Protective Effects of ω-3 PUFA in Anthracycline-Induced Cardiotoxicity: A Critical Review
Abstract
:1. Introduction
2. ω-3 PUFA and Cardiovascular (CV) Diseases
3. ω-3 PUFA and Cancer
4. Potential Adjuvant Role of ω-3 PUFA in Combination with Antineoplastic Drugs
5. Can Anthracyclines (ATC)-Induced Cardiotoxicity Be Prevented by ω-3 PUFA?
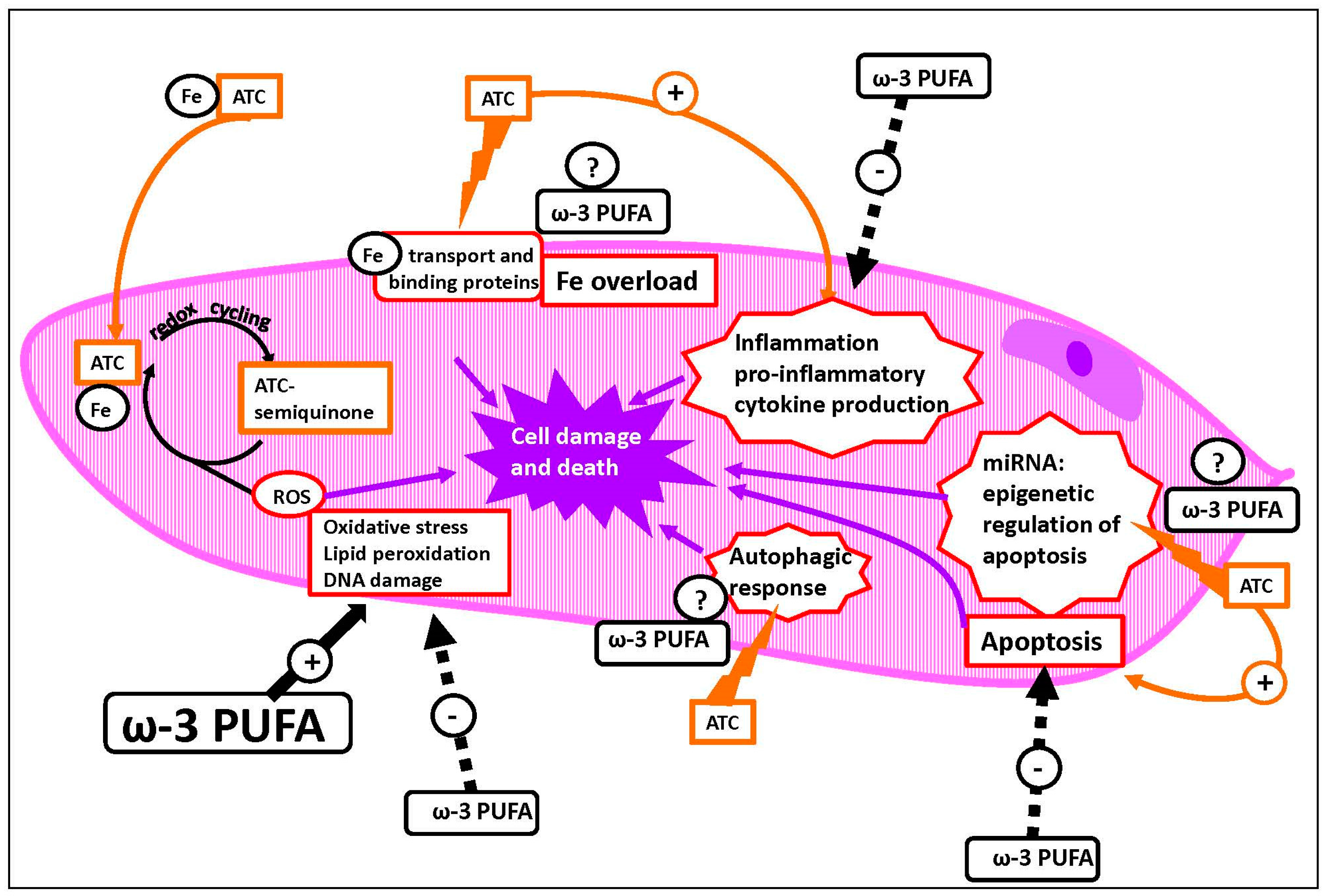
5.1. Mechanisms of ATC-Induced Toxicity
5.2. Available Evidence for ω-3 Prevention of ATC-Mediated Cardiotoxicity
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lee, J.Y.; Sim, T.B.; Lee, J.E.; Na, H.K. Chemopreventive and chemotherapeutic effects of fish oil derived ω-3 polyunsaturated fatty acids on colon carcinogenesis. Clin. Nutr. Res. 2017, 6, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, D.W. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients 2014, 6, 5184–5223. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Arita, M. Cardioprotective mechanism of ω-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Ω-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Su, H.M.; Weylandt, K.H.; Fasano, E.; Serini, S.; Cittadini, A. Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: Their potential role in inflammatory, neurodegenerative, and neoplastic diseases. BioMed Res. Int. 2013, 2013, 743171. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.W.; Seo, J.; Davidson, L.A.; Callaway, E.S.; Fan, Y.Y.; Lupton, J.R.; Chapkin, R.S. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004, 18, 1040–1042. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. Epigenetic regulation of gene expression and M2 macrophage polarization as new potential ω-3 polyunsaturated fatty acid targets in colon inflammation and cancer. Expert Opin. Ther. Targets 2016, 20, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Calviello, G. Reduction of oxidative/nitrosative stress in brain and its involvement in the neuroprotective effect of n-3 PUFA in Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Eltweri, A.M.; Thomas, A.L.; Metcalfe, M.; Calder, P.C.; Dennison, A.R.; Bowrey, D.J. Potential applications of fish oils rich in ω-3 polyunsaturated fatty acids in the management of gastrointestinal cancer. Clin. Nutr. 2017, 36, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Serini, S.; Piccioni, E. n-3 polyunsaturated fatty acids and the prevention of colorectal cancer: Molecular mechanisms involved. Curr. Med. Chem. 2007, 14, 3059–3069. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Calviello, G. Modulation of Ras/ERK and phosphoinositide signaling by long-chain n-3 PUFA in breast cancer and their potential complementary role in combination with targeted drugs. Nutrients 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Piccioni, E.; Merendino, N.; Calviello, G. Dietary polyunsaturated fatty acids as inducers of apoptosis: Implications for cancer. Apoptosis 2009, 14, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. How plausible is the use of dietary n-3 PUFA in the adjuvant therapy of cancer? Nutr. Res. Rev. 2016, 29, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Merendino, N.; Costantini, L.; Manzi, L.; Molinari, R.; D’Eliseo, D.; Velotti, F. Dietary ω-3 polyunsaturated fatty acid DHA: A potential adjuvant in the treatment of cancer. BioMed Res. Int. 2013, 2013, 310186. [Google Scholar] [CrossRef] [PubMed]
- Ghigo, A.; Li, M.; Hirsch, E. New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochim. Biophys. Acta 2016, 1863, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 7 November 2017).
