Profile of Polyphenol Compounds of Five Muscadine Grapes Cultivated in the United States and in Newly Adapted Locations in China
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic Content (TPC)
2.2. Polyphenol Composition and Accumulation
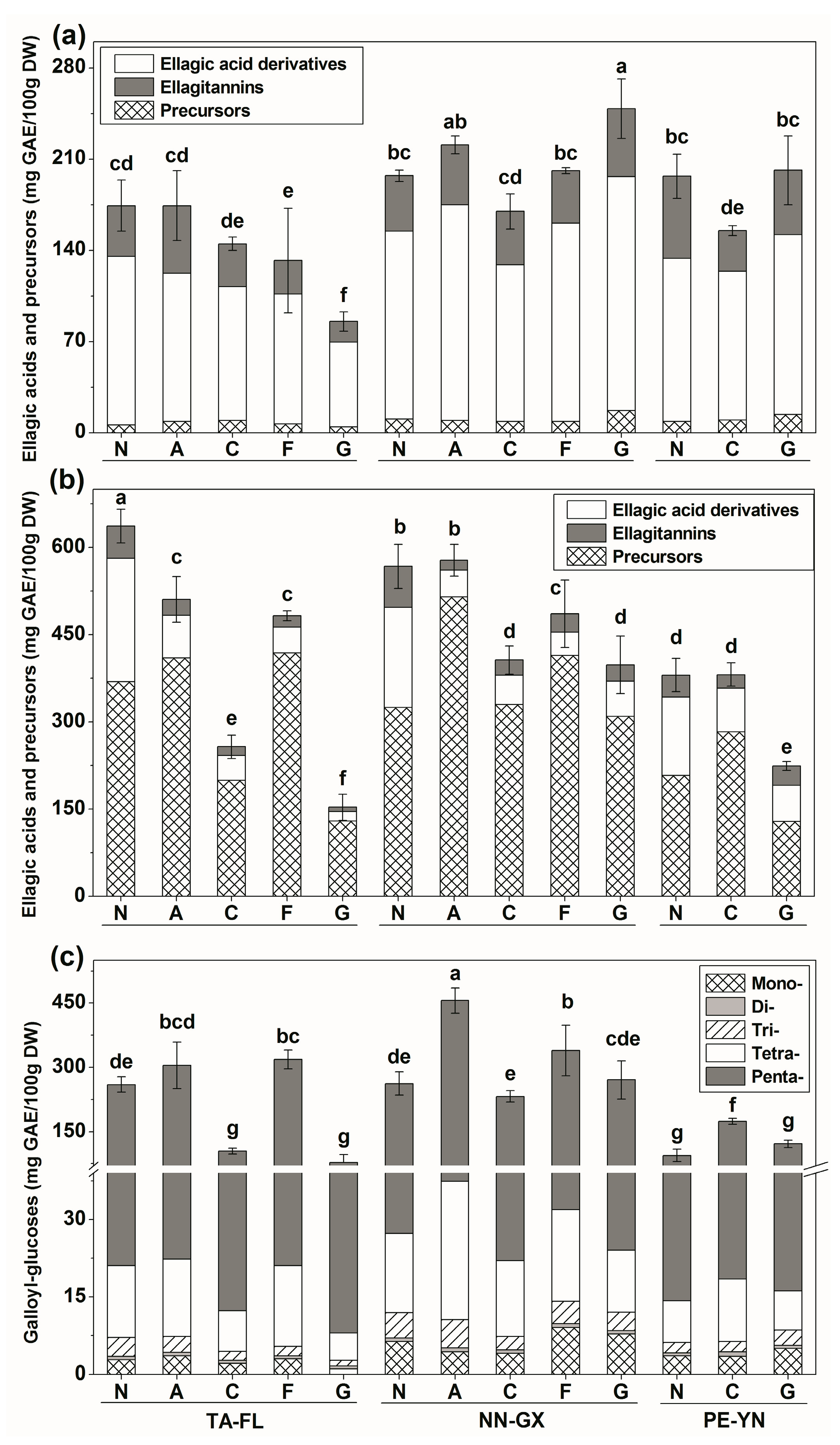
2.2.1. Ellagic Acids and Precursors Profiles
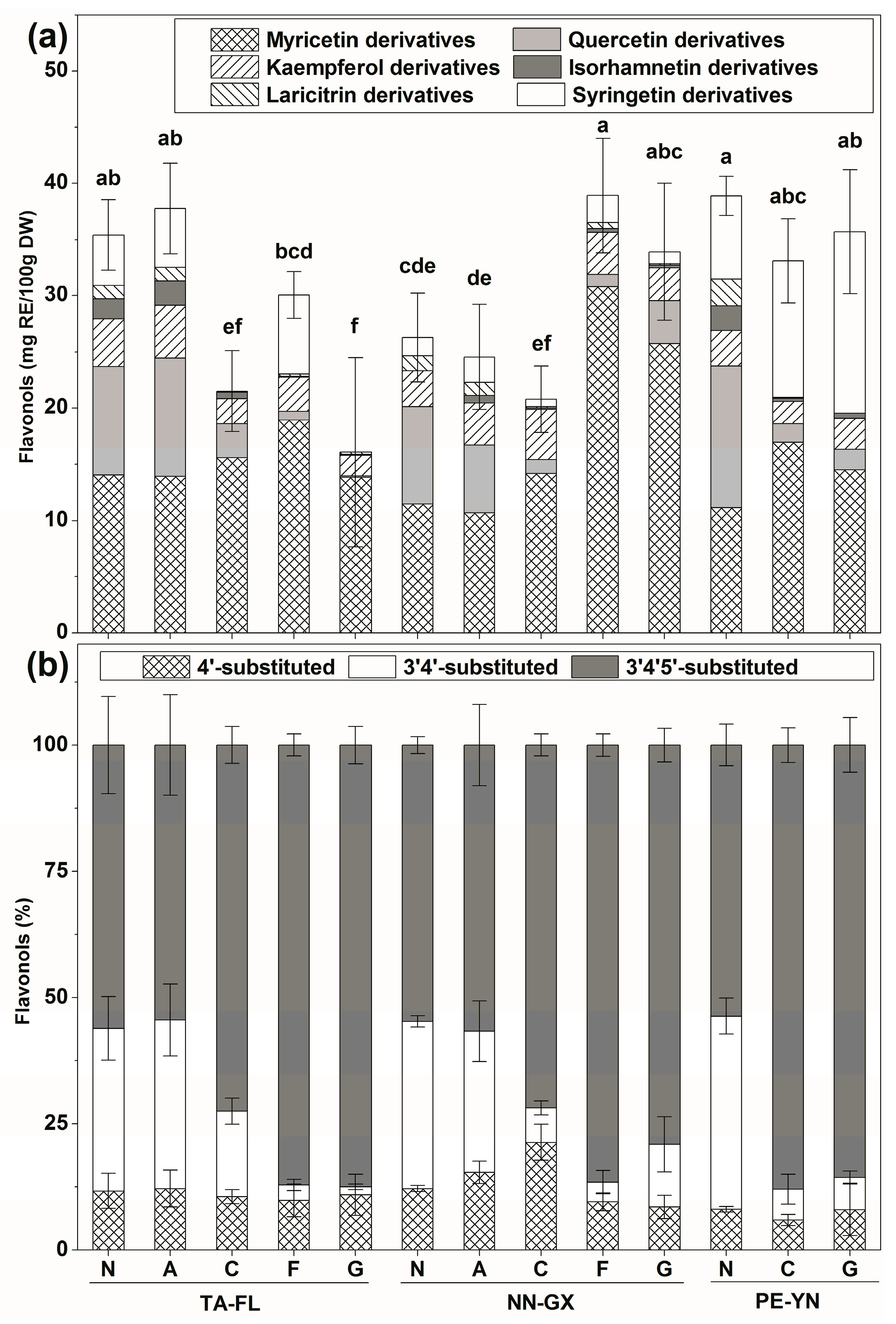
2.2.2. Flavonols Profiles
