Therapeutic Potential of Targeting the Ghrelin Pathway
Abstract
:1. Introduction
2. Molecular Structure and Synthesis of Ghrelin
3. Ghrelin and Inflammation
4. Ghrelin GI Motility
5. Anorexia/Cachexia
6. Cardiovascular Disorders
7. Sarcopenia
8. Renal Failure
9. Neurodegenerative Disorders
9.1. Dementia and Alzheimer’s Disease
9.2. Parkinson’s Disease
9.3. Multiple Sclerosis
9.4. Amyotrophic Lateral Sclerosis
10. Pulmonary Disease
11. Metabolic Disease
11.1. Obesity
11.2. Prader Willi Syndrome
11.3. Diabetes and Hyperglycemia
11.4. Novel Approaches to Target the Ghrelin System in Metabolic Disease
12. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bowers, C.Y.; Momany, F.; Reynolds, G.A.; Chang, D.; Hong, A.; Chang, K. Structure-activity relationships of a synthetic pentapeptide that specifically releases growth hormone in vitro. Endocrinology 1980, 106, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Momany, F.A.; Bowers, C.Y.; Reynolds, G.A.; Chang, D.; Hong, A.; Newlander, K. Design, synthesis, and biological activity of peptides which release growth hormone in vitro. Endocrinology 1981, 108, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Dickson, S.L.; Leng, G.; Robinson, I.C. Systemic administration of growth hormone-releasing peptide activates hypothalamic arcuate neurons. Neuroscience 1993, 53, 303–306. [Google Scholar] [CrossRef]
- Blake, A.D.; Smith, R.G. Desensitization studies using perifused rat pituitary cells show that growth hormone-releasing hormone and His-d-Trp-Ala-Trp-d-Phe-Lys-NH2 stimulate growth hormone release through distinct receptor sites. J. Endocrinol. 1991, 129, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bowers, C.Y.; Reynolds, G.A.; Durham, D.; Barrera, C.M.; Pezzoli, S.S.; Thorner, M.O. Growth hormone (GH)-releasing peptide stimulates GH release in normal men and acts synergistically with GH-releasing hormone. J. Clin. Endocrinol. Metab. 1990, 70, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Chan, W.W.; Butler, B.; Barreto, A.; Smith, R.G. Evidence for a role of protein kinase-C in His-d-Trp-Ala-Trp-d-Phe-Lys-NH2-induced growth hormone release from rat primary pituitary cells. Endocrinology 1991, 129, 3337–3342. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.D.; Feighner, S.D.; Cully, D.F.; Arena, J.P.; Liberator, P.A.; Rosenblum, C.I.; Hamelin, M.; Hreniuk, D.L.; Palyha, O.C.; Anderson, J.; et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996, 273, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef]
- Kohno, D.; Gao, H.-Z.; Muroya, S.; Kikuyama, S.; Yada, T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 2003, 52, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Trumbauer, M.E.; Chen, A.S.; Weingarth, D.T.; Adams, J.R.; Frazier, E.G.; Shen, Z.; Marsh, D.J.; Feighner, S.D.; Guan, X.-M.; et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 2004, 145, 2607–2612. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Tanaka, T.; Inomata, N.; Ohnuma, N.; Tanaka, S.; Itoh, Z.; Hosoda, H.; Kojima, M.; Kangawa, K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem. Biophys. Res. Commun. 2000, 276, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-K.; Martin, B.; Kim, W.; White, C.M.; Ji, S.; Sun, Y.; Smith, R.G.; Sévigny, J.; Tschöp, M.H.; Maudsley, S.; et al. Ghrelin Is Produced in Taste Cells and Ghrelin Receptor Null Mice Show Reduced Taste Responsivity to Salty (NaCl) and Sour (Citric Acid) Tastants. PLoS ONE 2010, 5, e12729. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.P.; Monzón, M.E.; Varas, M.M.; Cragnolini, A.B.; Schiöth, H.B.; Scimonelli, T.N.; de Barioglio, S.R. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem. Biophys. Res. Commun. 2002, 299, 739–743. [Google Scholar] [CrossRef]
- Steiger, A. Ghrelin and sleep-wake regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R573–R574. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Asnicar, M.; Saha, P.K.; Chan, L.; Smith, R.G. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006, 3, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Masaki, T.; Kakuma, T.; Yoshimatsu, H. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci. Lett. 2003, 349, 75–78. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Solenberg, P.J.; Perkins, D.R.; Willency, J.A.; Knierman, M.D.; Jin, Z.; Witcher, D.R.; Luo, S.; Onyia, J.E.; Hale, J.E. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA 2008, 105, 6320–6325. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.-J.; Liang, G.; Li, R.L.; Xie, X.; Sleeman, M.W.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Goldstein, J.L.; Brown, M.S. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7467–7472. [Google Scholar] [CrossRef] [PubMed]
- Neary, N.M.; Druce, M.R.; Small, C.J.; Bloom, S.R. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut 2006, 55, 135. [Google Scholar] [PubMed]
- Toshinai, K.; Yamaguchi, H.; Sun, Y.; Smith, R.G.; Yamanaka, A.; Sakurai, T.; Date, Y.; Mondal, M.S.; Shimbara, T.; Kawagoe, T.; et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 2006, 147, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.M.; Gill, D.A.S.; Davies, R.; Loveridge, N.; Houston, P.A.; Robinson, I.C.A.F.; Wells, T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 2004, 145, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chai, B.; Li, J.; Wang, H.; Mulholland, M.W. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology 2008, 149, 4710–4716. [Google Scholar] [CrossRef] [PubMed]
- Inhoff, T.; Mönnikes, H.; Noetzel, S.; Stengel, A.; Goebel, M.; Dinh, Q.T.; Riedl, A.; Bannert, N.; Wisser, A.-S.; Wiedenmann, B.; et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides 2008, 29, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, A.; Inui, A.; Fujimiya, M.; Sakamaki, R.; Shinfuku, N.; Ueta, Y.; Meguid, M.M.; Kasuga, M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 2005, 54, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Filigheddu, N.; Gnocchi, V.F.; Coscia, M.; Cappelli, M.; Porporato, P.E.; Taulli, R.; Traini, S.; Baldanzi, G.; Chianale, F.; Cutrupi, S.; et al. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol. Biol. Cell 2007, 18, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Baldanzi, G.; Filigheddu, N.; Cutrupi, S.; Catapano, F.; Bonissoni, S.; Fubini, A.; Malan, D.; Baj, G.; Granata, R.; Broglio, F.; et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell Biol. 2002, 159, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Delhanty, P.J.; Neggers, S.J.; van der Lely, A.J. Des-acyl ghrelin: A metabolically active peptide. Endocr. Dev. 2013, 25, 112–121. [Google Scholar] [PubMed]
- De Vriese, C.; Gregoire, F.; Lema-Kisoka, R.; Waelbroeck, M.; Robberecht, P.; Delporte, C. Ghrelin degradation by serum and tissue homogenates: Identification of the cleavage sites. Endocrinology 2004, 145, 4997–5005. [Google Scholar] [CrossRef] [PubMed]
- Akamizu, T.; Takaya, K.; Irako, T.; Hosoda, H.; Teramukai, S.; Matsuyama, A.; Tada, H.; Miura, K.; Shimizu, A.; Fukushima, M.; et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur. J. Endocrinol. 2004, 150, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.M.; Yu, H.; Palyha, O.C.; McKee, K.K.; Feighner, S.D.; Sirinathsinghji, D.J.; Smith, R.G.; Van der Ploeg, L.H.; Howard, A.D. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 1997, 48, 23–29. [Google Scholar] [CrossRef]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988. [Google Scholar] [CrossRef] [PubMed]
- Zigman, J.M.; Jones, J.E.; Lee, C.E.; Saper, C.B.; Elmquist, J.K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006, 494, 528–548. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-B.; Leung, P.-K.; Wise, H.; Cheng, C.H.K. Signal transduction mechanism of the seabream growth hormone secretagogue receptor. FEBS Lett. 2004, 577, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.A.; Langmead, C.J.; Wise, A.; Milligan, G. Growth hormone secretagogues and growth hormone releasing peptides act as orthosteric super-agonists but not allosteric regulators for activation of the G protein Gα(o1) by the Ghrelin receptor. Mol. Pharmacol. 2009, 76, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Mary, S.; Damian, M.; Louet, M.; Floquet, N.; Fehrentz, J.-A.; Marie, J.; Martinez, J.; Banères, J.-L. Ligands and signaling proteins govern the conformational landscape explored by a G protein-coupled receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 8304–8309. [Google Scholar] [CrossRef] [PubMed]
- Rak-Mardyla, A.; Gregoraszczuk, E.L. ERK 1/2 and PI-3 kinase pathways as a potential mechanism of ghrelin action on cell proliferation and apoptosis in the porcine ovarian follicular cells. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2010, 61, 451–458. [Google Scholar]
- Chen, X.; Chen, Q.; Wang, L.; Li, G. Ghrelin induces cell migration through GHSR1a-mediated PI3K/Akt/eNOS/NO signaling pathway in endothelial progenitor cells. Metabolism 2013, 62, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Grey, C.L.; Chang, J.P. Ghrelin-induced growth hormone release from goldfish pituitary cells involves voltage-sensitive calcium channels. Gen. Comp. Endocrinol. 2009, 160, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kohno, D.; Sone, H.; Minokoshi, Y.; Yada, T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem. Biophys. Res. Commun. 2008, 366, 388–392. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Lage, R.; Saha, A.K.; Pérez-Tilve, D.; Vázquez, M.J.; Varela, L.; Sangiao-Alvarellos, S.; Tovar, S.; Raghay, K.; Rodríguez-Cuenca, S.; et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008, 7, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Andrews, Z.B.; Liu, Z.-W.; Walllingford, N.; Erion, D.M.; Borok, E.; Friedman, J.M.; Tschöp, M.H.; Shanabrough, M.; Cline, G.; Shulman, G.I.; et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 2008, 454, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Lin, S.-L.; Chen, Y.-M.; Wu, V.-C.; Yang, W.-S.; Wu, K.-D. A low-salt diet increases the expression of renal sirtuin 1 through activation of the ghrelin receptor in rats. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, D.A.; Martínez, G.; Romero, A.; Vázquez, M.J.; Boit, K.D.; Dopeso-Reyes, I.G.; López, M.; Vidal, A.; Nogueiras, R.; Diéguez, C. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 2011, 60, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.O.; Antunes, C.; Geliang, G.; Liu, Z.-W.; Borok, E.; Nie, Y.; Xu, A.W.; Souza, D.O.; Gao, Q.; Diano, S.; et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: Roles for Sirt1 in neuronal firing and synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 11815–11825. [Google Scholar] [CrossRef] [PubMed]