- Ravera, A.; Carubelli, V.; Sciatti, E.; Bonadei, I.; Gorga, E.; Cani, D.; Vizzardi, E.; Metra, M.; Lombardi, C. Nutrition and cardiovascular disease: Finding the perfect recipe for cardiovascular health. Nutrients 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.M.; Heidecker, B.; Ganz, P. Behavioral cardiovascular risk factors-effect of physical activity and cardiorespiratory fitness on cardiovascular outcomes. Circ. J. 2016, 80, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Ω-3 fatty acids and cardiovascular disease: Are there benefits? Curr. Treat. Options Cardiovasc. Med. 2016, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Ω-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Emma-Okon, B.; Remaley, A.T. Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: A mini review. Lipids Health Dis. 2016, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, G.; Busnelli, M.; Manzini, S.; Parolini, C. Nutraceuticals and bioactive components from fish for dyslipidemia and cardiovascular risk reduction. Mar. Drugs 2016, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Weisman, D.; Beinart, R.; Erez, A.; Koren-Morag, N.; Goldenberg, I.; Eldar, M.; Glikson, M.; Luria, D. Effect of supplemented intake of ω-3 fatty acids on arrhythmias in patients with ICD: Fish oil therapy may reduce ventricular arrhythmia. J. Interv. Card. Electrophysiol. 2017, 49, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Phang, M.; Lincz, L.F.; Garg, M.L. Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. J. Nutr. 2013, 143, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballantyne, L.L.; Che, X.; Mewburn, J.D.; Kang, J.X.; Barkley, R.M.; Murphy, R.C.; Yu, Y.; Funk, C.D. Endogenously generated ω-3 fatty acids attenuate vascular inflammation and neointimal hyperplasia by interaction with free fatty acid receptor 4 in mice. J. Am. Heart. Assoc. 2015, 4, e001856. [Google Scholar] [CrossRef] [PubMed]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of ω-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L.A. Impact of ω-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sauder, K.A.; Skulas-Ray, A.C.; Campbell, T.S.; Johnson, J.A.; Kris-Etherton, P.M.; West, S.G. Effects of ω-3 fatty acid supplementation on heart rate variability at rest and during acute stress in adults with moderate hypertriglyceridemia. Psychosom. Med. 2013, 75, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Dahl, L.; Olson, G.; Thornton, D.; Graff, I.E.; Frøyland, L.; Thayer, J.F.; Pallesen, S. Fish consumption, sleep, daily functioning, and heart rate variability. J. Clin. Sleep Med. 2014, 10, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Biondo, P.D.; Brindley, D.N.; Sawyer, M.B.; Field, C.J. The potential for treatment with dietary long-chain polyunsaturated n-3 fatty acids during chemotherapy. J. Nutr. Biochem. 2008, 19, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Piccioni, E.; Calviello, G. ω-3 PUFAs and colon cancer: Experimental studies and human interventional trials. In Dietary Ω-3 Polyunsaturated Fatty Acids and Cancer; Calviello, G., Serini, S., Eds.; Springer: New York, NY, USA, 2010; Volume 1, pp. 67–89. ISBN 978-90-481-3578-3. [Google Scholar]
- Stillwell, W.; Jenski, L.J.; Crump, F.T.; Ehringer, W. Effect of docosahexaenoic acid on mouse mitochondrial membrane properties. Lipids 1997, 32, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Serini, S.; Palozza, P. n-3 polyunsaturated fatty acids as signal transduction modulators and therapeutical agents in cancer. Curr. Signal Transdust. Ther. 2006, 1, 255–271. [Google Scholar] [CrossRef]
- Gillet, L.; Roger, S.; Bougnoux, P.; Le Guennec, J.Y.; Besson, P. Beneficial effects of ω-3 long-chain fatty acids in breast cancer and cardiovascular diseases: Voltage-gated sodium channels as a common feature? Biochimie 2011, 93, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, G.R.; Redondo, I.; Zhu, J.; Murphy, M.G. Differential effects of docosahexaenoic acid on contractions and L-type Ca2+ current in adult cardiac myocytes. Cardiovasc. Res. 2002, 54, 601–610. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Spite, M. Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ. Res. 2016, 119, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Mottola, G.; Schaller, M.; Upchurch, G.R., Jr.; Conte, M.S. Resolution of vascular injury: Specialized lipid mediators and their evolving therapeutic implications. Mol. Asp. Med. 2017, 58, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.M.; Telis, N.; German, J.B.; Hammock, B.D. Dietary ω-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif. Agric. 2011, 65, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. The role of marine ω-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol. Nutr. Food Res. 2012, 56, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.T.; Chan, D.C.; Ooi, E.M.; Ng, T.W.; Watts, G.F.; Barrett, P.H. Ω-3 fatty acid ethyl ester supplementation decreases very-low-density lipoprotein triacylglycerol secretion in obese men. Clin. Sci. Lond. 2013, 125, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Takahashi, N.; Lin, S.; Goto, T.; Murota, K.; Nakata, R.; Inoue, H.; Kawada, T. DHA attenuates postprandial hyperlipidemia via activating PPARα in intestinal epithelial cells. J. Lipid Res. 2013, 54, 3258–3268. [Google Scholar] [CrossRef] [PubMed]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of ω-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. A critical review on the effect of docosahexaenoic acid (DHA) on cancer cell cycle. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef] [PubMed]
