Usefulness of Amino Acid Profiling in Ovarian Cancer Screening with Special Emphasis on Their Role in Cancerogenesis
Abstract
:1. Introduction
2. Results
2.1. Serum-Free Amino Acid Profiles
2.2. Ovarian Cancer versus the Healthy Control Group
2.3. Ovarian Cancer versus Combined Benign Ovarian Tumor and Healthy Control Groups
3. Discussion
4. Materials and Methods
4.1. Patients and Sample Collection
4.2. Determination of Free Amino Acid Profiles
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| OC | Ovarian cancer |
| FIGO | International Federation of Gynecology and Obstetrics |
| CA125 | Cancer antigen 125 |
| HE4 | Human epididymis protein 4 |
| BOT | Benign ovarian tumor(s) |
| HC | Healthy control(s) |
| LC-MS/MS | Liquid chromatography/tandem mass spectrometry |
| MRM | Multiple reaction monitoring |
| LOQ | Limit of quantitation |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| CI | Confidence interval |
| PLS-DA | Partial least squares-discriminant analysis |
| VIP | Variable importance in projection |
| DFA | Discriminant function analysis |
| HPLC-MS/MS | High-performance liquid chromatography/tandem mass spectrometry |
| DART/TOF-MS | Direct analysis in real time mass spectrometry |
| UPLC-QTOF-MS | Ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry |
| UPLC-MS/MS | Ultra-performance liquid chromatography-tandem mass spectrometry |
| LC-QTOF-MS | Liquid chromatography-quadrupole time-of-flight mass spectrometry |
| GCxGC-TOF-MS | Two-dimensional gas chromatography with time-of-flight mass spectrometer |
| IDO | Indoleamine-(2,3)-dioxygenase |
| PRPP | 5-Phosphoribosyl-1-pyrophosphate |
| HFBA | Heptafluorobutyric acid |
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Lin, Y.; Yin, H.; Wang, S.; Sun, Q.; Zhang, P.; Bi, W. Correlation analysis of urine metabolites and clinical staging in patients with ovarian cancer. Int. J. Clin. Exp. Med. 2015, 8, 18165–18171. [Google Scholar] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11; International Agency for Research on Cancer: Lyon, France, 2013; Available online: http://globocan.iarc.fr (accessed on 5 August 2017).
