using A Specific Assay for Methylglyoxal. Eur. J. Biochem. 1993, 212, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Smuda, M.; Glomb, M.A. Maillard Degradation Pathways of Vitamin C. Angew. Chem. 2013, 52, 4887–4891. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. Where Does Plasma Methylglyoxal Originate From? Diabetes Res. Clin. Pract. 2013, 99, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Wells-Knecht, K.J.; Zyzak, D.V.; Litchfield, J.E.; Thorpe, S.R.; Baynes, J.W. Mechanism of Autoxidative Glycosylation: Identification of Glyoxal and Arabinose As Intermediates in the Autoxidative Modification of Proteins by Glucose. Biochemistry 1995, 34, 3702–3709. [Google Scholar] [CrossRef] [PubMed]
- Glomb, M.A.; Monnier, V.M. Mechanism of Protein Modification by Glyoxal and Glycolaldehyde, Reactive Intermediates of the Maillard Reaction. J. Biol. Chem. 1995, 270, 10017–10026. [Google Scholar] [PubMed]
- Mlakar, A.; Spiteller, G. Previously Unknown Aldehydic Lipid Peroxidation Compounds of Arachidonic Acid. Chem. Phys. Lipids 1996, 79, 47–53. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Richardson, J.M.; Thorpe, S.R.; Baynes, J.W. Formation of Reactive Intermediates From Amadori Compounds Under Physiological Conditions. Arch. Biochem. Biophys. 1995, 316, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Szwergold, B.S.; Kappler, F.; Brown, T.R. Identification of Fructose 3-Phosphate in the Lens of Diabetic Rats. Science 1990, 247, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Delpierre, G.; Van Schaftingen, E. Fructosamine 3-Kinase, An Enzyme Involved in Protein Deglycation. Biochem. Soc. Trans. 2003, 31, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Slatter, D.A.; Bolton, C.H.; Bailey, A.J. The Importance of Lipid-Derived Malondialdehyde in Diabetes Mellitus. Diabetologia 2000, 43, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Slatter, D.A.; Avery, N.C.; Bailey, A.J. Identification of A New Cross-Link and Unique Histidine Adduct From Bovine Serum Albumin Incubated with Malondialdehyde. J. Biol. Chem. 2004, 279, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Requena, J.R.; Fu, M.X.; Ahmed, M.U.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. Quantification of Malondialdehyde and 4-Hydroxynonenal Adducts to Lysine Residues in Native and Oxidized Human Low-Density Lipoprotein. Biochem. J. 1997, 322, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Weisser, J.; Ctortecka, C.; Busch, C.J.; Austin, S.R.; Nowikovsky, K.; Uchida, K.; Binder, C.J.; Bennett, K.L. A Comprehensive Analytical Strategy to Identify Malondialdehyde-Modified Proteins and Peptides. Anal. Chem. 2017, 89, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Kumazawa, S.; Sakurai, T.; Nakayama, T.; Uchida, K. Mass Spectroscopic Characterization of Protein Modification by Malondialdehyde. Chem. Res. Toxicol. 2006, 19, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.W.; Westwood, M.E.; Mclellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and Modification of Proteins by Methylglyoxal under Physiological Conditions. A Kinetic and Mechanistic Study with Nα-Acetylarginine, Nα-Acetylcysteine, and Nα-Acetyllysine, and Bovine Serum Albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar] [PubMed]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative Screening of Advanced Glycation Endproducts in Cellular and Extracellular Proteins by Tandem Mass Spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Smuda, M.; Henning, C.; Raghavan, C.T.; Johar, K.; Vasavada, A.R.; Nagaraj, R.H.; Glomb, M.A. Comprehensive Analysis of Maillard Protein Modifications in Human Lenses: Effect of Age and Cataract. Biochemistry 2015, 54, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Bohme, D.; Singer, D.; Frolov, A. Specific Tandem Mass Spectrometric Detection of Age-Modified Arginine Residues in Peptides. J. Mass Spectrom. 2015, 50, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Pischetsrieder, M.; Seidel, W.; Münch, G.; Schinzel, R. N2-(1-Carboxyethyl)Deoxyguanosine, A Nonenzymatic Glycation Adduct of DNA, Induces Single-Strand Breaks and Increases Mutation Frequencies. Biochem. Biophys. Res. Commun. 1999, 264, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Frischmann, M.; Bidmon, C.; Angerer, J.; Pischetsrieder, M. Identification of DNA Adducts of Methylglyoxal. Chem. Res. Toxicol. 2005, 18, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Biemel, K.M.; Conrad, J.; Lederer, M.O. Unexpected Carbonyl Mobility in Aminoketoses: The Key to Major Maillard Crosslinks. Angew. Chem. 2002, 41, 801–804. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J. Advanced Glycation Endproducts: What Is Their Relevance to Diabetic Complications? Diabetes Obes. Metab. 2007, 9, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Monnier, V.M. Structure Elucidation of A Senescence Cross-Link From Human Extracellular Matrix. Implication of Pentoses in the Aging Process. J. Biol. Chem. 1989, 264, 21597–21602. [Google Scholar] [PubMed]
