Combined Effects of Simulated Microgravity and Radiation Exposure on Osteoclast Cell Fusion
Abstract
1. Introduction
2. Results
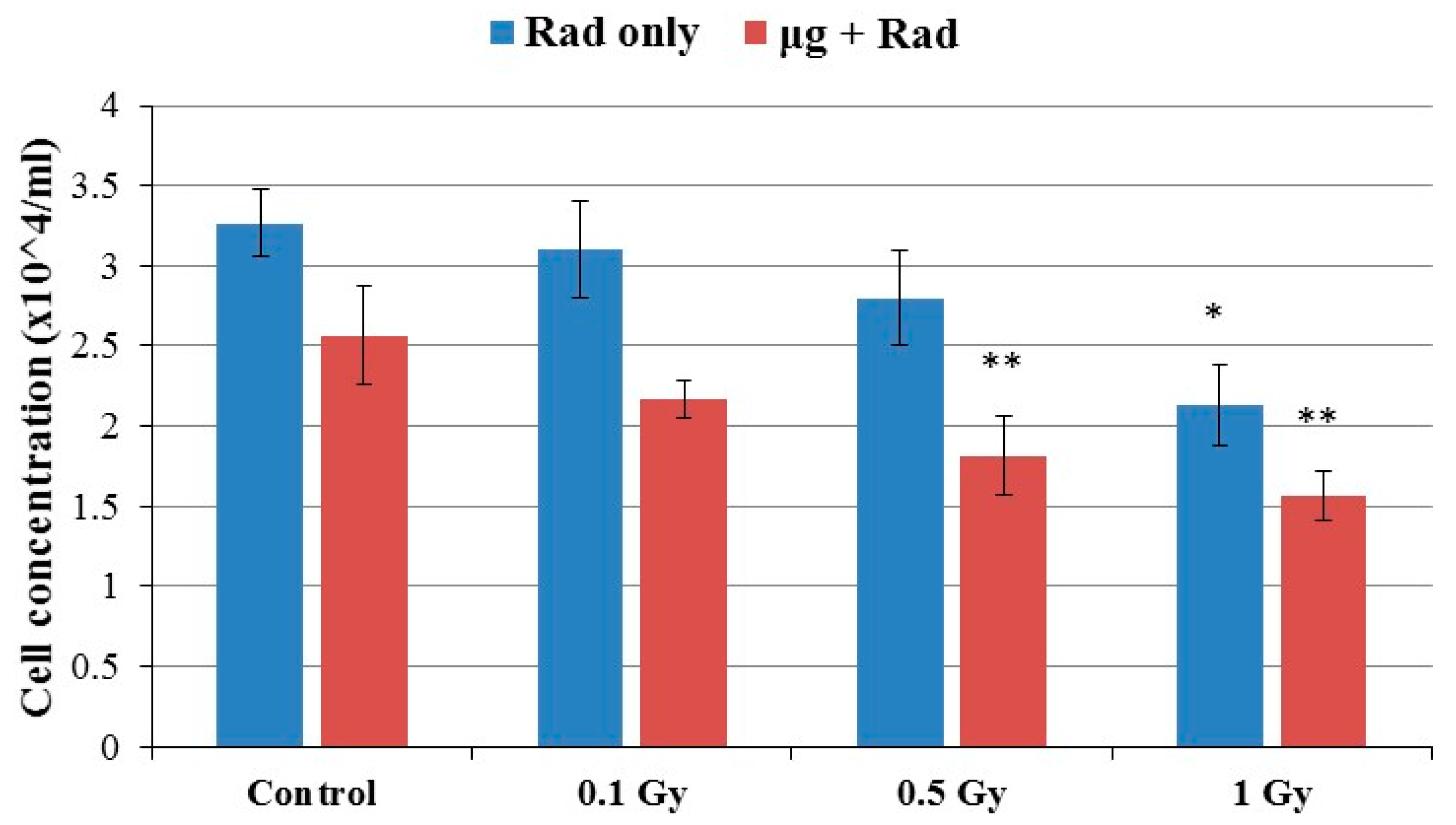