- Song, E.A.; Kim, H. Docosahexaenoic acid induces oxidative DNA damage and apoptosis, and enhances the chemosensitivity of cancer cells. Int. J. Mol. Sci. 2016, 17, 1257. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.; Mann, C.; Metcalfe, M.; Webb, M.; Pollard, C.; Spencer, D.; Berry, D.; Steward, W.; Dennison, A. The effect of ω-3 FAs on tumour angiogenesis and their therapeutic potential. Eur. J. Cancer 2009, 45, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Velotti, F. Ω-3 fatty acids and cancer cell cytotoxicity: Implications for multi-targeted cancer therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R. Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J. Nutr. Biochem. 2012, 23, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Schley, P.D.; Brindley, D.N.; Field, C.J. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J. Nutr. 2007, 137, 548–553. [Google Scholar] [PubMed]
- Schley, P.D.; Jijon, H.B.; Robinson, L.E.; Field, C.J. Mechanisms of ω-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2005, 92, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Q.; Liu, B.; Vaught, J.L.; Palmiter, R.D.; Lind, S.E. Clioquinol and docosahexaenoic acid act synergistically to kill tumor cells. Mol. Cancer Ther. 2006, 5, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Choudhury, T.; Mandal, C.C.; Woodruff, K.; St Clair, P.; Fernandes, G.; Choudhury, G.G.; Ghosh-Choudhury, N. Fish oil targets PTEN to regulate NFκB for downregulation of anti-apoptotic genes in breast tumor growth. Breast Cancer Res. Treat. 2009, 118, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Y.; Meng, M.; Cheng, D.; Wang, C. Eicosapentaenoic acid induced SKOV-3 cell apoptosis through ERK1/2-mTOR-NF-κB pathways. Anticancer Drugs 2016, 27, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Mazière, C.; Conte, M.A.; Degonville, J.; Ali, D.; Mazière, J.C. Cellular enrichment with polyunsaturated fatty acids induces an oxidative stress and activates the transcription factors AP1 and NFκB. Biochem. Biophys. Res. Commun. 1999, 265, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.; Hong, M.Y.; Fan, Y.Y.; Davidson, L.A.; Sanders, L.M.; Henderson, C.E.; Barhoumi, R.; Burghardt, R.C.; Turner, N.D.; Lupton, J.R. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids 2002, 37, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Evidence in support of potential applications of lipid peroxidation products in cancer treatment. Oxid. Med. Cell Longev. 2013, 2013, 931251. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.P.; Garcia, C.C.; Sena, F.; Freitas, F.P.; Miyamoto, S.; Medeiros, M.H.; Di Mascio, P. Lipid hydroperoxide-induced and hemoglobin-enhanced oxidative damage to colon cancer cells. Free Radic. Biol. Med. 2011, 51, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Falconer, J.S.; Ross, J.A.; Fearon, K.C.; Hawkins, R.A.; O’Riordain, M.G.; Carter, D.C. Effect of eicosapentaenoic acid and other fatty acids on the growth in vitro of human pancreatic cancer cell lines. Br. J. Cancer 1994, 69, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Bagga, D.; Wang, L.; Farias-Eisner, R.; Glaspy, J.A.; Reddy, S.T. Differential effects of prostaglandin derived from ω-6 and ω-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, V.C.; Hassing, M.R.; Lewandowski, P.A. Marine polyunsaturated fatty acids and cancer therapy. Br. J. Cancer 2013, 108, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Sunpaweravong, S.; Puttawibul, P.; Ruangsin, S.; Laohawiriyakamol, S.; Sunpaweravong, P.; Sangthawan, D.; Pradutkanchana, J.; Raungkhajorn, P.; Geater, A. Randomized study of antiinflammatory and immune-modulatory effects of enteral immunonutrition during concurrent chemoradiotherapy for esophageal cancer. Nutr. Cancer 2014, 66, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhelev, Z.; Ivanova, D.; Lazarova, D.; Aoki, I.; Bakalova, R.; Saga, T. Docosahexaenoic acid sensitizes leukemia lymphocytes to Barasertib and Everolimus by ROS-dependent mechanism without affecting the level of ROS and viability of normal lymphocytes. Anticancer Res. 2016, 36, 1673–1682. [Google Scholar] [PubMed]