- Lage, R.; Vázquez, M.J.; Varela, L.; Saha, A.K.; Vidal-Puig, A.; Nogueiras, R.; Diéguez, C.; López, M. Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Fernández-Mallo, D.; Novelle, M.G.; Vázquez, M.J.; Tena-Sempere, M.; Nogueiras, R.; López, M.; Diéguez, C. Hypothalamic mTOR signaling mediates the orexigenic action of ghrelin. PLoS ONE 2012, 7, e46923. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Small, C.J.; Ward, H.L.; Murphy, K.G.; Dakin, C.L.; Taheri, S.; Kennedy, A.R.; Roberts, G.H.; Morgan, D.G.; Ghatei, M.A.; et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000, 141, 4325–4328. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.J.; Xu, L.; Clarke, M.A.; Lemus, M.; Reichenbach, A.; Geenen, B.; Kozicz, T.; Andrews, Z.B. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol. Psychiatry 2012, 72, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Naleid, A.M.; Grace, M.K.; Cummings, D.E.; Levine, A.S. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 2005, 26, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Faulconbridge, L.F.; Cummings, D.E.; Kaplan, J.M.; Grill, H.J. Hyperphagic effects of brainstem ghrelin administration. Diabetes 2003, 52, 2260–2265. [Google Scholar] [CrossRef] [PubMed]
- Luquet, S.; Phillips, C.T.; Palmiter, R.D. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 2007, 28, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Natalucci, G.; Riedl, S.; Gleiss, A.; Zidek, T.; Frisch, H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: Maintenance of a meal-related pattern. Eur. J. Endocrinol. 2005, 152, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Toshinai, K.; Mondal, M.S.; Nakazato, M.; Date, Y.; Murakami, N.; Kojima, M.; Kangawa, K.; Matsukura, S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem. Biophys. Res. Commun. 2001, 281, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Wawarta, R.; Riepl, R.L.; Friedrich, S.; Bidlingmaier, M.; Landgraf, R.; Folwaczny, C. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Investig. 2001, 24, RC19–RC21. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Prudom, C.E.; Nass, R.; Pezzoli, S.S.; Oliveri, M.C.; Johnson, M.L.; Veldhuis, P.; Gordon, D.A.; Howard, A.D.; Witcher, D.R.; et al. Novel Ghrelin Assays Provide Evidence for Independent Regulation of Ghrelin Acylation and Secretion in Healthy Young Men. J. Clin. Endocrinol. Metab. 2008, 93, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Govoni, N.; Iasio, R.D.; Cocco, C.; Parmeggiani, A.; Galeati, G.; Pagotto, U.; Brancia, C.; Spinaci, M.; Tamanini, C.; Pasquali, R.; Ferri, G.-L.; Seren, E. Gastric immunolocalization and plasma profiles of acyl-ghrelin in fasted and fasted-refed prepuberal gilts. J. Endocrinol. 2005, 186, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Córdoba-Chacón, J.; Salvatori, R.; Castaño, J.P.; Kineman, R.D.; Luque, R.M. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol. Cell. Endocrinol. 2010, 317, 154. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, H.; Gutierrez, J.A.; Solenberg, P.J.; Pfluger, P.T.; Czyzyk, T.A.; Willency, J.A.; Schurmann, A.; Joost, H.G.; Jandacek, R.; Hale, J.E.; et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat. Med. 2009, 15, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Theander-Carrillo, C.; Wiedmer, P.; Cettour-Rose, P.; Nogueiras, R.; Perez-Tilve, D.; Pfluger, P.; Castaneda, T.R.; Muzzin, P.; Schürmann, A.; Szanto, I.; et al. Ghrelin action in the brain controls adipocyte metabolism. J. Clin. Investig. 2006, 116, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhao, J.; Yang, J.; Zhang, Z.; Du, J.; Tang, C. Therapeutic effects of ghrelin on endotoxic shock in rats. Eur. J. Pharmacol. 2003, 473, 171–176. [Google Scholar] [CrossRef]
- Chang, L.; Du, J.-B.; Gao, L.-R.; Pang, Y.-Z.; Tang, C.-S. Effect of ghrelin on septic shock in rats. Acta Pharmacol. Sin. 2003, 24, 45–49. [Google Scholar] [PubMed]
- Dixit, V.D.; Schaffer, E.M.; Pyle, R.S.; Collins, G.D.; Sakthivel, S.K.; Palaniappan, R.; Lillard, J.W.; Taub, D.D. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Investig. 2004, 114, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Li, W.G.; Gavrila, D.; Liu, X.; Wang, L.; Gunnlaugsson, S.; Stoll, L.L.; McCormick, M.L.; Sigmund, C.D.; Tang, C.; Weintraub, N.L. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation 2004, 109, 2221–2226. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.; Priego, T.; Martín, A.I.; Villanúa, M.A.; López-Calderón, A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E486–E492. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 2006, 130, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Engel, M.; Burnat, G.; Gaca, P.; Kwiecien, S.; Pajdo, R.; Konturek, S.J. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60, 41–47. [Google Scholar]
- Kasımay, O.; Işeri, S.O.; Barlas, A.; Bangir, D.; Yeğen, C.; Arbak, S.; Yeğen, B.C. Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2006, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Ashitani, J.-I.; Matsumoto, N.; Kangawa, K.; Nakazato, M. Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm. Pharmacol. Ther. 2008, 21, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Theil, M.-M.; Miyake, S.; Mizuno, M.; Tomi, C.; Croxford, J.L.; Hosoda, H.; Theil, J.; von Hörsten, S.; Yokote, H.; Chiba, A.; et al. Suppression of experimental autoimmune encephalomyelitis by ghrelin. J. Immunol. 2009, 183, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Kyoraku, I.; Shiomi, K.; Kangawa, K.; Nakazato, M. Ghrelin reverses experimental diabetic neuropathy in mice. Biochem. Biophys. Res. Commun. 2009, 389, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-X.; Yuan, M.-J.; Huang, H.; Wu, G.; Liu, Y.; Yu, S.-B.; Li, H.-T.; Wang, T. Ghrelin inhibits post-infarct myocardial remodeling and improves cardiac function through anti-inflammation effect. Peptides 2009, 30, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Erşahin, M.; Toklu, H.Z.; Erzik, C.; Cetinel, S.; Akakin, D.; Velioğlu-Oğünç, A.; Tetik, S.; Ozdemir, Z.N.; Sener, G.; Yeğen, B.C. The anti-inflammatory and neuroprotective effects of ghrelin in subarachnoid hemorrhage-induced oxidative brain damage in rats. J. Neurotrauma 2010, 27, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Cheyuo, C.; Wu, R.; Zhou, M.; Jacob, A.; Coppa, G.; Wang, P. Ghrelin suppresses inflammation and neuronal nitric oxide synthase in focal cerebral ischemia via the vagus nerve. Shock 2011, 35, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Zanetti, M.; Semolic, A.; Cattin, M.R.; Pirulli, A.; Cattin, L.; Guarnieri, G. High-fat diet with acyl-ghrelin treatment leads to weight gain with low inflammation, high oxidative capacity and normal triglycerides in rat muscle. PLoS ONE 2011, 6, e26224. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hai, J.; Li, L.; Chen, X.; Peng, H.; Cao, M.; Zhang, Q. Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine 2013, 43, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Semolic, A.; Cattin, M.R.; Zanetti, M.; Guarnieri, G. Acylated ghrelin limits fat accumulation and improves redox state and inflammation markers in the liver of high-fat-fed rats. Obesity 2014, 22, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Gortan Cappellari, G.; Zanetti, M.; Semolic, A.; Vinci, P.; Ruozi, G.; Falcione, A.; Filigheddu, N.; Guarnieri, G.; Graziani, A.; Giacca, M.; et al. Unacylated Ghrelin Reduces Skeletal Muscle Reactive Oxygen Species Generation and Inflammation and Prevents High-Fat Diet-Induced Hyperglycemia and Whole-Body Insulin Resistance in Rodents. Diabetes 2016, 65, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Maekura, R.; Nagaya, N.; Nakazato, M.; Kimura, H.; Murakami, S.; Ohnishi, S.; Hiraga, T.; Miki, M.; Kitada, S.; et al. Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: A multicenter, randomized, double-blind, placebo-controlled trial. PLoS ONE 2012, 7, e35708. [Google Scholar] [CrossRef] [PubMed]
- Takata, A.; Takiguchi, S.; Miyazaki, Y.; Miyata, H.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Mori, M.; Kangawa, K.; et al. Randomized Phase II Study of the Anti-inflammatory Effect of Ghrelin During the Postoperative Period of Esophagectomy. Ann. Surg. 2015, 262, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; Inui, A.; Asakawa, A.; Kihara, N.; Fujimura, M.; Fujimiya, M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J. Physiol. 2003, 550, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Miyano, Y.; Sakata, I.; Kuroda, K.; Aizawa, S.; Tanaka, T.; Jogahara, T.; Kurotani, R.; Sakai, T. The role of the vagus nerve in the migrating motor complex and ghrelin- and motilin-induced gastric contraction in suncus. PLoS ONE 2013, 8, e64777. [Google Scholar] [CrossRef] [PubMed]
- Swartz, E.M.; Browning, K.N.; Travagli, R.A.; Holmes, G.M. Ghrelin increases vagally mediated gastric activity by central sites of action. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2014, 26, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Edholm, T.; Levin, F.; Hellström, P.M.; Schmidt, P.T. Ghrelin stimulates motility in the small intestine of rats through intrinsic cholinergic neurons. Regul. Pept. 2004, 121, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Depoortere, I.; Bisschops, R.; Delporte, C.; Coulie, B.; Meulemans, A.; Janssens, J.; Peeters, T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut 2006, 55, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Nahata, M.; Muto, S.; Oridate, N.; Ohnishi, S.; Nakagawa, K.; Sadakane, C.; Saegusa, Y.; Hattori, T.; Asaka, M.; Takeda, H. Impaired ghrelin signaling is associated with gastrointestinal dysmotility in rats with gastroesophageal reflux disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G42–G53. [Google Scholar] [CrossRef] [PubMed]
- Checchi, S.; Montanaro, A.; Pasqui, L.; Ciuoli, C.; Cevenini, G.; Sestini, F.; Fioravanti, C.; Pacini, F. Serum ghrelin as a marker of atrophic body gastritis in patients with parietal cell antibodies. J. Clin. Endocrinol. Metab. 2007, 92, 4346–4351. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Nakazato, M.; Ueno, H.; Date, Y.; Nishi, Y.; Mukae, H.; Mizuta, Y.; Ohtsuru, A.; Yamashita, S.; Kohno, S. Low plasma ghrelin levels in patients with Helicobacter pylori-associated gastritis. Am. J. Med. 2004, 117, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Cha, D.Y.; Cheon, S.J.; Yeo, M.; Cho, S.W. Plasma ghrelin levels and their relationship with gastric emptying in patients with dysmotility-like functional dyspepsia. Digestion 2009, 80, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Shindo, T.; Futagami, S.; Hiratsuka, T.; Horie, A.; Hamamoto, T.; Ueki, N.; Kusunoki, M.; Miyake, K.; Gudis, K.; Tsukui, T.; et al. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non-erosive reflux disease. Digestion 2009, 79, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Trudel, L.; Tomasetto, C.; Rio, M.C.; Bouin, M.; Plourde, V.; Eberling, P.; Poitras, P. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G948–G952. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Malik, N.M.; Sanger, G.J.; Andrews, P.L.R. Ghrelin alleviates cancer chemotherapy-associated dyspepsia in rodents. Cancer Chemother. Pharmacol. 2006, 58, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.-C.; Wang, Z.-G.; Wang, W.-G.; Yan, J.; Zheng, Q. Gastric motor effects of ghrelin and growth hormone releasing peptide 6 in diabetic mice with gastroparesis. World J. Gastroenterol. 2008, 14, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.-C.; Wang, Z.-G.; Lv, R.; Wang, W.-G.; Han, X.-D.; Yan, J.; Wang, Y.; Zheng, Q.; Ai, K.-X. Ghrelin improves delayed gastrointestinal transit in alloxan-induced diabetic mice. World J. Gastroenterol. 2008, 14, 2572–2577. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Wang, Z.; Wang, W.; Yan, J.; Zheng, Q. Therapeutic effects of ghrelin and growth hormone releasing peptide 6 on gastroparesis in streptozotocin-induced diabetic guinea pigs in vivo and in vitro. Chin. Med. J. 2008, 121, 1183–1188. [Google Scholar] [PubMed]