2.1. Cell Growth
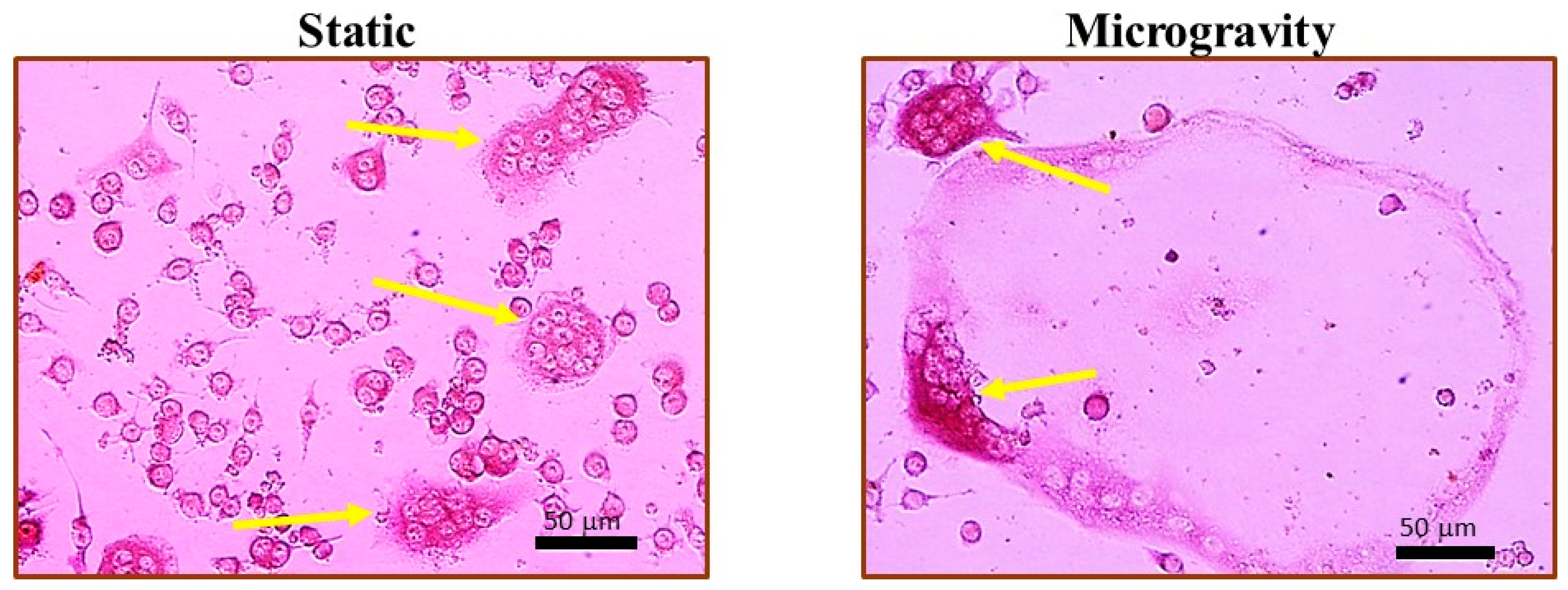
2.2. Simulated Microgravity Increases Osteoclast Fusion
2.3. Radiation Exposure Increases Osteoclast Fusion
