Cellular Midpalatal Suture Changes after Rapid Maxillary Expansion in Growing Subjects: A Case Report
Abstract
:1. Introduction
2. Results
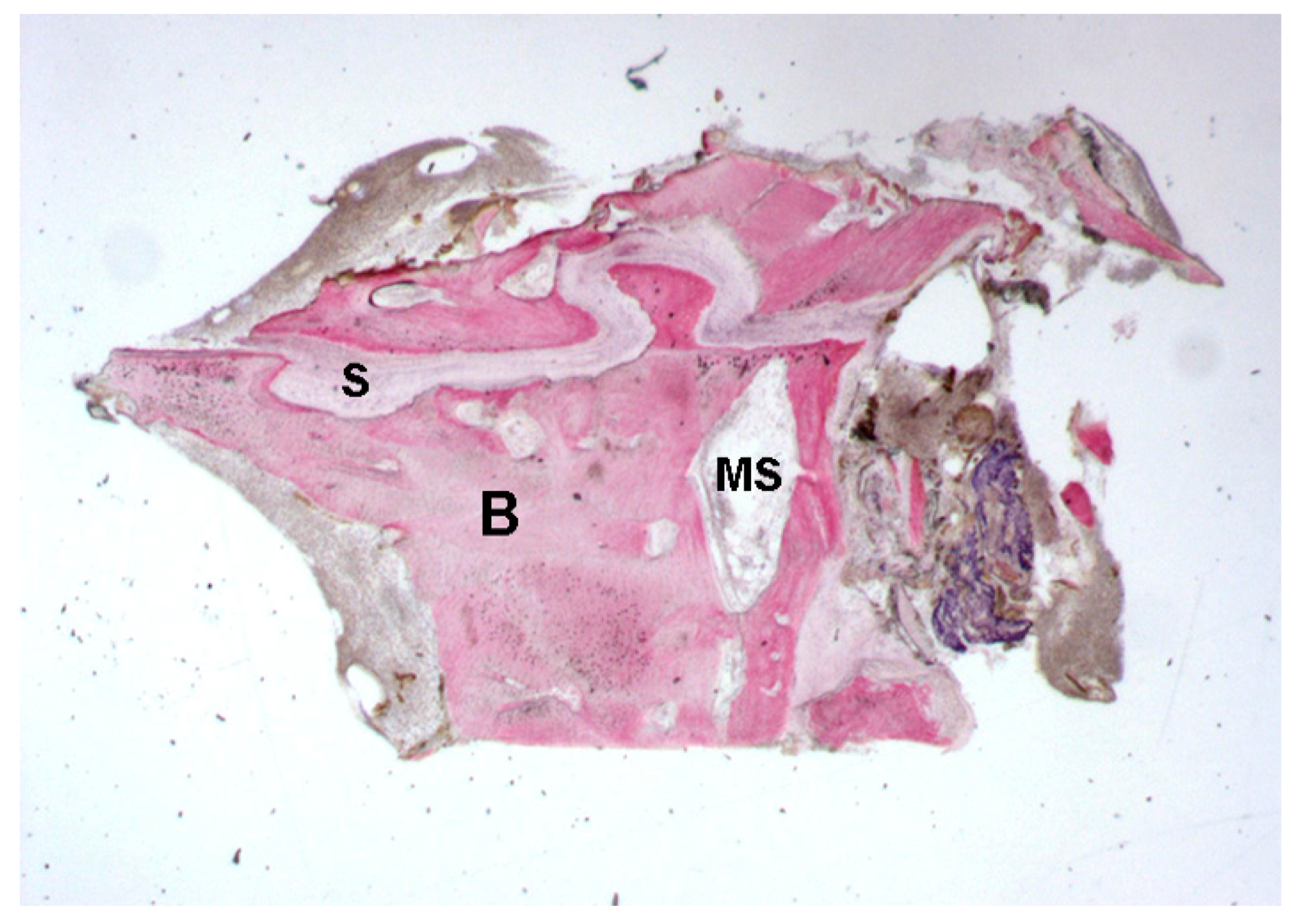
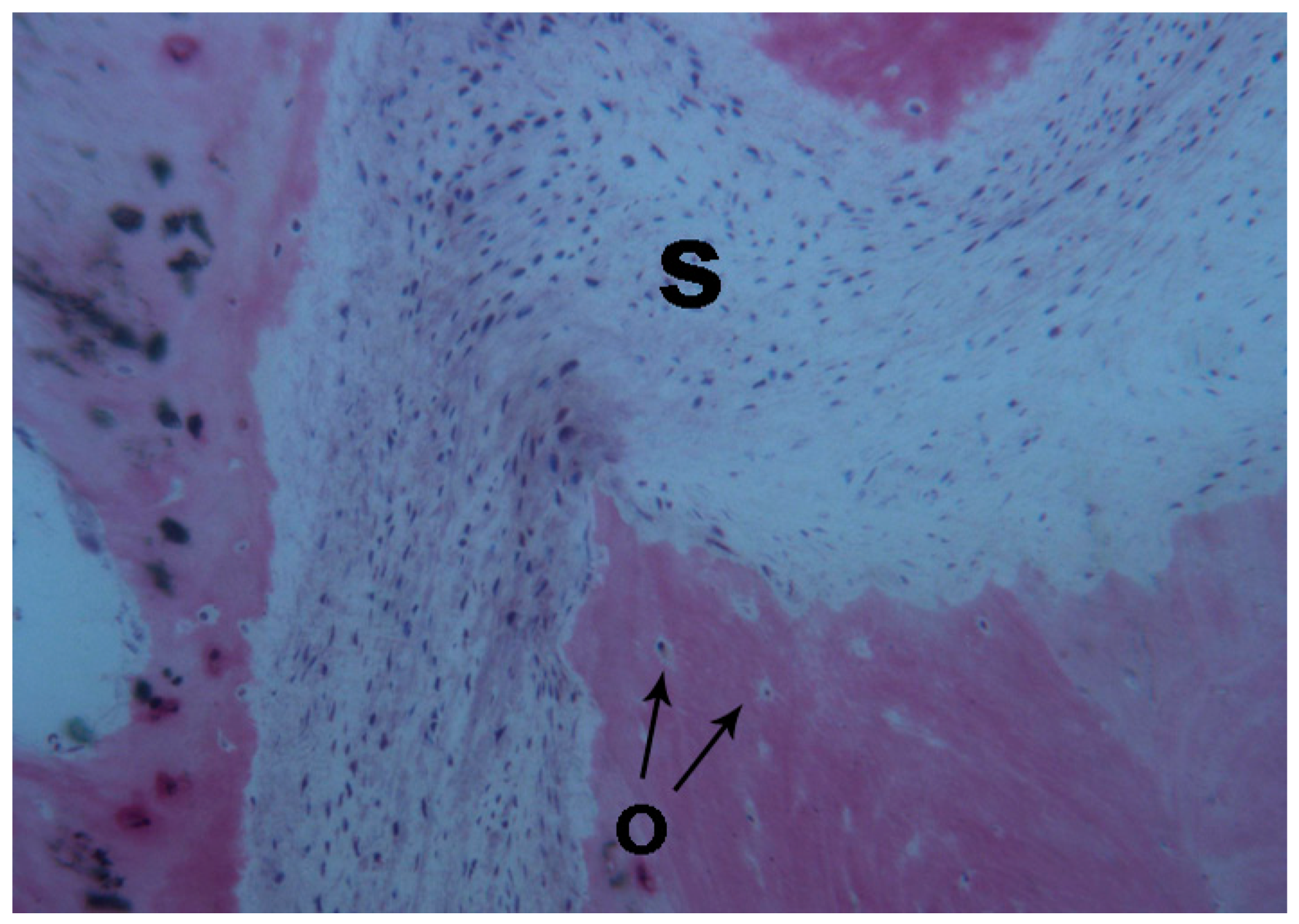
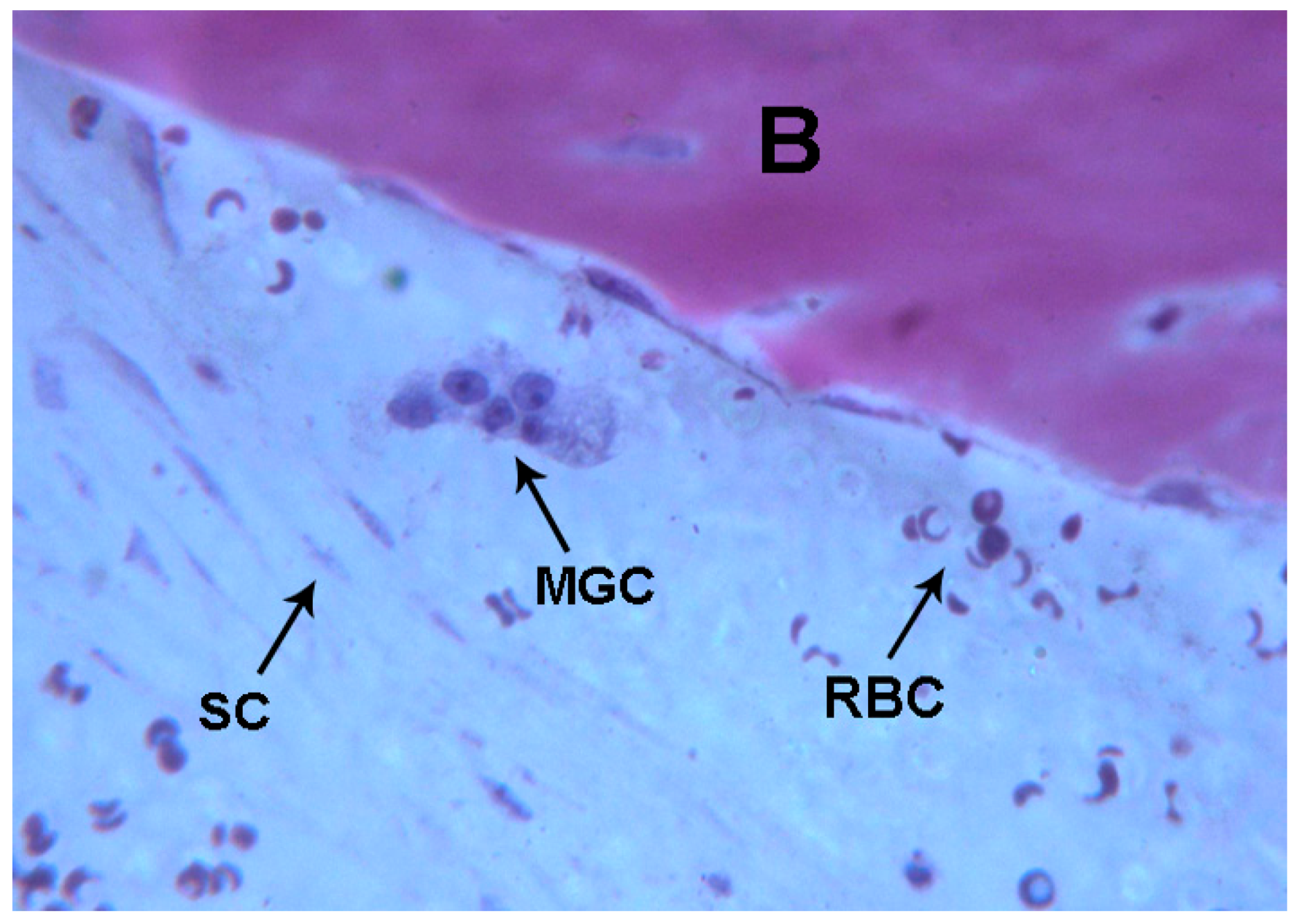
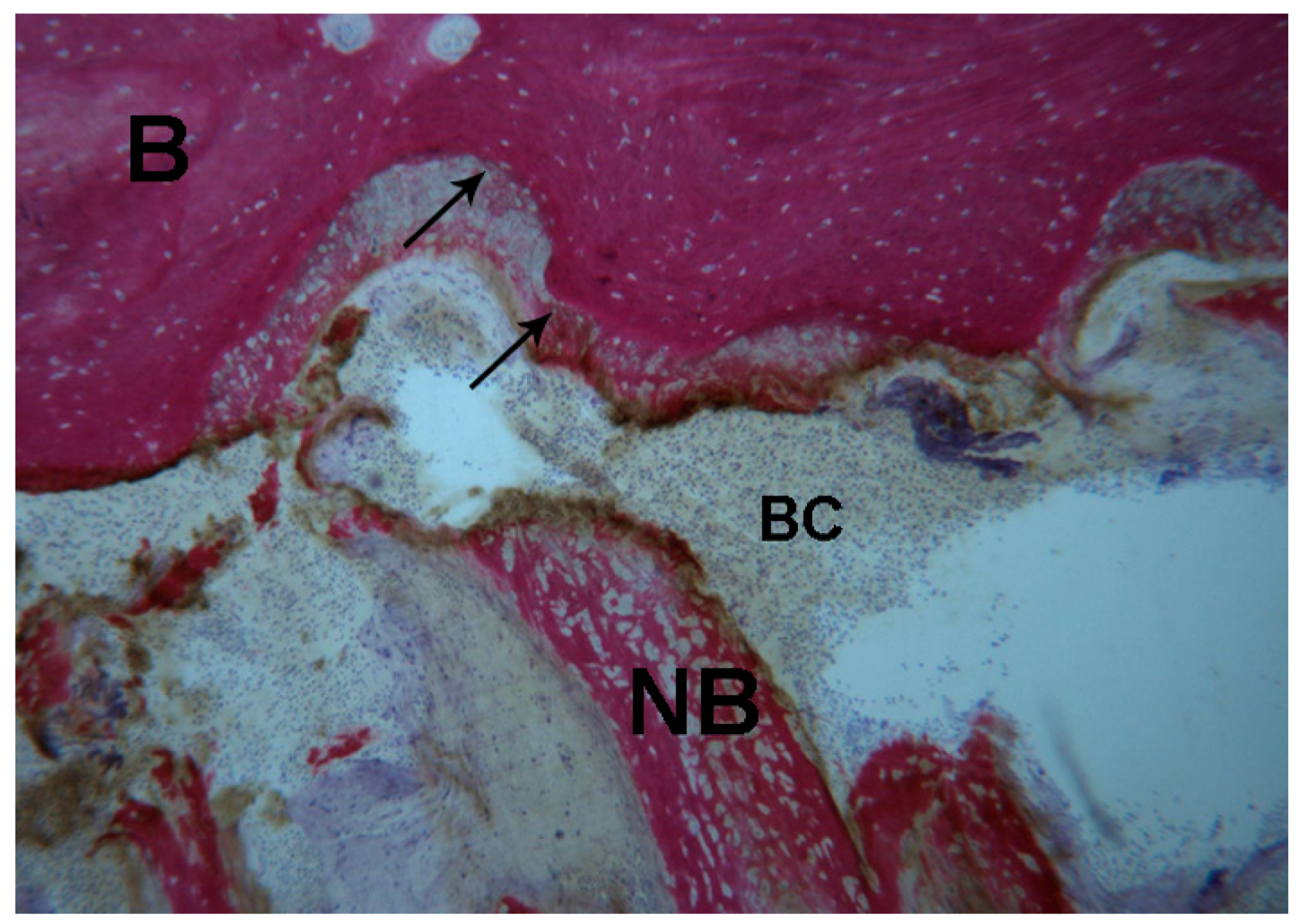
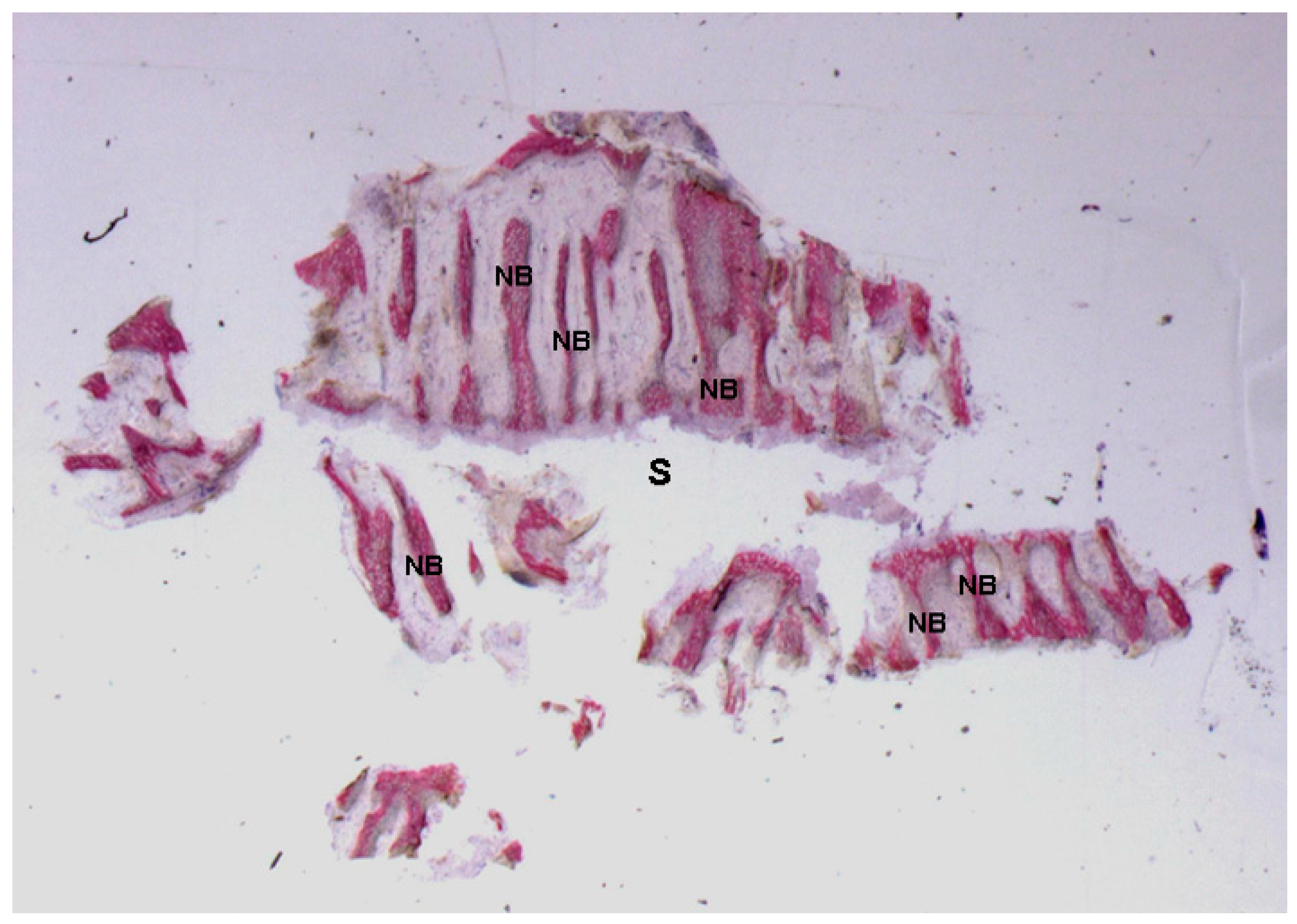
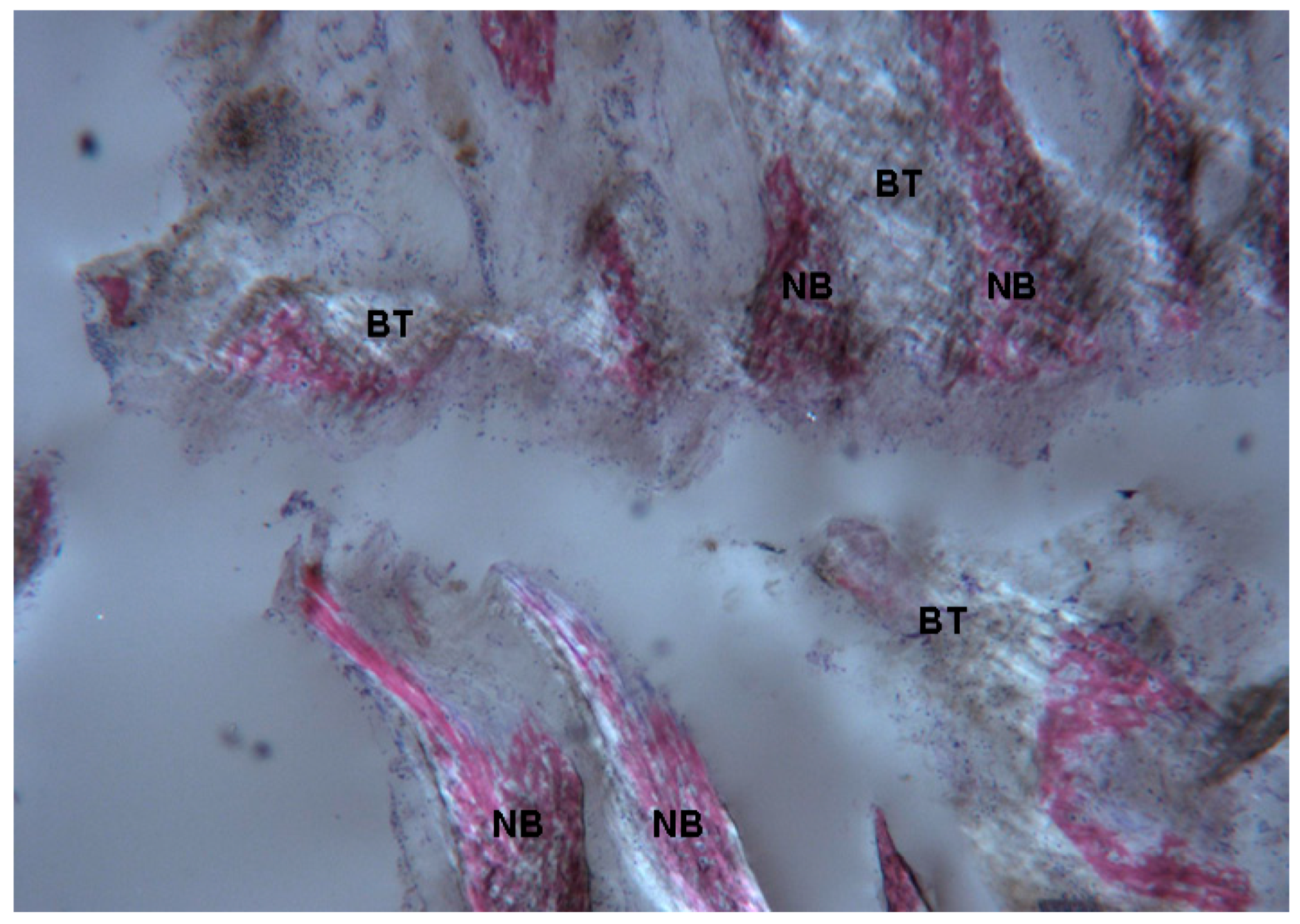
2.1. Histological Results
2.1.1. Control (Subject 3, Male)
2.1.2. Test 7-Days (Subject 1, Female)
2.1.3. Test at 30 Days (Subject 2, Male)
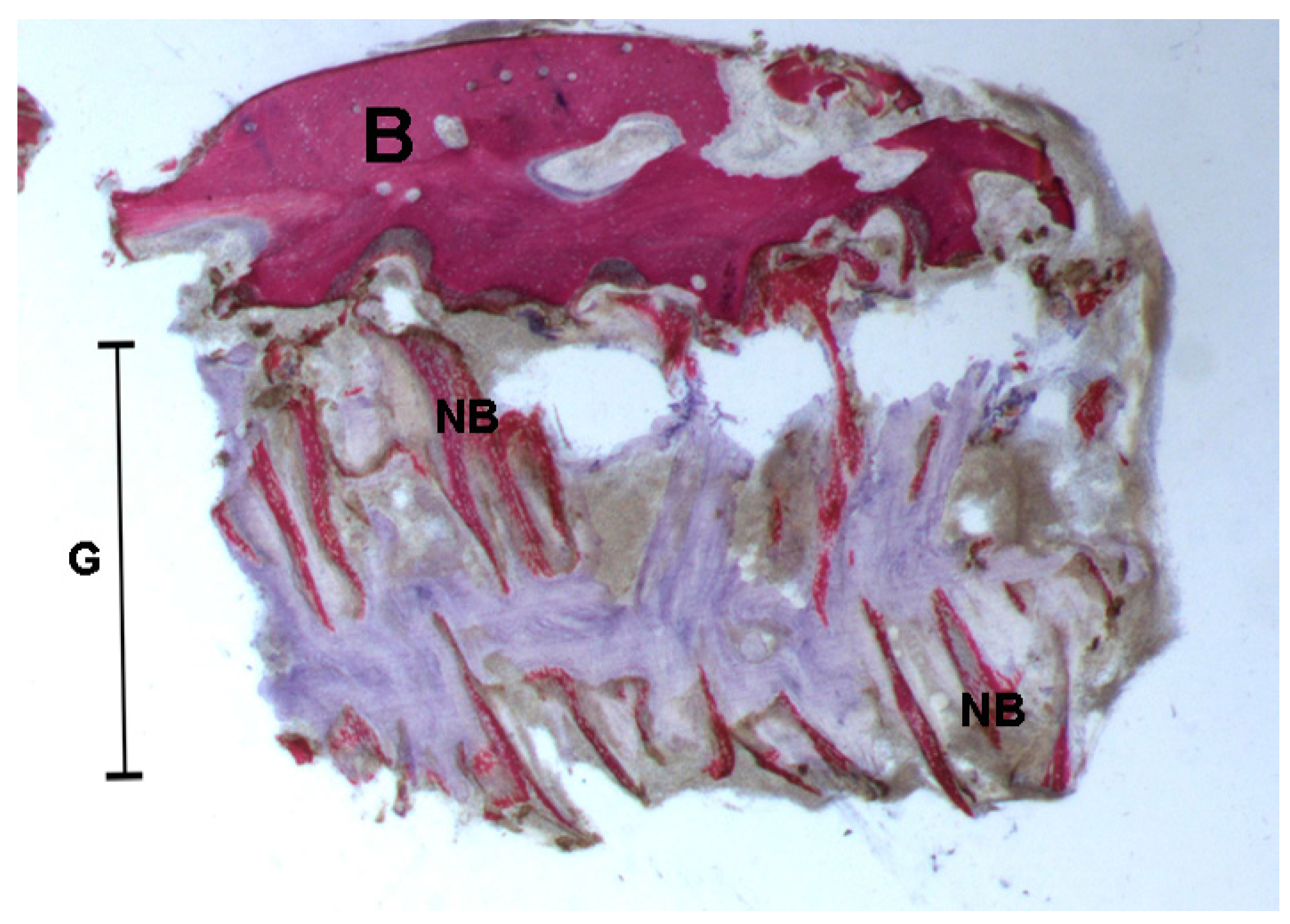
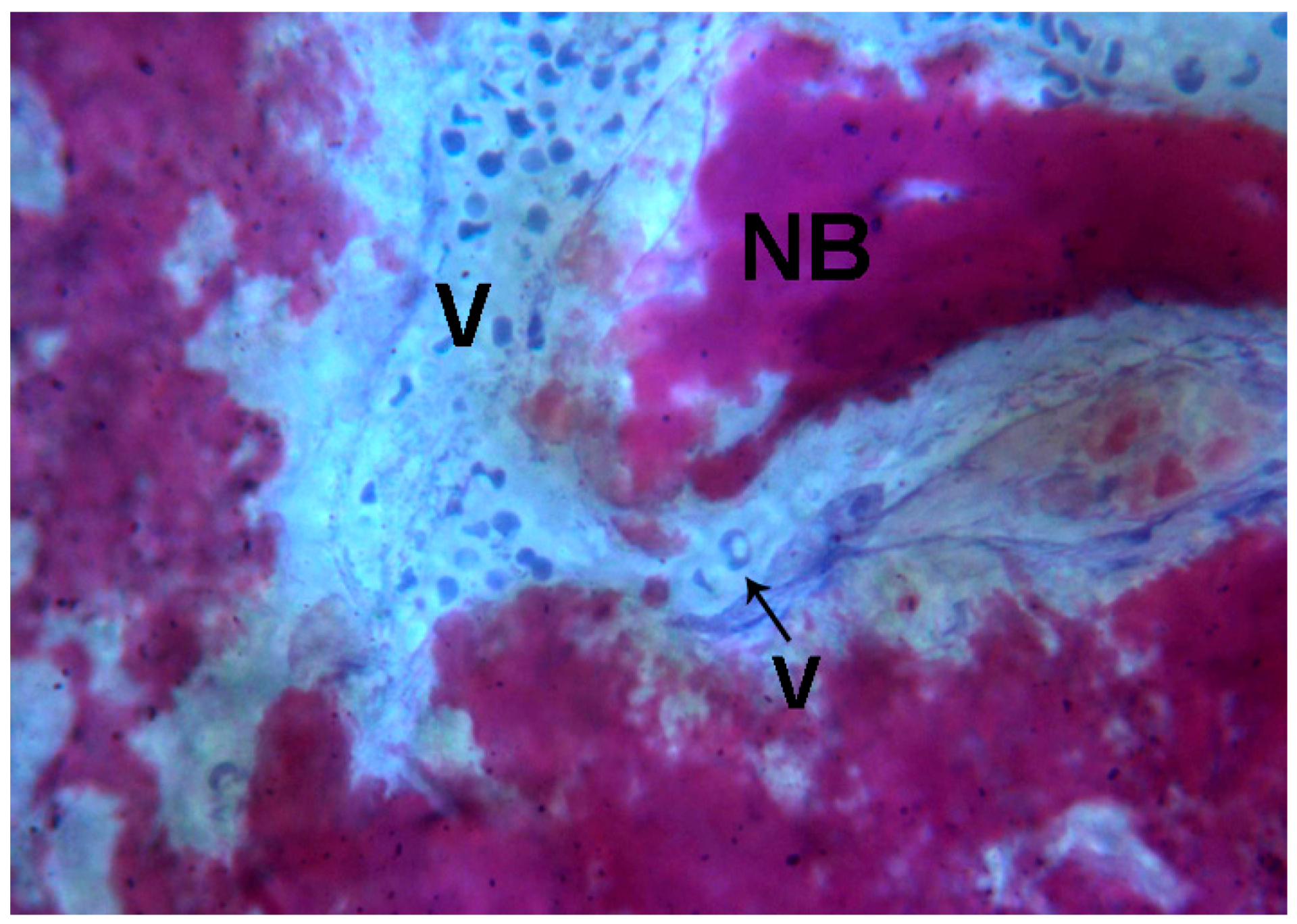
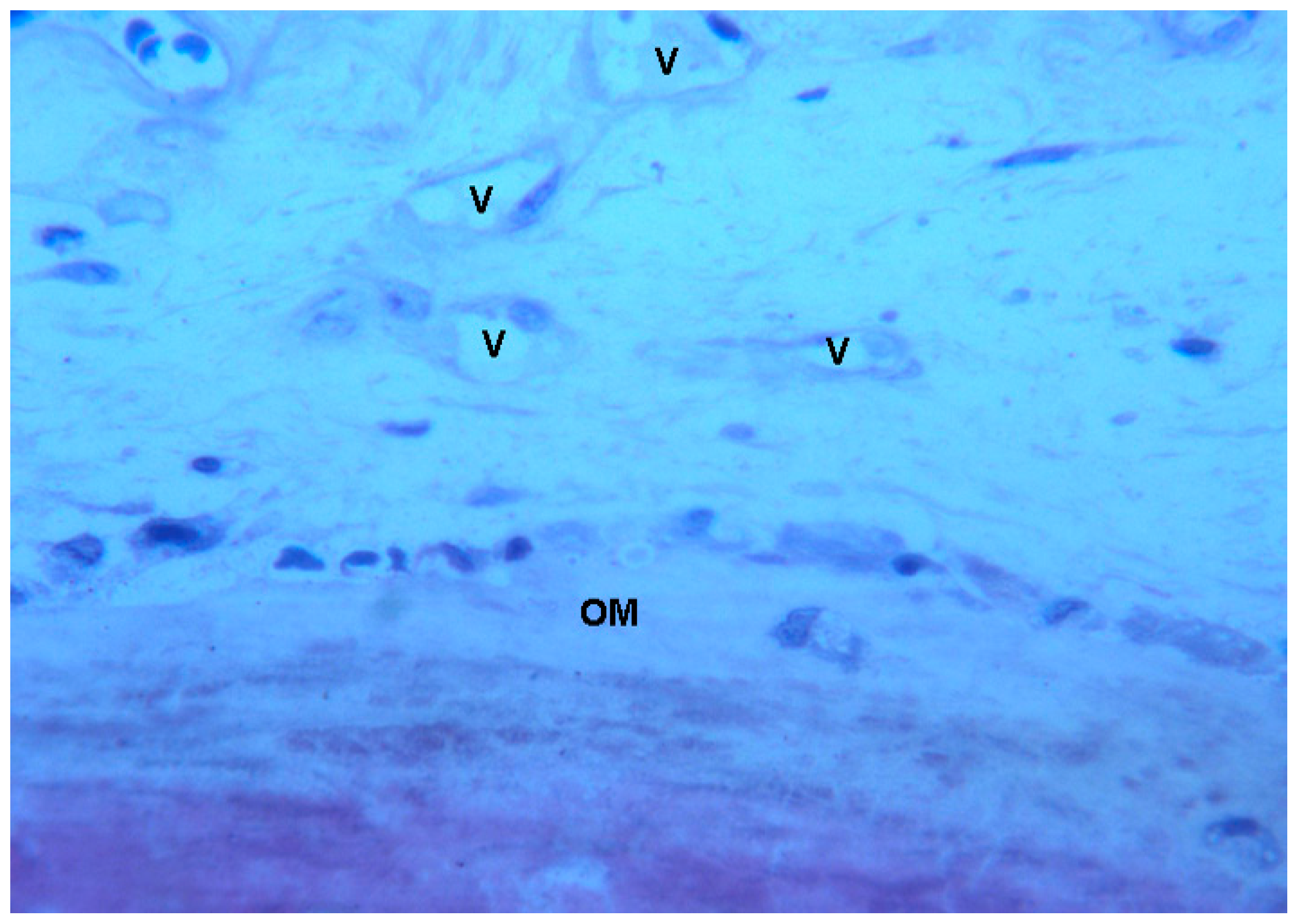
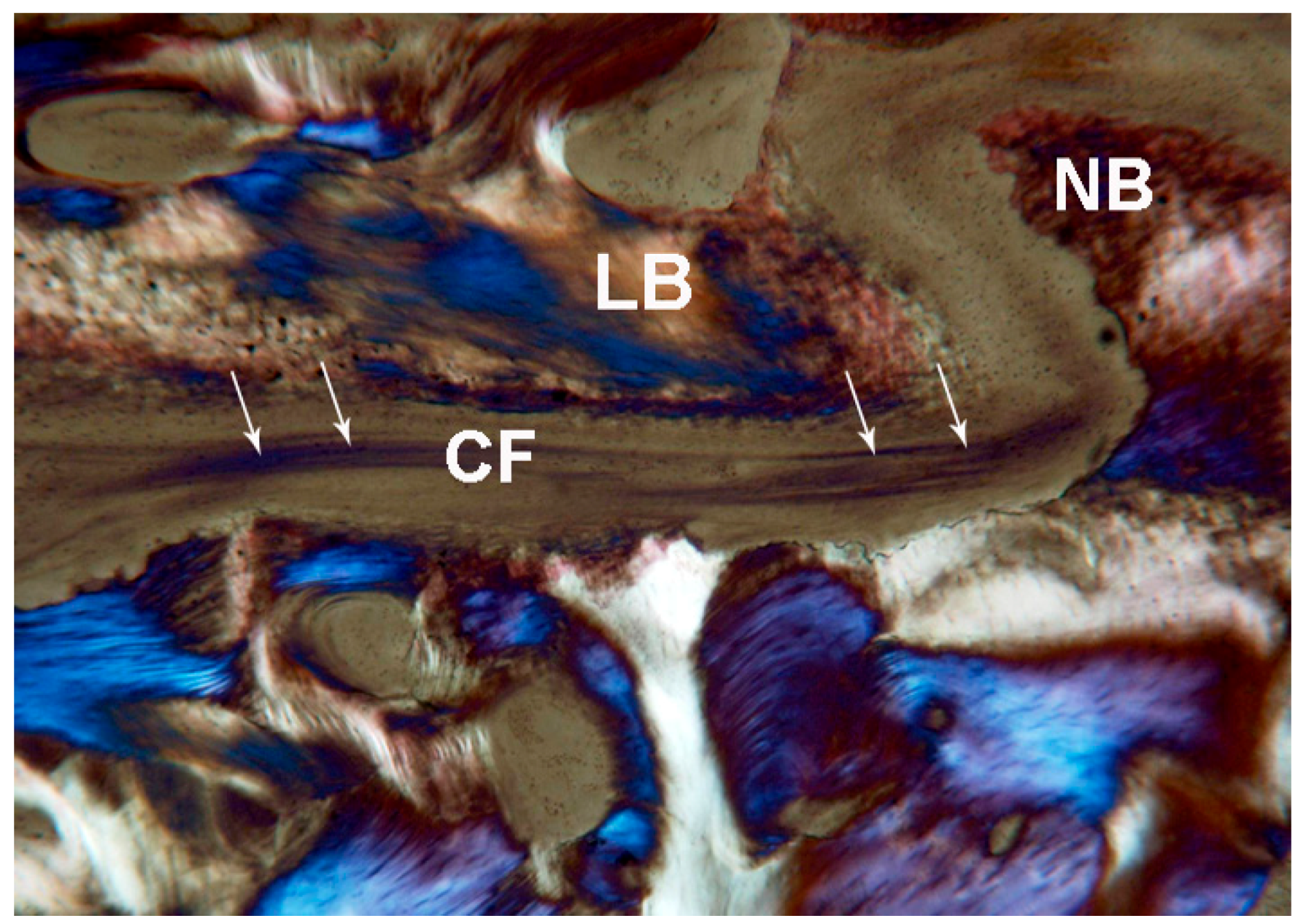
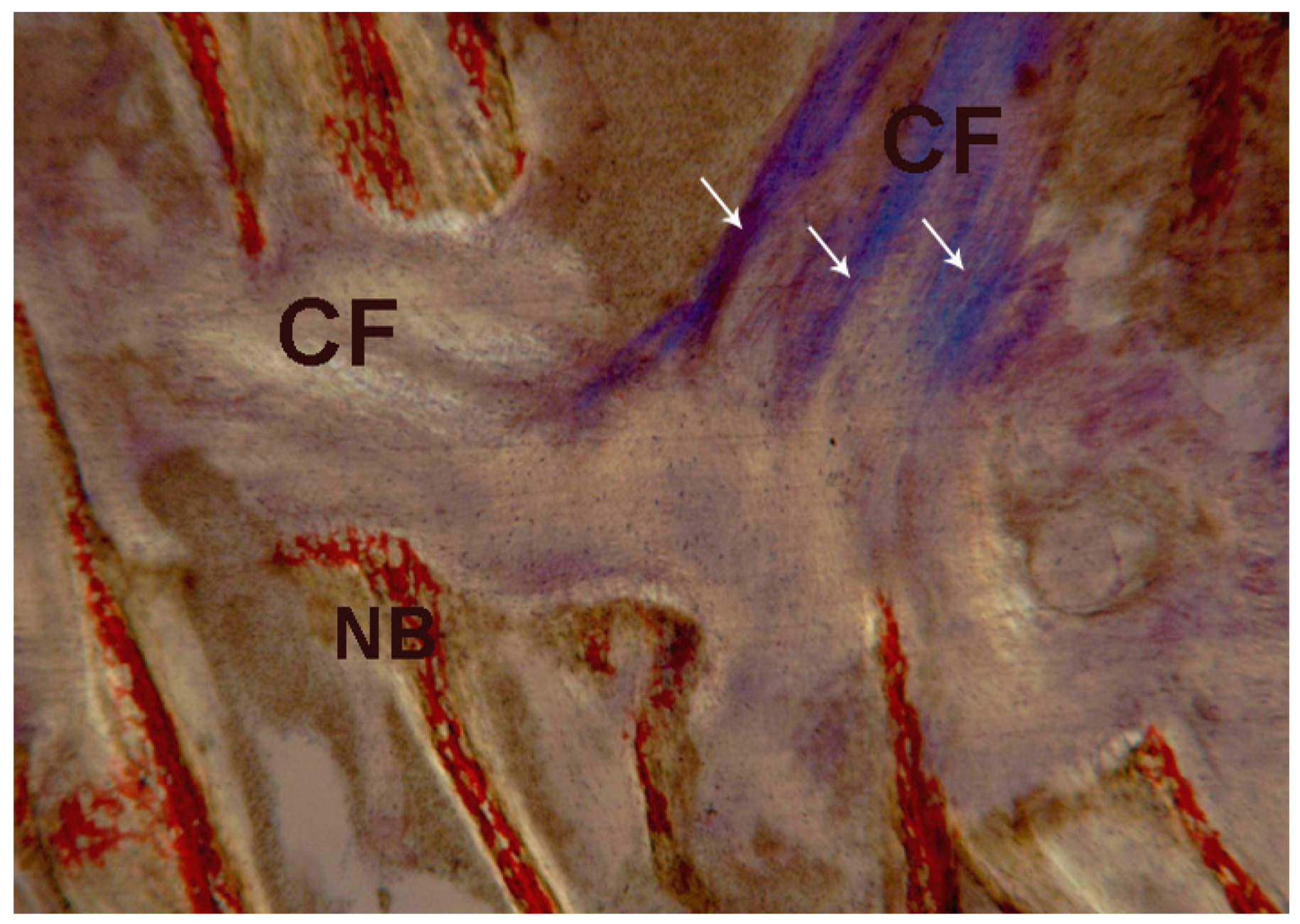
2.2. Polarized Light
2.2.1. Control (Subject 3, Male)
2.2.2. Test at 7 Days (Subject 1, Female)
2.2.3. Test at 30 Days (Subject 2, Male)
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Biopsy Procedure of the Midpalatal Suture
4.3. Histological Processing
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RME | Rapid Maxillary Expansion |
| CBCT | Cone Beam Computer Tomography |
References