- Turkoglu, O.; Zeb, A.; Graham, S.; Szyperski, T.; Szender, J.B.; Odunsi, K.; Bahado-Singh, R. Metabolomics of biomarker discovery in ovarian cancer: a systematic review of the current literature. Metabolomics 2016, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yin, M.; Ke, C.; Ge, T.; Zhang, G.; Zhang, W.; Zhou, X.; Lou, G.; Li, K. Use of Plasma Metabolomics to Identify Diagnostic Biomarkers for Early Stage Epithelial Ovarian Cancer. J. Cancer 2016, 7, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, H.; Li, A.; Cheng, J.; Yang, K.; Wang, J.; Wang, W.; Zhang, F.; Li, Z.; Dhillon, H.S.; et al. Distinct plasma lipids profiles of recurrent ovarian cancer by liquid chromatography-mass spectrometry. Oncotarget 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, X.; Yin, M.; Fan, L.; Zhang, H.; Zhao, F.; Zhang, W.; Ke, C.; Zhang, G.; Hou, Y.; et al. Discrimination between malignant and benign ovarian tumors by plasma metabolomic profiling using ultra performance liquid chromatography/mass spectrometry. Clin. Chim. Acta 2012, 413, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Buas, M.F.; Gu, H.; Djukovic, D.; Zhu, J.; Drescher, C.W.; Urban, N.; Raftery, D.; Li, C.I. Identification of novel candidate plasma metabolite biomarkers for distinguishing serous ovarian carcinoma and benign serous ovarian tumors. Gynecol. Oncol. 2016, 140, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Klupczynska, A.; Dereziński, P.; Dyszkiewicz, W.; Pawlak, K.; Kasprzyk, M.; Kokot, Z.J. Evaluation of serum amino acid profiles’ utility in non-small cell lung cancer detection in Polish population. Lung Cancer 2016, 100, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, Y.; Higashiyama, M.; Gochi, A.; Akaike, M.; Ishikawa, T.; Miura, T.; Saruki, N.; Bando, E.; Kimura, H.; Imamura, F.; et al. Plasma Free Amino Acid Profiling of Five Types of Cancer Patients and Its Application for Early Detection. PLoS ONE 2011, 6, e24143. [Google Scholar] [CrossRef] [PubMed]