2.2.3. Benzoic Acid Profiles
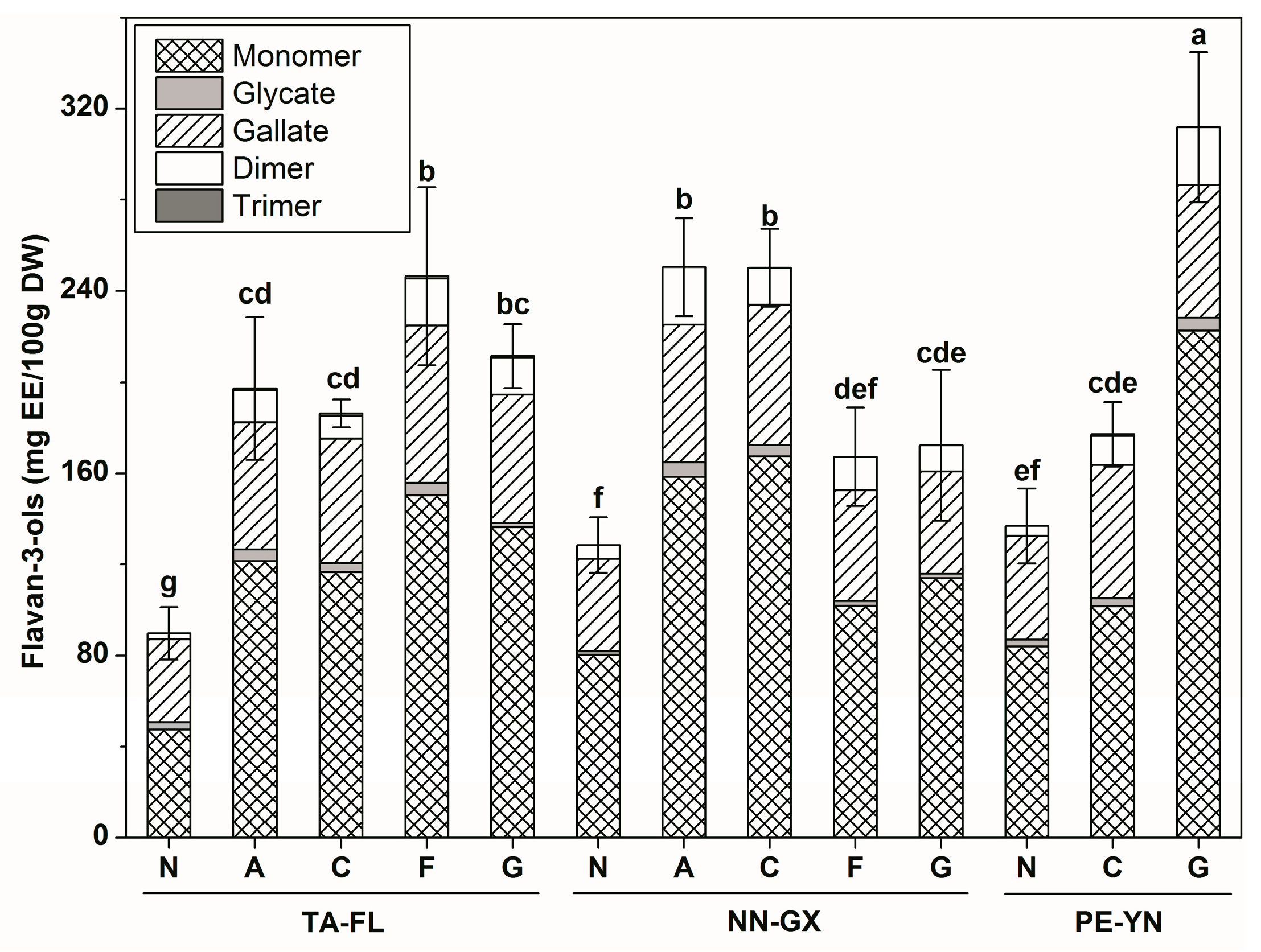
2.2.4. Flavan-3-ols Profiles
2.2.5. Stilbenes and Cinnamon Acids Profiles
2.3. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Chemicals
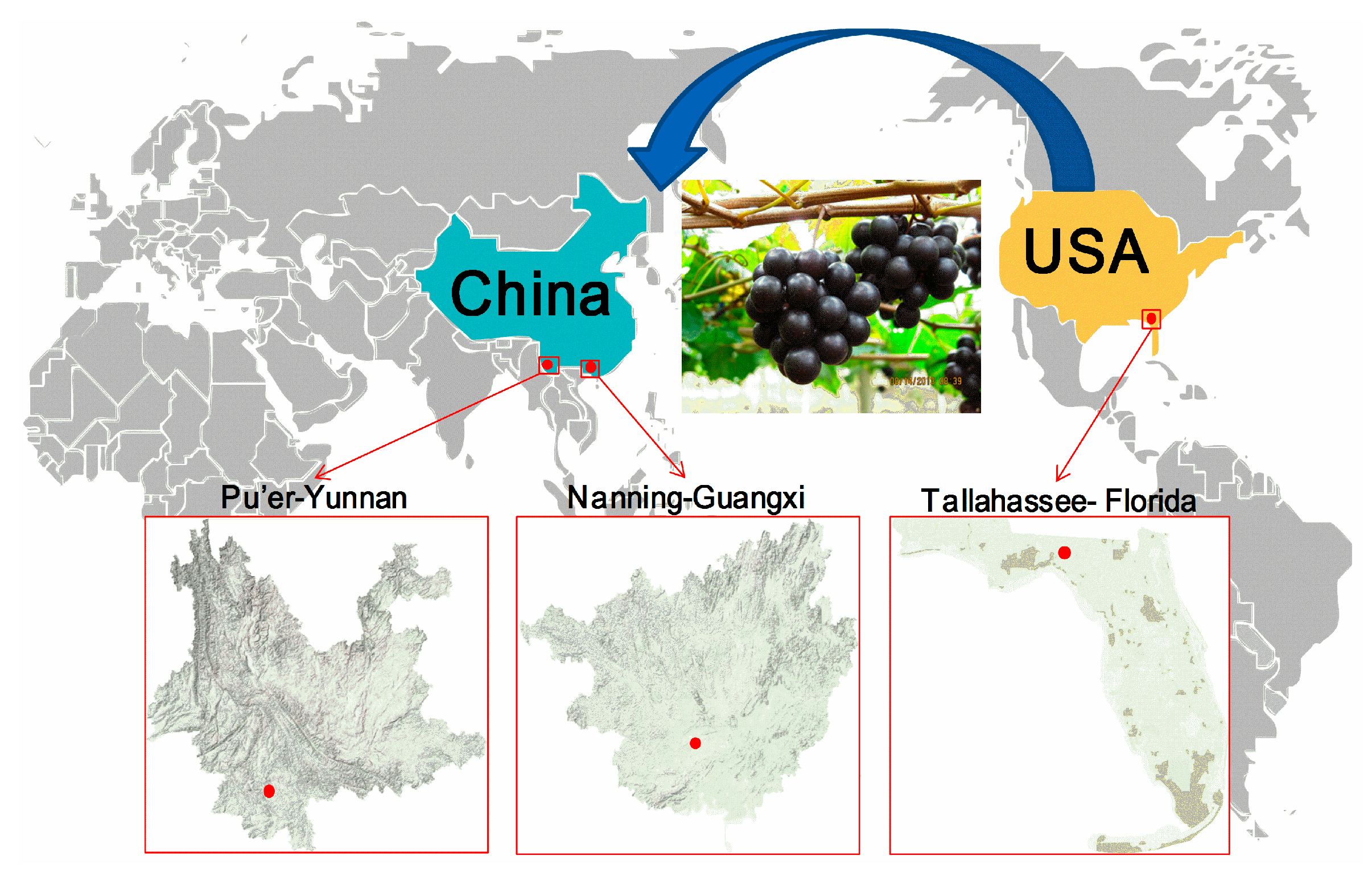
4.2. Grape Materials
4.3. Extraction and Determination of Polyphenol in Muscadine Grapes
4.3.1. Preparation of Berries Extraction
4.3.2. Extraction of Polyphenols in Muscadine Grapes
4.3.3. Determination of TPC
4.3.4. UPLC-Triple TOF-MS/MS Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Conner, P.J. Characteristics of promising muscadine grape (Vitis rotundifolia Michx.) selections from the University of Georgia (USA) Breeding Program. In Proceedings of the X International Conference on Grapevine Breeding and Genetics, 2014; Volume 1046, pp. 303–307.