- Angell, E.C. Treatment of irregularities of the permanent or adult teeth. Dent. Cosmos. 1860, 1, 540–544. [Google Scholar]
- Haas, A.J. The treatment of maxillary deficiency by opening the midpalatal suture. Angle Orthod. 1965, 35, 200–217. [Google Scholar] [PubMed]
- Pullen, H.A. Expansion of the dental arch and opening the maxillary suture in relation to the development of the internal and external face. Dent. Cosmos. 1912, 54, 509–528. [Google Scholar]
- McNamara, J.A., Jr.; Lione, R.; Franchi, L.; Angelieri, F.; Cevidanes, L.H.; Darendeliler, M.A.; Cozza, P. The role of rapid maxillary expansion in the promotion of oral and general health. Prog. Orthod. 2015, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, M.; Baumgartner, S. The impact of rapid palatal expansion on children’s general health: A literature review. Eur. J. Paediatr. Dent. 2014, 15, 67–71. [Google Scholar] [PubMed]
- Fastuca, R.; Perinetti, G.; Zecca, P.A.; Nucera, R.; Caprioglio, A. Airway compartments volume and oxygen saturation changes after rapid maxillary expansion: A longitudinal correlation study. Angle Orthod. 2015, 85, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Fastuca, R.; Zecca, P.A.; Caprioglio, A. Role of mandibular displacement and airway size in improving breathing after rapid maxillary expansion. Prog. Orthod. 2014, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, A.; Meneghel, M.; Fastuca, R.; Zecca, P.A.; Nucera, R.; Nosetti, L. Rapid maxillary expansion in growing patients: Correspondence between 3-dimensional airway changes and polysomnography. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Fastuca, R.; Meneghel, M.; Zecca, P.A.; Mangano, F.; Antonello, M.; Nucera, R.; Caprioglio, A. Multimodal airway evaluation in growing patients after rapid maxillary expansion. Eur. J. Paediatr. Dent. 2015, 16, 129–134. [Google Scholar] [PubMed]
- Dewey, M. Development as a result of mechanical force: report on further treatment in attempting the opening of the intermaxillary suture in animals. Items Interest 1914, 36, 420–438. [Google Scholar]
- Da Silva Filho, O.G.; Montes, L.A.; Torelly, L.F. Rapid maxillary expansion in the deciduous and mixed dentition evaluated through posteroanterior cephalometric analysis. Am. J. Orthod. Dentofacial. Orthop. 1995, 107, 268–275. [Google Scholar] [CrossRef]
