Radiation and Thyroid Cancer
Abstract
:1. Introduction
2. Sensitivity of the Thyroid Gland to Radiation and Cancer Development
3. Genes and Proteins Involved in Radiation-Induced Cancer
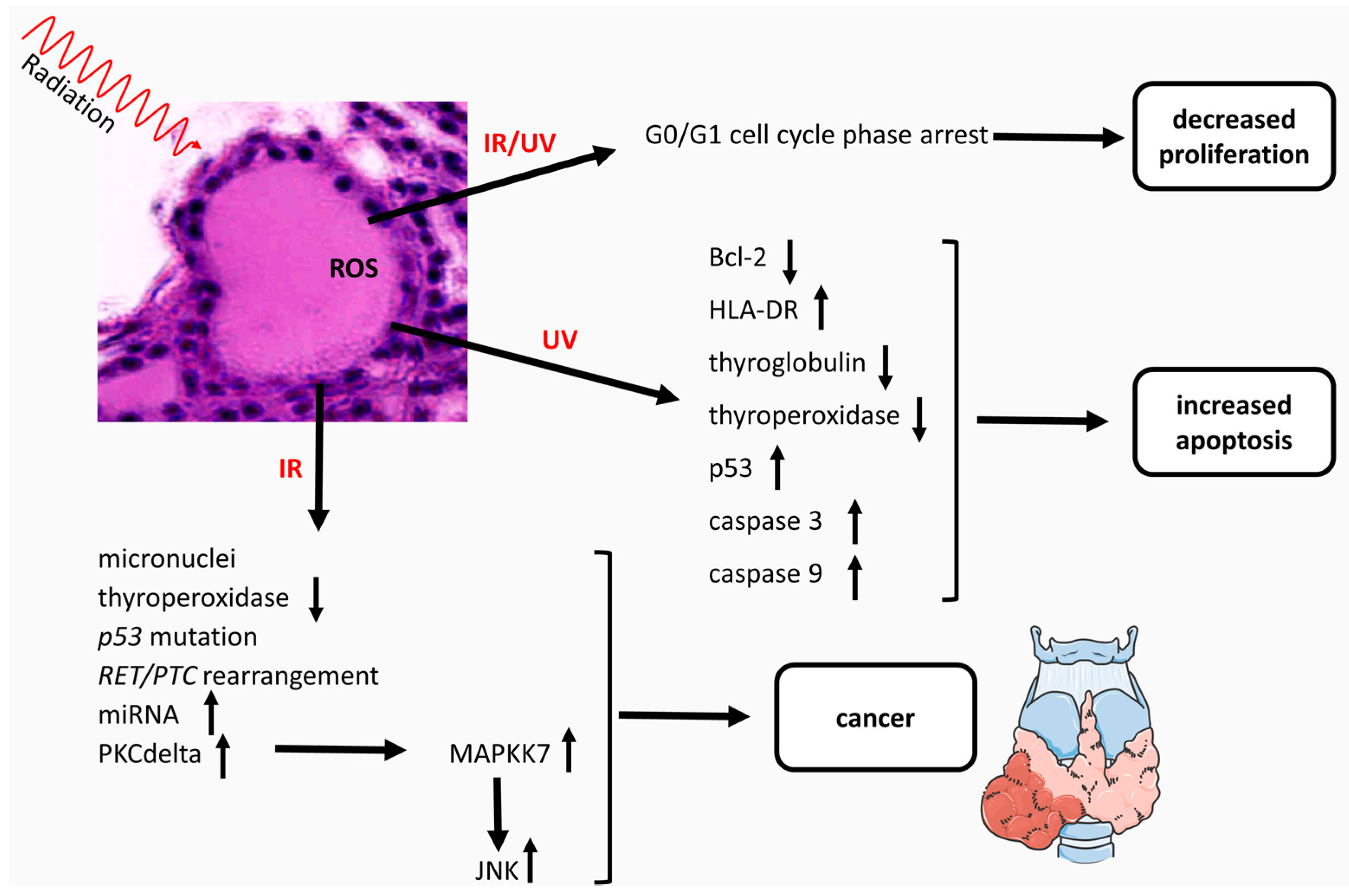
3.1. Ionizing Radiations
3.2. Non-Ionizing Radiations
4. Lipids as Regulators of Radiation-Induced Cancer
4.1. Ionizing Radiation
4.2. Non-Ionizing Radiation
5. Biomarkers of Thyroid Damage
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| A-SMase | Acid-sphingomyelinase |
| DAG | Diacylglycerol |
| HLA-DR | Human leukocyte antigen-DR |
| IR | Ionizing radiation |
| JNK | c-Jun NH2-terminal kinases |
| MAPKK7 | Mitogen-activated protein kinase kinase 7 |
| N-SMase | Neutral-sphingomyelinase |
| PC | Phosphatidylcholine |
| PC-PLC | Phosphatidylcholine-specific phospholipase C |
| PI | Phosphatidylinositol |
| PI-PLC | Phosphatidylinositol-specific phospholipase C |
| PKC | Protein kinase C |
| PTC | Papillary thyroid carcinoma |
| RET | Rearranged during transfection |
| RSM-synthase | Reverse sphingomyelin-synthase |
| SM | Sphingomyelin |
| SM-synthase | Sphingomyelin-synthase |
| UV | Ultraviolet rays |
References
- Port, M.; Herodin, F.; Valente, M.; Drouet, M.; Ullmann, R.; Doucha-Senf, S.; Lamkowski, A.; Majewski, M.; Abend, M. MicroRNA expression for early prediction of late occurring hematologic acute radiation syndrome in baboons. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Kazzi, Z.; Buzzell, J.; Bertelli, L.; Christensen, D. Emergency department management of patients internally contaminated with radioactive material. Emerg. Med. Clin. N. Am. 2015, 33, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Yamoah, K.; Johnstone, P.A. Proton beam therapy: Clinical utility and current status in prostate cancer. OncoTargets Ther. 2016, 16, 5721–5727. [Google Scholar] [CrossRef]
- Frush, D. MO-FG-207A-03: Radiation and cancer perspectives from the trenches: Are we providing care or promoting scare? Med. Phys. 2016, 43, 3714. [Google Scholar] [CrossRef]
- Orton, C.; Borras, C.; Carlson, D. Radiation biology for radiation therapy physicists. Med. Phys. 2014, 41, 532. [Google Scholar] [CrossRef]
- Dent, P.; Yacoub, A.; Contessa, J.; Caron, R.; Amorino, G.; Valerie, K.; Hagan, M.P.; Grant, S.; Schmidt-Ullrich, R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat. Res. 2003, 159, 283–300. [Google Scholar] [CrossRef]
- Rubin, P.; Casarett, G.W. Clinical radiation pathology as applied to curative radiotherapy. Cancer 1968, 22, 767–780. [Google Scholar]
- Paro, J.N.; Zavisić, B.K. Iodine and thyroid gland with or without nuclear catastrophe. Med. Pregl. 2012, 65, 489–495. [Google Scholar]