- Siddiqui, R.A.; Harvey, K.A.; Xu, Z.; Bammerlin, E.M.; Walker, C.; Altenburg, J.D. Docosahexaenoic acid: A natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors 2011, 37, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, G.; Corsetto, P.A.; Campia, I.; Montorfano, G.; Kopecka, J.; Castella, B.; Gazzano, E.; Ghigo, D.; Rizzo, A.M.; Riganti, C. Omega 3 fatty acids chemosensitize multidrug resistant colon cancer cells by down-regulating cholesterol synthesis and altering detergent resistant membranes composition. Mol. Cancer 2013, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Hannafon, B.N.; Zhang, R.R.; Fung, K.M.; Ding, W.Q. Docosahexaenoic acid and disulfiram act in concert to kill cancer cells: A mutual enhancement of their anticancer actions. Oncotarget 2017, 8, 17908–17920. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Liang, Q.; Zhao, Z.H.; Li, Y.F.; Wang, S.F. Synergistic anticancer properties of docosahexaenoic acid and 5-fluorouracil through interference with energy metabolism and cell cycle arrest in human gastric cancer cell line AGS cells. World J. Gastroenterol. 2016, 22, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Chen, X.; Liu, B.; Li, P.; Cao, W. Ω-3 polyunsaturated fatty acids enhance cisplatin efficacy in gastric cancer cells by inducing apoptosis via ADORA1. Anticancer Agents Med. Chem. 2016, 16, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, L.; Goupille, C.; Blanc, C.; Pinault, M.; Domingo, I.; Guimaraes, C.; Bougnoux, P.; Chevalier, S.; Mahéo, K. Long chain n-3 polyunsaturated fatty acids increase the efficacy of docetaxel in mammary cancer cells by downregulating Akt and PKCε/δ-induced ERK pathways. Biochim. Biophys. Acta 2016, 1861, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Abdi, J.; Garssen, J.; Faber, J.; Redegeld, F.A. Ω-3 fatty acids, EPA and DHA induce apoptosis and enhance drug sensitivity in multiple myeloma cells but not in normal peripheral mononuclear cells. J. Nutr. Biochem. 2014, 25, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.K.; Fu, M.; Chen, J.; Thompson, L.U. Flaxseed oil enhances the effectiveness of trastuzumab in reducing the growth of HER2-overexpressing human breast tumors (BT-474). J. Nutr. Biochem. 2015, 26, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced non-small cell lung cancer. Cancer 2011, 117, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Yano, M.; Yasuda, T.; Yamasaki, M.; Murakami, K.; Makino, T.; Nishiki, K.; Sugimura, K.; Motoori, M.; Shiraishi, O.; et al. Randomized study of the clinical effects of ω-3 fatty acid-containing enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Nutrition 2017, 33, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Okugawa, Y.; Hishida, A.; Ogawa, A.; Okamoto, K.; Shintani, M.; Morimoto, Y.; Nishikawa, R.; Yokoe, T.; Tanaka, K.; et al. Fish oil-enriched nutrition combined with systemic chemotherapy for gastrointestinal cancer patients with cancer cachexia. Sci. Rep. 2017, 7, 4826. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.L.; Shao, L.; Zhao, Y.T.; Yu, X.; Zhang, D.F.; Zhang, H. The beneficial effect of n-3 polyunsaturated fatty acids on doxorubicin-induced chronic heart failure in rats. J. Int. Med. Res. 2010, 38, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Uygur, R.; Aktas, C.; Tulubas, F.; Alpsoy, S.; Topcu, B.; Ozen, O.A. Cardioprotective effects of fish ω-3 fatty acids on doxorubicin-induced cardiotoxicity in rats. Hum. Exp. Toxicol. 2014, 33, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Chen, C.Y.; Chen, M.F. N-3 polyunsaturated fatty acids decrease levels of doxorubicin-induced reactive oxygen species in cardiomyocytes—Involvement of uncoupling protein UCP2. J. Biomed. Sci. 2014, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Ren, W.; Denkinger, M.; Schlotzer, E.; Wischmeyer, P.E. Nutrition modulation of cardiotoxicity and anticancer efficacy related to Doxorubicin chemotherapy by glutamine and ω-3 polyunsaturated fatty acids. J. Parenter. Enter. Nutr. 2016, 40, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Chen, M.T.; Zhang, R.; Zhang, Y.; Li, W.; Li, Y.G. Docosahexaenoic acid attenuates doxorubicin-induced cytotoxicity and inflammation by suppressing NF-κB/iNOS/NO signaling pathway activation in H9C2 cardiac cells. J. Cardiovasc. Pharmacol. 2016, 67, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, K.V.; Ajeesh Kumar, K.K.; Chatterjee, N.S.; Lekshmi, R.G.K.; Sreerekha, P.R.; Mathew, S.; Ravishankar, C.N. Sardine oil loaded vanillic acid grafted chitosan microparticles, a new functional food ingredient: Attenuates myocardial oxidative stress and apoptosis in cardiomyoblast cell lines (H9c2). Cell Stress Chaperones 2017. [Google Scholar] [CrossRef] [PubMed]