- Akamizu, T.; Iwakura, H.; Ariyasu, H.; Hosoda, H.; Murayama, T.; Yokode, M.; Teramukai, S.; Seno, H.; Chiba, T.; Noma, S.; et al. Repeated administration of ghrelin to patients with functional dyspepsia: Its effects on food intake and appetite. Eur. J. Endocrinol. 2008, 158, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.M.; Ejskjaer, N.; Hellström, P.M.; Malik, R.A.; Pezzullo, J.C.; Shaughnessy, L.; Charlton, P.; Kosutic, G.; McCallum, R.W. Randomised clinical trial: Ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting—Randomised clinical study subset data. Aliment. Pharmacol. Ther. 2011, 33, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Matsumura, T.; Tsuchiya, N.; Sadakane, C.; Inami, R.; Suzuki, T.; Yoshikawa, M.; Imazeki, F.; Yokosuka, O. Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepatogastroenterology 2012, 59, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Finan, B.; Clemmensen, C.; van der Ploeg, L.H.T.; Tschöp, M.H.; Müller, T.D. The Pentapeptide RM-131 Promotes Food Intake and Adiposity in Wildtype Mice but Not in Mice Lacking the Ghrelin Receptor. Front. Nutr. 2014, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Camilleri, M.; Busciglio, I.; Burton, D.; Stoner, E.; Noonan, P.; Gottesdiener, K.; Smith, S.A.; Vella, A.; Zinsmeister, A.R. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: Pharmacokinetics and pharmacodynamics. Diabetes Care 2013, 36, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Camilleri, M.; McCallum, R.; Sastre, R.; Breton, C.; Spence, S.; White, J.; Currie, M.; Gottesdiener, K.; Stoner, E.; et al. Relamorelin Reduces Vomiting Frequency and Severity and Accelerates Gastric Emptying in Adults with Diabetic Gastroparesis. Gastroenterology 2016, 151, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Perez-Tilve, D.; Tong, J.; Pfluger, P.T.; Tschöp, M.H. Ghrelin and its potential in the treatment of eating/wasting disorders and cachexia. J. Cachexia Sarcopenia Muscle 2010, 1, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Chance, W.T.; Dayal, R.; Friend, L.A.; Thomas, I.; Sheriff, S. Continuous intravenous infusion of ghrelin does not stimulate feeding in tumor-bearing rats. Nutr. Cancer 2008, 60, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Andersson, M.; Iresjö, B.-M.; Lönnroth, C.; Lundholm, K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int. J. Oncol. 2006, 28, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Toshinai, K.; Kajimura, N.; Nara-Ashizawa, N.; Tsukada, T.; Hayashi, Y.; Osuye, K.; Kangawa, K.; Matsukura, S.; Nakazato, M. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem. Biophys. Res. Commun. 2003, 301, 275–279. [Google Scholar] [CrossRef]
- Tsubouchi, H.; Yanagi, S.; Miura, A.; Matsumoto, N.; Kangawa, K.; Nakazato, M. Ghrelin relieves cancer cachexia associated with the development of lung adenocarcinoma in mice. Eur. J. Pharmacol. 2014, 743, 1–10. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Zhu, X.X.; Levasseur, P.; Meguid, M.M.; Suzuki, S.; Inui, A.; Taylor, J.E.; Halem, H.A.; Dong, J.Z.; Datta, R.; et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology 2007, 148, 3004–3012. [Google Scholar] [CrossRef] [PubMed]
- Perboni, S.; Bowers, C.; Kojima, S.; Asakawa, A.; Inui, A. Growth hormone releasing peptide 2 reverses anorexia associated with chemotherapy with 5-fluoruracil in colon cancer cell-bearing mice. World J. Gastroenterol. 2008, 14, 6303–6305. [Google Scholar] [CrossRef] [PubMed]
- Northrup, R.; Kuroda, K.; Duus, E.M.; Barnes, S.R.; Cheatham, L.; Wiley, T.; Pietra, C. Effect of ghrelin and anamorelin (ONO-7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2013, 21, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Borner, T.; Loi, L.; Pietra, C.; Giuliano, C.; Lutz, T.A.; Riediger, T. The ghrelin receptor agonist HM01 mimics the neuronal effects of ghrelin in the arcuate nucleus and attenuates anorexia-cachexia syndrome in tumor-bearing rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R89–R96. [Google Scholar] [CrossRef] [PubMed]
- Deboer, M.D.; Zhu, X.; Levasseur, P.R.; Inui, A.; Hu, Z.; Han, G.; Mitch, W.E.; Taylor, J.E.; Halem, H.A.; Dong, J.Z.; et al. Ghrelin treatment of chronic kidney disease: Improvements in lean body mass and cytokine profile. Endocrinology 2008, 149, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Uematsu, M.; Kojima, M.; Ikeda, Y.; Yoshihara, F.; Shimizu, W.; Hosoda, H.; Hirota, Y.; Ishida, H.; Mori, H.; et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation 2001, 104, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, A.; Joshi, R.; Su, C.; Friend, L.A.; Sheriff, S.; Kagan, R.J.; James, J.H. Ghrelin inhibits skeletal muscle protein breakdown in rats with thermal injury through normalizing elevated expression of E3 ubiquitin ligases MuRF1 and MAFbx. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R893–R901. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Yamaki, A.; Furuya, M.; Inomata, N.; Minamitake, Y.; Ohsuye, K.; Kangawa, K. Ghrelin improves body weight loss and skeletal muscle catabolism associated with angiotensin II-induced cachexia in mice. Regul. Pept. 2012, 178, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L.; Heiman, M.L.; Lehnert, P.; Fichter, M.; Tschöp, M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, 669–673. [Google Scholar] [PubMed]
- Tolle, V.; Kadem, M.; Bluet-Pajot, M.-T.; Frere, D.; Foulon, C.; Bossu, C.; Dardennes, R.; Mounier, C.; Zizzari, P.; Lang, F.; et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J. Clin. Endocrinol. Metab. 2003, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Hosoda, H.; Nin, K.; Ooya, C.; Hayashi, H.; Akamizu, T.; Kangawa, K. Plasma levels of active form of ghrelin during oral glucose tolerance test in patients with anorexia nervosa. Eur. J. Endocrinol. 2003, 149, R1–R3. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Naruo, T.; Yasuhara, D.; Tatebe, Y.; Nagai, N.; Shiiya, T.; Nakazato, M.; Matsukura, S.; Nozoe, S. Fasting plasma ghrelin levels in subtypes of anorexia nervosa. Psychoneuroendocrinology 2003, 28, 829–835. [Google Scholar] [CrossRef]
- Soriano-Guillén, L.; Barrios, V.; Campos-Barros, A.; Argente, J. Ghrelin levels in obesity and anorexia nervosa: Effect of weight reduction or recuperation. J. Pediatr. 2004, 144, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Uematsu, M.; Kojima, M.; Date, Y.; Nakazato, M.; Okumura, H.; Hosoda, H.; Shimizu, W.; Yamagishi, M.; Oya, H.; et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: Relationships between ghrelin and anabolic/catabolic factors. Circulation 2001, 104, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Nagaya, N.; Isobe, T.; Imazu, M.; Okumura, H.; Hosoda, H.; Kojima, M.; Kangawa, K.; Kohno, N. Increased Plasma Ghrelin Level in Lung Cancer Cachexia. Clin. Cancer Res. 2003, 9, 774–778. [Google Scholar] [PubMed]
- Miljic, D.; Pekic, S.; Djurovic, M.; Doknic, M.; Milic, N.; Casanueva, F.F.; Ghatei, M.; Popovic, V. Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. J. Clin. Endocrinol. Metab. 2006, 91, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Ohwada, R.; Akamizu, T.; Shibasaki, T.; Takano, K.; Kangawa, K. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: A pilot study. Endocr. J. 2009, 56, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Haruta, I.; Fuku, Y.; Kinoshita, K.; Yoneda, K.; Morinaga, A.; Amitani, M.; Amitani, H.; Asakawa, A.; Sugawara, H.; Takeda, Y.; et al. One-year intranasal application of growth hormone releasing peptide-2 improves body weight and hypoglycemia in a severely emaciated anorexia nervosa patient. J. Cachexia Sarcopenia Muscle 2015, 6, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Neary, N.M.; Small, C.J.; Wren, A.M.; Lee, J.L.; Druce, M.R.; Palmieri, C.; Frost, G.S.; Ghatei, M.A.; Coombes, R.C.; Bloom, S.R. Ghrelin increases energy intake in cancer patients with impaired appetite: Acute, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2004, 89, 2832–2836. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Itoh, T.; Murakami, S.; Oya, H.; Uematsu, M.; Miyatake, K.; Kangawa, K. Treatment of cachexia with ghrelin in patients with COPD. Chest 2005, 128, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Strasser, F.; Lutz, T.A.; Maeder, M.T.; Thuerlimann, B.; Bueche, D.; Tschöp, M.; Kaufmann, K.; Holst, B.; Brändle, M.; von Moos, R.; et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: A randomised, placebo-controlled, double-blind, double-crossover study. Br. J. Cancer 2008, 98, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Pietra, C.; Takeda, Y.; Tazawa-Ogata, N.; Minami, M.; Yuanfeng, X.; Duus, E.M.; Northrup, R. Anamorelin HCl (ONO-7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia-cachexia syndrome: Preclinical profile. J. Cachexia Sarcopenia Muscle 2014, 5, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Boccia, R.V.; Graham, C.D.; Yan, Y.; Duus, E.M.; Allen, S.; Friend, J. Anamorelin for patients with cancer cachexia: An integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015, 16, 108–116. [Google Scholar] [CrossRef]
- Temel, J.S.; Abernethy, A.P.; Currow, D.C.; Friend, J.; Duus, E.M.; Yan, Y.; Fearon, K.C. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016, 17, 519–531. [Google Scholar] [CrossRef]
- Takayama, K.; Katakami, N.; Yokoyama, T.; Atagi, S.; Yoshimori, K.; Kagamu, H.; Saito, H.; Takiguchi, Y.; Aoe, K.; Koyama, A.; et al. Anamorelin (ONO-7643) in Japanese patients with non-small cell lung cancer and cachexia: Results of a randomized phase 2 trial. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2016, 24, 3495–3505. [Google Scholar] [CrossRef] [PubMed]