- Verzijl, N.; Degroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.; Lafeber, F.P.; Baynes, J.W.; Tekoppele, J.M. Effect of Collagen Turnover on the Accumulation of Advanced Glycation End Products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, T.; Barto, R.; Ten Brink, H.J.; Schalkwijk, C.G. Measurement of Nε-(Carboxymethyl)lysine and Nε-(Carboxyethyl)lysine in Human Plasma Protein by Stable-Isotope-Dilution Tandem Mass Spectrometry. Clin. Chem. 2004, 50, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Klopfer, A.; Spanneberg, R.; Glomb, M.A. Formation of Arginine Modifications in A Model System of Nα-tert-Butoxycarbonyl (Boc)-Arginine with Methylglyoxal. J. Agric. Food Chem. 2011, 59, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Argirov, O.K.; Minhas, H.S.; Cordeiro, C.A.; Thornalley, P.J. Assay of Advanced Glycation Endproducts (Ages): Surveying Ages by Chromatographic Assay with Derivatization by 6-Aminoquinolyl-N-Hydroxysuccinimidyl-Carbamate and Application to Nε-Carboxymethyl-Lysine- and Nε-(1-Carboxyethyl)lysine-Modified Albumin. Biochem. J. 2002, 364, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J. Peptide Mapping of Human Serum Albumin Modified Minimally by Methylglyoxal in Vitro and in Vivo. Ann. N. Y. Acad. Sci. 2005, 1043, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Iberg, N.; Fluckiger, R. Nonenzymatic Glycosylation of Albumin in Vivo. Identification of Multiple Glycosylated Sites. J. Biol. Chem. 1986, 261, 13542–13545. [Google Scholar] [PubMed]
- Wolff, S.P.; Dean, R.T. Glucose Autoxidation and Protein Modification. The Potential Role of ‘Autoxidative Glycosylation’ in Diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Dunn, J.A.; Walla, M.D.; Thorpe, S.R.; Baynes, J.W. Oxidative Degradation of Glucose Adducts to Protein. Formation of 3-(Nε-Lysino)-Lactic Acid From Model Compounds and Glycated Proteins. J. Biol. Chem. 1988, 263, 8816–8821. [Google Scholar] [PubMed]
- Dunn, J.A.; Mccance, D.R.; Thorpe, S.R.; Lyons, T.J.; Baynes, J.W. Age-Dependent Accumulation of Nε -(Carboxymethyl)Lysine and Nε-(Carboxymethyl)Hydroxylysine in Human Skin Collagen. Biochemistry 1991, 30, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Genuth, S.; Sell, D.R. The Pecking Order of Skin Advanced Glycation Endproducts (AGEs) As Long-Term Markers of Glycemic Damage and Risk Factors for Micro- and Subclinical Macrovascular Disease Progression in Type 1 Diabetes. Glycoconj. J. 2016, 33, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Flamini, R.; Dalla Vedova, A.; Senesi, A.; Reitano, R.; Fedele, D.; Basso, E.; Seraglia, R.; Traldi, P. Glyoxal and Methylglyoxal Levels in Diabetic Patients: Quantitative Determination by A New GC/MS Method. Clin. Chem. Lab. Med. 2003, 41, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Odani, H.; Shinzato, T.; Matsumoto, Y.; Usami, J.; Maeda, K. Increase in Three α,β-Dicarbonyl Compound Levels in Human Uremic Plasma: Specific in Vivo Determination of Intermediates in Advanced Maillard Reaction. Biochem. Biophys. Res. Commun. 1999, 256, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.; Schalkwijk, C.G. Quantification of Glyoxal, Methylglyoxal and 3-Deoxyglucosone in Blood and Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry: Evaluation of Blood Specimen. Clin. Chem. Lab. Med. 2014, 52, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Randell, E.; Vasdev, S.; Gill, V.; Gadag, V.; Newhook, L.A.; Grant, M.; Hagerty, D. Plasma Methylglyoxal and Glyoxal Are Elevated and Related to Early Membrane Alteration in Young, Complication-Free Patients with Type 1 Diabetes. Mol. Cell. Biochem. 2007, 305, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Ma, M.Z.; Huang, K.; Qin, L.; Zhang, H.M.; Yang, Z.; Li, X.Y.; Su, Q. Increased Plasma Levels of the Methylglyoxal in Patients with Newly Diagnosed Type 2 Diabetes 2. J. Diabetes 2014, 6, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Fleming, T.; Stoyanov, S.; Leffler, A.; Babes, A.; Neacsu, C.; Sauer, S.K.; Eberhardt, M.; Schnolzer, M.; Lasitschka, F.; et al. Methylglyoxal Modification of Nav1.8 Facilitates Nociceptive Neuron Firing and Causes Hyperalgesia in Diabetic Neuropathy. Nat. Med. 2012, 18, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, H.; Miyata, S.; Ohara, T.; Liu, B.F.; Uriuhara, A.; Kojima, H.; Suzuki, K.; Miyazaki, H.; Yamashita, Y.; Inaba, K.; et al. Relation Between Serum 3-Deoxyglucosone and Development of Diabetic Microangiopathy. Diabetes Care 2003, 26, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Wells-Knecht, K.J.; Lyons, T.J.; Mccance, D.R.; Thorpe, S.R.; Feather, M.S.; Baynes, J.W. 3-Deoxyfructose Concentrations Are Increased in Human Plasma and Urine in Diabetes. Diabetes 1994, 43, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Zhang, S.; Choong, Y.S.; Hogl, S.; Middleditch, M.; Kamalov, M.; Brimble, M.A.; Gong, D.; Cooper, G.J. Diabetes-Induced Alterations in Tissue Collagen and Carboxymethyllysine in Rat Kidneys: Association with