- Edwardson, D.W.; Narendrula, R.; Chewchuk, S.; Mispel-Beyer, K.; Mapletoft, J.P.; Parissenti, A.M. Role of drug metabolism in the cytotoxicity and clinical efficacy of anthracyclines. Curr. Drug Metab. 2015, 16, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, F.M. From the pigments of the actinomycetes to third generation antitumor anthracyclines. Biochimie 1998, 80, 201–206. [Google Scholar] [CrossRef]
- Preobrazhenskaya, M.N.; Tevyashova, A.N.; Olsufyeva, E.N.; Huang, H.-F.; Huang, H.S. Second generation drugs—Derivatives of natural anti-tumor anthracycline antibiotics Daunorubicin, Doxorubicin and Carminomycin. J. Med. Sci. 2006, 26, 119–128. [Google Scholar]
- Cortes-Funes, H.; Coronado, C. Role of anthracyclines in the era of targeted therapy. Cardiovasc. Toxicol. 2007, 7, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Fumoleau, P.; Roche, H.; Kerbrat, P.; Bonneterre, J.; Romestaing, P.; Fargeot, P.; Namer, M.; Monnier, A.; Montcuquet, P.; Goudier, M.J.; et al. Long-term cardiac toxicity after adjuvant epirubicin-based chemotherapy in early breast cancer: French Adjuvant Study Group results. Ann. Oncol. 2006, 17, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.J.; Moasser, M.M. Cellular mechanisms of resistance to anthracyclines and taxanes in cancer: Intrinsic and acquired. Semin. Oncol. 2008, 35, S1–S14. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.J.; Trivedi, P.C.; Pulinilkunnil, T. Autophagic dysregulation in doxorubicin cardiomyopathy. J. Mol. Cell. Cardiol. 2017, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Maccarinelli, F.; Buratti, P.; Recalcati, S.; Cairo, G. The role of iron in anthracycline cardiotoxicity. Front. Pharmacol. 2014, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Vejpongsa, P.; Yeh, E.T. Prevention of anthracycline-induced cardiotoxicity: Challenges and opportunities. J. Am. Coll. Cardiol. 2014, 64, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Link, G.; Tirosh, R.; Pinson, A.; Hershko, C. Role of iron in the potentiation of anthracycline cardiotoxicity: Identification of heart cell mitochondria as a major site of iron-anthracycline interaction. J. Lab. Clin. Med. 1996, 127, 272–278. [Google Scholar] [CrossRef]
- Van Dalen, E.C.; Caron, H.N.; Dickinson, H.O.; Kremer, L.C. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst. Rev. 2011, 6, CD003917. [Google Scholar]
- Dresdale, A.R.; Barr, L.H.; Bonow, R.O.; Mathisen, D.J.; Myers, C.E.; Schwartz, D.E.; D’Angelo, T.; Rosenberg, S.A. Prospective randomized study of the role of N-acetyl cysteine in reversing doxorubicin-induced cardiomyopathy. Am. J. Clin. Oncol. 1982, 5, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Panjrath, G.S.; Patel, V.; Valdiviezo, C.I.; Narula, N.; Narula, J.; Jain, D. Potentiation of Doxorubicin cardiotoxicity by iron loading in a rodent model. J. Am. Coll. Cardiol. 2007, 49, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Hasinoff, B.B.; Schnabl, K.L.; Marusak, R.A.; Patel, D.; Huebner, E. Dexrazoxane (ICRF-187) protects cardiac myocytes against doxorubicin by preventing damage to mitochondria. Cardiovasc. Toxicol. 2003, 3, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Thougaard, A.V.; Grauslund, M.; Jensen, P.B.; Bjorkling, F.; Hasinoff, B.B.; Tjørnelund, J.; Sehested, M.; Jensen, L.H. Evaluation of the topoisomerase II-inactive bisdioxopiperazine ICRF-161 as a protectant against doxorubicin-induced cardiomyopathy. Toxicology 2009, 255, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, K.C.; Nitiss, J.L. Twisting and ironing: Doxorubicin cardiotoxicity by mitochondrial DNA damage. Clin. Cancer Res. 2014, 20, 4737–4739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, T.A.; Mohamed, R.H.; Pasha, H.F.; Abdel-Aziz, H.R. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin. Exp. Med. 2012, 12, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Lin, J.; Xu, W.; Shen, N.; Mo, L.; Zhang, C.; Feng, J. Hydrogen sulfide attenuates doxorubicin-induced cardiotoxicity by inhibition of the p38 MAPK pathway in H9c2 cells. Int. J. Mol. Med. 2013, 31, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Del Pizzo, M.; Marzocco, S.; Sorrentino, R.; Ciccarelli, M.; Iaccarino, G.; Pinto, A.; Popolo, A. Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol. Appl. Pharmacol. 2016, 293, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, G.; Synnergren, J.; Andersson, C.X.; Lindahl, A.; Sartipy, P. MicroRNAs as potential biomarkers for doxorubicin-induced cardiotoxicity. Toxicol. In Vitro 2016, 34, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Saddic, L.A.; Muehlschlegel, J.D. Sarco “MiR” friend or foe: A perspective on the mechanisms of doxorubicin-induced cardiomyopathy. Ann. Transl. Med. 2016, 4, 203. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Piccioni, E.; Toesca, A.; Monego, G.; Cittadini, A.R.