- Wynne, K.; Giannitsopoulou, K.; Small, C.J.; Patterson, M.; Frost, G.; Ghatei, M.A.; Brown, E.A.; Bloom, S.R.; Choi, P. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: A randomized, placebo-controlled trial. J. Am. Soc. Nephrol. 2005, 16, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Ashby, D.R.; Ford, H.E.; Wynne, K.J.; Wren, A.M.; Murphy, K.G.; Busbridge, M.; Brown, E.A.; Taube, D.H.; Ghatei, M.A.; Tam, F.W.K.; et al. Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int. 2009, 76, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Lacerda-Miranda, G.; Soares, V.M.; Vieira, A.K.G.; Lessa, J.G.; Rodrigues-Cunha, A.C.S.; Cortez, E.; Garcia-Souza, E.P.; Moura, A.S. Ghrelin signaling in heart remodeling of adult obese mice. Peptides 2012, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Beiras-Fernandez, A.; Kreth, S.; Weis, F.; Ledderose, C.; Pöttinger, T.; Dieguez, C.; Beiras, A.; Reichart, B. Altered myocardial expression of ghrelin and its receptor (GHSR-1a) in patients with severe heart failure. Peptides 2010, 31, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, E.T.; Andersen, N.H.; Hansen, T.K.; Rasmussen, L.M.; Moller, N.; Sorensen, K.E.; Sloth, E.; Jorgensen, J.O.L. Cardiovascular effects of intravenous ghrelin infusion in healthy young men. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3020–H3026. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.N.; do Carmo, J.M.; Adi, A.H.; da Silva, A.A. Chronic central ghrelin infusion reduces blood pressure and heart rate despite increasing appetite and promoting weight gain in normotensive and hypertensive rats. Peptides 2013, 42, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Soeki, T.; Kishimoto, I.; Schwenke, D.O.; Tokudome, T.; Horio, T.; Yoshida, M.; Hosoda, H.; Kangawa, K. Ghrelin suppresses cardiac sympathetic activity and prevents early left ventricular remodeling in rats with myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H426–H432. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Ren, Y.; Liu, X.; Li, W.G.; Yang, J.; Geng, B.; Weintraub, N.L.; Tang, C. Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J. Cardiovasc. Pharmacol. 2004, 43, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Khatib, M.N.; Shankar, A.; Kirubakaran, R.; Agho, K.; Simkhada, P.; Gaidhane, S.; Saxena, D.; Gode, D.; Gaidhane, A.; Zahiruddin, S.Q.; et al. Effect of ghrelin on mortality and cardiovascular outcomes in experimental rat and mice models of heart failure: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0126697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-G.; Cai, H.-Q.; Li, Y.-H.; Sui, Y.-B.; Zhang, J.-S.; Chang, J.-R.; Ning, M.; Wu, Y.; Tang, C.-S.; Qi, Y.-F.; et al. Ghrelin protects heart against ERS-induced injury and apoptosis by activating AMP-activated protein kinase. Peptides 2013, 48, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, H.; Shen, X.; Jiang, H. Ghrelin protects MES23.5 cells against rotenone via inhibiting mitochondrial dysfunction and apoptosis. Neuropeptides 2016, 56, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Liu, H.; Li, N.; Sun, Y.; Liu, Z.; Yang, P. Ghrelin protects H9c2 cardiomyocytes from angiotensin II-induced apoptosis through the endoplasmic reticulum stress pathway. J. Cardiovasc. Pharmacol. 2012, 59, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Moriya, J.; Yasumura, Y.; Uematsu, M.; Ono, F.; Shimizu, W.; Ueno, K.; Kitakaze, M.; Miyatake, K.; Kangawa, K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 2004, 110, 3674–3679. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, M.; Schinzari, F.; Iantorno, M.; Rizza, S.; Melina, D.; Lauro, D.; Cardillo, C. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 2005, 112, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Pincelli, A.I.; Corrà, B.; Viarengo, R.; Bonomo, S.M.; Galimberti, D.; Scacchi, M.; Scarpini, E.; Cavagnini, F.; Müller, E.E. Plasma ghrelin concentrations in elderly subjects: Comparison with anorexic and obese patients. J. Endocrinol. 2002, 175, R1–R5. [Google Scholar] [CrossRef] [PubMed]
- Akamizu, T.; Murayama, T.; Teramukai, S.; Miura, K.; Bando, I.; Irako, T.; Iwakura, H.; Ariyasu, H.; Hosoda, H.; Tada, H.; et al. Plasma ghrelin levels in healthy elderly volunteers: The levels of acylated ghrelin in elderly females correlate positively with serum IGF-I levels and bowel movement frequency and negatively with systolic blood pressure. J. Endocrinol. 2006, 188, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Farhy, L.S.; Liu, J.; Pezzoli, S.S.; Johnson, M.L.; Gaylinn, B.D.; Thorner, M.O. Age-dependent decline in acyl-ghrelin concentrations and reduced association of acyl-ghrelin and growth hormone in healthy older adults. J. Clin. Endocrinol. Metab. 2014, 99, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Serra-Prat, M.; Papiol, M.; Monteis, R.; Palomera, E.; Cabré, M. Relationship between Plasma Ghrelin Levels and Sarcopenia in Elderly Subjects: A Cross-Sectional Study. J. Nutr. Health Aging 2015, 19, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-A.; Splenser, A.; Guillory, B.; Luo, J.; Mendiratta, M.; Belinova, B.; Halder, T.; Zhang, G.; Li, Y.-P.; Garcia, J.M. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: Characterization of multiple mechanisms involved. J. Cachexia Sarcopenia Muscle 2015, 6, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Asakawa, A.; Morinaga, A.; Amitani, M.S.; Amitani, H.; Katsuura, G.; Sawada, Y.; Sudo, Y.; Uezono, Y.; Mochiki, E.; et al. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol. Psychiatry 2016, 21, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Ki, K.H.; Yang, J.-Y.; Jang, B.Y.; Song, J.A.; Baek, W.-Y.; Kim, J.H.; An, J.H.; Kim, S.W.; Kim, S.Y.; et al. Chronic central administration of Ghrelin increases bone mass through a mechanism independent of appetite regulation. PLoS ONE 2013, 8, e65505. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Pezzoli, S.S.; Oliveri, M.C.; Patrie, J.T.; Harrell, F.E.; Clasey, J.L.; Heymsfield, S.B.; Bach, M.A.; Vance, M.L.; Thorner, M.O. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: A randomized trial. Ann. Intern. Med. 2008, 149, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, A.; Mori, K.; Sugawara, A.; Mukoyama, M.; Yahata, K.; Suganami, T.; Takaya, K.; Hosoda, H.; Kojima, M.; Kangawa, K.; et al. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J. Am. Soc. Nephrol. 2002, 13, 2748–2752. [Google Scholar] [CrossRef] [PubMed]
- Nüsken, K.-D.; Gröschl, M.; Rauh, M.; Stöhr, W.; Rascher, W.; Dötsch, J. Effect of renal failure and dialysis on circulating ghrelin concentration in children. Nephrol. Dial. Transplant. 2004, 19, 2156–2157. [Google Scholar] [CrossRef] [PubMed]
- Jarkovská, Z.; Rosická, M.; Krsek, M.; Sulková, S.; Haluzík, M.; Justová, V.; Lacinová, Z.; Marek, J. Plasma ghrelin levels in patients with end-stage renal disease. Physiol. Res. 2005, 54, 403–408. [Google Scholar] [PubMed]
- Elsayed, N.M.; Hamed, S.T.; El-Khatib, M.M.; El-Shehaby, A.M. The relation between dual energy X-ray absorptiometry measurement of body fat composition and plasma ghrelin in patients with end-stage renal disease. Saudi Med. J. 2009, 30, 109–115. [Google Scholar] [PubMed]
- Rodriguez Ayala, E.; Pecoits-Filho, R.; Heimbürger, O.; Lindholm, B.; Nordfors, L.; Stenvinkel, P. Associations between plasma ghrelin levels and body composition in end-stage renal disease: A longitudinal study. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2004, 19, 421–426. [Google Scholar] [CrossRef]
- Takeda, R.; Nishimatsu, H.; Suzuki, E.; Satonaka, H.; Nagata, D.; Oba, S.; Sata, M.; Takahashi, M.; Yamamoto, Y.; Terauchi, Y.; et al. Ghrelin improves renal function in mice with ischemic acute renal failure. J. Am. Soc. Nephrol. 2006, 17, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Khowailed, A.; Younan, S.M.; Ashour, H.; Kamel, A.E.; Sharawy, N. Effects of ghrelin on sepsis-induced acute kidney injury: One step forward. Clin. Exp. Nephrol. 2015, 19, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-X.; Ding, R.; Li, M.; Guo, Y.; Fan, L.-P.; Yue, L.-S.; Li, L.-Y.; Zhao, M. Ghrelin attenuates renal fibrosis and inflammation of obstructive nephropathy. J. Urol. 2015, 193, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Szentirmai, E.; Kapás, L.; Krueger, J.M. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R575–R585. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A.; Liu, Z.-W.; Andrews, Z.B.; Shanabrough, M.; Borok, E.; Elsworth, J.D.; Roth, R.H.; Sleeman, M.W.; Picciotto, M.R.; Tschöp, M.H.; et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Investig. 2006, 116, 3229–3239. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.P.; Perez, M.F.; Salde, E.; Schiöth, H.B.; Ramirez, O.A.; de Barioglio, S.R. Ghrelin induced memory facilitation implicates nitric oxide synthase activation and decrease in the threshold to promote LTP in hippocampal dentate gyrus. Physiol. Behav. 2010, 101, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xing, T.; Wang, M.; Miao, Y.; Tang, M.; Chen, J.; Li, G.; Ruan, D.-Y. Local infusion of ghrelin enhanced hippocampal synaptic plasticity and spatial memory through activation of phosphoinositide 3-kinase in the dentate gyrus of adult rats. Eur. J. Neurosci. 2011, 33, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.A.; Beynon, A.L.; Hornsby, A.K.E.; Bekinschtein, P.; Bussey, T.J.; Davies, J.S.; Saksida, L.M. The orexigenic hormone acyl-ghrelin increases adult hippocampal neurogenesis and enhances pattern separation. Psychoneuroendocrinology 2015, 51, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, P.S.; Xie, D.; Liu, K.; Chen, L. Ghrelin reduces injury of hippocampal neurons in a rat model of cerebral ischemia/reperfusion. Chin. J. Physiol. 2006, 49, 244–250. [Google Scholar] [PubMed]
- Miao, Y.; Xia, Q.; Hou, Z.; Zheng, Y.; Pan, H.; Zhu, S. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochem. Biophys. Res. Commun. 2007, 359, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, E.; Lee, D.H.; Seo, S.; Ju, S.; Lee, D.; Kim, H.; Park, S. Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology 2007, 148, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Beynon, A.L.; Brown, M.R.; Wright, R.; Rees, M.I.; Sheldon, I.M.; Davies, J.S. Ghrelin inhibits LPS-induced release of IL-6 from mouse dopaminergic neurones. J. Neuroinflammation 2013, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, V.V.; Rodrigues, A.L.S.; De Lima, T.C.; de Barioglio, S.R.; Raisman-Vozari, R.; Prediger, R.D. Ghrelin as a neuroprotective and palliative agent in Alzheimer’s and Parkinson’s disease. Curr. Pharm. Des. 2013, 19, 6773–6790. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Du, X.; Jiang, H.; Xie, J. Ghrelin and Neurodegenerative Disorders-a Review. Mol. Neurobiol. 2017, 54, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Proto, C.; Romualdi, D.; Cento, R.M.; Spada, R.S.; Di Mento, G.; Ferri, R.; Lanzone, A. Plasma levels of neuropeptides in Alzheimer’s disease. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2006, 22, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Córdoba-Chacón, J.; Kineman, R.D.; Luque, R.M.; Castaño, J.P. Role of ghrelin system in neuroprotection and cognitive functions: Implications in Alzheimer’s disease. Peptides 2011, 32, 2225–2228. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Ohnuma, T.; Kuerban, B.; Komatsu, M.; Arai, H. Genetic association between ghrelin polymorphisms and Alzheimer’s disease in a Japanese population. Dement. Geriatr. Cogn. Disord. 2011, 32, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Mansego, M.L.; Serra-Prat, M.; Palomera, E.; Boquet, X.; Chaves, J.F.; Puig-Domingo, M.; Mataró Ageing Study Group. Glucose impairment and ghrelin gene variants are associated to cognitive dysfunction. Aging Clin. Exp. Res. 2014, 26, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, M.B.; Benitez, A.; Updegraff, J.; Potter, V.; Alexander, T.; Glickman, E.; Gunstad, J. Serum ghrelin is inversely associated with cognitive function in a sample of non-demented elderly. Psychiatry Clin. Neurosci. 2010, 64, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Kunath, N.; Müller, N.C.J.; Tonon, M.; Konrad, B.N.; Pawlowski, M.; Kopczak, A.; Elbau, I.; Uhr, M.; Kühn, S.; Repantis, D.; et al. Ghrelin modulates encoding-related brain function without enhancing memory formation in humans. NeuroImage 2016, 142, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, C.-P.; Li, C.-R.; Wang, W.; Zhang, D.; Han, L.-L.; Zhang, X.-Q.; Kim, A.; Kim, S.; Liu, G.-L. Ghrelin modulates insulin sensitivity and tau phosphorylation in high glucose-induced hippocampal neurons. Biol. Pharm. Bull. 2010, 33, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, I.; Tamiazzo, L.; Bresciani, E.; Rapetti, D.; Caporali, S.; Lattuada, D.; Locatelli, V.; Torsello, A. Desacyl-ghrelin and synthetic GH-secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by beta-amyloid fibrils. J. Neurosci. Res. 2009, 87, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Choi, J.G.; Nam, D.W.; Hong, H.-S.; Choi, Y.-J.; Oh, M.S.; Mook-Jung, I. Ghrelin ameliorates cognitive dysfunction and neurodegeneration in intrahippocampal amyloid-β1-42 oligomer-injected mice. J. Alzheimers Dis. 2011, 23, 147–159. [Google Scholar] [PubMed]