- Cahoon, E.K.; Nadirov, E.A.; Polanskaya, O.N.; Yauseyenka, V.V.; Velalkin, I.V.; Yeudachkova, T.I.; Maskvicheva, T.I.; Minenko, V.F.; Liu, W.; Drozdovitch, V.; et al. Risk of thyroid nodules in residents of Belarus exposed to Chernobyl fallout as children and adolescents. J. Clin. Endocrinol. Metab. 2017. [Google Scholar] [CrossRef]
- Imaizumi, M.; Ohishi, W.; Nakashima, E.; Sera, N.; Neriishi, K.; Yamada, M.; Tatsukawa, Y.; Takahashi, I.; Fujiwara, S.; Sugino, K.T.; et al. Association of radiation dose with prevalence of thyroid nodules among atomic bomb survivors exposed in childhood (2007–2011). JAMA Intern. Med. 2015, 175, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Nagataki, S. Thyroid consequences of the Fukushima nuclear reactor accident. Eur. Thyroid J. 2012, 1, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Preston, D.; Funamoto, S.; Yonehara, S.; Ito, M.; Tokuoka, S.; Sugiyama, H.; Soda, M.; Ozasa, K.; Mabuchi, K. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 Years after exposure. Int. J. Cancer 2013, 132, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Lagarde, F.; Tsuda, N.; Funamoto, S.; Preston, D.L.; Koyama, K.; Mabuchi, K.; Ron, E.; Kodama, K.; Tokuoka, S. Papillary microcarcinoma of the thyroid among atomic bomb survivors: Tumor characteristics and radiation risk. Cancer 2010, 116, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.B. Exposure to ionizing radiation in adulthood and thyroid cancer incidence. Epidemiology 2009, 20, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.; Lubin, J.H.; Shore, R.E.; Mabuchi, K.; Modan, B.; Pottern, L.M.; Schneider, A.B.; Tucker, M.A.; Boice, J.D. Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. J. Radiat. Res. 1995, 141, 259–277. [Google Scholar] [CrossRef]
- Holm, L.E. Radiation-induced thyroid neoplasia. Sozial und Präventivmedizin 1991, 36, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Cardis, E.; Kesminiene, A.; Ivanov, V.; Malakhova, I.; Shibata, Y.; Khrouch, V.; Drozdovitch, V.; Maceika, E.; Zvonova, I.; Vlassov, O.; et al. Risk of thyroid cancer after exposure to 131I in childhood. J. Natl. Cancer Inst. 2005, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.; Schneider, A.B. Thyroid cancer following exposure to radioactive iodine. Rev. Endocr. Metab. Disord. 2000, 3, 197–203. [Google Scholar] [CrossRef]
- Tonorezos, E.S.; Barnea, D.; Moskowitz, C.S.; Chou, J.F.; Sklar, C.A.; Elkin, E.B.; Wong, R.J.; Li, D.; Tuttle, R.M.; Korenstein, D.; et al. Screening for thyroid cancer in survivors of childhood and young adult cancer treated with neck radiation. J. Cancer Surviv. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Gooi, Z.; Mydlarz, W.K.; Agrawal, N. Risk of thyroid malignancy following an index head and neck squamous cell carcinoma: A population-based study. Ear Nose Throat J. 2016, 95, E7–E11. [Google Scholar] [PubMed]