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim. Biophys. Acta 2012, 1822, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Dirks-Naylor, A.J.; Kouzi, S.A.; Yang, S.; Tran, N.T.; Bero, J.D.; Mabolo, R.; Phan, D.T.; Whitt, S.D.; Taylor, H.N. Can short-term fasting protect against doxorubicin-induced cardiotoxicity? World J. Biol. Chem. 2014, 5, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, M.; Troncoso, R.; Martinez, G.J.; Chiong, M.; Castro, P.F.; Lavandero, S. Basal autophagy protects cardiomyocytes from doxorubicin-induced toxicity. Toxicology 2016, 370, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.; du Toit, A.; Hofmeyr, J.H. Defining and measuring autophagosome flux-concept and reality. Autophagy 2014, 10, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, D.; Rossi, F.; Piegari, E.; Quaini, F.; Berrino, L.; Urbanek, K.; De Angelis, A. Doxorubicin targets multiple players: A new view of an old problem. Pharmacol. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Morishima, I.; Hayashi, K.; Kamiya, H.; Saburi, Y.; Okumura, K. Dietary fish oil does not prevent doxorubicin-induced cardiomyopathy in rats. Can. J. Cardiol. 2002, 18, 279–286. [Google Scholar] [PubMed]
- Carbone, A.; Psaltis, P.J.; Nelson, A.J.; Metcalf, R.; Richardson, J.D.; Weightman, M.; Thomas, A.; Finnie, J.W.; Young, G.D.; Worthley, S.G. Dietary ω-3 supplementation exacerbates left ventricular dysfunction in an ovine model of anthracycline-induced cardiotoxicity. J. Card. Fail. 2012, 18, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Germain, E.; Lavandier, F.; Chajès, V.; Schubnel, V.; Bonnet, P.; Lhuillery, C.; Bougnoux, P. Dietary n-3 polyunsaturated fatty acids and oxidants increase rat mammary tumor sensitivity to epirubicin without change in cardiac toxicity. Lipids 1999, 34, S203. [Google Scholar] [CrossRef] [PubMed]
- Germain, E.; Bonnet, P.; Aubourg, L.; Grangeponte, M.C.; Chajès, V.; Bougnoux, P. Anthracycline-induced cardiac toxicity is not increased by dietary ω-3 fatty acids. Pharmacol. Res. 2003, 47, 111–117. [Google Scholar] [CrossRef]
- Schjøtt, J.; Brurok, H.; Jynge, P.; Bjerve, K.S. Effects of eicosapentaenoic acid and docosahexaenoic acid diet supplement on tolerance to the cardiotoxicity of epirubicin and to ischaemia reperfusion in the isolated rat heart. Pharmacol. Toxicol. 1996, 79, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cui, L.; Zhang, Z.; Zhao, Q.; Li, S. α-Linolenic acid attenuates doxorubicin-induced cardiotoxicity in rats through suppression of oxidative stress and apoptosis. Acta Biochim. Biophys. Sin. 2013, 45, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Hajjaji, N.; Besson, P.; Bougnoux, P. Tumor and non-tumor tissues differential oxidative stress response to supplemental DHA and chemotherapy in rats. Cancer Chemother. Pharmacol. 2012, 70, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Vitelli, M.R.; Filippelli, A.; Rinaldi, B.; Rossi, S.; Palazzo, E.; Rossi, F.; Berrino, L. Effects of docosahexaenoic acid on [Ca2+]i increase induced by doxorubicin in ventricular rat cardiomyocytes. Life Sci. 2002, 71, 1905–1916. [Google Scholar] [CrossRef]
- Lau, D.H.; Psaltis, P.J.; Carbone, A.; Kelly, D.J.; Mackenzie, L.; Worthington, M.; Metcalf, R.G.; Kuklik, P.; Nelson, A.J.; Zhang, Y.; et al. Atrial protective effects of n-3 polyunsaturated fatty acids: A long-term study in ovine chronic heart failure. Heart Rhythm 2011, 8, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Cittadini, A.; Calviello, G. Long-chain n-3 PUFA against breast and prostate cancer: Which are the appropriate doses for intervention studies in animals and humans? Crit. Rev. Food Sci. Nutr. 2017, 57, 2245–2262. [Google Scholar] [CrossRef] [PubMed]
- Menna, P.; Salvatorelli, E. Primary prevention strategies for anthracycline cardiotoxicity: A brief overview. Chemotherapy 2017, 62, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T. Efficiency of conversion of α-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Colas, S.; Mahéo, K.; Denis, F.; Goupille, C.; Hoinard, C.; Champeroux, P.; Tranquart, F.; Bougnoux, P. Sensitization by dietary docosahexaenoic acid of rat mammary carcinoma to anthracycline: A role for tumor vascularization. Clin. Cancer Res. 2006, 12, 5879–5886. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.; Calviello, G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem. Res. Toxicol. 2011, 24, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Cetrullo, S.; Tantini, B.; Flamigni, F.; Pazzini, C.; Facchini, A.; Stefanelli, C.; Caldarera, C.M.; Pignatti, C. Antiapoptotic and antiautophagic effects of eicosapentaenoic acid in cardiac myoblasts exposed to palmitic acid. Nutrients 2012, 4, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Chen, C.Y.; Chiang, C.H.; Chen, M.F. Eicosapentaenoic acid attenuated oxidative stress-induced cardiomyoblast apoptosis by activating adaptive autophagy. Eur. J. Nutr. 2014, 53, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Gwon, D.H.; Hwang, T.W.; Ro, J.Y.; Kang, Y.J.; Jeong, J.Y.; Kim, D.K.; Lim, K.; Kim, D.W.; Choi, D.E.; Kim, J.J. High endogenous accumulation of ω-3 polyunsaturated fatty acids protect against ischemia-reperfusion renal injury through AMPK-mediated autophagy in Fat-1 mice. Int. J. Mol. Sci. 2017, 18, 2081. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, K.; Monsen, V.T.; Hakvåg Pettersen, C.H.; Overland, H.B.; Pettersen, G.; Samdal, H.; Tesfahun, A.N.; Lundemo, A.G.; Bjørkøy, G.; Schønberg, S.A. DHA-induced stress response in human colon cancer cells—Focus on oxidative stress and autophagy. Free Radic. Biol. Med. 2016, 90, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Vedin, I.; Freund Levi, Y.; Basun, H.; Faxén Irving, G.; Eriksdotter, M.; Wahlund, L.O.; Schultzberg, M.; Hjorth, E.; Cederholm, T.; et al. DHA-rich n-3 fatty acid supplementation decreases DNA methylation in blood leukocytes: The ΩD study. Am. J. Clin. Nutr. 2017, 106, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Rincón-Cervera, M.Á.; Echeverría, F.; Barrera, C.; Espinosa, A.; Hernández-Rodas, M.C.; Ortiz, M.; Valenzuela, A.; Videla, L.A. Iron-induced pro-oxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition 2018, 45, 49–58. [Google Scholar] [CrossRef] [PubMed]
| Experimental Model | ATC Treatment | ω-3 PUFA Treatment | Treatment Combined with ω-3 PUFA | Control Condition (Alternative to ω-3 PUFA or/and ATC Treatments) | ω-3 PUFA-Induced Morphological/Functional Effects at CV Level | ω-3 PUFA-Induced Biochemical and Molecular Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Rats bearing NMNU-induced mammary tumors | EPI (3 mg/kg/week; IP) for 3 weeks | Diet containing 15% SO (6 weeks) | ±LP inducers * in drinking water or ± vitamin E (100 IU/kg diet) | none | NO EFFECTS SO ± LP inducers * or SO ± vit E: no variation in EPI-induced increase in LVEDP in almost 10% of rats in all groups | n.d. | [114] |
| Hearts isolated from rats supplemented with ω-3 PUFA and perfused ex vivo with EPI | EPI heart perfusion ex vivo: 0.2 mg/min/10 min | 1.0 mL (300 mg) EPA or DHA ethylesters suspended in 0.5% carboxymethylcellulose by gavage (1st week); 1.5 mL (450 mg) (2nd week) | none | 1.0 mL (1st week) or 1.5 mL (2nd week) olive oil (alternative to EPA or DHA treatments) | PROTECTIVE EFFECTS Heart from EPA- or DHA-treated rats perfused with EPI: lower aortic pressure (index of coronary resistance) than in olive oil-treated hearts | No difference in the heart release of LDH among groups during and after EPI infusion | [116] |
| Sprague-Dawley rats | DOX (cumulative dose): 15 mg/kg after 4 weeks of FO treatments | Diet containing 10% MO (4 weeks prior and 3 weeks after DOX treatment) | none | 0.28 M dextrose solution (alternative to DOX treatment) | HARMFUL EFFECTS FO diet: -highest mortality; -further reduction in cardiac LVFS | FO diet: increase in myocardial LP and decrease in vit E level | [112] |
| Sprague–Dawley rats | After oil treatments: EPI (weekly, cumulative doses): 9 mg/kg (exp. 1) 15 mg/kg (exp. 2) | Diet containing 15% SO or DHASCO oil (% not reported) (at least 3 weeks before EPI treatment) | ±LP inducers in drinking water or vitamin E (100 IU/kg diet) | Palm oil (alternative to DHASCO oil treatment) | NO EFFECTS SO or DHASCO oils (± anti- or pro-oxidants): no changes in EPI-induced: -mortality; -alterations of LVEDP and LVSP and left ventricular systolic pressure; -histological damages | n.d. | [115] |
| Male Sprague-Dawley rats | IP DOX injection (2 mg/kg/week) (8 weeks) | FO § (0.6% of body weight) daily, by gavage (for 8 weeks, after DOX treatment) | none | 0.9% normal saline (alternative to FO) | PROTECTIVE EFFECTS FO supplementation: -Lower LVEDD and LVESD -Higher LVEF and LVFS | n.d. | [78] |
| Merino wether sheep | Intracoronary DOX infusions (1.0 mg/kg, every other week) (for 3–4 weeks) | Oral supplementation: 1.8 g EPA + 1.2 g DHA/day ° (1 week prior and 13–15 weeks after the last DOX infusion) | none | No supplementations, sham operated; (Alternative to ω-3 and DOX treatments) Olive oil (10 mL) | PROTECTIVE EFFECTS EPA + DHA supplementation, suppression of DOX-induced: -left atrial dilation and interstitial fibrosis; -alterations in atrial conduction | n.d. | [120] |
| Merino wether sheep | Intracoronary DOX infusion (1.2 mg/kg, every other week) (for 3 weeks) | Oral supplementation: ω 18/12 FO (23 mL) (3 times/week for 3 weeks prior and 16 weeks during and post DOX treatment | none | Oral supplementation with 23 mL olive oil (alternative to FO) | HARMFUL EFFECTS FO supplementation: left ventricular dilatation; greater decline in ejection fraction | More frequent elevation of serum troponin-T after DOX treatment in ω-3 treated sheep | [113] |
| Female Sprague-Dawley rats | IV EPI injection (0.8 mg/kg once a week) (for 6 weeks) | Oral supplementation DHASCO oil 80 g/kg diet (45% DHA) for 12–13 weeks prior and 6 weeks during EPI treatment | none | Palm oil-based diet | n.d. | In cardiac tissue: no changes in LP and total antioxidant activity; increased antioxidant enzyme (GPx, SOD) activity | [118] |