- Kang, S.; Moon, N.R.; Kim, D.S.; Kim, S.H.; Park, S. Central acylated ghrelin improves memory function and hippocampal AMPK activation and partly reverses the impairment of energy and glucose metabolism in rats infused with β-amyloid. Peptides 2015, 71, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, E.J.; Allison, D.B.; van Groen, T.; Kadish, I. Hunger in the absence of caloric restriction improves cognition and attenuates Alzheimer’s disease pathology in a mouse model. PLoS ONE 2013, 8, e60437. [Google Scholar] [CrossRef] [PubMed]
- Kunath, N.; van Groen, T.; Allison, D.B.; Kumar, A.; Dozier-Sharpe, M.; Kadish, I. Ghrelin agonist does not foster insulin resistance but improves cognition in an Alzheimer’s disease mouse model. Sci. Rep. 2015, 5, 11452. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Cha, M.-Y.; Mook-Jung, I. Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. J. Alzheimers Dis. 2014, 41, 233–241. [Google Scholar] [PubMed]
- Jiang, H.; Li, L.-J.; Wang, J.; Xie, J.-X. Ghrelin antagonizes MPTP-induced neurotoxicity to the dopaminergic neurons in mouse substantia nigra. Exp. Neurol. 2008, 212, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Kim, H.G.; Hwang, L.; Seo, J.-H.; Kim, S.; Hwang, S.; Kim, S.; Lee, D.; Chung, H.; Oh, M.S.; et al. Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease by blocking microglial activation. Neurotox. Res. 2009, 15, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Andrews, Z.B.; Erion, D.; Beiler, R.; Liu, Z.-W.; Abizaid, A.; Zigman, J.; Elsworth, J.D.; Savitt, J.M.; DiMarchi, R.; Tschoep, M.; et al. Ghrelin promotes and protects nigrostriatal dopamine function via an UCP2-dependent mitochondrial mechanism. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 14057–14065. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.A.; Lemus, M.B.; Stark, R.; Santos, V.V.; Thompson, A.; Rees, D.J.; Galic, S.; Elsworth, J.D.; Kemp, B.E.; Davies, J.S.; et al. Ghrelin-AMPK Signaling Mediates the Neuroprotective Effects of Calorie Restriction in Parkinson’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 3049–3063. [Google Scholar] [CrossRef] [PubMed]
- Marrinan, S.; Emmanuel, A.V.; Burn, D.J. Delayed gastric emptying in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, H.; Pietra, C.; Giuliano, C.; Garcia-Rubio, S.; Xu, X.; Yakabi, S.; Taché, Y.; Wang, L. New ghrelin agonist, HM01 alleviates constipation and L-dopa-delayed gastric emptying in 6-hydroxydopamine rat model of Parkinson’s disease. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2014, 26, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Möller, J.C.; Mankel, K.; Eggert, K.M.; Bohne, K.; Bodden, M.; Stiasny-Kolster, K.; Kann, P.H.; Mayer, G.; Tebbe, J.J.; et al. Postprandial ghrelin response is reduced in patients with Parkinson’s disease and idiopathic REM sleep behaviour disorder: A peripheral biomarker for early Parkinson’s disease? J. Neurol. 2011, 258, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.M. Multiple sclerosis, an autoimmune inflammatory disease: Prospects for its integrative management. Altern. Med. Rev. J. Clin. Ther. 2001, 6, 540–566. [Google Scholar]
- Lee, J.Y.; Chung, H.; Yoo, Y.S.; Oh, Y.J.; Oh, T.H.; Park, S.; Yune, T.Y. Inhibition of apoptotic cell death by ghrelin improves functional recovery after spinal cord injury. Endocrinology 2010, 151, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Oertel, W.H.; Tackenberg, B. Cerebrospinal fluid concentrations of ghrelin in patients with multiple sclerosis. Neuro Endocrinol. Lett. 2013, 34, 14–17. [Google Scholar] [PubMed]
- Berilgen, M.S.; Bulut, S.; Ustundag, B.; Tekatas, A.; Ayar, A. Patients with multiple sclerosis have higher levels of serum ghrelin. Neuro Endocrinol. Lett. 2005, 26, 819–822. [Google Scholar] [PubMed]
- Eftekhari, E.; Etemadifar, M.; Ebrahimi, A.; Baradaran, S. The relation between peptide hormones and sex hormone in patients with multiple sclerosis. Iran. J. Neurol. 2013, 12, 60–65. [Google Scholar] [PubMed]
- Rey, L.K.; Wieczorek, S.; Akkad, D.A.; Linker, R.A.; Chan, A.; Hoffjan, S. Polymorphisms in genes encoding leptin, ghrelin and their receptors in German multiple sclerosis patients. Mol. Cell. Probes 2011, 25, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Souza-Moreira, L.; Delgado-Maroto, V.; Morell, M.; O’Valle, F.; Del Moral, R.G.; Gonzalez-Rey, E. Therapeutic effect of ghrelin in experimental autoimmune encephalomyelitis by inhibiting antigen-specific Th1/Th17 responses and inducing regulatory T cells. Brain Behav. Immun. 2013, 30, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Holm, T.; Maier, A.; Wicks, P.; Lang, D.; Linke, P.; Münch, C.; Steinfurth, L.; Meyer, R.; Meyer, T. Severe Loss of Appetite in Amyotrophic Lateral Sclerosis Patients: Online Self-Assessment Study. Interact. J. Med. Res. 2013, 2, e8. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.; Robberecht, W. Neuroinflammation in amyotrophic lateral sclerosis: Role of glial activation in motor neuron disease. Lancet Neurol. 2011, 10, 253–263. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.J.; Huang, L.; Mantovani, S.; Pfluger, C.M.M.; Woodruff, T.M.; O’Sullivan, J.D.; Henderson, R.D.; McCombe, P.A. Altered expression of metabolic proteins and adipokines in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 2015, 357, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Czell, D.; Baldinger, R.; Schneider, U.; Neuwirth, C.; Weber, M. The role of the SenseWear device and ghrelin for metabolism in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Lee, S.; Li, E.; Kim, Y.; Park, S. Ghrelin protects spinal cord motoneurons against chronic glutamate-induced excitotoxicity via ERK1/2 and phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3β pathways. Exp. Neurol. 2011, 230, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.; Li, E.; Park, S. Ghrelin Protects Spinal Cord Motoneurons against Chronic Glutamate Excitotoxicity by Inhibiting Microglial Activation. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2012, 16, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Murayama, N.; Ogino, R.; Inomata, N.; Inoue, T.; Furuya, M.; Matsuo, T.; Murayama, N.; Ogino, R.; Inomata, N.; et al. Ghrelin attenuates disease progression in a mouse model of amyotrophic lateral sclerosis. F1000Research 2014, 5, 223. [Google Scholar]
- Itoh, T.; Nagaya, N.; Yoshikawa, M.; Fukuoka, A.; Takenaka, H.; Shimizu, Y.; Haruta, Y.; Oya, H.; Yamagishi, M.; Hosoda, H.; et al. Elevated plasma ghrelin level in underweight patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 170, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Cai, B.; Ma, Y.; Zhu, H.; Sun, Q.; Song, A. Circulating leptin and ghrelin in patients with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 2007, 30, 182–185. (In Chinese) [Google Scholar] [PubMed]
- Ying, B.W.; Song, X.B.; Fan, H.; Wang, L.L.; Li, Y.S.; Cheng, Z.; Cheng, H.; Wen, F.Q. Plasma ghrelin levels and weight loss in Chinese Uygur patients with chronic obstructive pulmonary disease. J. Int. Med. Res. 2008, 36, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bao, Z.; Wang, Z.; Yang, G.; Zhu, D.; Zhang, L.; Tan, R. The changes of ghrelin, growth hormone, growth hormone releasing hormone and their clinical significances in patients with chronic obstructive pulmonary disease. Zhonghua Nei Ke Za Zhi 2012, 51, 536–539. (In Chinese) [Google Scholar] [PubMed]
- Luo, F.-M.; Liu, X.-J.; Li, S.-Q.; Wang, Z.-L.; Liu, C.-T.; Yuan, Y.-M. Circulating ghrelin in patients with chronic obstructive pulmonary disease. Nutrition 2005, 21, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Deveci, Y.; Deveci, F.; Ilhan, N.; Karaca, I.; Turgut, T.; Muz, M.H. Serum ghrelin, IL-6 and TNF-α levels in patients with chronic obstructive pulmonary disease. Tuberk. Ve Toraks 2010, 58, 162–172. [Google Scholar]
- Uzum, A.K.; Aydin, M.M.; Tutuncu, Y.; Omer, B.; Kiyan, E.; Alagol, F. Serum ghrelin and adiponectin levels are increased but serum leptin level is unchanged in low weight Chronic Obstructive Pulmonary Disease patients. Eur. J. Intern. Med. 2014, 25, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, C.; Galli-Tsinopoulou, A.; Koliakos, G.; Fotoulaki, M.; Nousia-Arvanitakis, S. Ghrelin and leptin levels in young adults with cystic fibrosis: Relationship with body fat. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2007, 6, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, C.-T.; Yoon, H.I.; Song, J.; Shin, W.G.; Lee, J.H. Relation of ghrelin, leptin and inflammatory markers to nutritional status in active pulmonary tuberculosis. Clin. Nutr. Edinb. Scotl. 2010, 29, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, A.; Wang, Q.; Han, X.; Cai, J.; Schouten, E.G.; Kok, F.J.; Li, Y. Relation of leptin, ghrelin and inflammatory cytokines with body mass index in pulmonary tuberculosis patients with and without type 2 diabetes mellitus. PLoS ONE 2013, 8, e80122. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.I.; Tsang, D.; Koenig, S.; Wilson, D.; McCloskey, T.; Chandra, S. Plasma ghrelin and leptin in adult cystic fibrosis patients. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2008, 7, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Cassoni, P.; Allia, E.; Marrocco, T.; Ghè, C.; Ghigo, E.; Muccioli, G.; Papotti, M. Ghrelin and cortistatin in lung cancer: Expression of peptides and related receptors in human primary tumors and in vitro effect on the H345 small cell carcinoma cell line. J. Endocrinol. Investig. 2006, 29, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.C.; Sá, S.V.; Goldbaum, T.S.; Catania, M.; Campos, V.C.; Corrêa-Giannella, M.L.; Giannella-Neto, D.; Salgado, L.R. In vivo response to growth hormone-releasing peptide-6 in adrenocorticotropin-dependent Cushing’s syndrome by lung carcinoid tumor is associated with growth hormone secretagogue receptor type 1a mRNA expression. J. Endocrinol. Investig. 2007, 30, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, D.; Pan, W.; Zhang, L.; Ge, J. Circulating ghrelin was negatively correlated with pulmonary arterial pressure in atrial septal defect patients. Chin. Med. J. 2013, 126, 3936–3939. [Google Scholar] [PubMed]