- Borek, C. Molecular mechanisms in cancer induction and prevention. Environ. Health Perspect. 1993, 101, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Radiation interactions with biological system. Int. J. Radiat. Biol. 2017, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, O.V.; Pavlov, A.V.; Korableva, T.V. Cytogenetic effects in follicular epithelium of thyroid gland under prolonged exposure to gamma-radiation at low-doses. Radiat. Biol. Radioecol. 2008, 48, 160–166. [Google Scholar]
- Christov, K. Effect of irradiation on the proliferation kinetics of thyroid follicular cells in infant rats. Exp. Pathol. 1982, 21, 117–122. [Google Scholar] [CrossRef]
- Russo, E.; Guerra, A.; Marotta, V.; Faggiano, A.; Colao, A.; del Vecchio, S.; Tonacchera, M.; Vitale, M. Radioiodide induces apoptosis in human thyroid tissue in culture. Endocrine 2013, 44, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Blasko, I.; Sztankay, A.; Lukas, P.; Grubeck-Loebenstein, B. Decreased thyroid peroxidase expression in cultured thyrocytes after external gamma irradiation. Exp. Clin. Endocrinol. Diabetes 2000, 108, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Guernsey, D.L.; Ong, A.; Borek, C. Thyroid hormone modulation of X ray-induced in vitro neoplastic transformation. Nature 1980, 288, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Kyoizumi, S.; Suzuki, T.; Iwamoto, K.S.; Seyama, T. Continued expression of a tissue specific activated oncogene in the early steps of radiation-induced human thyroid carcinogenesis. Oncogene 1997, 15, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Gamble, S.C.; Cook, M.C.; Riches, A.C.; Herceg, Z.; Bryant, P.E.; Arrand, J.E. p53 mutations in tumors derived from irradiated human thyroid epithelial cells. Mutat. Res. 1999, 425, 231–238. [Google Scholar] [CrossRef]
- Hara, T.; Namba, H.; Yang, T.T.; Nagayama, Y.; Fukata, S.; Kuma, K.; Ishikawa, N.; Ito, K.; Yamashita, S. Ionizing radiation activates c-Jun NH2-terminal kinase (JNK/SAPK) via a PKC-dependent pathway in human thyroid cells. Biochem. Biophys. Res. Commun. 1998, 244, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Mitsutake, N.; Namba, H.; Shklyaev, S.S.; Tsukazaki, T.; Ohtsuru, A.; Ohba, M.; Kuroki, T.; Ayabe, H.; Yamashita, S. PKC delta mediates ionizing radiation-induced activation of c-Jun NH2-terminal kinase through MKK7 in human thyroid cells. Oncogene 2001, 20, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Caudill, C.M.; Zhu, Z.; Ciampi, R.; Stringer, J.R.; Nikiforov, Y.E. Dose-dependent generation of RET/papillary thyroid carcinoma in human thyroid cells after in vitro exposure to γ-radiation: A model of carcinogenic chromosomal rearrangement induced by ionizing radiation. J. Clin. Endocrinol. Metab. 