- Talcott, S.T.; Lee, J. Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J. Agric. Food Chem. 2002, 50, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
- Louime, C.; Lu, J.; Onokpise, O.; Vasanthaiah, H.K.N.; Kambiranda, D.; Basha, S.M.; Yun, H.K. Resistance to Elsinoe Ampelina and expression of related resistant genes in Vitis Rotundifolia Michx. grapes. Int. J. Mol. Sci. 2011, 12, 3473–3488. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.A.; Stringer, S.J.; Spiers, J.D. Stilbene, ellagic acid, flavonol, and phenolic content of muscadine grape (Vitis rotundifolia Michx.) cultivars. Pharm. Crops 2012, 3, 69–77. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Fu, S.; Yin, L.; Zhang, Y.; Lu, J. Callose synthase family genes involved in the grapevine defense response to downy mildew disease. Phytopathology 2016, 106, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W. Antimicrobial and Antioxidant Activity of Muscadine (Vitis rotundifolia Michx.) Extracts as Influenced by Solvent Extraction Methods and Cultivars. Master’s Thesis, Mississippi State University, Starkville, MI, USA, 2011. [Google Scholar]
- Xu, C.; Yagiz, Y.; Hsu, W.; Simonne, A.; Lu, J.; Marshall, M.R. Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J. Agric. Food Chem. 2014, 62, 6640–6649. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yavuz, Y.; Marshall, S.; Li, Z.; Simonne, A.H.; Lu, J.; Marshall, M.R. Application of muscadine grape (Vitis rotundifolia) pomace extract to reduce carcinogenic acrylamide. Food Chem. 2015, 182, 200–208. [Google Scholar] [CrossRef] [PubMed]
- You, Q. Biological Properties Evaluation and Chemical Profiles of Phenolic Compounds in Muscadine Grapes (Vitis rotundifolia). Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2012. [Google Scholar]
- Xu, C.; Zhang, Y.; Zhu, L.; Huang, Y.; Lu, J. Influence of growing season on phenolic compounds and antioxidant properties of grape berries from vines grown in subtropical climate. J. Agric. Food Chem. 2011, 59, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.; Lu, J. Phenolic contents and compositions in skins of red wine grape cultivars among various genetic backgrounds and originations. Int. J. Mol. Sci. 2012, 13, 3492–3510. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.K.; Gu, L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (Muscadine Grapes) as determined by HPLC-DAD-ESI-MS(n). J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Johnson, J.V.; Talcott, S.T. Identification of ellagic acid conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J. Agric. Food Chem. 2005, 53, 6003–6010. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, B.; Chira, K.; Teissedre, P. Phenolic composition of Merlot and Cabernet-Sauvignon grapes from Bordeaux vineyard for the 2009-vintage: Comparison to 2006, 2007 and 2008 vintages. Food Chem. 2011, 126, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Narduzzi, L.; Stanstrup, J.; Mattivi, F. Comparing wild American grapes with Vitis vinifera: A metabolomics study of grape composition. J. Agric. Food Chem. 2011, 63, 6823–6834. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Shaw, D.A.; Stringer, S.J.; Sampson, B.J.; Spiers, J.D. Storage retention of stilbene, ellagic acid, flavonol, and phenolic content of muscadine grape (Vitis rotundifolia Michx.) cultivars. J. Food Chem. Nutr. 2014, 2, 81–92. [Google Scholar]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin composition of blackberry as determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Edit. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- García-ESstévez, I.; Andrés-García, P.; Alcalde-Eon, C.; Giacosa, S.; Rolle, L.; Rivas-Gonzalo, J.C.; Quijada-Morín, N.; Escribano-Bailón, M.T. Relationship between agronomic parameters, phenolic composition of grape skin, and texture properties of Vitis vinifera L. cv. Tempranillo. J. Agric. Food Chem. 2015, 63, 7663–7669. [Google Scholar] [CrossRef] [PubMed]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Viticult. 2006, 57, 257–268. [Google Scholar]
