1. Introduction
Gastrointestinal stromal tumors (GISTs) are epidemiologically analyzed to be the most common form of the mesenchymal tumor of the gastrointestinal tract, as the worldwide incidence and prevalence are estimated to be approximately 1 to 1.5 per 100,000 per year and 13 per 100,000, respectively [
1]. At the present time, the combined application of surgery resection and molecular targeted drugs therapy is the most effective treatment for patients with the middle or high risk GISTs. Imatinib mesylate (IM) is a first-line targeted therapy for inoperable, metastatic, or recurrent
KIT-positive GIST and for the adjuvant treatment of patients following resection of primary
KIT-positive GIST [
2]. After the clinical introduction of IM, the patient outcomes for GIST have been dramatically improved, with an impressive impact on both quality of life and long-term prognosis. However, there are large individual variations in clinical efficacy and adverse reactions of IM [
3,
4,
5]. Ten to fifteen percent of patients who underwent IM treatment after 3–6 months progressed rapidly to widespread metastatic disease [
6], about 30% of patients who developed serious adverse reactions had to stop taking medication [
7]. Recently, the relationship between IM plasma concentrations and efficacy and toxicity have been described [
8,
9], minimal plasma concentration thresholds (1100 ng/mL) have been established, under which a substantial increase in treatment failure and drug resistance was observed [
10]. Thus, inter-individual variations in IM pharmacokinetics may, therefore, have important clinical consequences. This implicates that timely monitoring of IM plasma concentration is warranted in GIST patients.
It has been found that genetic polymorphisms of main drug-metabolizing enzymes and transporters may significantly influence the inter-individual variations in drug reaction and disposition [
11]. IM is orally administrated and was mainly metabolized in the liver by cytochrome P450 3A4 (CYP3A4), and effluxed by ATP Binding Cassette Subfamily B Member 1(ABCB1, P-glycoprotein), ATP Binding Cassette Subfamily G Member 2 (ABCG2, BCRP), etc. The current studies of individualized therapy for IM mainly focused on the genetic polymorphisms of main drug-metabolizing enzymes (CYP3A4) [
12] and transporters (ABCB1, ABCG2) [
13,
14]. However, the variant differences in IM clinical effects and adverse reactions remains controversial, suggesting that there are some other genetic factors neglected. In addition,
CYP3A4 rs2242480, the highest frequency single nucleotide polymorphisms (SNPs) in the Chinese population [
15] was found to be correlated with the increased activity of CYP3A4, indicating that it is likely to be associated with IM pharmacokinetics.
Recently, nuclear receptors, as domain transcriptional regulators, have been found to play a crucial role in regulating the expression of relevant drug metabolic enzymes and transporters. Pregnane X Receptor (PXR, encoded by
NR1I2), a member of the nuclear receptor superfamily, was increasingly found in simultaneously inducing the expression of CYP3A4 [
16,
17,
18,
19] and ABCB1 [
17,
18,
20], predicting that the genetic polymorphisms of
NRI12 may have an effect on IM pharmacokinetics.
Based on these observations, six SNPs (CYP3A4 rs2242480; ABCB1 rs1045642; ABCG2 rs2231137; NRI12 rs3814055, rs6785049, rs2276706), which have been associated with the expression and/or function of the above drug-metabolizing enzyme and transporters genes and/or proteins in IM pharmacokinetics pathway, and with the minor allele frequency higher than 10% in Asian, were chosen as candidate SNPs, in order to investigate the influence of CYP3A4, ABCB1, ABCG2 and NR1I2 genetic polymorphisms on the steady-state IM dose-adjusted trough plasma concentrations and related adverse reactions in Chinese GIST patients. The results can be served as valuable fundamental knowledge for IM therapy, in order to make the antitumor treatment more successfully, and increase the safety and long-term tolerability of IM in Chinese GIST patients.
3. Discussion
In the past decade, imatinib mesylate, the first molecular-targeted drug with a known mechanism of efficacy, has radically changed the life expectancy of patients with GISTs. However, a large number of researchers have focused on the peculiar proto-oncogene of GISTs, and it can only partially explain the inter-individual variances of clinical response rate. While the germline DNA of patients remains important, as it dictates drug pharmacokinetics that may indirectly determine efficacy and toxicity. Based on these previous observations, we have involved the most relevant pharmacogenetic parameters, such as genetic polymorphisms of drug metabolizing enzymes, transporters and nuclear receptors, in order to investigate their influences on the steady-state IM dose-adjusted trough plasma concentrations and adverse reactions, for further explanation of the inter-individual variances on IM pharmacokinetics.