2005, 90, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Ameziane-El-Hassani, R.; Boufraqech, M.; Lagente-Chevallier, O.; Weyemi, U.; Talbot, M.; Métivier, D.; Courtin, F.; Bidart, J.M.; El Mzibri, M.; Schlumberger, M.; et al. Role of H2O2 in RET/PTC1 chromosomal rearrangement produced by ionizing radiation in human thyroid cells. Cancer Res. 2010, 70, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, A.; D’Agati, V.; Larsson-Blomberg, L.; Costantini, F.; Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 1994, 367, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Hamatani, K.; Eguchi, H.; Ito, R.; Mukai, M.; Takahashi, K.; Taga, M.; Imai, K.; Cologne, J.; Soda, M.; Arihiro, K.; et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008, 68, 7176–7182. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Iwamoto, K.S.; Kyoizumi, S.; Nagamura, H.; Shinohara, T.; Koyama, K.; Seyama, T.; Hamatani, K. Preferential induction of RET/PTC1 rearrangement by X-ray irradiation. Oncogene 2000, 19, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Daliri, M.; Abbaszadegan, M.R.; Bahar, M.M.; Arabi, A.; Yadollahi, M.; Ghafari, A.; Taghehchian, N.; Zakavi, S.R. The role of BRAF V600E mutation as a potential marker for prognostic stratification of papillary thyroid carcinoma: A long-term follow-up study. Endocr. Res. 2014, 39, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Ji, M.; Bao, R.; Yu, H.; Wang, Y.; Hou, P.; Zhang, Y.; Shan, Z.; Teng, W.; Xing, M. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2009, 94, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Kondo, T.; Nakazawa, T.; Mochizuki, K.; Inoue, T.; Kasai, K.; Tahara, I.; Yabuta, T.; Hirokawa, M.; Miyauchi, A.; et al. Frequent BRAF V600E and absence of TERT promoter mutations characterize sporadic pediatric papillary thyroid carcinomas in Japan. Endocr. Pathol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cordioli, M.I.; Moraes, L.; Bastos, A.U.; Besson, P.; Alves, M.T.; Delcelo, R.; Monte, O.; Longui, C.; Cury, A.N.; Cerutti, J.M. Fusion Oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid 2017, 27, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Tronko, M.; Bogdanova, T.; Voskoboynyk, L.; Zurnadzhy, L.; Shpak, V.; Gulak, L. Radiation induced thyroid cancer: Fundamental and applied aspects. Exp. Oncol. 2010, 32, 200–204. [Google Scholar] [PubMed]