- Sandhu, A.K.; Gray, D.J.; Lu, J.; Gu, L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skin. Food Chem. 2011, 126, 982–988. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, Y.; Zhao, L.; Jiang, X.; Li, M.; Wang, Y.; Xu, Y.; Gao, L.; Xia, T. Effect of low-intensity white light mediated de-etiolation on the biosynthesis of polyphenols in tea seedslings. Plant Physiol. Biochem. 2014, 80, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Artem, V.; Antoce, A.O.; Namolosanu, I.; Ranca, A.; Petrescu, A. The influence of the vine cultivation technology on the phenolic composition of red grapes. Horticulture 2015, 59, 117–122. [Google Scholar]
- Zhu, L.; Zhang, Y.; Zhang, W.; Lu, J. Effects of exogenous abscisic acid on phenolic characteristics of red Vitis vinifera grapes and wines. Food Sci. Biotechnol. 2016, 25, 361–370. [Google Scholar] [CrossRef]
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Câmara, J.S. Establishment of the varietal profile of Vitis vinifera L. grape varieties from different geographical regions based on HS-SPME/GC-qMS combined with chemometric tools. Microchem. J. 2014, 116, 107–117. [Google Scholar] [CrossRef]
- Silva, J.K.; Cazarin, C.B.B.; Correa, L.C.; Batista, A.G.; Furlan, C.P.B.; Biasoto, A.C.T.; Pereira, G.E.; Camargo, A.C.; Maróstica Junior, M.R. Bioactive compounds of juices from two Brazilian grape cultivars. J. Sci. Food Agric. 2016, 96, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Heras-Roger, J.; Díaz-Romero, C.; Darias-Martín, J. A comprehensive study of red wine properties according to variety. Food Chem. 2016, 196, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Camp, M.J. Temperatures after bloom affect plant growth and fruit quality of strawberry. Sci. Hortic. 2000, 85, 183–199. [Google Scholar] [CrossRef]
- Li, X.; Wasila, H.; Liu, L.; Yuan, T.; Gao, Z.; Zhao, B.; Ahmad, I. Physicochemical characteristics, polyphenol compositions and antioxidant potential of pomegranate juices from 10 Chinese cultivars and the environmental factors analysis. Food Chem. 2015, 175, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Viticult. 2012, 53, 171–182. [Google Scholar]
- Sun, X.; Li, L.; Ma, T.; Liu, X.; Huang, W.; Zhan, J. Profiles of phenolic acids and flavan-3-ols for select Chinese red wines: A comparison and differentiation according to geographic origin and grape variety. J. Food Sci. 2015, 80, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Shibayama, K. Effects of trunk girdling and crop load levels on fruit quality and root elongation in ‘Aki Queen’ grapevines. J. Jap. Soc. Hortic. Sci. 2006, 75, 439–444. [Google Scholar] [CrossRef]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factor in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine R. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Terry, L.A.; Chope, G.A.; Giné Bordonaba, J. Effect of water deficit irrigation and inoculation with: Botrytis cinerea on strawberry (Fragaria×ananassa) fruit quality. J. Agric. Food Chem. 2007, 55, 10812–10819. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Matthews, M.A.; Gaspero, G.D.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Doumett, S.; Fibbi, D.; Cincinelli, A.; Giordani, E.; Nin, S.; Del Bubba, M. Comparison of nutritional and nutraceutical properties in cultivated fruits of Fragaria vesca L. produced in Italy. Food Res. Int. 2011, 44, 1209–1216. [Google Scholar] [CrossRef]
- Guerrero-Chavez, G.; Scampicchio, M.; Andreotti, C. Influence of the site altitude on strawberry phenolic composition and quality. Sci. Hortic. 2015, 192, 21–28. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, Y.; Huang, Y.; Zhang, Y.; Lu, J. Optimization of ultrasound-assisted extraction of ellagic acid and total phenols from muscadine (Vitis rotundifolia) by response surface methodology. Food Sci. 2015, 36, 29–35. (In Chinese) [Google Scholar]

| Cultivar | Region | Year | TPC (mg GAE/g DW) | ||