In our study, there was nearly a 16-fold variance in the steady-state IM trough plasma concentrations (from 272.22 to 4365.96 ng/mL) with IM doses ranging from 300 to 600 mg daily, which is much larger than the previous reports [
10,
23]. In addition, 47.06% of patients in the study, whose IM trough plasma concentrations had not reached the predefined minimal plasma concentration thresholds (1100 ng/mL), may be at the higher risk of disease progression and treatment failure.
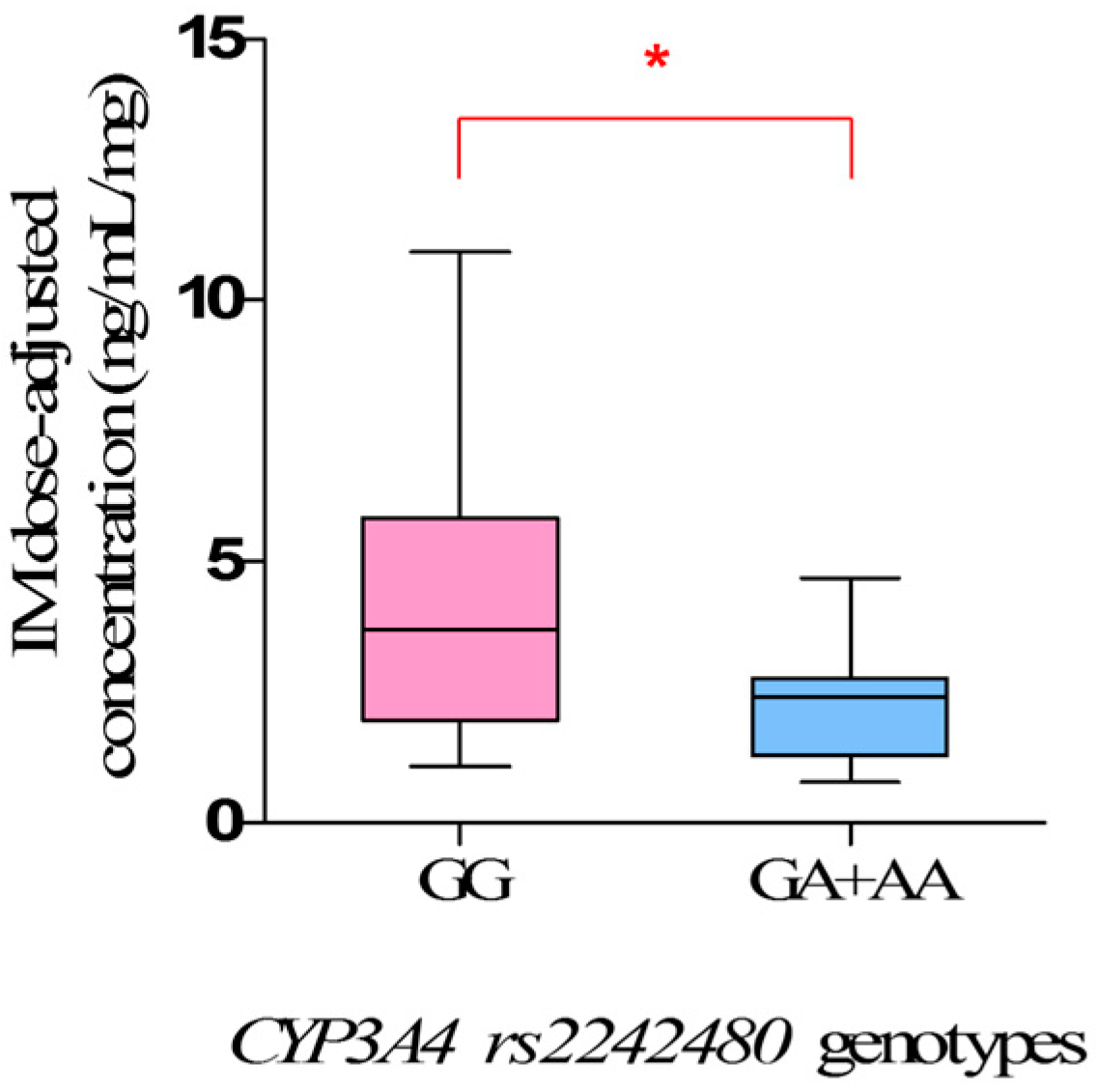
Our study clearly showed the clinical impact of
CYP3A4 rs2242480 polymorphisms on IM plasma levels, and the steady-state IM dose-adjusted trough plasma concentrations in mutant allele
A carriers (
GA +
AA) were significantly lower than that in wild-types (
GG) (
p = 0.0171). However, the influence of
rs2242480 polymorphisms on IM plasma levels in Chinese GIST patients has not been investigated previously.