- Selmansberger, M.; Braselmann, H.; Hess, J.; Bogdanova, T.; Abend, M.; Tronko, M.; Brenner, A.; Zitzelsberger, H.; Unger, K. Genomic copy number analysis of Chernobyl papillary thyroid carcinoma in the Ukrainian-American Cohort. Carcinogenesis 2015, 36, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Eguchi, H.; Arihiro, K.; Ito, R.; Koyama, K.; Soda, M.; Cologne, J.; Hayashi, Y.; Nakata, Y.; Nakachi, K.; et al. The presence of BRAF point mutation in adult papillary thyroid carcinomas from atomic bomb survivors correlates with radiation dose. Mol. Carcinog. 2007, 46, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Mitsutake, N.; Fukushima, T.; Matsuse, M.; Rogounovitch, T.; Saenko, V.; Uchino, S.; Ito, M.; Suzuki, K.; Suzuki, S.; Yamashita, S. BRAFV600E mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: A different oncogenic profile from Chernobyl. Sci. Rep. 2015, 5, 16976. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Tseng, G.C.; Steward, D.; Diorio, D.; Nikiforov, Y.E. MicroRNA expression profiling of thyroid tumors: Biological significance and diagnostic utility. J. Clin. Endocrinol. Metab. 2008, 93, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, X.; Lim, L.P.; de Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Gandhi, M.; Kelly, L.; Nikiforov, Y.E. MicroRNA dysregulation in human thyroidcells following exposure to ionizing radiation. Thyroid 2011, 21, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Del Terra, E.; Francesconi, A.; Meli, A.; Ambesi-Impiombato, F.S. Radiation-dependent apoptosis on cultured thyroid cells. Phys. Med. 2001, 17 (Suppl. S1), 261–263. [Google Scholar] [PubMed]
- Kostic, I.; Toffoletto, B.; Toller, M.; Beltrami, C.A.; Ambesi-Impiombato, F.S.; Curcio, F. UVC radiation-induced effect on human primary thyroid cell proliferation and HLA-DR expression. Horm. Metab. Res. 2010, 42, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; D’Armiento, M.; Sorrenti, S.; del Sordo, M.; Mocini, R.; Morrone, S.; Gnessi, L.; Curcio, F.; Ulisse, S. Effects of ultravioletradiation on FRTL-5 cell growth and thyroid-specific gene expression. Astrobiology 2013, 13, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Del Terra, E.; Francesconi, A.; Donnini, D.; Curcio, F.; Ambesi-Impiombato, F.S. Thyrotropin effects on ultravioletradiation-dependent apoptosis in FRTL-5 cells. Thyroid 2003, 13, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Cataldi, S.; Rossi, G.; Viola Magni, M.; Toller, M.; Casani, S.; Perrella, G. The nuclear ceramide/diacylglycerol balance depends on the physiological state of thyroidcells and changes during UV-C radiation-induced apoptosis. Arch. Biochem. Biophys. 