|---|---|---|---|---|---|
| Skins | Seeds | Skins + Seeds | |||
| Red cultivars | |||||
| Noble (N) | TA-FL | 2012 | 38.96 ± 2.57 d | 96.65 ± 3.30 a | 135.61 ± 5.17 c |
| NN-GX | 2012 | 42.06 ± 2.00 c | 82.10 ± 5.15 b | 124.16 ± 6.55 d | |
| 2013 | 48.08 ± 0.67 b | 95.07 ± 1.07 a | 143.14 ± 0.95 b | ||
| PE-YN | 2012 | 41.40 ± 1.93 c | 85.44 ± 3.40 b | 126.84 ± 4.32 d | |
| 2013 | 52.68 ± 1.91 a | 97.55 ± 3.04 a | 150.23 ± 3.19 a | ||
| Sig. a | ** | *** | *** | ||
| Alachua (A) | TA-FL | 2012 | 36.21 ± 1.70 b | 118.59 ± 2.51 a | 154.80 ± 3.33 b |
| NN-GX | 2012 | 45.03 ± 1.35 a | 107.05 ± 3.48 b | 152.08 ± 4.28 b | |
| 2013 | 46.12 ± 2.82 a | 119.41 ± 4.14 a | 165.53 ± 1.49 a | ||
| Sig. a | *** | *** | ns | ||
| Sig. b | **, ** | ***, *** | ***, *** | ||
| Bronze cultivars | |||||
| Carlos (C) | TA-FL | 2012 | 30.86 ± 4.88 c | 103.74 ± 4.50 b | 134.60 ± 7.92 c |
| NN-GX | 2012 | 35.98 ± 1.58 b | 90.32 ± 5.06 c | 126.30 ± 5.38 d | |
| 2013 | 56.44 ± 1.37 a | 122.55 ± 1.61 a | 178.99 ± 1.96 a | ||
| PE-YN | 2012 | 30.22 ± 1.50 c | 83.83 ± 4.80 d | 114.05 ± 4.94 e | |
| 2013 | 57.33 ± 2.07 a | 102.87 ± 3.01 b | 160.20 ± 3.58 b | ||
| Sig. a | *** | *** | *** | ||
| Fry (F) | TA-FL | 2012 | 27.43 ± 4.25 b | 118.74 ± 3.47 a | 146.17 ± 6.69 b |
| NN-GX | 2012 | 38.68 ± 1.83 a | 77.88 ± 3.81 c | 116.56 ± 3.54 c | |
| 2013 | 39.80 ± 2.02 a | 113.49 ± 2.78 b | 153.29 ± 3.70 a | ||
| Sig. a | *** | *** | *** | ||
| Granny Val (G) | TA-FL | 2012 | 34.44 ± 2.02 b | 76.56 ± 2.28 d | 111.00 ± 3.83 c |
| NN-GX | 2012 | 29.34 ± 2.41 c | 81.14 ± 1.73 c | 110.48 ± 3.21 c | |
| 2013 | 44.92 ± 1.08 a | 116.36 ± 1.21 a | 161.28 ± 0.94 a | ||
| PE-YN | 2012 | 20.53 ± 0.78 d | 85.78 ± 2.94 b | 106.31 ± 3.24 d | |
| 2013 | 35.38 ± 1.08 b | 114.13 ± 3.46 a | 149.51 ± 3.85 b | ||
| Sig. a | *** | *** | * | ||
| Sig. b | ***, *** | ***, *** | ***, *** | ||
| Cultivar | Region | Year | Ellagic Acids and Precursors (mg GAE/100 g DW) | Flavonols (mg RE/100 g DW) | Benzoic Acids (mg GAE/100 g DW) | Flavan-3-ols (mg EE/100 g DW) | Stilbenes (mg REE/100 g DW) | Cinnamic Acids (mg CAE/100 g DW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skins | Seeds | Skins | Seeds | Skins | Seeds | Skins | Seeds | Skins | Seeds | Skins | Seeds | |||
| Red cultivars | ||||||||||||||
| Noble (N) | TA-FL | 2012 | 174.30 b | 636.75 a | 35.42 c | 18.96 a | 19.49 b | 4.20 d | nd | 89.72 c | 0.06 b | nd | nd | nd |
| NN-GX | 2012 | 197.25 b | 557.76 b | 26.28 d | 7.03 c | 36.60 a | 19.10 b | 0.08 a | 128.43 b | nd | nd | nd | nd | |
| 2013 | 255.66 a | 670.73 a | 57.52 b | 13.03 b | 39.00 a | 34.90 a | nd | 216.25 a | nd | nd | nd | nd | ||
| PE-YN | 2012 | 196.85 b | 380.61 d | 38.89 c | 10.48 b,c | 18.00 b | 7.18 c,d | 0.05 b | 136.88 b | 0.06 b | nd | nd | nd | |
| 2013 | 299.12 a | 468.22 c | 66.00 a | 14.76 a,b | 40.36 a | 10.96 c | nd | 230.45 a | 0.11 a | nd | nd | nd | ||
| Sig. a | ns | *** | ** | *** | *** | ** | n/a | * | n/a | n/a | n/a | n/a | ||
| Alachua (A) | TA-FL | 2012 | 174.31 c | 510.76 b | 37.74 b | 21.26 a | 17.04 a | 6.75 b | 0.02 b | 197.61 b | nd | nd | nd | nd |
| NN-GX | 2012 | 220.99 b | 577.89 a | 24.54 c | 9.50 b | 7.51 b | 19.11 a | 0.05 a | 250.96 b | 0.06 a | nd | nd | nd | |
| 2013 | 294.57 a | 512.46 b | 55.20 a | 28.85 a | 9.72 b | 0.36 c | 0.03 a,b | 369.42 a | 0.06 a | nd | nd | nd | ||
| Sig. a | * | ns | * | * | * | ** | ** | ns | n/a | n/a | n/a | n/a | ||
| Sig. b | ns, ** | *, ns | ns, ns | ns, * | ns, *** | *, ns | ***, ** | **, *** | n/a, n/a | n/a, n/a | n/a, n/a | n/a, n/a | ||
| Bronze cultivars | ||||||||||||||
| Carlos (C) | TA-FL | 2012 | 144.94 d | 257.21 c | 21.52 c | 20.69 c | 12.28 b,c | 4.93 b,c | 0.05 b | 186.56 b | nd | nd | nd | nd |
| NN-GX | 2012 | 169.89 c | 406.42 b | 20.79 c | 10.52 d | 21.42 a | 7.22 a | 0.08 b | 250.65 a | 0.18 a | nd | 0.14 b | nd | |
| 2013 | 212.85 b | 457.55 a,b | 31.66 b | 41.60 a | 16.12 b | 4.34 c | 0.24 a | 266.21 a | 0.24 a | nd | nd | nd | ||