Increased Collagen-Degrading Proteinases and Amelioration by Cu(II)-Selective Chelation. Biochim. Biophys. Acta 2015, 1852, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Van Eupen, M.G.; Schram, M.T.; Colhoun, H.M.; Hanssen, N.M.; Niessen, H.W.; Tarnow, L.; Parving, H.H.; Rossing, P.; Stehouwer, C.D.; Schalkwijk, C.G. The Methylglyoxal-Derived AGE Tetrahydropyrimidine Is Increased in Plasma of Individuals with Type 1 Diabetes Mellitus and in Atherosclerotic Lesions and Is Associated with Svcam-1. Diabetologia 2013, 56, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Van Eupen, M.G.; Schram, M.T.; Colhoun, H.M.; Scheijen, J.L.; Stehouwer, C.D.; Schalkwijk, C.G. Plasma Levels of Advanced Glycation Endproducts Are Associated with Type 1 Diabetes and Coronary Artery Calcification. Cardiovasc. Diabetol. 2013, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Drummond, K.S.; Nelson, R.G.; Howell, S.K.; Szwergold, B.S.; Mauer, M. Susceptibility to Diabetic Nephropathy Is Related to Dicarbonyl and Oxidative Stress. Diabetes 2005, 54, 3274–3281. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.; Cuny, J.; Nawroth, G.; Djuric, Z.; Humpert, P.M.; Zeier, M.; Bierhaus, A.; Nawroth, P.P. Is Diabetes An Acquired Disorder of Reactive Glucose Metabolites and Their Intermediates? Diabetologia 2012, 55, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; Niessen, P.M.; Miyata, T.; Ostergaard, J.A.; Flyvbjerg, A.; Peutz-Kootstra, C.J.; Sieber, J.; Mundel, P.H.; Brownlee, M.; Janssen, B.J.; et al. Glyoxalase-1 Overexpression Reduces Endothelial Dysfunction and Attenuates Early Renal Impairment in A Rat Model of Diabetes. Diabetologia 2014, 57, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Sveen, K.A.; Karime, B.; Jorum, E.; Mellgren, S.I.; Fagerland, M.W.; Monnier, V.M.; Dahl-Jorgensen, K.; Hanssen, K.F. Small- and Large-Fiber Neuropathy After 40 Years of Type 1 Diabetes: Associations with Glycemic Control and Advanced Protein Glycation: The Oslo Study. Diabetes Care 2013, 36, 3712–3717. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.S.; Jensen, T.M.; Jensen, J.S.; Nawroth, P.; Fleming, T.; Witte, D.R.; Lauritzen, T.; Sandbaek, A.; Charles, M.; Fleischer, J.; et al. The Role of Serum Methylglyoxal on Diabetic Peripheral and Cardiovascular Autonomic Neuropathy: The Addition Denmark Study. Diabet. Med. 2015, 32, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Karachalias, N.; Babaei-Jadidi, R.; Ahmed, N.; Thornalley, P.J. Accumulation of Fructosyl-Lysine and Advanced Glycation End Products in the Kidney, Retina and Peripheral Nerve of Streptozotocin-Induced Diabetic Rats. Biochem. Soc. Trans. 2003, 31, 1423–1425. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Measurement of Methylglyoxal by Stable Isotopic Dilution Analysis LC–MS/MS with Corroborative Prediction in Physiological Samples. Nat. Protoc. 2014, 9, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Beeri, M.S.; Moshier, E.; Schmeidler, J.; Godbold, J.; Uribarri, J.; Reddy, S.; Sano, M.; Grossman, H.T.; Cai, W.; Vlassara, H.; et al. Serum Concentration of An Inflammatory Glycotoxin, Methylglyoxal, Is Associated with Increased Cognitive Decline in Elderly Individuals. Mech. Ageing Dev. 2011, 132, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.M.; Vistisen, D.; Fleming, T.; Nawroth, P.P.; Rossing, P.; Jørgensen, M.E.; Lauritzen, T.; Sandbæk, A.; Witte, D.R. Methylglyoxal Is Associated with Changes in Kidney Function Among Individuals with Screen-Detected Type 2 Diabetes Mellitus. Diabet. Med. 2016, 33, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Randell, E.; Han, Y.; Adeli, K.; Krahn, J.; Meng, Q.H. Increased Plasma Methylglyoxal Level, Inflammation, and Vascular Endothelial Dysfunction in Diabetic Nephropathy. Clin. Biochem. 2011, 44, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Howell, S.K.; Russell, G.B.; Miller, M.E.; Rich, S.S.; Mauer, M. Early Progression of Diabetic Nephropathy Correlates with Methylglyoxal-Derived Advanced Glycation End Products. Diabetes Care 2013, 36, 3234–3239. [Google Scholar] [CrossRef] [PubMed]
- Neuman, R.E.; Logan, M.A. The Determination of Collagen and Elastin in Tissues. J. Biol. Chem. 1950, 186, 549–556. [Google Scholar] [PubMed]
- Peters, T., Jr. Serum Albumin. In Advances in Protein Chemistry; Anfinsen, C.B.J.T.E., Frederic, M.R., Eds.; Academic Press: Cambridge, MA, USA, 1985; Volume 37, pp. 161–245. [Google Scholar]