- Hasim, A.; Aili, A.; Maimaiti, A.; Mamtimin, B.; Abudula, A.; Upur, H. Plasma-free amino acid profiling of cervical cancer and cervical intraepithelial neoplasia patients and its application for early detection. Mol. Biol. Rep. 2013, 40, 5853–5859. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, T.; Fu, S.; Sun, X.; Wang, L.; Wang, J.; Lu, Y.; Ding, S.; Ruan, G.; Teng, L.; et al. Perioperative dynamics and significance of amino acid profiles in patients with cancer. J. Transl. Med. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Bachmayr-Heyda, A.; Aust, S.; Auer, K.; Meier, S.M.; Schmetterer, K.G.; Dekan, S.; Gerner, C.; Pils, D. Integrative Systemic and Local Metabolomics with Impact on Survival in High Grade Serous Ovarian Cancer. Clin. Cancer Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Guan, W.; Walker, L.D.; Mezencev, R.; Benigno, B.B.; Gray, A.; Fernández, F.M.; McDonald, J.F. Rapid Mass Spectrometric Metabolic Profiling of Blood Sera Detects Ovarian Cancer with High Accuracy. Cancer Epidemiol. Prev. Biomark. 2010, 19, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Hou, Y.; Zhang, H.; Fan, L.; Ge, T.; Guo, B.; Zhang, F.; Yang, K.; Wang, J.; Lou, G.; et al. Large-scale profiling of metabolic dysregulation in ovarian cancer. Int. J. Cancer 2014, 136. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; de Santiago, I.; Gopalacharyulu, P.; Schmitt, W.D.; Budczies, J.; Kuhberg, M.; Dietel, M.; Aittokallio, T.; Markowetz, F.; Denkert, C.; et al. Accumulated Metabolites of Hydroxybutyric Acid Serve as Diagnostic and Prognostic Biomarkers of Ovarian High-Grade Serous Carcinomas. Cancer Res. 2016, 76, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Simińska, E.; Koba, M. Amino acid profiling as a method of discovering biomarkers for early diagnosis of cancer. Amino Acids 2016, 48, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nishiumi, S.; Ikeda, A.; Yoshie, T.; Sakai, A.; Matsubara, A.; Izumi, Y.; Tsumura, H.; Tsuda, M.; Nishisaki, H.; et al. Anovel serum metabolomics-based diagnostic approach to pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2013. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Ke, C.; Yin, M.; Li, Z.; Fan, L.; Zhang, W.; Zhang, H.; Zhao, F.; Zhou, X.; et al. Identification of Potential Biomarkers for Ovarian Cancer by Urinary Metabolomic Profiling. J. Proteome Res. 2013, 12, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Gaul, D.A.; Mezencev, R.; Long, T.Q.; Jones, C.M.; Benigno, B.B.; Gray, A.; Fernández, F.M.; McDonald, J.F. Highly-accurate metabolomic detection of early-stage ovarian cancer. Sci. Rep. 2015, 5, 16351. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Nikaido, T.; Ochiai, K.; Takakura, S.; Saito, M.; Aoki, Y.; Ishii, N.; Yanaihara, N.; Yamada, K.; Takikawa, O.; et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin. Cancer Res. 2005, 11, 6030–6039. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C. Cancer: Why tumours eat tryptophan. Nature 2011, 478, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.V.; Pan, J.; Rai, S.N.; Galandiuk, S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery 2016, 159, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 143–202. [Google Scholar]
- Tong, X.; Zhao, F.; Thompson, C.B. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr. Opin. Genet. Dev. 2009, 19, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Ananieva, E. Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World J. Biol. Chem. 2015, 6, 281. [Google Scholar] [CrossRef] [PubMed]
- Barzał, J.A.; Szczylik, C.; Rzepecki, P.; Jaworska, M.; Anuszewska, E. Plasma citrulline level as a biomarker for cancer therapy-induced small bowel mucosal damage. Acta Biochim. Pol. 2014, 61, 615–631. [Google Scholar] [PubMed]
- Ma, Q.; Wang, Z.; Zhang, M.; Hu, H.; Li, J.; Zhang, D.; Guo, K.; Sha, H. Targeting the l-arginine-nitric oxide pathway for cancer treatment. Curr. Pharm. Des. 2010, 16, 392–410. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Dereziński, P.; Klupczynska, A.; Matysiak, J.; Kaczmarek, E.; Kokot, Z.J. Effects of a honeybee sting on the serum free amino acid profile in humans. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S.; Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 14.10.1–14.10.91. [Google Scholar]
- Frolkis, A.; Knox, C.; Lim, E.; Jewison, T.; Law, V.; Hau, D.D.; Liu, P.; Gautam, B.; Ly, S.; Guo, A.C.; et al. SMPDB: The small molecule pathway database. Nucleic Acids Res. 2009, 38. [Google Scholar] [CrossRef] [PubMed]