| PE-YN | 2012 | 155.07 c,d | 381.49 b | 33.10 b | 14.67 c,d | 6.81 d | 6.14 a,b | 0.06 b | 177.49 b | nd | nd | 0.22 a | nd | |
| 2013 | 264.76 a | 514.50 a | 54.75 a | 29.06 b | 7.77 c,d | 1.71 d | 0.02 b | 258.52 a | 0.03 b | nd | nd | nd | ||
| Sig. a | * | *** | ** | *** | ** | ** | * | *** | n/a | n/a | n/a | n/a | ||
| Fry (F) | TA-FL | 2012 | 132.30 c | 482.50 a | 30.06 c | 27.89 b | 13.31 b | 9.14 a | 0.02 b | 246.94 a | nd | nd | 0.11 | nd |
| NN-GX | 2012 | 201.26 b | 486.00 a | 39.95 b | 7.98 c | 22.37 a | 6.62 b | 0.07 a | 167.81 b | nd | nd | nd | nd | |
| 2013 | 273.25 a | 475.86 a | 60.60 a | 38.97 a | 6.59 c | 5.50 b | nd | 240.49 a | 0.12 | nd | nd | nd | ||
| Sig. a | * | ns | * | ** | ** | * | *** | * | n/a | n/a | n/a | n/a | ||
| Granny Val (G) | TA-FL | 2012 | 85.42 d | 153.14 d | 16.07 c | 10.17 b | 16.41 b,c | 4.93 b | 0.02 c,d | 212.01 b,c | 0.06 a | nd | 0.19 b | nd |
| NN-GX | 2012 | 248.73 b | 398.08 b | 33.90 b | 9.82 b | 33.97 a | 19.65 a | 0.04 b,c | 172.55 c | 0.06 a | nd | nd | nd | |
| 2013 | 295.44 a | 582.68 a | 44.83 a,b | 31.56 a | 14.80 b,c | 11.66 a,b | 0.06 a,b | 250.59 b | 0.09 a | nd | nd | nd | ||
| PE-YN | 2012 | 201.41 c | 224.19 c | 35.69 a,b | 12.13 b | 11.90 a | 18.83 a | 0.09 a | 312.45 a | 0.06 a | nd | 0.59 a | nd | |
| 2013 | 268.67 a,b | 281.51 c | 47.86 a | 23.60 a | 23.53 a,b | 10.83 a,b | nd | 319.56 a | nd | nd | nd | nd | ||
| Sig. a | *** | *** | * | ns | * | ns | ** | ** | ns | n/a | n/a | n/a | ||
| Sig. b | *, ** | ***, ns | ns, ** | ***, ns | ns, ns | ***, ns | ***, ** | ns, * | n/a, n/a | n/a, n/a | n/a, n/a | n/a, n/a | ||
| No. | Compounds | Abbreviation | Skins | Seeds | ||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group A | Group B | Group C | |||
| 1 | Total phenolic content | TPC | −0.4043 | −1.2307 | 1.4201 | −0.1302 | 0.4540 | −0.2132 |
| 2 | Ellagic acids and precursors | EPs | −4.0271 | 2.4873 | 0.3189 | 2.5816 | 1.8111 | −2.6843 |
| 3 | Ellagic acid derivatives | EDs | −3.0158 | 2.5967 | 0.1427 | 5.5202 | −1.1154 | −0.2678 |
| 4 | Ellagic acid | E | −2.7306 | 1.4526 | 0.2713 | 4.8190 | −1.0040 | −0.2151 |
| 5 | Methyl-ellagic acids (1–3) | Me-Es | −0.1053 | 0.5372 | −0.2334 | 1.3958 | −0.2875 | −0.0571 |
| 6 | Ducheside A | Du-A | −0.0512 | 0.1191 | 0.0386 | 0.9210 | −0.2086 | −0.0345 |
| 7 | Ducheside B | Du-B | 0.1316 | 1.5802 | −1.0522 | 1.0886 | −0.1932 | −0.0910 |
| 8 | Ellagic acid glucoside | E-g | −0.8667 | 0.8782 | −0.0100 | 0.3727 | −0.0648 | −0.0317 |
| 9 | Ellagic acid-dihexoside | E-dig | −0.0232 | 0.0385 | −0.0194 | − | − | − |
| 10 | Ellagic acid glu/diglu | E-g/dig | −1.6530 | 1.4763 | 0.0737 | − | − | − |
| 11 | Ellagitannins | ETs | −1.9865 | 0.2552 | 1.1640 | 1.6039 | −0.2092 | −0.1179 |
| 12 | Methyl brevifolin carboxylate | Me-B-C | −0.9633 | 0.4421 | 0.6616 | 0.8561 | −0.0601 | −0.0858 |
| 13 | HHDP-glucose | H-g | 0.0546 | 0.0342 | −0.1208 | 0.7131 | −0.1203 | −0.0299 |
| 14 | Pedunculagin α/β isomer (Di-HHDP-glucose) | Di-H-g | − | − | − | 0.1157 | −0.0191 | −0.0138 |
| 15 | Mono-/di-HHDP-glucose | Mo/di-H-g | − | − | − | − | − | − |
| 16 | Galloyl-HHDP-glucose (Corilagin, Strictinin) | G-H-g | −0.0325 | 0.1229 | −0.0254 | 0.4223 | −0.0227 | −0.0541 |
| 17 | Galloyl-bis-HHDP-glucose (Casuarinin) | G-diH-g | − | − | − | 0.2189 | −0.0866 | 0.00002 |
| 18 | Galloyl-HHDP-glucose/Galloyl-bis-HHDP-glucose | G-H-g/G-diH-g | − | − | − | 0.4264 | −0.1078 | −0.0077 |
| 19 | HHDP-galloyl-glucose (Isostrictinin) | H-G-g | −0.0325 | 0.1229 | −0.0254 | 0.4223 | −0.0227 | −0.0541 |
| 20 | Tellimagrain I (Digalloyl-HHDP-glucose) | DiG-H-g | −0.0307 | 0.0334 | 0.0139 | 0.8165 | −0.0951 | −0.0365 |
| 21 | Tellimagrain II (Trigalloyl-HHDP-glucose) | TriG-H-g | − | − | − | 0.1536 | 0.0236 | −0.0365 |
| 22 | Precursors (Gallic acid derivatives) | GDs | −0.4620 | 0.9006 | −0.1423 | 0.5890 | 2.7441 | −2.3172 |
| 23 | Gallic acid | G | 0.0934 | 0.0532 | −0.1932 | 0.5468 | 0.3028 | −0.3943 |
| 24 | Methyl gallate | Me-G | −0.6455 | 0.6317 | 0.0035 | −0.1558 | −0.0158 | 0.0232 |