- Franchi, L.; Baccetti, T.; Lione, R.; Fanucci, E.; Cozza, P. Modifications of midpalatal sutural density induced by rapid maxillary expansion: A low-dose computed-tomography evaluation. Am. J. Orthod. Dentofacial. Orthop. 2010, 137, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Acar, Y.B.; Motro, M.; Everdi, N. A new indicator showing maxillary resistance in rapid maxillary expansion cases? Angle Orthod. 2015, 85, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Acar, Y.B.; Motro, M.; Everdi, N. Three-dimensional densitometric analysis of maxillary sutural changes induced by rapid maxillary expansion. Dentomaxillofac. Radiol. 2013, 42, 71798010. [Google Scholar]
- Storey, E. Bone changes associated with tooth movement: A histological study of the effect of force in the rabbit, guinea pig and rat. Aust. Dent. J. 1955, 59, 147. [Google Scholar]
- Cleall, J.F.; Bayne, D.I.; Posen, J.M.; Subtenly, J.D. Expansion of the midpalatal suture in the monkey. Angle Orthod. 1965, 35, 23–35. [Google Scholar] [PubMed]
- Murray, J.M.; Cleall, J.F. Early tissue response to rapid maxillary expansion in the midpalatal suture of the rhesus moneky. J. Dent. Res. 1971, 50, 1654. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, O. Effect of lateral expansion force on the maxillary structure in Cynomolgus monkey. J. Osaka Dent. Univ. 1972, 6, 11–50. [Google Scholar]
- Melsen, B. Palatal growth studied on human autopsy material. Am. J. Orthod. 1975, 68, 42–54. [Google Scholar] [CrossRef]
- Melsen, B. A histological study of the influence of sutural morphology and skeletal maturation on rapid palatal expansion in children. Trans. Eur. Orthod. Sot. 1972, 499–507. [Google Scholar]
- Liu, S.; Xu, T.; Zou, W. Effects of rapid maxillary expansion on the midpalatal suture: A systematic review. Eur. J. Orthod. 2015, 37, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Fukai, N.; Olsen, B.R. Mechanical force-induced midpalatal suture remodeling in mice. Bone 2007, 40, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Giuliano Maino, G.; Pagin, P.; Di Blasio, A. Success of miniscrews used as anchorage for orthodontic treatment: analysis of different factors. Prog. Orthod. 2012, 13, 202–209. [Google Scholar] [CrossRef]