- Henriques-Coelho, T.; Correia-Pinto, J.; Roncon-Albuquerque, R.; Baptista, M.J.; Lourenço, A.P.; Oliveira, S.M.; Brandão-Nogueira, A.; Teles, A.; Fortunato, J.M.; Leite-Moreira, A.F. Endogenous production of ghrelin and beneficial effects of its exogenous administration in monocrotaline-induced pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2885–H2890. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, D.O.; Tokudome, T.; Shirai, M.; Hosoda, H.; Horio, T.; Kishimoto, I.; Kangawa, K. Exogenous ghrelin attenuates the progression of chronic hypoxia-induced pulmonary hypertension in conscious rats. Endocrinology 2008, 149, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, D.O.; Gray, E.A.; Pearson, J.T.; Sonobe, T.; Ishibashi-Ueda, H.; Campillo, I.; Kangawa, K.; Umetani, K.; Shirai, M. Exogenous ghrelin improves blood flow distribution in pulmonary hypertension-assessed using synchrotron radiation microangiography. Pflugers Arch. 2011, 462, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-P.; Zhu, J.-J.; Cheng, F.; Jiang, K.-W.; Gu, W.-Z.; Shen, Z.; Wu, Y.-D.; Liang, L.; Du, L.-Z. Ghrelin ameliorates hypoxia-induced pulmonary hypertension via phospho-GSK3 β/β-catenin signaling in neonatal rats. J. Mol. Endocrinol. 2011, 47, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, Z.; Zhang, H.; Luo, Q. Ghrelin protects human pulmonary artery endothelial cells against hypoxia-induced injury via PI3-kinase/Akt. Peptides 2013, 42, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei Bavil, F.; Alipour, M.R.; Keyhanmanesh, R.; Alihemmati, A.; Ghiyasi, R.; Mohaddes, G. Ghrelin Decreases Angiogenesis, HIF-1α and VEGF Protein Levels in Chronic Hypoxia in Lung Tissue of Male Rats. Adv. Pharm. Bull. 2015, 5, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Dong, W.; Zhou, M.; Zhang, F.; Marini, C.P.; Ravikumar, T.S.; Wang, P. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am. J. Respir. Crit. Care Med. 2007, 176, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhu, Y.; Zhang, Y.; Wilhelmsen, K.; Jia, C.; Jin, J.; Xue, Q.; Feng, X.; Zhang, F.; Yu, B. Effects of ghrelin on pulmonary NOD2 mRNA expression and NF-κB activation when protects against acute lung injury in rats challenged with cecal ligation and puncture. Int. Immunopharmacol. 2012, 13, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; He, W.; Li, L.; Li, B.; Luo, L.; Huang, X.; Guan, K.; Chen, W. Ghrelin attenuates sepsis-associated acute lung injury oxidative stress in rats. Inflammation 2015, 38, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Shu, Q.; Li, S.; Luo, F. Ghrelin attenuates lipopolysaccharide-induced acute lung injury through NO pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2008, 14, BR141–BR146. [Google Scholar]
- Li, G.; Li, J.; Zhou, Q.; Song, X.; Liang, H.; Huang, L. Growth hormone releasing peptide-2, a ghrelin agonist, attenuates lipopolysaccharide-induced acute lung injury in rats. Tohoku J. Exp. Med. 2010, 222, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zeng, M.; He, W.; Huang, X.; Luo, L.; Zhang, H.; Deng, D.Y.B. Ghrelin protects alveolar macrophages against lipopolysaccharide-induced apoptosis through growth hormone secretagogue receptor 1a-dependent c-Jun N-terminal kinase and Wnt/β-catenin signaling and suppresses lung inflammation. Endocrinology 2015, 156, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xue, C. Ghrelin attenuates acute pancreatitis-induced lung injury and inhibits substance P expression. Am. J. Med. Sci. 2010, 339, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, R.; Wang, F.; Zhao, G.; Lu, Z.; Qiu, Q. Protective effect of ghrelin against paraquat-induced acute lung injury in mice. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2014, 32, 190–194. (In Chinese) [Google Scholar] [PubMed]
- Imazu, Y.; Yanagi, S.; Miyoshi, K.; Tsubouchi, H.; Yamashita, S.-I.; Matsumoto, N.; Ashitani, J.-I.; Kangawa, K.; Nakazato, M. Ghrelin ameliorates bleomycin-induced acute lung injury by protecting alveolar epithelial cells and suppressing lung inflammation. Eur. J. Pharmacol. 2011, 672, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Guven, B.; Gokce, M.; Saydam, O.; Can, M.; Bektas, S.; Yurtlu, S. Effect of ghrelin on inflammatory response in lung contusion. Kaohsiung J. Med. Sci. 2013, 29, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kamiide, Y.; Inomata, N.; Furuya, M.; Yada, T. Ghrelin ameliorates catabolic conditions and respiratory dysfunction in a chronic obstructive pulmonary disease model of chronic cigarette smoke-exposed rats. Eur. J. Pharmacol. 2015, 755, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, T.; Shen, Y.; Wan, C.; Li, X.; Li, D.; Liu, Y.; Wang, T.; Xu, D.; Wen, F.; et al. Ghrelin Inhibits Interleukin-6 Production Induced by Cigarette Smoke Extract in the Bronchial Epithelial Cell Via NF-κB Pathway. Inflammation 2016, 39, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Maekura, R.; Nagaya, N.; Kitada, S.; Miki, M.; Yoshimura, K.; Tateishi, Y.; Motone, M.; Hiraga, T.; Mori, M.; et al. Effects of ghrelin treatment on exercise capacity in underweight COPD patients: A substudy of a multicenter, randomized, double-blind, placebo-controlled trial of ghrelin treatment. BMC Pulm. Med. 2013, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Maekura, R.; Nagaya, N.; Miki, M.; Kitada, S.; Yoshimura, K.; Mori, M.; Kangawa, K. Effects of ghrelin treatment on exertional dyspnea in COPD: An exploratory analysis. J. Physiol. Sci. JPS 2015, 65, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Miki, K.; Tsubouchi, H.; Sakamoto, A.; Arimura, Y.; Yanagi, S.; Iiboshi, H.; Yoshida, M.; Souma, R.; Ishimoto, H.; et al. Ghrelin administration for chronic respiratory failure: A randomized dose-comparison trial. Lung 2015, 193, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Maekura, R.; Nakazato, M.; Matsumoto, N.; Kitada, S.; Miki, M.; Yoshimura, K.; Mori, M.; Kangawa, K. Randomized, dose-finding trial of ghrelin treatment for chronic respiratory failure. Clin. Respir. J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, A.; Inui, A.; Kaga, T.; Katsuura, G.; Fujimiya, M.; Fujino, M.A.; Kasuga, M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 2003, 52, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ahmed, S.; Smith, R.G. Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell. Biol. 2003, 23, 7973–7981. [Google Scholar] [CrossRef] [PubMed]
- Wortley, K.E.; Anderson, K.D.; Garcia, K.; Murray, J.D.; Malinova, L.; Liu, R.; Moncrieffe, M.; Thabet, K.; Cox, H.J.; Yancopoulos, G.D.; et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc. Natl. Acad. Sci. USA 2004, 101, 8227–8232. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Butte, N.F.; Garcia, J.M.; Smith, R.G. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 2008, 149, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Wortley, K.E.; del Rincon, J.-P.; Murray, J.D.; Garcia, K.; Iida, K.; Thorner, M.O.; Sleeman, M.W. Absence of ghrelin protects against early-onset obesity. J. Clin. Investig. 2005, 115, 3573–3578. [Google Scholar] [CrossRef] [PubMed]
- Kouno, T.; Akiyama, N.; Ito, T.; Okuda, T.; Nanchi, I.; Notoya, M.; Oka, S.; Yukioka, H. Ghrelin O-acyltransferase knockout mice show resistance to obesity when fed high-sucrose diet. J. Endocrinol. 2016, 228, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zigman, J.M.; Nakano, Y.; Coppari, R.; Balthasar, N.; Marcus, J.N.; Lee, C.E.; Jones, J.E.; Deysher, A.E.; Waxman, A.R.; White, R.D.; et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Investig. 2005, 115, 3564–3572. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Saha, P.K.; Ma, X.; Henshaw, I.O.; Shao, L.; Chang, B.H.J.; Buras, E.D.; Tong, Q.; Chan, L.; McGuinness, O.P.; et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell 2011, 10, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lin, L.; Xu, P.; Saito, K.; Wei, Q.; Meadows, A.G.; Bongmba, O.Y.N.; Pradhan, G.; Zheng, H.; Xu, Y.; et al. Neuronal Deletion of Ghrelin Receptor Almost Completely Prevents Diet-Induced Obesity. Diabetes 2016, 65, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, P.T.; Kirchner, H.; Günnel, S.; Schrott, B.; Perez-Tilve, D.; Fu, S.; Benoit, S.C.; Horvath, T.; Joost, H.-G.; Wortley, K.E.; et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G610–G618. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, E.P.; Iwasaki, S.; Moss, J.A.; Chang, J.; Otsuji, J.; Inoue, K.; Meijler, M.M.; Janda, K.D. Vaccination against weight gain. Proc. Natl. Acad. Sci. USA 2006, 103, 13226–13231. [Google Scholar] [CrossRef] [PubMed]
- Shearman, L.P.; Wang, S.-P.; Helmling, S.; Stribling, D.S.; Mazur, P.; Ge, L.; Wang, L.; Klussmann, S.; Macintyre, D.E.; Howard, A.D.; et al. Ghrelin neutralization by a ribonucleic acid-SPM ameliorates obesity in diet-induced obese mice. Endocrinology 2006, 147, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Esler, W.P.; O’Connor, S.; Coish, P.D.G.; Wickens, P.L.; Brands, M.; Bierer, D.E.; Bloomquist, B.T.; Bondar, G.; Chen, L.; et al. Quinazolinone Derivatives as Orally Available Ghrelin Receptor Antagonists for the Treatment of Diabetes and Obesity. J. Med. Chem. 2007, 50, 5202–5216. [Google Scholar] [CrossRef] [PubMed]
- Esler, W.P.; Rudolph, J.; Claus, T.H.; Tang, W.; Barucci, N.; Brown, S.-E.; Bullock, W.; Daly, M.; DeCarr, L.; Li, Y.; et al. Small-Molecule Ghrelin Receptor Antagonists Improve Glucose Tolerance, Suppress Appetite, and Promote Weight Loss. Endocrinology 2007, 148, 5175–5185. [Google Scholar] [CrossRef] [PubMed]
- Maletínská, L.; Matyšková, R.; Maixnerová, J.; Sýkora, D.; Pýchová, M.; Spolcová, A.; Blechová, M.; Drápalová, J.; Lacinová, Z.; Haluzík, M.; et al. The Peptidic GHS-R antagonist [d-Lys(3)]GHRP-6 markedly improves adiposity and related metabolic abnormalities in a mouse model of postmenopausal obesity. Mol. Cell. Endocrinol. 2011, 343, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.; Zhu, L.; Anini, Y.; Wang, Q. Neutralizing circulating ghrelin by expressing a growth hormone secretagogue receptor-based protein protects against high-fat diet-induced obesity in mice. Gene Ther. 2015, 22, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.P.; Hwang, Y.; Taylor, M.S.; Kirchner, H.; Pfluger, P.T.; Bernard, V.; Lin, Y.; Bowers, E.M.; Mukherjee, C.; Song, W.-J.; et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science 2010, 330, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-C.; Xu, J.; Chinookoswong, N.; Liu, S.; Steavenson, S.; Gegg, C.; Brankow, D.; Lindberg, R.; Véniant, M.; Gu, W. An acyl-ghrelin-specific neutralizing antibody inhibits the acute ghrelin-mediated orexigenic effects in mice. Mol. Pharmacol. 2009, 75, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Pinho, F.; Ribeiro, A.M.; Carreira, M.; Casanueva, F.F.; Roy, P.; Monteiro, M.P. Immunization against active ghrelin using virus-like particles for obesity treatment. Curr. Pharm. Des. 2013, 19, 6551–6558. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.; Istrate, N.; Wang, L.; Nichols, A.J.; Tozzo, E.; Stricker-Krongrad, A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: Reversal upon weight loss. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2004, 28, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.I.; Enriori, P.J.; Lemus, M.B.; Cowley, M.A.; Andrews, Z.B. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 2010, 151, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Naznin, F.; Toshinai, K.; Waise, T.M.Z.; NamKoong, C.; Md Moin, A.S.; Sakoda, H.; Nakazato, M. Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J. Endocrinol. 2015, 226, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Phase I/IIa Clinical Trial with Obese Individuals Shows no Effect of CYT009-GhrQb on Weight Loss. Available online: http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=103189 (accessed on 3 January 2015).