| 25 | Mono-O-galloyl-glucose | Mo-G-g | −0.0028 | 0.0315 | −0.0143 | 0.0113 | 0.0393 | −0.0362 |
| 26 | Di-O-galloyl-glucose | Di-G-g | 0.0170 | 0.2667 | −0.1653 | −0.0016 | 0.0059 | −0.0037 |
| 27 | Tri-O-galloyl-glucose | Tri-G-g | 0.0035 | 0.0996 | −0.0766 | 0.1246 | 0.0980 | −0.1513 |
| 28 | Tetra-O-galloyl-glucose | Tetra-G-g | − | − | − | 0.1164 | 0.5409 | −0.4237 |
| 29 | Penta-O-galloyl-glucose | Penta-G-g | − | − | − | 0.5180 | 2.7015 | −2.0699 |
| 30 | Galloyl-glucoses (1–5) | G-gs | 0.0137 | 0.2838 | −0.1641 | 0.5533 | 2.7601 | −2.1336 |
| 31 | Flavonols | Fla | −0.5623 | 1.0174 | 0.0858 | −0.0143 | 0.0376 | −0.0142 |
| 32 | Myricetin derivatives | MDs | 0.0022 | 1.0660 | −0.7312 | 0.2699 | −0.0538 | −0.0153 |
| 33 | Quercetin derivatives | QDs | −0.3407 | −0.3765 | 1.1126 | −0.0641 | 0.0734 | −0.0118 |
| 34 | Kaempferol derivatives | KDs | −0.2456 | −0.0330 | 0.2954 | −0.0013 | −0.0213 | 0.0107 |
| 35 | Isorhamnetin derivatives | IDs | −0.0266 | −0.0245 | 0.3417 | 0.0296 | 0.0184 | −0.0067 |
| 36 | Laricitrin derivatives | LDs | −0.1156 | −0.1750 | 0.4492 | 0.0073 | −0.0007 | −0.0028 |
| 37 | Syringetin derivatives | SDs | 0.0174 | 0.5389 | −0.3337 | − | − | − |
| 38 | 4'-substituted flavonol | 4'-s | −0.2456 | −0.0330 | 0.2954 | −0.0013 | −0.0213 | 0.0107 |
| 39 | 3'4'-substituted flavonol | 3'4'-s | −0.3238 | −0.3563 | 1.1856 | −0.0317 | 0.0497 | −0.0140 |
| 40 | 3'4'5'-substituted flavonol | 3'4'5'-s | −0.0401 | 2.3605 | −1.1655 | 0.2033 | −0.0183 | −0.0211 |
| 41 | Flavonol aglycone | Agl | −0.0720 | 0.2056 | 0.0156 | −0.0351 | 0.0521 | −0.0141 |
| 42 | Flavonol monoglucoside | Mog | −0.6273 | −0.1335 | 0.7024 | 0.1012 | −0.0196 | −0.0228 |
| 43 | Flavonol diglucoside | Dig | −0.1058 | −0.2083 | 0.6461 | − | − | − |
| 44 | Flavonol acylation | Acy | −0.2102 | −0.4368 | 0.9267 | −0.0109 | −0.0100 | 0.0072 |
| 45 | Benzoic acids | BAs | −0.5820 | 0.5540 | 0.2280 | 0.0510 | 0.0038 | −0.0110 |
| 46 | Flavan-3-ols | Fla-3-o | −0.0175 | 0.0228 | −0.0020 | −3.6329 | 0.7254 | 0.1494 |
| 47 | Flavan-3-ol monomer | Mo | −0.0021 | −0.0014 | 0.0037 | −2.7396 | 0.3694 | 0.1878 |
| 48 | Flavan-3-ol glycate | Gly | − | − | − | −0.2402 | 0.1468 | −0.0084 |
| 49 | Flavan-3-ol gallate | Gal | −0.0077 | 0.1030 | −0.0425 | −1.5488 | 0.4488 | 0.0222 |
| 50 | Flavan-3-ol dimer | Di | − | − | − | −0.4692 | 0.1855 | −0.00004 |
| 51 | Flavan-3-ol trimer | Tri | − | − | − | −0.2596 | 0.0910 | 0.0051 |
| 52 | Flavan-3-ol monomer/gallate | Mo/Gal | −0.0103 | 0.1002 | −0.0349 | −0.2456 | 0.0016 | 0.0313 |
| 53 | Stilbenes | Sti | −0.0164 | −0.0003 | 0.0271 | − | − | − |
| 54 | Cinnamic acids | CAs | 0.0181 | 0.1248 | −0.0987 | − | − | − |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Luo, J.; Huang, Y.; Guo, W.; Zhang, Y.; Guan, H.; Xu, C.; Lu, J. Profile of Polyphenol Compounds of Five Muscadine Grapes Cultivated in the United States and in Newly Adapted Locations in China. Int. J. Mol. Sci. 2017, 18, 631. https://doi.org/10.3390/ijms18030631
Wei Z, Luo J, Huang Y, Guo W, Zhang Y, Guan H, Xu C, Lu J. Profile of Polyphenol Compounds of Five Muscadine Grapes Cultivated in the United States and in Newly Adapted Locations in China. International Journal of Molecular Sciences. 2017; 18(3):631. https://doi.org/10.3390/ijms18030631
Chicago/Turabian StyleWei, Zheng, Jianming Luo, Yu Huang, Wenfeng Guo, Yali Zhang, Huan Guan, Changmou Xu, and Jiang Lu. 2017. "Profile of Polyphenol Compounds of Five Muscadine Grapes Cultivated in the United States and in Newly Adapted Locations in China" International Journal of Molecular Sciences 18, no. 3: 631. https://doi.org/10.3390/ijms18030631
APA StyleWei, Z., Luo, J., Huang, Y., Guo, W., Zhang, Y., Guan, H., Xu, C., & Lu, J. (2017). Profile of Polyphenol Compounds of Five Muscadine Grapes Cultivated in the United States and in Newly Adapted Locations in China. International Journal of Molecular Sciences, 18(3), 631. https://doi.org/10.3390/ijms18030631