| Male Sprague-Dawley rats | DOX (2.5 mg/kg, IP) (from the 4th day, every other day) (for 7 times) | Pretreatment with ALA (500 µg/kg body weight) by gavage (3 days); from the 4th day: every other day (for 7 days) | none | Oral supplementation with normal saline throughout the experiments (alternative to ALA and DOX treatments) | PROTECTIVE EFFECTS ALA supplementation, suppression of DOX-induced: -cardiac histopathological alterations, -reduction of LVEDV, SV, and EF, -increase in HW/BW, -cardiomyocyte apoptosis | ALA prevented DOX-induced: -in serum: increase in BNP, CK-MB, LDH, cTnI levels; -in cardiac tissue: LP increase and antioxidant enzymes ** decrease; -caspase-3 activation and changed BAX and BCL2 expression; -p-AKT and p-ERK decreased expression | [117] |
| Sprague-Dawley rats | Single IP DOX dose (30 mg/kg, after 30 day ω-3 PUFA treatment) | Pretreatment with ω-3 PUFA capsules (New Life EFA S-1200 (400 mg/kg/d, by gavage) (30 days before DOX injections) | none | 0.4 mL/kg saline by gavage (alternative to ω-3 PUFA and DOX treatments) | PROTECTIVE EFFECTS PUFA treatment: -improved cardiac histological appearance; -reduction of apoptotic index (Tunel-positive cardiomyocytes) | Decrease in MDA levels; increase in SOD and GPx activities | [79] |
| Female Fisher 344 rats bearing syngeneic MatBIII mammary adenocarcinoma xenograft | DOX (1 mg/kg, IV) starting as tumor mass =1.2 cm3 (for 6 days and then weekly for 6 weeks) | Parenteral solution (tail vein) containing 0.19 g/kg EPA + 0.18 g/kg DHA) every other day (6 days before DOX treatment until day 50) | ±Parenteral solution containing glutamine (0.35 g/kg) ± EPA + DHA | Parenteral saline (alternative to ω-3 PUFA, DOX) | MIXED EFFECTS EPA + DHA solution: -No modifications in DOX-induced: increase in LVEDD, LVESD and apoptosis; -Partially improved LV dilation and function (no statistical significance); -Reversion of glutamine positive cardiovascular effects | EPA + DHA parenteral solution prevents DOX-induced elevation of plasma cTnI levels; No effect on DOX-induced lipid peroxidation and enzymatic and non-enzymatic antioxidants in cardiac tissue | [81] |
| Experimental Model | ATC Treatment | ω-3 PUFA Treatment | Additional Treatments | Control Condition | ω-3 PUFA Effect on DOX-Induced Cardiac Cell Viability | Effects of ω-3 PUFA in Combination with DOX at Biochemical and Molecular Levels | Ref. |
|---|---|---|---|---|---|---|---|
| Isolated adult rat cardiomyocytes perfused with CaCl2 Krebs solution | 100 µM DOX (after 20 min DHA treatment) | Pre-treatment with 10 µM DHA (20 min) | none | No treatment with DHA ± treatment with DOX | n.d. | PROTECTIVE DHA pretreatment: Inhibition of DOX-induced (Ca2+)i increase | [119] |
| H9C2 cardiomyoblast cell line | 1 μM DOX in DMEM-10% FBS (24 h) after 24 h EPA/DHA treatment | Pre-treatment with 100 μM EPA or 50 μM DHA in DMEM-0.1% BSA (24 h) | none | No treatment with ω-3 PUFA ± treatment with DOX | n.d. | PROTECTIVE EPA or DHA pretreatment: prevention of DOX-induced: -decrease in UCP2 levels -increase in ROS production -MMP decrease | [80] |
| H9C2 cardiomyoblast cell line | 5 μM DOX in DMEM-10% FBS (4 h) | Co-treatment with 10 μM DHA-FFA (4 h) | none | No treatment with DHA ± treatment with DOX | Increased viability | PROTECTIVE DHA co-treatment: suppression of DOX-induced: -ROS production; -expression of TNF-α, IL-6, MCP-1, iNOS, and IL-1β; -phosphorylation of IκB-αand NF-κB/P65 | [82] |
| H9C2 cardiomyoblast cell line | 20 mM DOX in DMEM-10% FBS (1 h) | After DOX treatment: 1.25 mg/mL SO-loaded Va-g-Ch microparticles (SO-M) | none | No treatments | n.d. * | PROTECTIVE SO-M treatment: suppression of DOX-induced: -caspase-3 activation; -increased expression of NF-κB | [83] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serini, S.; Ottes Vasconcelos, R.; Nascimento Gomes, R.; Calviello, G. Protective Effects of ω-3 PUFA in Anthracycline-Induced Cardiotoxicity: A Critical Review. Int. J. Mol. Sci. 2017, 18, 2689. https://doi.org/10.3390/ijms18122689
Serini S, Ottes Vasconcelos R, Nascimento Gomes R, Calviello G. Protective Effects of ω-3 PUFA in Anthracycline-Induced Cardiotoxicity: A Critical Review. International Journal of Molecular Sciences. 2017; 18(12):2689. https://doi.org/10.3390/ijms18122689
Chicago/Turabian StyleSerini, Simona, Renata Ottes Vasconcelos, Renata Nascimento Gomes, and Gabriella Calviello. 2017. "Protective Effects of ω-3 PUFA in Anthracycline-Induced Cardiotoxicity: A Critical Review" International Journal of Molecular Sciences 18, no. 12: 2689. https://doi.org/10.3390/ijms18122689
APA StyleSerini, S., Ottes Vasconcelos, R., Nascimento Gomes, R., & Calviello, G. (2017). Protective Effects of ω-3 PUFA in Anthracycline-Induced Cardiotoxicity: A Critical Review. International Journal of Molecular Sciences, 18(12), 2689. https://doi.org/10.3390/ijms18122689