2008, 478, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Lazzarini, R.; Viola Magni, M. Phosphatidylcholine/sphingomyelinmetabolismcrosstalk inside the nucleus. Biochem. J. 2008, 410, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Rossi, G.; Maraldi, N.M.; Viola Magni, M.; Cataldi, S.; Solimando, L.; Zini, N. Involvement of nuclear phosphatidylinositol-dependent phospholipases C in cell cycle progression during rat liver regeneration. J. Cell. Physiol. 2003, 197, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.; Takamura, N.; Shklyaev, S.; Nagayama, Y.; Ohtsuru, A.; Namba, H.; Yamashita, S. Ceramide-induced apoptosis of human thyroid cancer cells resistant to apoptosis by irradiation. Thyroid 2000, 10, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Perrella, G.; Lazzarini, A.; Cataldi, S.; Lazzarini, R.; Floridi, A.; Ambesi-Impiombato, F. S.; Curcio, F. Critical role for the protons in FRTL-5 thyroid cells: Nuclear sphingomyelinase induced-damage. Int. J. Mol. Sci. 2014, 15, 11555–11565. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Viola Magni, M.P. The role of intranuclear lipids. Biol. Cell 2004, 96, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Cascianelli, G.; Villani, M.; Tosti, M.; Marini, F.; Bartoccini, E.; Magni, M.V.; Albi, E. Lipid microdomains in cell nucleus. Mol. Biol. Cell 2008, 19, 5289–5295. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Villani, M. Nuclear lipid microdomains regulate cell function. Commun. Integr. Biol. 2009, 2, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Lazzarini, A.; Lazzarini, R.; Floridi, A.; Damaskopoulou, E.; Curcio, F.; Cataldi, S. Nuclear lipid microdomain as place of interaction between sphingomyelin and DNA during liver regeneration. Int. J. Mol. Sci. 2013, 14, 6529–6541. [Google Scholar] [CrossRef] [PubMed]
- Bartoccini, E.; Marini, F.; Damaskopoulou, E.; Lazzarini, R.; Cataldi, S.; Cascianelli, G.; Gil Garcia, M.; Albi, E. Nuclear lipid microdomain sregulate nuclear vitamin D3 uptake and influence embryonic hippocampal cell differentiation. Mol. Biol. Cell 2011, 22, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Codini, M.; Cascianelli, G.; Tringali, S.; Tringali, A.R.; Lazzarini, A.; Floridi, A.; Bartoccini, E.; Garcia-Gil, M.; Lazzarini, R.; et al. Nuclear lipid microdomain as resting place of dexamethasone to impair cell proliferation. Int. J. Mol. Sci. 2014, 15, 19832–19846. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Cataldi, S.; Villani, M.; Perrella, G. Nuclear phosphatidylcholine and sphingomyelin metabolism of thyroid cells changes during stratospheric balloon flight. J. Biomed. Biotechnol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Iarmarcovai, G.; Ceppi, M.; Botta, A.; Orsière, T.; Bonassi, S. Micronuclei frequency in peripheral blood lymphocytes of cancer patients: A meta-analysis. Mutat. Res. 2008, 659, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Vrndić, O.B.; Milošević-Djordjević, O.M.; Mijatović Teodorović, L.C.; Jeremić, M.Z.; Stošić, I.M.; Grujicić, D.V.; Zivancević Simonović, S.T. Correlation betweenmicronuclei frequency in peripheral blood lymphocytes and retention of 131-I in thyroid cancer patients. Tohoku J. Exp. Med. 2013, 229, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Dom, G.; Tarabichi, M.; Unger, K.; Thomas, G.; Oczko-Wojciechowska, M.; Bogdanova, T.; Jarzab, B.; Dumont, J.E.; Detours, V.; Maenhaut, C. A gene expression signature distinguishes normal tissues of sporadic and radiation-induced papillary thyroid carcinomas. Br. J. Cancer 2012, 107, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Port, M.; Boltze, C.; Wang, Y.; Röper, B.; Meineke, V.; Abend, M. A radiation-induced gene signature distinguishes post-Chernobyl from sporadic papillary thyroid cancers. Radiat. Res. 2007, 168, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Maenhaut, C.; Detours, V.; Dom, G.; Handkiewicz-Junak, D.; Oczko-Wojciechowska, M.; Jarzab, B. Gene expression profiles for radiation-induced thyroid cancer. Clin. Oncol. 2011, 23, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.C.; Meckbach, R.; Eidemüller, M.; Selmansberger, M.; Unger, K.; Shpak, V.; Blettner, M.; Zitzelsberger, H.; Jacob, P. Integration of a radiation biomarker into modeling of thyroid carcinogenesis and post-Chernobyl risk assessment. Carcinogenesis 2016, 37, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Selmansberger, M.; Kaiser, J.C.; Hess, J.; Güthlin, D.; Likhtarev, I.; Shpak, V.; Tronko, M.; Brenner, A.; Abend, M.; Blettner, M.; et al. Dose-dependent expression of CLIP2 in post-Chernobyl papillary thyroid carcinomas. Carcinogenesis 2015, 36, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Selmansberger, M.; Feuchtinger, A.; Zurnadzhy, L.; Michna, A.; Kaiser, J.C.; Abend, M.; Brenner, A.; Bogdanova, T.; Walch, A.; Unger, K.; et al. CLIP2 as radiation biomarker in papillary thyroid carcinoma. Oncogene 2015, 34, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Das, S.C.; Isichei, U.P. Serum and thyroid tissue lipids in patients with thyroid tumors in euthyroidism. Indian J. Exp. Biol. 1989, 27, 538–544. [Google Scholar] [PubMed]
- Raffelt, K.; Moka, D.; Süllentrop, F.; Dietlein, M.; Hahn, J.; Schicha, H. Systemic alterations in phospholipid concentrations of blood plasma in patients with thyroid carcinoma: An in-vitro 31P high-resolution NMR study. NMR Biomed. 2000, 13, 8–13. [Google Scholar] [CrossRef]