- Jewison, T.; Su, Y.; Disfany, F.M.; Liang, Y.; Knox, C.; Maciejewski, A.; Poelzer, J.; Huynh, J.; Zhou, Y.; Arndt, D.; et al. SMPDB 2.0: Big improvements to the small molecule pathway database. Nucleic Acids Res. 2014, 42. [Google Scholar] [CrossRef] [PubMed]
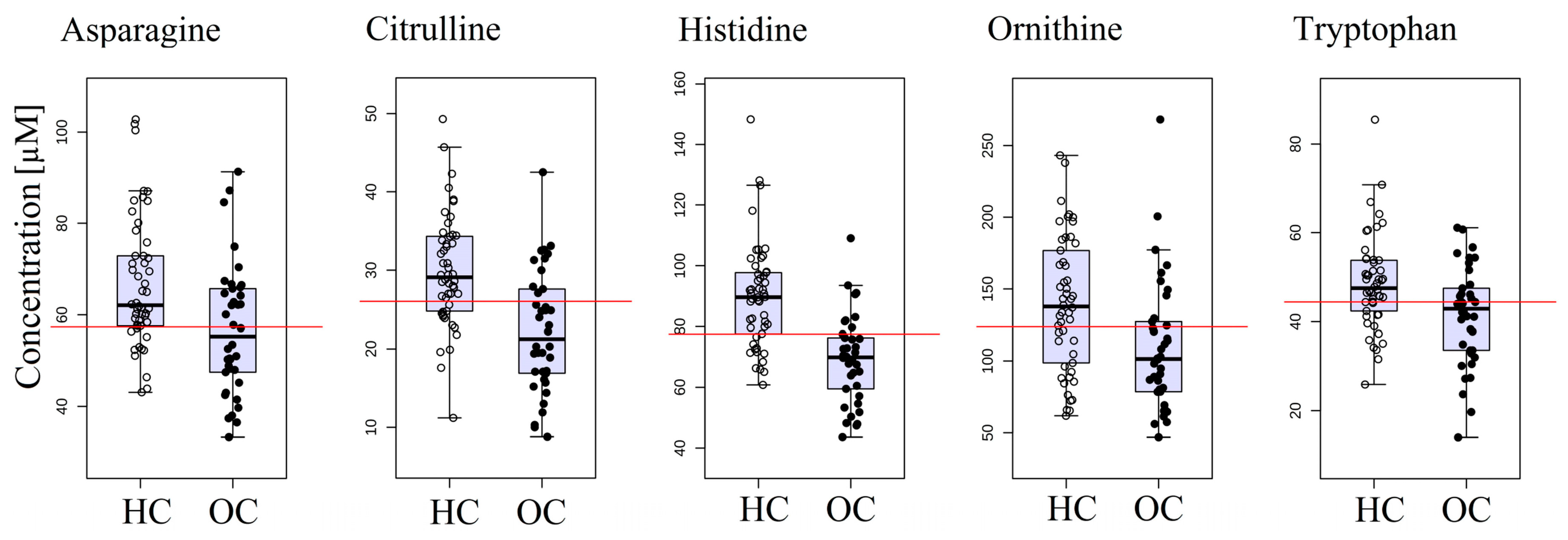
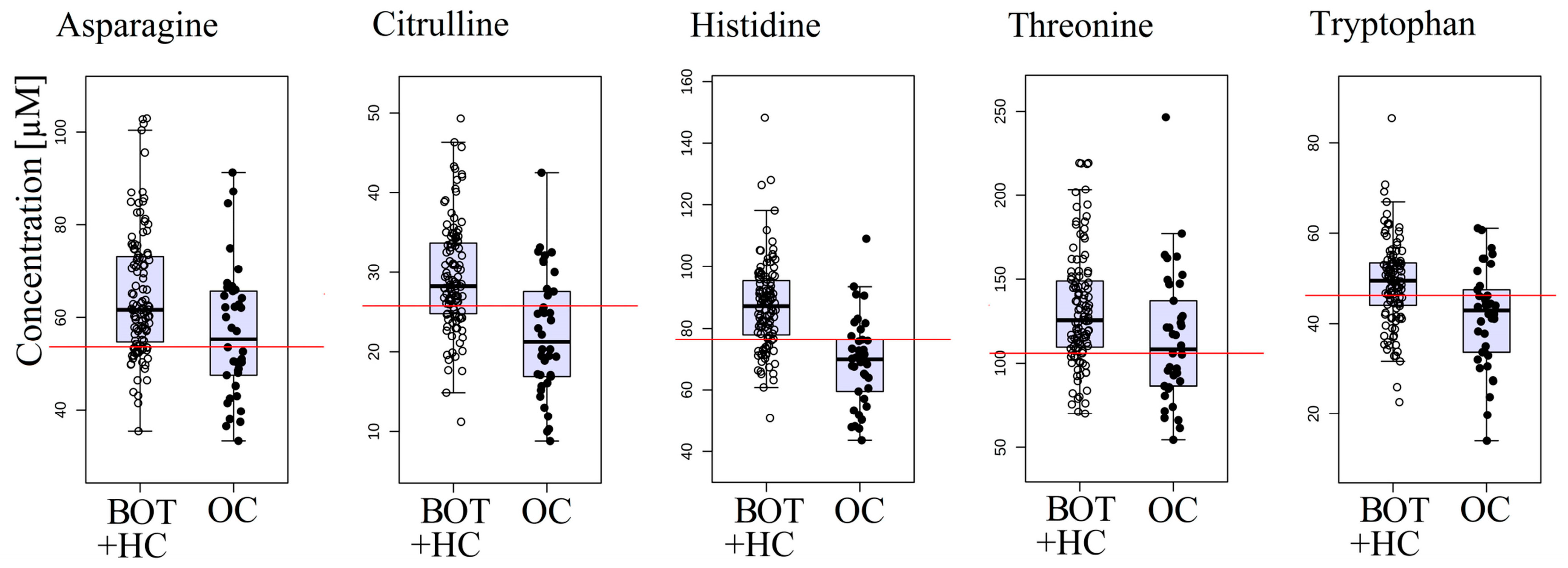
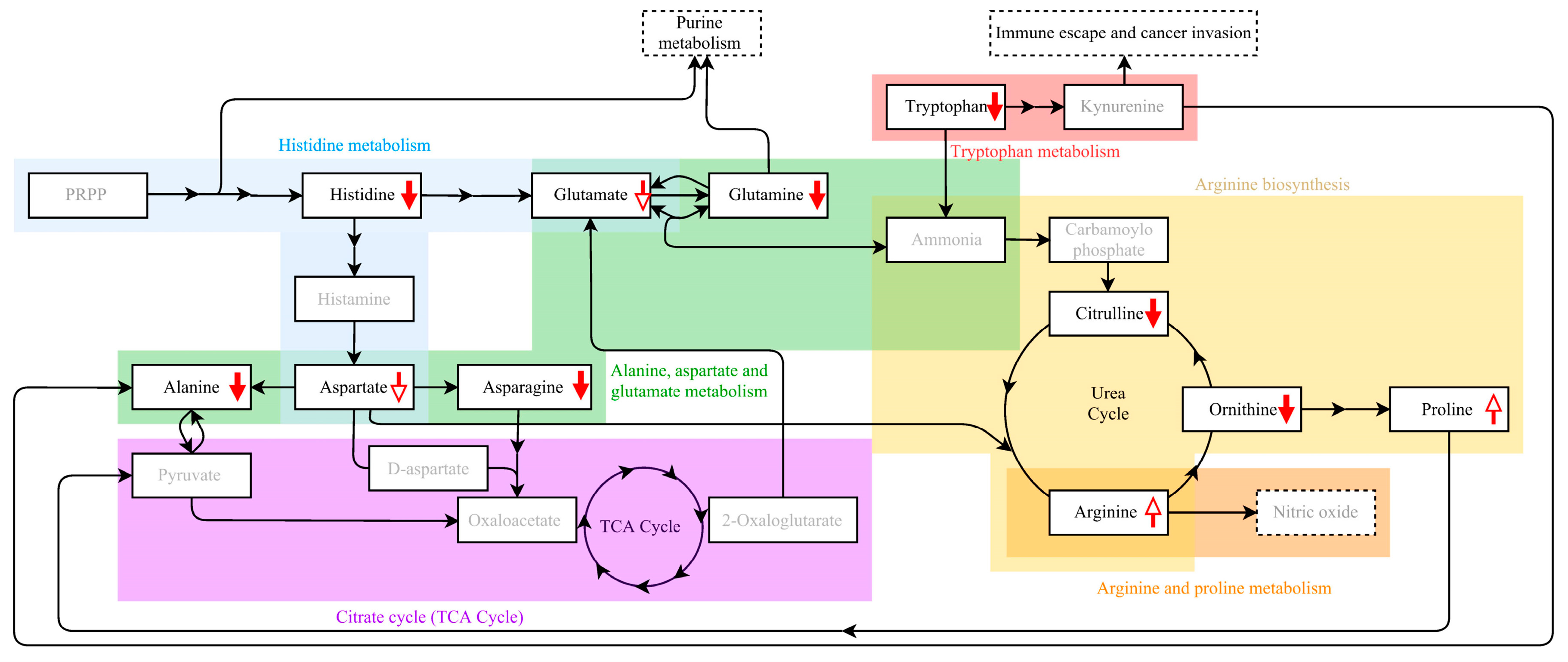
| Type of Comparison | Model 1 | Model 2 |
|---|---|---|
| OC vs. HC | OC vs. (BOT + HC) | |
| Compounds in the model | histidine, ornithine | histidine, citrulline, alanine, asparagine, ornithine |
| Wilks’ Lambda | 0.63367 | 0.57495 |
| p-value | p < 0.0001 | p < 0.0001 |
| Sensitivity (%) | 76.32 | 60.53 |
| Specificity (%) | 80.00 | 94.64 |
| Total Group Membership Classification (%) | 78.41 | 86.00 |
| Authors | Biological Matrix | Groups | Technique | Metabolites/Groups of Metabolites Identified as Potential Biomarkers |
|---|---|---|---|---|
| Zhou, M., 2010 [14] | Human serum | 44 serous papillary ovarian cancers, 50 controls | DART/TOF-MS | Metabolites involved in: amines and amino acids metabolism, eicosanoids |
| Zhang, T., 2012 [7] | Human plasma | 80 epithelial ovarian cancers, 90 benign ovarian tumors | UPLC-QTOF-MS | Tryptophan, lysoPC(18:3), lysoPC(14:0), 2-piperidinone |
| Zhang, T., 2013 [19] | Human urine | 40 preoperative epithelial ovarian cancers, 62 benign ovarian tumors, 54 healthy controls | UPLC-QTOF-MS | 22 metabolites involved in: nucleotide metabolism, histidine metabolism, tryptophan metabolism, mucin metabolism |
| Ke, C., 2014 [15] | Human plasma | 40 epithelial ovarian cancers, 158 benign ovarian tumors, 150 uterine fibroids | UPLC-QTOF-MS | 53 metabolites involved in: phospholipid metabolism, tryptophan catabolism, fatty acid b-oxidation, metabolism of piperidine derivatives |
| Gaul, D.A., 2015 [20] | Human serum | 46 early-stage (I/II) ovarian cancers, 49 healthy controls | UPLC-MS/MS | 16 metabolites involved in lipids and fatty acids metabolism (phospholipids, lysophospholipids, sphingolipids) |
| Buas, F., 2016 [8] | Human plasma | 50 serous ovarian cancers, 50 controls | LC-QTOF-MS; LC-MS/MS | Global lipidomics: 34 metabolites (glycerophospholipids, glycerolipids, sphingolipid, sterol lipid) Targeted profiling: alanine |