rs2242480, characterized by a G to A substitution in intron 10 of
CYP3A4, is the most frequent SNP of
CYP3A4 in the Chinese population. The frequency of mutant allele
A in the study was 17.58%, similar to Healthy Han Chinese and Asian [
15,
24], but significantly different from Caucasian (7.34%) and African (85.71%). The mutant allele
A was reported to be associated with a higher CYP3A4 metabolic activity [
25,
26,
27], thus increasing the clearance of IM, which was mainly metabolized by CYP3A4, leading the lower IM plasma levels. Therefore, the mutant allele
A of
rs2242480 is a meaningful risk factor for predicting inadequate clinical efficacy of IM, and patients who carry mutant allele
A of
rs2242480 may be suggested to have a higher dose therapy.
In addition, our study obviously showed the clinical impact of
ABCB1 rs1045642 polymorphisms on IM plasma levels, and the steady-state IM dose-adjusted trough plasma concentrations in mutate allele
T carriers (
CT +
TT) were significantly higher than that in wild-types (
CC) (
p = 0.0066). Interestingly, the same association has been reported in Chronic Myelogenous Leukemia (CML) [
28] patients under IM treatment, corroborating the importance of IM pharmacokinetics. The frequency of mutant allele
T in the study was 36.76%, similar to Healthy Han Chinese and Asian [
21], but significantly different from Caucasian (51.79%) and African (11.06%). The
ABCB1 rs1045642 mutant allele
T was reported to reduce the transcript levels of
ABCB1 mRNA in vivo by as much as two- to four-fold when compared with allele
C [
29]. Thus, the mutant allele