- Ogawa, S.; Nakayama, K.; Nakayama, M.; Mori, T.; Matsushima, M.; Okamura, M.; Senda, M.; Nako, K.; Miyata, T.; Ito, S. Methylglyoxal Is A Predictor in Type 2 Diabetic Patients of Intima-Media Thickening and Elevation of Blood Pressure. Hypertension 2010, 56, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Nin, J.W.; Jorsal, A.; Ferreira, I.; Schalkwijk, C.G.; Prins, M.H.; Parving, H.H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D. Higher Plasma Levels of Advanced Glycation End Products Are Associated with Incident Cardiovascular Disease and All-Cause Mortality in Type 1 Diabetes: A 12-Year Follow-Up Study. Diabetes Care 2011, 34, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Nakamura, S.; Miyazaki, S.; Morita, T.; Suzuki, M.; Pischetsrieder, M.; Niwa, T. N2-Carboxyethyl-2′-Deoxyguanosine, A DNA Glycation Marker, in Kidneys and Aortas of Diabetic and Uremic Patients. Kidney Int. 2006, 69, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Li, H.; Adijiang, A.; Pischetsrieder, M.; Niwa, T. Pyridoxal Phosphate Prevents Progression of Diabetic Nephropathy. Nephrol. Dial. Transplant. 2007, 22, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Breyer, V.; Frischmann, M.; Bidmon, C.; Schemm, A.; Schiebel, K.; Pischetsrieder, M. Analysis and Biological Relevance of Advanced Glycation End-Products of DNA in Eukaryotic Cells. FEBS J. 2008, 275, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Heizmann, C.W. The Mechanism by Which Dietary AGEs Are A Risk to Human Health Is Via Their Interaction with RAGE: Arguing Against the Motion. Mol. Nutr. Food Res. 2007, 51, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Arguing for the Motion: Yes, RAGE Is A Receptor for Advanced Glycation Endproducts. Mol. Nutr. Food Res. 2007, 51, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Szwergold, B.S.; Howell, S.; Beisswenger, P.J. Human Fructosamine-3-Kinase. Diabetes 2001, 50, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Henning, C.; Liehr, K.; Girndt, M.; Ulrich, C.; Glomb, M.A. Extending the Spectrum of α-Dicarbonyl Compounds in Vivo. J. Biol. Chem. 2014, 289, 28676–28688. [Google Scholar] [CrossRef] [PubMed]
- Gensberger-Reigl, S.; Huppert, J.; Pischetsrieder, M. Quantification of Reactive Carbonyl Compounds in Icodextrin-Based Peritoneal Dialysis Fluids by Combined UHPLC-DAD and -MS/MS Detection. J. Pharm. Biomed. Anal. 2016, 118, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Degen, J.; Vogel, M.; Richter, D.; Hellwig, M.; Henle, T. Metabolic Transit of Dietary Methylglyoxal. J. Agric. Food Chem. 2013, 61, 10253–10260. [Google Scholar] [CrossRef] [PubMed]
- Mclellan, A.C.; Phillips, S.A.; Thornalley, P.J. The Assay of Methylglyoxal in Biological Systems by Derivatization with 1,2-Diamino-4,5-Dimethoxybenzene. Anal. Biochem. 1992, 206, 17–23. [Google Scholar] [CrossRef]
- Morgenstern, J.; Fleming, T.; Schumacher, D.; Eckstein, V.; Freichel, M.; Herzig, S.; Nawroth, P. Loss of Glyoxalase 1 Induces Compensatory Mechanism to Achieve Dicarbonyl Detoxification in Mammalian Schwann Cells. J. Biol. Chem. 2017, 292, 3224–3238. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Ashour, A.; Thornalley, P.J. Mass Spectrometric Determination of Early and Advanced Glycation in Biology. Glycoconj. J. 2016, 33, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Waris, S.; Fleming, T.; Santarius, T.; Larkin, S.J.; Winklhofer-Roob, B.M.; Stratton, M.R.; Rabbani, N. Imidazopurinones Are Markers of Physiological Genomic Damage Linked to DNA Instability and Glyoxalase 1-Associated Tumour Multidrug Resistance. Nucleic Acids Res. 2010, 38, 5432–5442. [Google Scholar] [CrossRef] [PubMed]
- Synold, T.; Xi, B.; Wuenschell, G.E.; Tamae, D.; Figarola, J.L.; Rahbar, S.; Termini, J. Advanced Glycation End Products of DNA: Quantification of N2-(1-Carboxyethyl)-2′-Deoxyguanosine in Biological Samples by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Chem. Res. Toxicol. 2008, 21, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Rabbani, N. Detection of Oxidized and Glycated Proteins in Clinical Samples Using Mass Spectrometry—A User’s Perspective. Biochim. Biophys. Acta 2014, 1840, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Draghici, C.; Wang, T.; Spiegel, D.A. Concise Total Synthesis of Glucosepane. Science 2015, 350, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Koito, W.; Araki, T.; Horiuchi, S.; Nagai, R. Conventional Antibody Against Nε-(Carboxymethyl)Lysine (CML) Shows Cross-Reaction to Nε-(Carboxyethyl)Lysine (CEL): Immunochemical Quantification of CML with A Specific Antibody. J. Biochem. 2004, 136, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.; Smit, A.J. Simple Non-Invasive Assessment of Advanced Glycation Endproduct Accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Lutgers, H.L.; Links, T.P.; Graaff, R.; Baynes, J.W.; Gans, R.O.; Smit, A.J. Skin Autofluorescence Is A Strong Predictor of Cardiac Mortality in Diabetes. Diabetes Care 2007, 30, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Sousa Silva, M.; Gomes, R.A.; Ferreira, A.E.; Ponces Freire, A.; Cordeiro, C. The Glyoxalase Pathway: The First Hundred Years... and Beyond. Biochem. J. 2013, 453, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Dicarbonyl Proteome and Genome Damage in Metabolic and Vascular Disease. Biochem. Soc. Trans. 2014, 42, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Vander Jagt, D.L.; Han, L.P.; Lehman, C.H. Kinetic Evaluation of Substrate Specificity in the Glyoxalase-I-Catalyzed Disproportionation of -Ketoaldehydes. Biochemistry 1972, 11, 3735–3740. [Google Scholar] [CrossRef] [PubMed]