- Kanumakala, S.; Greaves, R.; Pedreira, C.C.; Donath, S.; Warne, G.L.; Zacharin, M.R.; Harris, M. Fasting ghrelin levels are not elevated in children with hypothalamic obesity. J. Clin. Endocrinol. Metab. 2005, 90, 2691–2695. [Google Scholar] [CrossRef] [PubMed]
- DelParigi, A.; Tschöp, M.; Heiman, M.L.; Salbe, A.D.; Vozarova, B.; Sell, S.M.; Bunt, J.C.; Tataranni, P.A. High circulating ghrelin: A potential cause for hyperphagia and obesity in prader-willi syndrome. J. Clin. Endocrinol. Metab. 2002, 87, 5461–5464. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Clement, K.; Purnell, J.Q.; Vaisse, C.; Foster, K.E.; Frayo, R.S.; Schwartz, M.W.; Basdevant, A.; Weigle, D.S. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat. Med. 2002, 8, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Kweh, F.A.; Miller, J.L.; Sulsona, C.R.; Wasserfall, C.; Atkinson, M.; Shuster, J.J.; Goldstone, A.P.; Driscoll, D.J. Hyperghrelinemia in Prader-Willi syndrome begins in early infancy long before the onset of hyperphagia. Am. J. Med. Genet. A 2015, 167A, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Haqq, A.M.; Stadler, D.D.; Rosenfeld, R.G.; Pratt, K.L.; Weigle, D.S.; Frayo, R.S.; LaFranchi, S.H.; Cummings, D.E.; Purnell, J.Q. Circulating ghrelin levels are suppressed by meals and octreotide therapy in children with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 3573–3576. [Google Scholar] [CrossRef] [PubMed]
- De Waele, K.; Ishkanian, S.L.; Bogarin, R.; Miranda, C.A.; Ghatei, M.A.; Bloom, S.R.; Pacaud, D.; Chanoine, J.-P. Long-acting octreotide treatment causes a sustained decrease in ghrelin concentrations but does not affect weight, behaviour and appetite in subjects with Prader-Willi syndrome. Eur. J. Endocrinol. 2008, 159, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kuppens, R.J.; Diène, G.; Bakker, N.E.; Molinas, C.; Faye, S.; Nicolino, M.; Bernoux, D.; Delhanty, P.J.D.; van der Lely, A.J.; Allas, S.; et al. Elevated ratio of acylated to unacylated ghrelin in children and young adults with Prader-Willi syndrome. Endocrine 2015, 50, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Beauloye, V.; Diene, G.; Kuppens, R.; Zech, F.; Winandy, C.; Molinas, C.; Faye, S.; Kieffer, I.; Beckers, D.; Nergårdh, R.; et al. High unacylated ghrelin levels support the concept of anorexia in infants with prader-willi syndrome. Orphanet J. Rare Dis. 2016, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Alamri, B.N.; Shin, K.; Chappe, V.; Anini, Y. The role of ghrelin in the regulation of glucose homeostasis. Horm. Mol. Biol. Clin. Investig. 2016, 26, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Arvat, E.; Benso, A.; Gottero, C.; Muccioli, G.; Papotti, M.; van der Lely, A.J.; Deghenghi, R.; Ghigo, E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5083–5086. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, E.T.; Jessen, N.; Møller, N.; Jørgensen, J.O.L. Acyl Ghrelin Induces Insulin Resistance Independently of GH, Cortisol, and Free Fatty Acids. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Gauna, C.; Meyler, F.M.; Janssen, J.A.M.J.L.; Delhanty, P.J.D.; Abribat, T.; van Koetsveld, P.; Hofland, L.J.; Broglio, F.; Ghigo, E.; van der Lely, A.J. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J. Clin. Endocrinol. Metab. 2004, 89, 5035–5042. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Dornonville de la Cour, C.; Håkanson, R.; Lundquist, I. Effects of ghrelin on insulin and glucagon secretion: A study of isolated pancreatic islets and intact mice. Regul. Pept. 2004, 118, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gauna, C.; Delhanty, P.J.D.; Hofland, L.J.; Janssen, J.A.M.J.L.; Broglio, F.; Ross, R.J.M.; Ghigo, E.; van der Lely, A.J. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J. Clin. Endocrinol. Metab. 2005, 90, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Özcan, B.; Neggers, S.J.; Miller, A.R.; Yang, H.-C.; Lucaites, V.; Abribat, T.; Allas, S.; Huisman, M.; Visser, J.A.; Themmen, A.P.N.; et al. Does des-acyl ghrelin improve glycemic control in obese diabetic subjects by decreasing acylated ghrelin levels? Eur. J. Endocrinol. 2014, 170, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Gottero, C.; Prodam, F.; Gauna, C.; Muccioli, G.; Papotti, M.; Abribat, T.; van der Lely, A.J.; Ghigo, E. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J. Clin. Endocrinol. Metab. 2004, 89, 3062–3065. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, K.; Sone, H.; Koizumi, M.; Nakata, M.; Kakei, M.; Nagai, H.; Hosoda, H.; Kangawa, K.; Yada, T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 2006, 55, 3486–3493. [Google Scholar] [CrossRef] [PubMed]