T was associated with a lower production of P-glycoprotein, leading to a lower drug clearance capability [
30,
31]. This might be responsible for the observation in the study that the mutant allele
T carriers (
CT +
TT) have the higher IM plasma levels. We suggest that the mutant allele
T of
rs1045642 is a significant risk factor for predicting excessive treatment of IM, and patients who carry the mutant allele
T of
rs1045642 may be suggested to have a lower dose therapy.
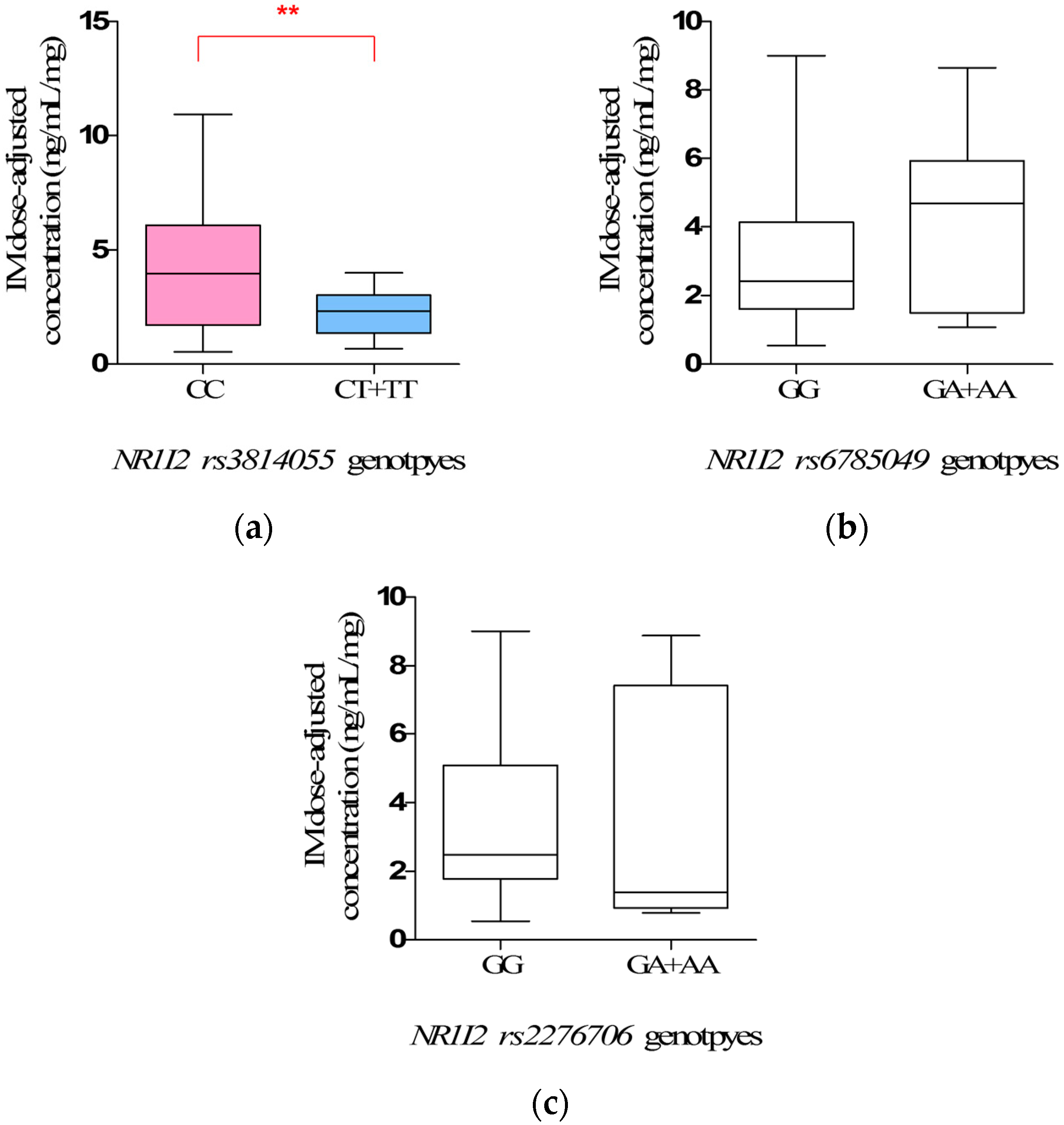
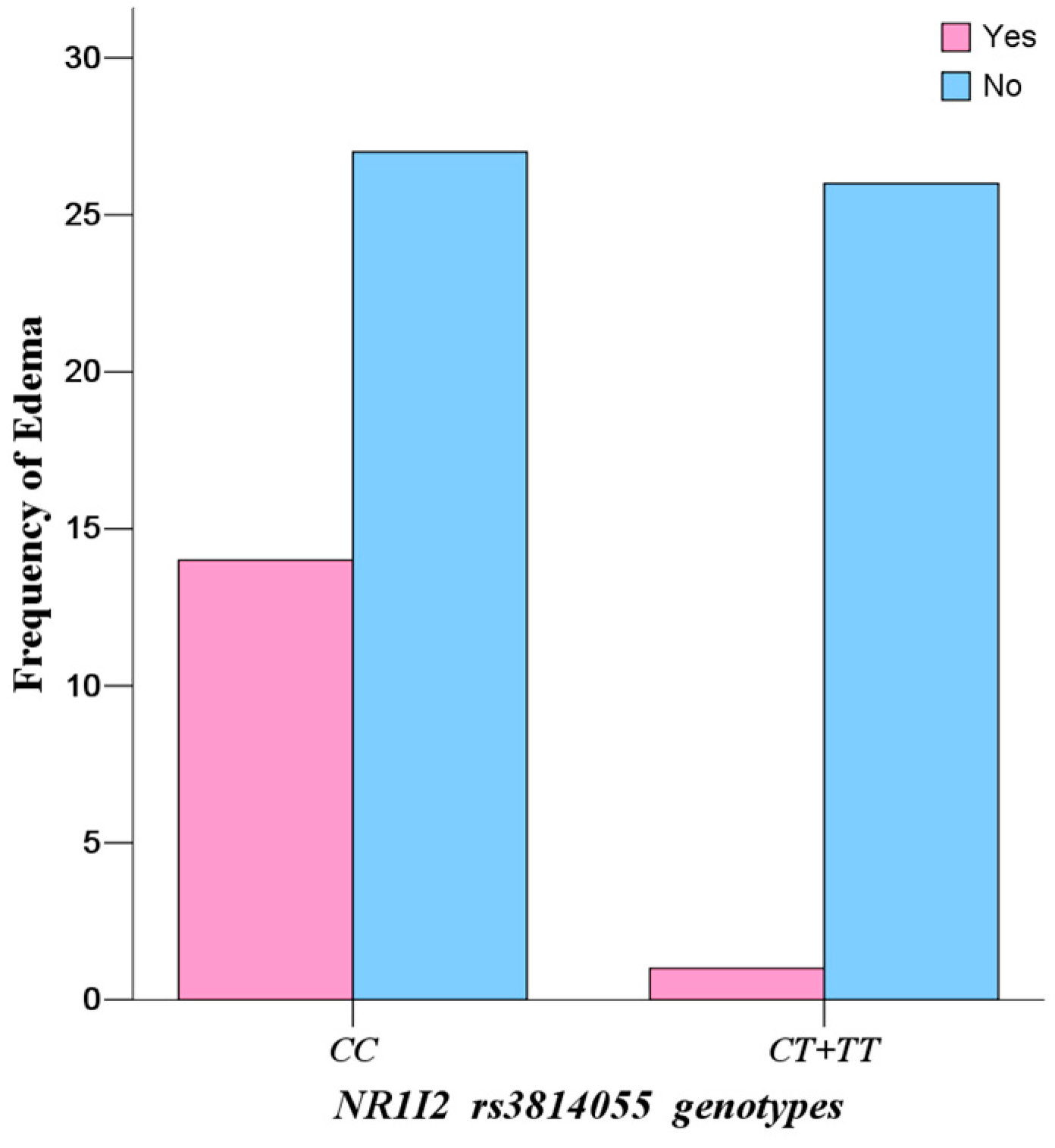
However, the most interesting finding is the influence of
NR1I2 rs3814055 polymorphisms on IM plasma levels and adverse reaction. The steady-state IM dose-adjusted trough plasma concentrations in mutate allele
T carriers (
CT +
TT) (2.34 ± 0.25 ng/mL/mg) were significantly lower than that in wild-types (
CC), and the incidence rate of edema in mutate allele
T carriers (
CT +
TT) was significantly lower than that in wild-types (
CC) (
p = 0.0030, OR = 13.48, 95% CI: 1.65–109.98). However, the influence of
rs3814055 polymorphisms on IM plasma levels and adverse reactions in Chinese GIST patients has not been reported previously, although a similar association has been reported in the studies on the pharmacokinetics of Tacrolimus in healthy subjects [
32]. It is known that PXR is a crucial nuclear receptor to simultaneously regulate the expression of CYP3A4 [
16,
17,
18,
19] and ABCB1 [
17,
18,
20].
rs3814055, located in 5′ Untranslated Regions of
NRI12, is the highest frequency SNP of
NR1I2 in the Chinese population. The frequency of mutant allele