- Vander Jagt, D.L.; Daub, E.; Krohn, J.A.; Han, L.P. Effects of pH and Thiols on the Kinetics of Yeast Glyoxalase I. An Evaluation of the Random Pathway Mechanism. Biochemistry 1975, 14, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Clelland, J.D.; Thornalley, P.J. S-2-Hydroxyacylglutathione-Derivatives: Enzymatic Preparation, Purification and Characterisation. J. Chem. Soc. Perkin Trans. 1 1991, 3009–3015. [Google Scholar] [CrossRef]
- Thornalley, P.J. Glyoxalase I—Structure, Function and A Critical Role in the Enzymatic Defence against Glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyls and Glyoxalase in Disease Mechanisms and Clinical Therapeutics. Glycoconj. J. 2016, 33, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Du, X.; D’agati, V.D.; Milne, R.; Sui, G.; Geoffrion, M.; Brownlee, M. Knockdown of Glyoxalase 1 Mimics Diabetic Nephropathy in Nondiabetic Mice. Diabetes 2014, 63, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Mclellan, A.C.; Thornalley, P.J.; Benn, J.; Sonksen, P.H. Glyoxalase System in Clinical Diabetes Mellitus and Correlation with Diabetic Complications. Clin. Sci. 1994, 87, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Hooper, N.I.; Jennings, P.E.; Florkowski, C.M.; Jones, A.F.; Lunec, J.; Barnett, A.H. The Human Red Blood Cell Glyoxalase System in Diabetes Mellitus. Diabetes Res. Clin. Pract. 1989, 7, 115–120. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in Diabetes, Obesity and Related Disorders. Semin. Cell. Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.M.; Nagaraj, R.H. Upregulation of Glyoxalase I Fails to Normalize Methylglyoxal Levels: A Possible Mechanism for Biochemical Changes in Diabetic Mouse Lenses. Mol. Cell. Biochem. 2006, 288, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Metzger, R.P.; Sauerheber, R.D.; Lyons, S.A.; Westall, J.R. The Effect of Streptozotocin Diabetes on the Levels of Glycolate and Lactate Excreted in Rat Urine. Arch. Biochem. Biophys. 1975, 169, 555–559. [Google Scholar] [CrossRef]
- Vander Jagt, D.L.; Hassebrook, R.K.; Hunsaker, L.A.; Brown, W.M.; Royer, R.E. Metabolism of the 2-Oxoaldehyde Methylglyoxal by Aldose Reductase and by Glyoxalase-I: Roles for Glutathione in Both Enzymes and Implications for Diabetic Complications. Chem. Biol. Interact. 2001, 130–132, 549–562. [Google Scholar] [CrossRef]
- Vander Jagt, D.L.; Hunsaker, L.A.; Young, B.S.; Brown, W.M. Aldo-Keto Reductase-Catalyzed Detoxication of Endogenous Aldehydes Associated with Diabetic Complications. In Aldo-Keto Reductases and Toxicant Metabolism; American Chemical Society: Washington, WA, USA, 2003; Volume 865, pp. 23–35. [Google Scholar]
- Kato, H.; Van Chuyen, N.; Shinoda, T.; Sekiya, F.; Hayase, F. Metabolism of 3-Deoxyglucosone, An Intermediate Compound in the Maillard Reaction, Administered Orally or Intravenously to Rats. Biochim. Biophys. Acta 1990, 1035, 71–76. [Google Scholar] [CrossRef]
- Suzuki, K.; Koh, Y.H.; Mizuno, H.; Hamaoka, R.; Taniguchi, N. Overexpression of Aldehyde Reductase Protects PC12 Cells From the Cytotoxicity of Methylglyoxal or 3-Deoxyglucosone. J. Biochem. 1998, 123, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.P.; Barski, O.A.; Ahmed, Y.; O’toole, T.E.; Conklin, D.J.; Bhatnagar, A.; Srivastava, S. Reductive Metabolism of Age Precursors: A Metabolic Route for Preventing Age Accumulation in Cardiovascular Tissue. Diabetes 2009, 58, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Vander Jagt, D.L. Methylglyoxal, Diabetes Mellitus and Diabetic Complications. Drug Metab. Drug Interact. 2008, 23, 93–124. [Google Scholar]
- Degen, J.; Beyer, H.; Heymann, B.; Hellwig, M.; Henle, T. Dietary Influence on Urinary Excretion of 3-Deoxyglucosone and Its Metabolite 3-Deoxyfructose. J. Agric. Food Chem. 2014, 62, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Fujii, E.; Iwase, H.; Ishii-Karakasa, I.; Yajima, Y.; Hotta, K. The Presence of 2-Keto-3-Deoxygluconic Acid and Oxoaldehyde Dehydrogenase Activity in Human Erythrocytes. Biochem. Biophys. Res. Commun. 1995, 210, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Collard, F.; Vertommen, D.; Fortpied, J.; Duester, G.; Van Schaftingen, E. Identification of 3-Deoxyglucosone Dehydrogenase as Aldehyde Dehydrogenase 1a1 (Retinaldehyde Dehydrogenase 1). Biochimie 2007, 89, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, V.J.; Giesbertz, P.; Wiemer, J.; Bethan, B.; Looser, R.; Liebenberg, V.; Ruiz Noppinger, P.; Daniel, H.; Rein, D. Glyoxylate, A New Marker Metabolite of Type 2 Diabetes. J. Diabetes Res. 2014, 2014, 685204. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, F.W.; Vernochet, C.; O’brien, P.; Spoerl, S.; Brown, J.D.; Nallamshetty, S.; Zeyda, M.; Stulnig, T.M.; Cohen, D.E.; Kahn, C.R.; et al. Retinaldehyde Dehydrogenase 1 Regulates a Thermogenic Program in White Adipose Tissue. Nat. Med. 2012, 18, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Veiga Da-Cunha, M.; Jacquemin, P.; Delpierre, G.; Godfraind, C.; Theate, I.; Vertommen, D.; Clotman, F.; Lemaigre, F.; Devuyst, O.; Van Schaftingen, E. Increased Protein Glycation in Fructosamine 3-Kinase-Deficient Mice. Biochem. J. 2006, 399, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Uchiki, T.; Weikel, K.A.; Jiao, W.; Shang, F.; Caceres, A.; Pawlak, D.; Handa, J.T.; Brownlee, M.; Nagaraj, R.; Taylor, A. Glycation-Altered Proteolysis as A Pathobiologic Mechanism That Links Dietary Glycemic Index, Aging, and Age-Related Disease (in Nondiabetics). Aging Cell 2012, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Knecht, R.; Leber, R.; Hasslacher, C. Degradation of Glomerular Basement Membrane in Diabetes. I. Susceptibility of Diabetic and Nondiabetic Basement Membrane to Proteolytic Degradation of Isolated Glomeruli. Res. Exp. Med. 1987, 187, 323–328. [Google Scholar] [CrossRef]
- Leber, R.; Knecht, R.; Hasslacher, C. Degradation of Glomerular Basement Membrane in Diabetes. II. Proteolytic Activity of Diabetic and Nondiabetic Glomeruli. Res. Exp. Med. 1987, 187, 347–352. [Google Scholar] [CrossRef]
- Grimm, S.; Horlacher, M.; Catalgol, B.; Hoehn, A.; Reinheckel, T.; Grune, T. Cathepsins D and L Reduce the Toxicity of Advanced Glycation End Products. Free Radic. Biol. Med. 2012, 52, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Babaei-Jadidi, R.; Howell, S.K.; Beisswenger, P.J.; Thornalley, P.J. Degradation Products of Proteins Damaged by Glycation, Oxidation and Nitration in Clinical Type 1 Diabetes. Diabetologia 2005, 48, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, R.; Morais, J.A.; Chevalier, S.; Pereira, S.; Lamarche, M.; Marliss, E.B. Determinants of Whole-Body Protein Metabolism in Subjects with and without Type 2 Diabetes. Diabetes Care 2008, 31, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hebert, S.L.; Nair, K.S. Protein and Energy Metabolism in Type 1 Diabetes. Clin. Nutr. 2010, 29, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycaemia in Type 2 Diabetes: A Patient-Centered Approach. Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012, 55, 1577–1596. [Google Scholar] [CrossRef] [PubMed]
- Borg, D.J.; Forbes, J.M. Targeting Advanced Glycation with Pharmaceutical Agents: Where Are We Now? Glycoconj. J. 2016, 33, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M.; Vlassara, H.; Kooney, A.; Ulrich, P.; Cerami, A. Aminoguanidine Prevents Diabetes-Induced Arterial Wall Protein Cross-Linking. Science 1986, 232, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Dhar, I.; Desai, K.M.; Wu, L. Methylglyoxal Scavengers Attenuate Endothelial Dysfunction Induced by Methylglyoxal and High Concentrations of Glucose. Br. J. Pharmacol. 2010, 161, 1843–1856. [Google Scholar] [CrossRef] [PubMed]
- Nyengaard, J.R.; Chang, K.; Berhorst, S.; Reiser, K.M.; Williamson, J.R.; Tilton, R.G. Discordant Effects of Guanidines on Renal Structure and Function and on Regional Vascular Dysfunction and Collagen Changes in Diabetic Rats. Diabetes 1997, 46, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Soulis, T.; Cooper, M.E.; Sastra, S.; Thallas, V.; Panagiotopoulos, S.; Bjerrum, O.J.; Jerums, G. Relative Contributions of Advanced Glycation and Nitric Oxide Synthase Inhibition to Aminoguanidine-Mediated Renoprotection in Diabetic Rats. Diabetologia 1997, 40, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, T.P.; Fu, M.X.; Voss, E.; Reiff, K.; Neidlein, R.; Strein, K.; Thorpe, S.R.; Baynes, J.W.; Reiter, R. Aminoguanidine Inhibits Albuminuria, But Not the Formation of Advanced Glycation End-Products in Skin Collagen of Diabetic Rats. Diabetes Res. Clin. Pract. 1999, 43, 81–89. [Google Scholar] [CrossRef]
- Hammes, H.P.; Martin, S.; Federlin, K.; Geisen, K.; Brownlee, M. Aminoguanidine Treatment Inhibits the Development of Experimental Diabetic Retinopathy. Proc. Natl. Acad. Sci. USA 1991, 88, 11555–11558. [Google Scholar] [CrossRef] [PubMed]
- Bolton, W.K.; Cattran, D.C.; Williams, M.E.; Adler, S.G.; Appel, G.B.; Cartwright, K.; Foiles, P.G.; Freedman, B.I.; Raskin, P.; Ratner, R.E.; et al. Randomized Trial of An Inhibitor of Formation of Advanced Glycation End Products in Diabetic Nephropathy. Am. J. Nephrol. 2004, 24, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.I.; Wuerth, J.P.; Cartwright, K.; Bain, R.P.; Dippe, S.; Hershon, K.; Mooradian, A.D.; Spinowitz, B.S. Design and Baseline Characteristics for the Aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control. Clin. Trials 1999, 20, 493–510. [Google Scholar] [CrossRef]
- Corbett, J.A.; Tilton, R.G.; Chang, K.; Hasan, K.S.; Ido, Y.; Wang, J.L.; Sweetland, M.A.; Lancaster, J.R., Jr.; Williamson, J.R.; Mcdaniel, M.L. Aminoguanidine, A Novel Inhibitor of Nitric Oxide Formation, Prevents Diabetic Vascular Dysfunction. Diabetes 1992, 41, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Bowman, M.A.; Simell, O.G.; Peck, A.B.; Cornelius, J.; Luchetta, R.; Look, Z.; Maclaren, N.K.; Atkinson, M.A. Pharmacokinetics of Aminoguanidine Administration and Effects on the Diabetes Frequency in Nonobese Diabetic Mice. J. Pharmacol. Exp. Ther. 1996, 279, 790–794. [Google Scholar] [PubMed]