- Brial, F.; Lussier, C.R.; Belleville, K.; Sarret, P.; Boudreau, F. Ghrelin Inhibition Restores Glucose Homeostasis in Hepatocyte Nuclear Factor-1α (MODY3)-Deficient Mice. Diabetes 2015, 64, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, M.R.; Brown, M.S.; Goldstein, J.L.; Zhao, T.-J. Induced Ablation of Ghrelin Cells in Adult Mice Does Not Decrease Food Intake, Body Weight, or Response to High Fat Diet. Cell Metab. 2014, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, C.; Finan, B.; Fischer, K.; Tom, R.Z.; Legutko, B.; Sehrer, L.; Heine, D.; Grassl, N.; Meyer, C.W.; Henderson, B.; et al. Dual melanocortin-4 receptor and GLP-1 receptor agonism amplifies metabolic benefits in diet-induced obese mice. EMBO Mol. Med. 2015, 7, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Finan, B.; Yang, B.; Ottaway, N.; Smiley, D.L.; Ma, T.; Clemmensen, C.; Chabenne, J.; Zhang, L.; Habegger, K.M.; Fischer, K.; et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015, 21, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Finan, B.; Clemmensen, C.; Zhu, Z.; Stemmer, K.; Gauthier, K.; Müller, L.; De Angelis, M.; Moreth, K.; Neff, F.; Perez-Tilve, D.; et al. Chemical Hybridization of Glucagon and Thyroid Hormone Optimizes Therapeutic Impact for Metabolic Disease. Cell 2016, 167, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Willesen, M.G.; Kristensen, P.; Rømer, J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 1999, 70, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Whiddon, B.B.; Palmiter, R.D. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc. Natl. Acad. Sci. USA 2012, 109, 3155–3160. [Google Scholar] [CrossRef] [PubMed]
- Skibicka, K.P.; Hansson, C.; Alvarez-Crespo, M.; Friberg, P.A.; Dickson, S.L. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 2011, 180, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.J.; Sun, B.; Chen, K.; Lv, B.; Luo, X.; Yan, J.Q. Ghrelin signaling in the ventral tegmental area mediates both reward-based feeding and fasting-induced hyperphagia on high-fat diet. Neuroscience 2015, 300, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Skibicka, K.P.; Shirazi, R.H.; Rabasa-Papio, C.; Alvarez-Crespo, M.; Neuber, C.; Vogel, H.; Dickson, S.L. Divergent circuitry underlying food reward and intake effects of ghrelin: Dopaminergic VTA-accumbens projection mediates ghrelin’s effect on food reward but not food intake. Neuropharmacology 2013, 73, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Crespo, M.; Skibicka, K.P.; Farkas, I.; Molnár, C.S.; Egecioglu, E.; Hrabovszky, E.; Liposits, Z.; Dickson, S.L. The amygdala as a neurobiological target for ghrelin in rats: Neuroanatomical, electrophysiological and behavioral evidence. PLoS ONE 2012, 7, e46321. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Fortin, S.M.; Ricks, K.M.; Grill, H.J. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol. Psychiatry 2013, 73, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Finan, B.; Yang, B.; Ottaway, N.; Stemmer, K.; Müller, T.D.; Yi, C.-X.; Habegger, K.; Schriever, S.C.; García-Cáceres, C.; Kabra, D.G.; et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 2012, 18, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
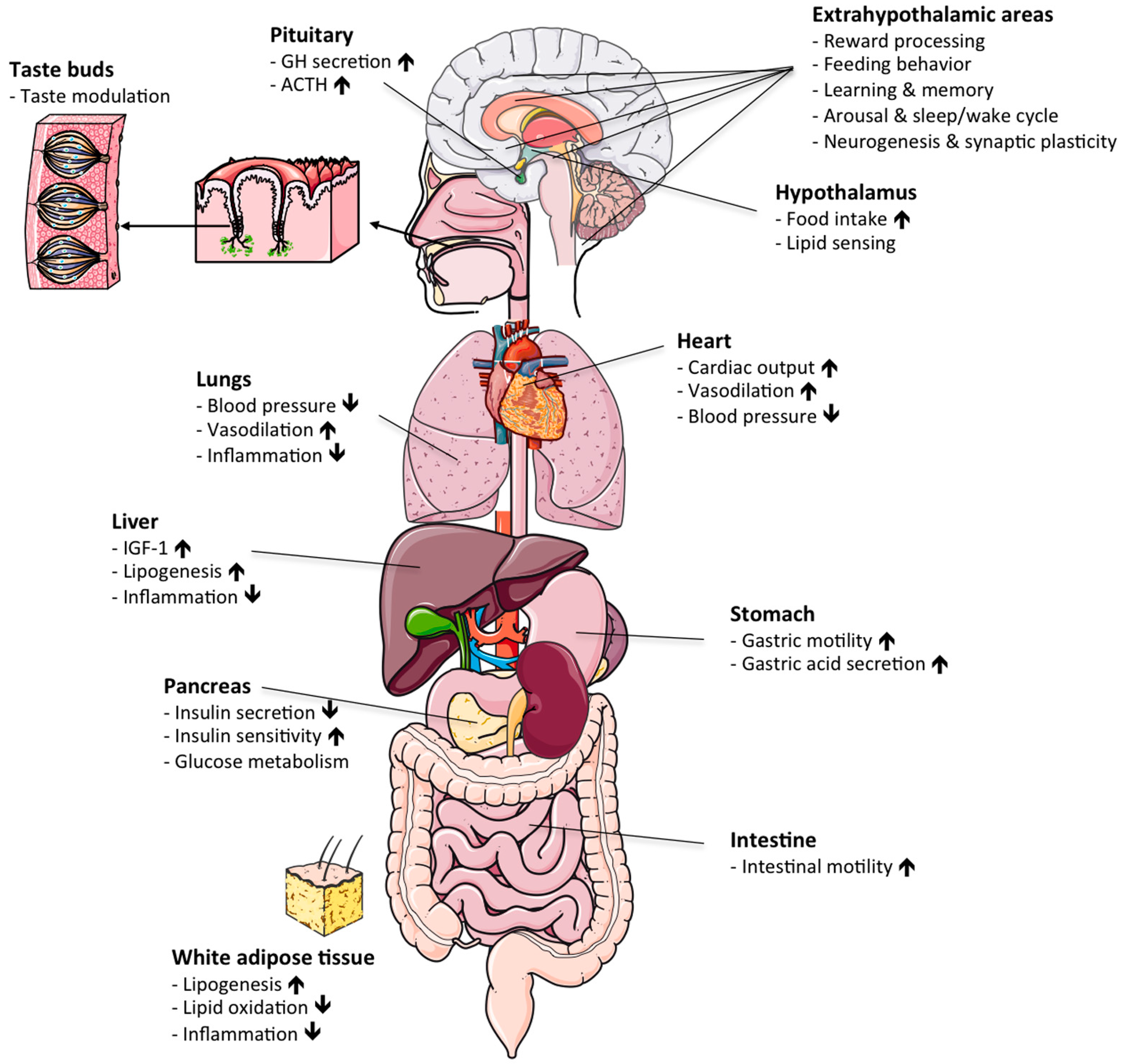
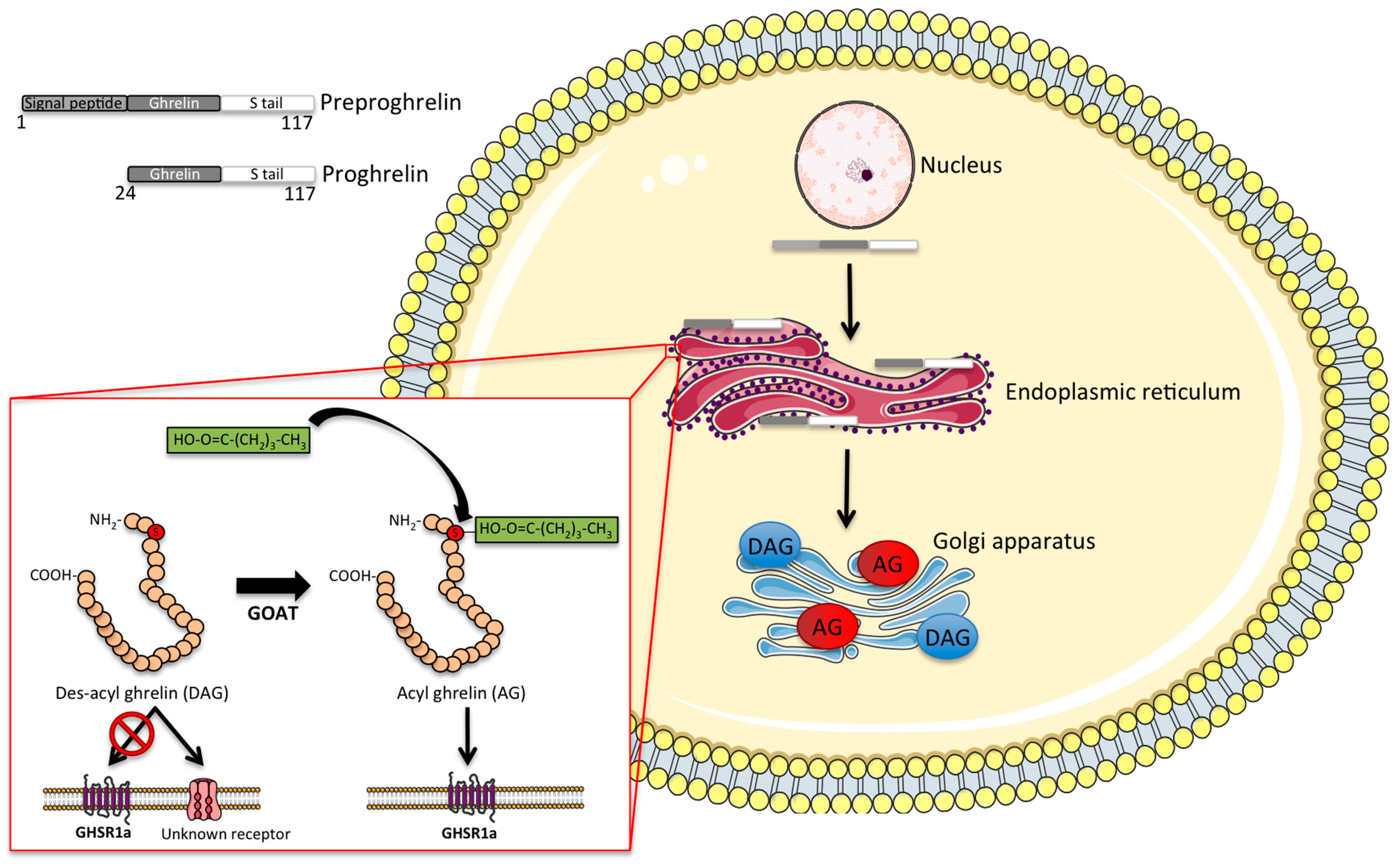
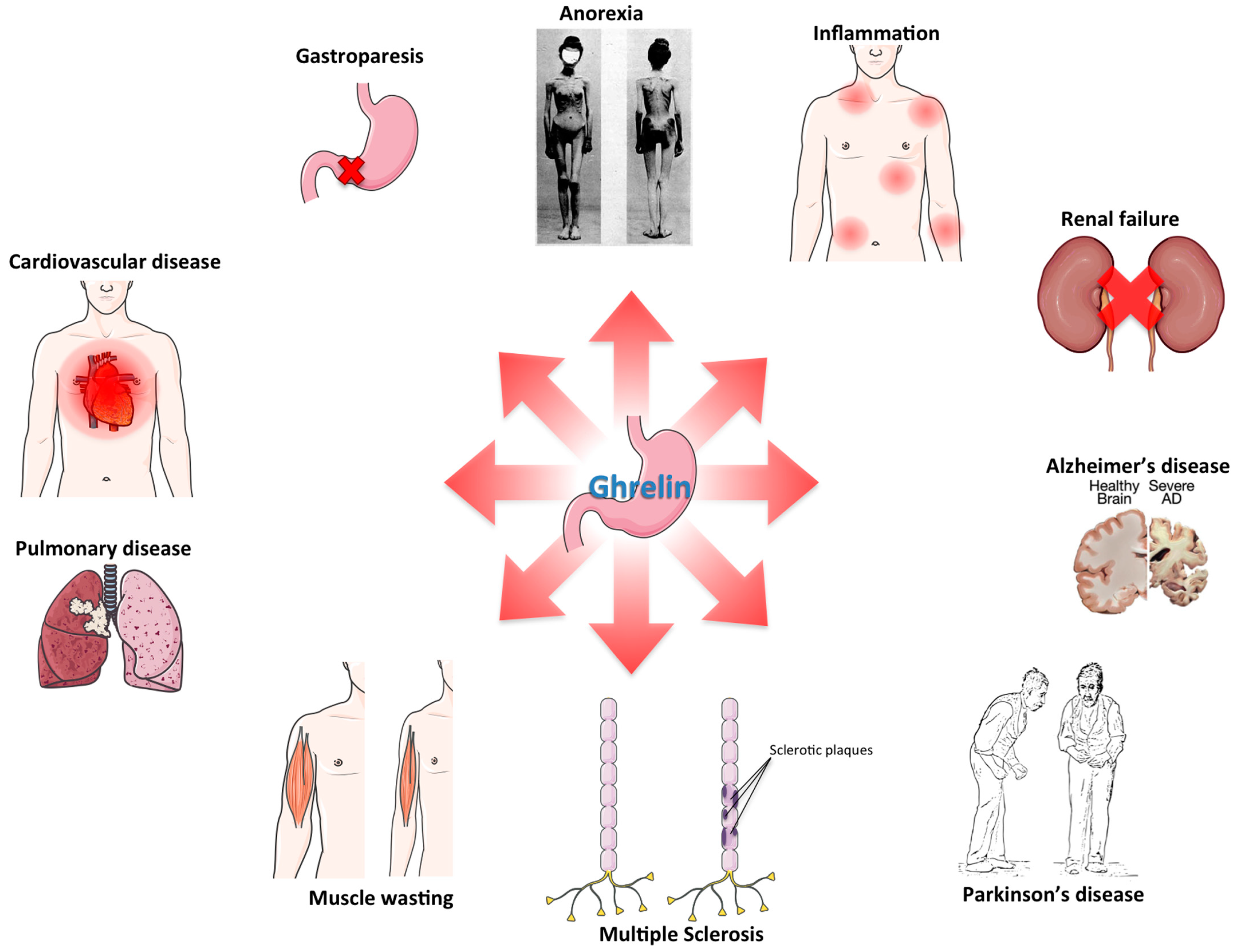
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colldén, G.; Tschöp, M.H.; Müller, T.D. Therapeutic Potential of Targeting the Ghrelin Pathway. Int. J. Mol. Sci. 2017, 18, 798. https://doi.org/10.3390/ijms18040798
Colldén G, Tschöp MH, Müller TD. Therapeutic Potential of Targeting the Ghrelin Pathway. International Journal of Molecular Sciences. 2017; 18(4):798. https://doi.org/10.3390/ijms18040798
Chicago/Turabian StyleColldén, Gustav, Matthias H. Tschöp, and Timo D. Müller. 2017. "Therapeutic Potential of Targeting the Ghrelin Pathway" International Journal of Molecular Sciences 18, no. 4: 798. https://doi.org/10.3390/ijms18040798
APA StyleColldén, G., Tschöp, M. H., & Müller, T. D. (2017). Therapeutic Potential of Targeting the Ghrelin Pathway. International Journal of Molecular Sciences, 18(4), 798. https://doi.org/10.3390/ijms18040798