T in the study was 23.53%, similar to Healthy Han Chinese and Asian [
22], but significantly different from Caucasian (36.58%) and African (30.64%). It is reported that the erythromycin breath test in mutant allele
T carriers (
CT +
TT) was two times stronger than wild-types (
CC) by the inducing of rifampicin, indicated that mutant allele
T was associated with a higher transcript levels of CYP3A4 mRNA and metabolic activity [
33,
34], thus increasing the clearance of IM, which was mainly metabolized by CYP3A4, leading to the lower IM plasma levels.
Continuous edema is the most common side effect of IM treatment, with incidence of 37.85% in the study population. Because severe and continuous edema (fluid retention) may result in interruption of IM, careful monitoring of severe edema is especially important in elderly patients (65 and older), patients with preexisting coronary artery disease or renal impairment and patients on higher doses of IM [
35,
36,
37]. However, it is considered to be a concentration-dependent and dose-limiting adverse reaction of IM [
38,
39,
40]—the higher the IM plasma concentrations, the higher the risk of incidence of the adverse reaction. This might be responsible for the observation in this study that the wild-types (
CC) in
NR1I2 rs3814055 have higher IM plasma levels, leading to a high incidence rate of continuous edema. Therefore, allele
C of
rs3814055 is a productive risk factor for predicting excessive treatment and high risk of adverse reaction of IM, and patients who carry the allele
C of
rs3814055 may be suggested to have a lower dose or adjuvant diuretic therapy.