| Bachmayr-Heyda, A., 2016 [13] | Preoperative and follow-up sera, ascites, and tumor tissues | 65 high-grade serous ovarian cancers, 62 healthy controls | LC-MS/MS | 43 glycerophospholipids, 5 amino acids |
| Fan, L., 2016 [5] | Human plasma | 21 early stage epithelial ovarian cancers, 31 healthy controls | UPLC-QTOF-MS | 18 metabolites including lysophospholipids, 2-piperidone, monoacylglycerol (18:2) |
| Hilvo, M., 2016 [16] | Human serum and tumor tissue | 158 high-grade serous ovarian cancers, 100 controls with benign or non-neoplastic lesions | GCxGC-TOF-MS | Tryptophan, 3-hydroxybutyric acid, 3,4-dihydroxybutyric acid |
| Li, J., 2017 [6] | Human plasma | 39 epithelial ovarian cancer recurrent patients, 31 non-recurrent patients | UPLC-QTOF-MS | 31 lipid metabolites including phosphatidylcholines, lysophosphatidylcholines, phosphatidylinositols |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plewa, S.; Horała, A.; Dereziński, P.; Klupczynska, A.; Nowak-Markwitz, E.; Matysiak, J.; Kokot, Z.J. Usefulness of Amino Acid Profiling in Ovarian Cancer Screening with Special Emphasis on Their Role in Cancerogenesis. Int. J. Mol. Sci. 2017, 18, 2727. https://doi.org/10.3390/ijms18122727
Plewa S, Horała A, Dereziński P, Klupczynska A, Nowak-Markwitz E, Matysiak J, Kokot ZJ. Usefulness of Amino Acid Profiling in Ovarian Cancer Screening with Special Emphasis on Their Role in Cancerogenesis. International Journal of Molecular Sciences. 2017; 18(12):2727. https://doi.org/10.3390/ijms18122727
Chicago/Turabian StylePlewa, Szymon, Agnieszka Horała, Paweł Dereziński, Agnieszka Klupczynska, Ewa Nowak-Markwitz, Jan Matysiak, and Zenon J. Kokot. 2017. "Usefulness of Amino Acid Profiling in Ovarian Cancer Screening with Special Emphasis on Their Role in Cancerogenesis" International Journal of Molecular Sciences 18, no. 12: 2727. https://doi.org/10.3390/ijms18122727
APA StylePlewa, S., Horała, A., Dereziński, P., Klupczynska, A., Nowak-Markwitz, E., Matysiak, J., & Kokot, Z. J. (2017). Usefulness of Amino Acid Profiling in Ovarian Cancer Screening with Special Emphasis on Their Role in Cancerogenesis. International Journal of Molecular Sciences, 18(12), 2727. https://doi.org/10.3390/ijms18122727