- Voziyan, P.A.; Metz, T.O.; Baynes, J.W.; Hudson, B.G. A Post-Amadori Inhibitor Pyridoxamine Also Inhibits Chemical Modification of Proteins by Scavenging Carbonyl Intermediates of Carbohydrate and Lipid Degradation. J. Biol. Chem. 2002, 277, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Voziyan, P.A.; Khalifah, R.G.; Thibaudeau, C.; Yildiz, A.; Jacob, J.; Serianni, A.S.; Hudson, B.G. Modification of Proteins in Vitro by Physiological Levels of Glucose: Pyridoxamine Inhibits Conversion of Amadori Intermediate to Advanced Glycation End-Products Through Binding of Redox Metal Ions. J. Biol. Chem. 2003, 278, 46616–46624. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Murray, D.B.; Metz, T.O.; Baynes, J.W. Chelation: A Fundamental Mechanism of Action of AGE Inhibitors, AGE Breakers, and Other Inhibitors of Diabetes Complications. Diabetes 2012, 61, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, M.; Gohda, T.; Kaneko, S.; Hagiwara, S.; Murakoshi, M.; Aoki, T.; Yamada, K.; Ito, T.; Matsumoto, M.; Horikoshi, S.; et al. Effect of Pyridoxamine (K-163), An Inhibitor of Advanced Glycation End Products, on Type 2 Diabetic Nephropathy in KK-A(y)/Ta Mice. Metab. Clin. Exp. 2007, 56, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, T.P.; Alderson, N.L.; Arrington, D.D.; Beattie, R.J.; Basgen, J.M.; Steffes, M.W.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine Inhibits Early Renal Disease and Dyslipidemia in the Streptozotocin-Diabetic Rat. Kidney Int. 2002, 61, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.; Gardiner, T.A.; Alderson, N.L.; Canning, P.; Frizzell, N.; Duffy, N.; Boyle, C.; Januszewski, A.S.; Chachich, M.; Baynes, J.W.; et al. The AGE Inhibitor Pyridoxamine Inhibits Development of Retinopathy in Experimental Diabetes. Diabetes 2002, 51, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Bolton, W.K.; Khalifah, R.G.; Degenhardt, T.P.; Schotzinger, R.J.; Mcgill, J.B. Effects of Pyridoxamine in Combined Phase 2 Studies of Patients with Type 1 and Type 2 Diabetes and Overt Nephropathy. Am. J. Nephrol. 2007, 27, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Greene, T.; Spitalewiz, S.; Blumenthal, S.; Berl, T.; Hunsicker, L.G.; Pohl, M.A.; Rohde, R.D.; Raz, I.; Yerushalmy, Y.; et al. Pyridorin in Type 2 Diabetic Nephropathy. J. Am. Soc. Nephrol. 2012, 23, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Adrover, M.; Vilanova, B.; Frau, J.; Munoz, F.; Donoso, J. A Comparative Study of the Chemical Reactivity of Pyridoxamine, Ac-Phe-Lys and Ac-Cys with Various Glycating Carbonyl Compounds. Amino Acids 2009, 36, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, R.H.; Sarkar, P.; Mally, A.; Biemel, K.M.; Lederer, M.O.; Padayatti, P.S. Effect of Pyridoxamine on Chemical Modification of Proteins by Carbonyls in Diabetic Rats: Characterization of A Major Product From the Reaction of Pyridoxamine and Methylglyoxal. Arch. Biochem. Biophys. 2002, 402, 110–119. [Google Scholar] [CrossRef]
- Kinsky, O.R.; Hargraves, T.L.; Anumol, T.; Jacobsen, N.E.; Dai, J.; Snyder, S.A.; Monks, T.J.; Lau, S.S. Metformin Scavenges Methylglyoxal to Form A Novel Imidazolinone Metabolite in Humans. Chem. Res. Toxicol. 2016, 29, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; De Buhr, S.; Beijer, B.; Lindner, T.; Wischnjow, A.; Kender, Z.; Peters, V.; Kopf, S.; Haberkorn, U.; et al. A Scavenger Peptide Prevents Methylglyoxal Induced Pain in Mice. Biochim. Biophys. Acta 2016, 1863, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin Reduces Systemic Methylglyoxal Levels in Type 2 Diabetes. Diabetes 1999, 48, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Battah, S.; Ahmed, N.; Thornalley, P.J. Kinetics and Mechanism of the Reaction of Metformin with Methylglyoxal. Int. Congr. Ser. 2002, 1245, 355–356. [Google Scholar] [CrossRef]
- Kender, Z.; Fleming, T.; Kopf, S.; Torzsa, P.; Grolmusz, V.; Herzig, S.; Schleicher, E.; Racz, K.; Reismann, P.; Nawroth, P.P. Effect of Metformin on Methylglyoxal Metabolism in Patients with Type 2 Diabetes. Exp. Clin. Endocrinol. Diabetes 2014, 122, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S.; Zhang, X.; Zhang, X.; Kapurniotu, A.; Bernhagen, J.; Teichberg, S.; Basgen, J.; Wagle, D.; Shih, D.; Terlecky, I.; et al. An Agent Cleaving Glucose-Derived Protein Crosslinks in Vitro and in Vivo. Nature 1996, 382, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Wolffenbuttel, B.H.; Boulanger, C.M.; Crijns, F.R.; Huijberts, M.S.; Poitevin, P.; Swennen, G.N.; Vasan, S.; Egan, J.J.; Ulrich, P.; Cerami, A.; et al. Breakers of Advanced Glycation End Products Restore Large Artery Properties in Experimental Diabetes. Proc. Natl. Acad. Sci. USA 1998, 95, 4630–4634. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Litchfield, J.E.; Baynes, J.W. AGE-Breakers Cleave Model Compounds, But Do Not Break Maillard Crosslinks in Skin and Tail Collagen From Diabetic Rats. Arch. Biochem. Biophys. 2003, 412, 42–46. [Google Scholar] [CrossRef]