- Bianchi, B.; Ferri, A.; Brevi, B.; Di Blasio, A.; Copelli, C.; Di Blasio, C.; Barbot, A.; Ferri, T.; Sesenna, E. Orthognathic surgery for the complete rehabilitation of Moebius patients: Principles, timing and our experience. J. Craniomaxillofac. Surg. 2013, 41, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Di Blasio, A.; Cassi, D.; Di Blasio, C.; Gandolfini, M. Temporomandibular joint dysfunction in Moebius syndrome. Eur. J. Paediatr. Dent. 2013, 14, 295–298. [Google Scholar] [PubMed]
- Biondi, K.; Lorusso, P.; Fastuca, R.; Mangano, A.; Zecca, P.A.; Bosco, M.; Caprioglio, A.; Levrini, L. Evaluation of 307 masseter muscle in different vertical skeletal patterns in growing patients. Eur. J. Paediatr. Dent. 2016, 308, 47–52. [Google Scholar]
- Anghinoni, M.L.; Magri, A.S.; Di Blasio, A.; Toma, L.; Sesenna, E. Midline mandibular osteotomy in an asymmetric patient. Angle Orthod. 2009, 79, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Cassi, D.; De Biase, C.; Tonni, I.; Gandolfini, M.; Di Blasio, A.; Piancino, M.G. Natural position of the head: review