- Guo, S.; Qiu, L.; Wang, Y.; Qin, X.; Liu, H.; He, M.; Zhang, Y.; Li, Z.; Chen, X. Tissue imaging and serum lipidomic profiling for screening potential biomarkers of thyroid tumors by matrix-assisted laser desorption/ionization-Fourier transform ion cyclotron resonance mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 4357–4370. [Google Scholar] [CrossRef] [PubMed]
- Rath, G.; Schneider, C.; Langlois, B.; Sartelet, H.; Morjani, H.; Btaouri, H.E.; Dedieu, S.; Martiny, L. De novo ceramide synthesis is responsible for the anti-tumor properties of camptothecin and doxorubicin in follicular thyroid carcinoma. Int. J. Biochem. Cell Biol. 2009, 41, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Törnquist, K. Sphingosine 1-phosphate and cancer: Lessons from thyroid cancer cells. Biomolecules 2013, 3, 303–315. [Google Scholar] [CrossRef] [PubMed]
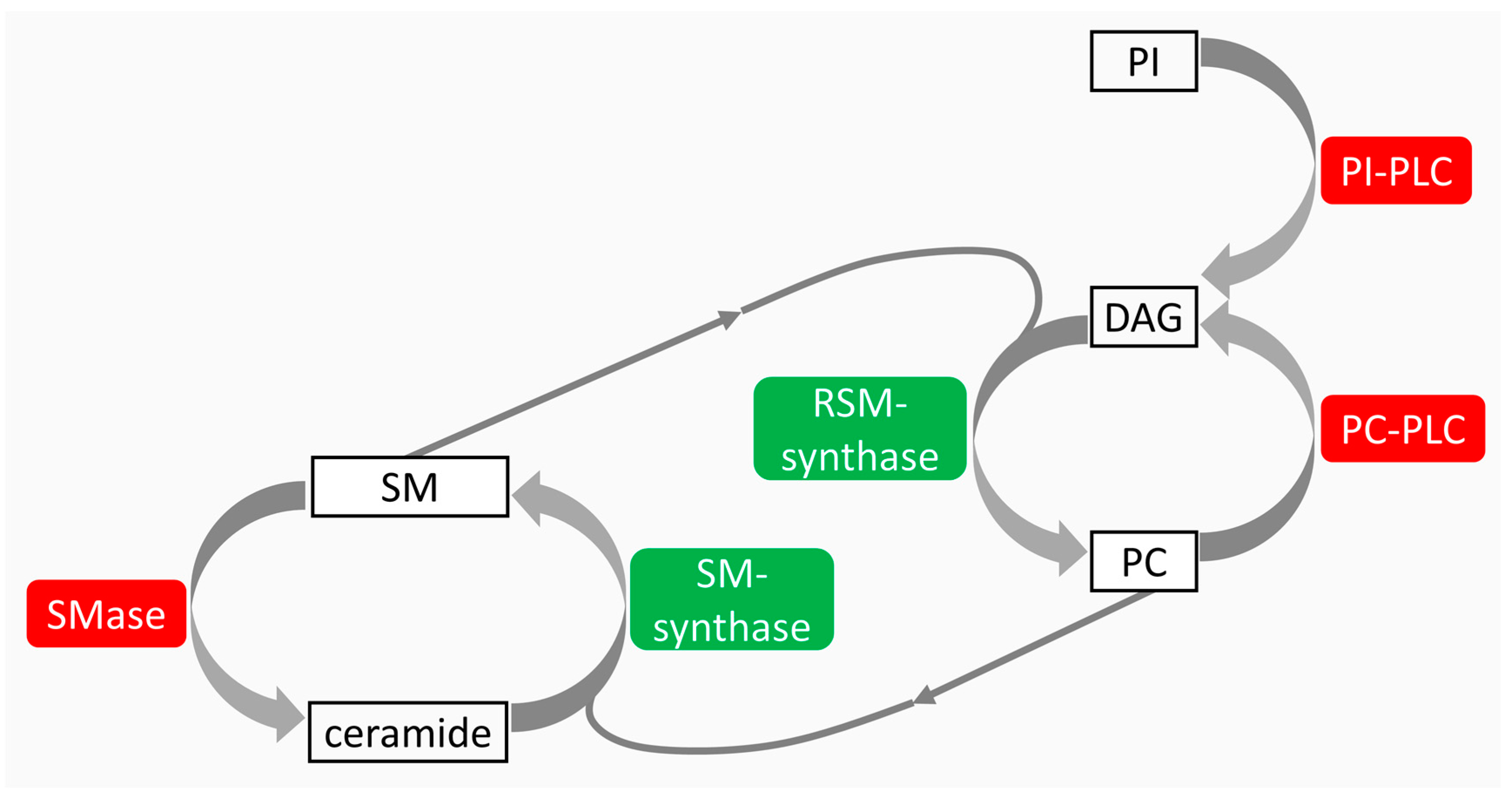
| FRTL-5 Nuclei | ||||
|---|---|---|---|---|
| Radiation | Proliferating Cells | Quiescent Cells | Proapoptotic Cells | References |
| UV | ↑ SMase ++ | ↑ SMase + | ↑ SMase ++ | [46] |
| ↑ RSMase ++ | ↑ RSMase ++ | ↑ RSMase ++ | ||
| ↓ SMsynthase ++ | ↓ SMsynthase + | ↓ SMsynthase ++ | ||
| ↓ PCPLC ++ | ↓ PCPLC + | ↓ PCPLC ++ | ||
| ↓ PIPLC ++ | ↓ PIPLC + | ↓ PIPLC ++ | ||
| Stratosphere | ↑ SMase ++ | ↑ SMase + | [57] | |
| ↑ RSMase ++ | ↑ RSMase ++ | |||
| ↓ SMsynthase ++ | ↓ SMsynthase ++ | |||
| ↓ PCPLC ++ | ↓ PCPLC + | |||
| Protons | ↑ SMase +++ | ↑ SMase = | [50] | |
| ↓ SMsynthase = | ↓ SMsynthase ++ | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albi, E.; Cataldi, S.; Lazzarini, A.; Codini, M.; Beccari, T.; Ambesi-Impiombato, F.S.; Curcio, F. Radiation and Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 911. https://doi.org/10.3390/ijms18050911
Albi E, Cataldi S, Lazzarini A, Codini M, Beccari T, Ambesi-Impiombato FS, Curcio F. Radiation and Thyroid Cancer. International Journal of Molecular Sciences. 2017; 18(5):911. https://doi.org/10.3390/ijms18050911
Chicago/Turabian StyleAlbi, Elisabetta, Samuela Cataldi, Andrea Lazzarini, Michela Codini, Tommaso Beccari, Francesco Saverio Ambesi-Impiombato, and Francesco Curcio. 2017. "Radiation and Thyroid Cancer" International Journal of Molecular Sciences 18, no. 5: 911. https://doi.org/10.3390/ijms18050911
APA StyleAlbi, E., Cataldi, S., Lazzarini, A., Codini, M., Beccari, T., Ambesi-Impiombato, F. S., & Curcio, F. (2017). Radiation and Thyroid Cancer. International Journal of Molecular Sciences, 18(5), 911. https://doi.org/10.3390/ijms18050911