- Dhar, A.; Desai, K.M.; Wu, L. Alagebrium Attenuates Acute Methylglyoxal-Induced Glucose Intolerance in Sprague-Dawley Rats. Br. J. Pharmacol. 2010, 159, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Spiegel, D.A. The Unique Reactivity of N-Phenacyl-Derived Thiazolium Salts Toward α-Dicarbonyl Compounds. Rejuvenation Res. 2013, 16, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Thallas-Bonke, V.; Lindschau, C.; Rizkalla, B.; Bach, L.A.; Boner, G.; Meier, M.; Haller, H.; Cooper, M.E.; Forbes, J.M. Attenuation of Extracellular Matrix Accumulation in Diabetic Nephropathy by the Advanced Glycation End Product Cross-Link Breaker ALT-711 Via A Protein Kinase C-α-Dependent Pathway. Diabetes 2004, 53, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M.; et al. Transcriptional Control of Glyoxalase 1 by Nrf2 Provides A Stress-Responsive Defence Against Dicarbonyl Glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved Glycemic Control and Vascular Function in Overweight and Obese Subjects by Glyoxalase 1 Inducer Formulation. Diabetes 2016, 65, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, X.K.; Wang, Y.; Cai, L. The Role of Zinc, Copper and Iron in the Pathogenesis of Diabetes and Diabetic Complications: Therapeutic Effects by Chelators. Hemoglobin 2008, 32, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Wells-Knecht, M.C.; Thorpe, S.R.; Baynes, J.W. Pathways of Formation of Glycoxidation Products During Glycation of Collagen. Biochemistry 1995, 34, 15134–15141. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Nagai, M.; Shimasaki, S.; Baynes, J.W.; Fujiwara, Y. Citric Acid Inhibits Development of Cataracts, Proteinuria and Ketosis in Streptozotocin (Type 1) Diabetic Rats. Biochem. Biophys. Res. Commun. 2010, 393, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.E.; Cotter, M.A. Neurovascular Dysfunction in Diabetic Rats. Potential Contribution of Autoxidation and Free Radicals Examined Using Transition Metal Chelating Agents. J. Clin. Investig. 1995, 96, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.E.; Cotter, M.A. Effects of An Extracellular Metal Chelator on Neurovascular Function in Diabetic Rats. Diabetologia 2001, 44, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Goertz, C.; Boineau, R.; Mark, D.B.; Rozema, T.; Nahin, R.L.; Lindblad, L.; Lewis, E.F.; Drisko, J.; Lee, K.L.; et al. Effect of Disodium EDTA Chelation Regimen on Cardiovascular Events in Patients with Previous Myocardial Infarction: The Tact Randomized Trial. JAMA 2013, 309, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Escolar, E.; Lamas, G.A.; Mark, D.B.; Boineau, R.; Goertz, C.; Rosenberg, Y.; Nahin, R.L.; Ouyang, P.; Rozema, T.; Magaziner, A.; et al. The Effect of An EDTA-Based Chelation Regimen on Patients with Diabetes Mellitus and Prior Myocardial Infarction in the Trial to Assess Chelation Therapy (TACT). Circ. Cardiovasc. Qual. Outcomes 2014, 7, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.J.; Young, A.A.; Gamble, G.D.; Occleshaw, C.J.; Dissanayake, A.M.; Cowan, B.R.; Brunton, D.H.; Baker, J.R.; Phillips, A.R.; Frampton, C.M.; et al. A Copper(II)-Selective Chelator Ameliorates Left-Ventricular Hypertrophy in Type 2 Diabetic Patients: A Randomised Placebo-Controlled Study. Diabetologia 2009, 52, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I. Application of Allometric Principles for the Prediction of Pharmacokinetics in Human and Veterinary Drug Development. Adv. Drug Deliv. Rev. 2007, 59, 1177–1192. [Google Scholar] [CrossRef] [PubMed]
- Gineyts, E.; Cloos, P.A.; Borel, O.; Grimaud, L.; Delmas, P.D.; Garnero, P. Racemization and Isomerization of Type I Collagen C-Telopeptides in Human Bone and Soft Tissues: Assessment of Tissue Turnover. Biochem. J. 2000, 345, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Szwergold, B.S. Fructosamine-6-Phosphates Are Deglycated by Phosphorylation to Fructosamine-3,6-Bisphosphates Catalyzed by Fructosamine-3-Kinase (FN3K) and/Or Fructosamine-3-Kinase-Related-Protein (FN3KRP). Med. Hypotheses 2007, 68, 37–45. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int. J. Mol. Sci. 2017, 18, 984. https://doi.org/10.3390/ijms18050984
Brings S, Fleming T, Freichel M, Muckenthaler MU, Herzig S, Nawroth PP. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. International Journal of Molecular Sciences. 2017; 18(5):984. https://doi.org/10.3390/ijms18050984
Chicago/Turabian StyleBrings, Sebastian, Thomas Fleming, Marc Freichel, Martina U. Muckenthaler, Stephan Herzig, and Peter P. Nawroth. 2017. "Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention" International Journal of Molecular Sciences 18, no. 5: 984. https://doi.org/10.3390/ijms18050984
APA StyleBrings, S., Fleming, T., Freichel, M., Muckenthaler, M. U., Herzig, S., & Nawroth, P. P. (2017). Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. International Journal of Molecular Sciences, 18(5), 984. https://doi.org/10.3390/ijms18050984