of two-dimensional and three-dimensional methods of recording. Br. J. Oral. Maxillofac. Surg. 2016, 54, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Di Blasio, A.; Mandelli, G.; Generali, I.; Gandolfini, M. Facial aesthetics and childhood. Eur. J. Paediatr. Dent. 2009, 10, 131–134. [Google Scholar] [PubMed]
- Zecca, P.A.; Fastuca, R.; Beretta, M.; Caprioglio, A.; Macchi, A. Correlation assessment between three-dimensional facial soft tissue scan and lateral cephalometric radiography in Orthodontic Diagnosis. Int. J. Dent. 2016, 2016, 1473918. [Google Scholar] [CrossRef] [PubMed]
- Boyde, A.; Riggs, C.M. The quantitative study of the orientation of collagen in compact bone slices. Bone 1990, 11, 35–39. [Google Scholar] [CrossRef]
- Martin, R.B.; Boardman, D.L. The effects of collagen fiber orientation, porosity, density and mineralization on bovine cortical bone bending properties. J. Biomech. 1993, 26, 1047–1054. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caprioglio, A.; Fastuca, R.; Zecca, P.A.; Beretta, M.; Mangano, C.; Piattelli, A.; Macchi, A.; Iezzi, G. Cellular Midpalatal Suture Changes after Rapid Maxillary Expansion in Growing Subjects: A Case Report. Int. J. Mol. Sci. 2017, 18, 615. https://doi.org/10.3390/ijms18030615
Caprioglio A, Fastuca R, Zecca PA, Beretta M, Mangano C, Piattelli A, Macchi A, Iezzi G. Cellular Midpalatal Suture Changes after Rapid Maxillary Expansion in Growing Subjects: A Case Report. International Journal of Molecular Sciences. 2017; 18(3):615. https://doi.org/10.3390/ijms18030615
Chicago/Turabian StyleCaprioglio, Alberto, Rosamaria Fastuca, Piero Antonio Zecca, Matteo Beretta, Carlo Mangano, Adriano Piattelli, Aldo Macchi, and Giovanna Iezzi. 2017. "Cellular Midpalatal Suture Changes after Rapid Maxillary Expansion in Growing Subjects: A Case Report" International Journal of Molecular Sciences 18, no. 3: 615. https://doi.org/10.3390/ijms18030615
APA StyleCaprioglio, A., Fastuca, R., Zecca, P. A., Beretta, M., Mangano, C., Piattelli, A., Macchi, A., & Iezzi, G. (2017). Cellular Midpalatal Suture Changes after Rapid Maxillary Expansion in Growing Subjects: A Case Report. International Journal of Molecular Sciences, 18(3), 615. https://doi.org/10.3390/ijms18030615