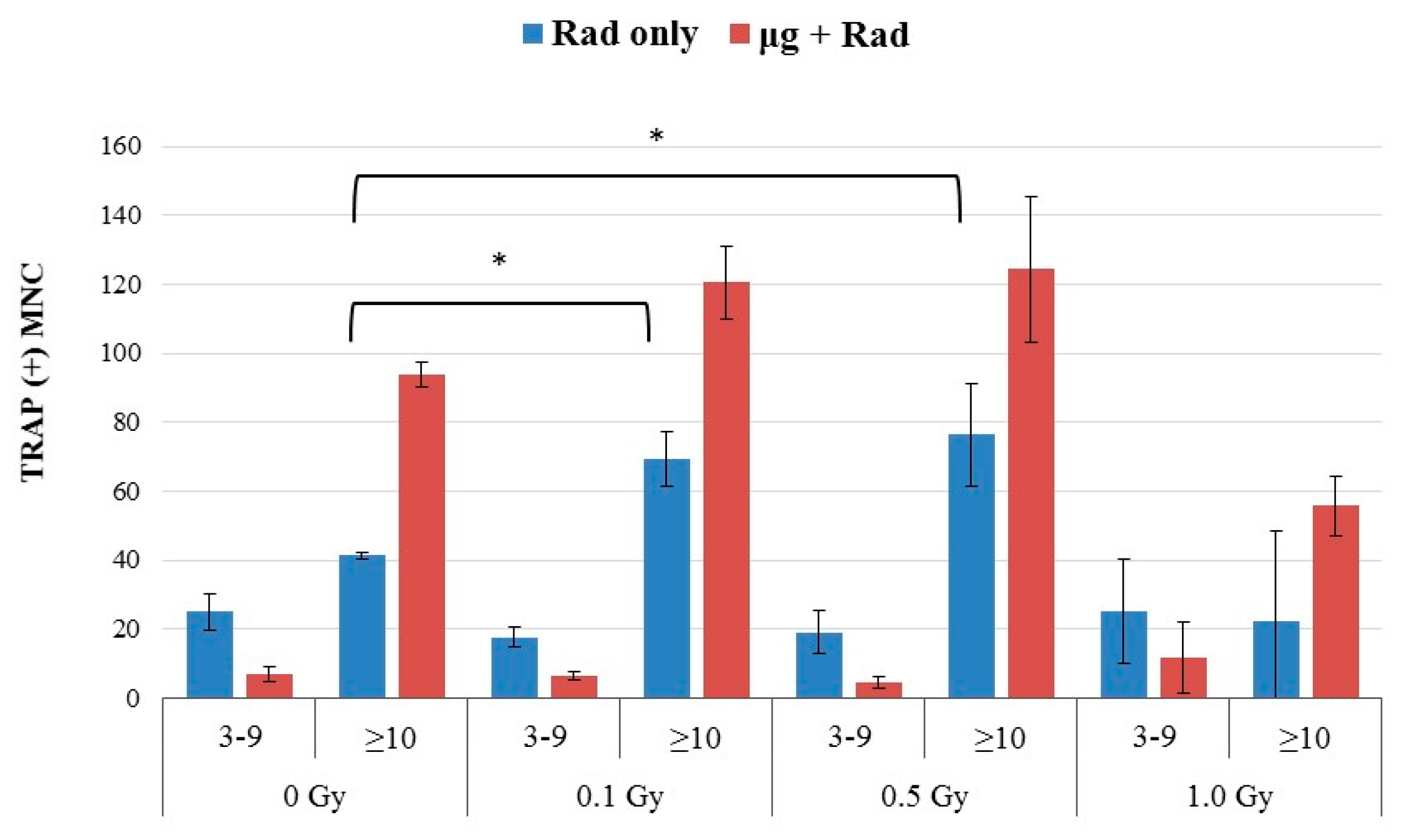
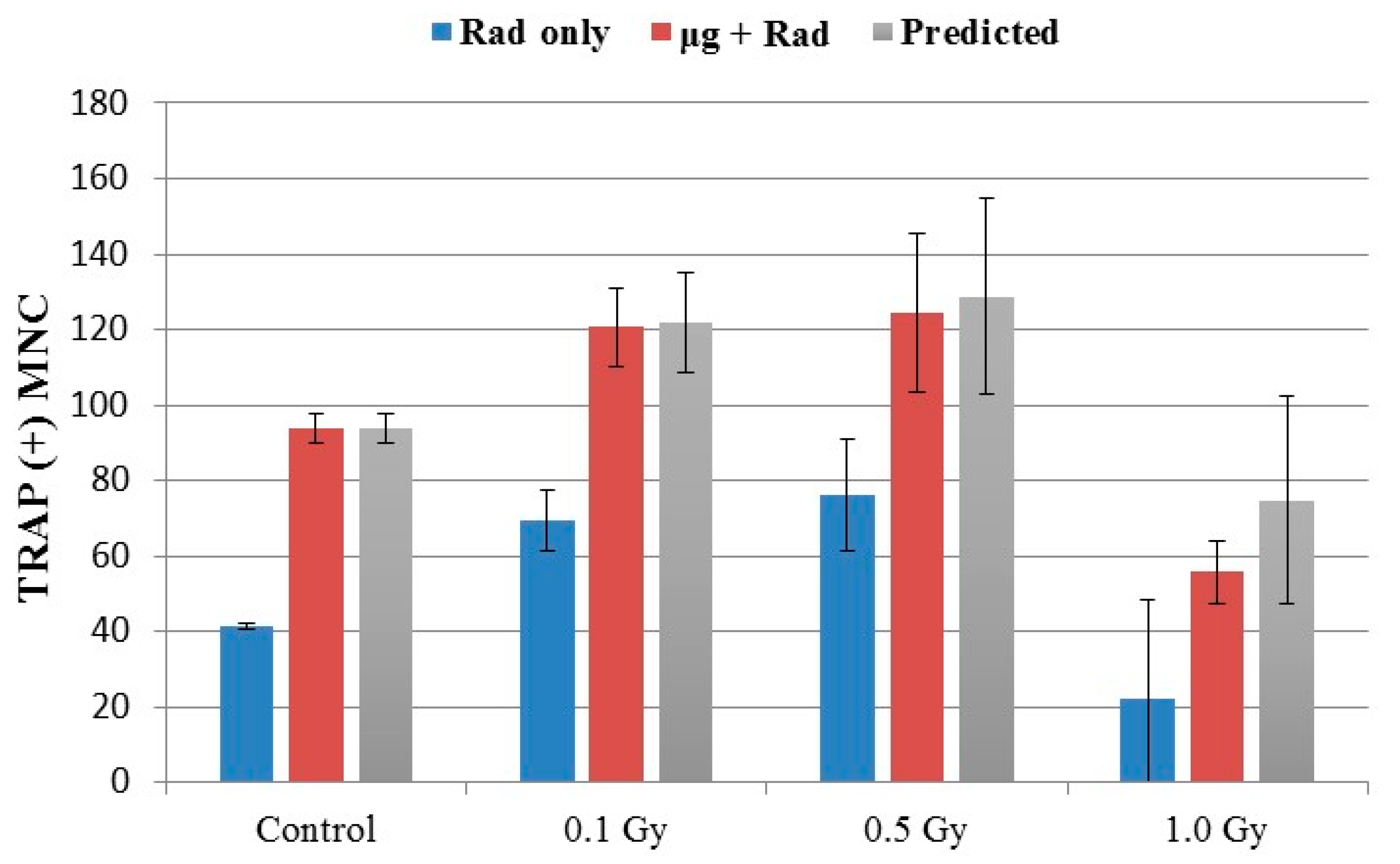
2.4. Effects of Combined Radiation Exposure and Simulated Microgravity
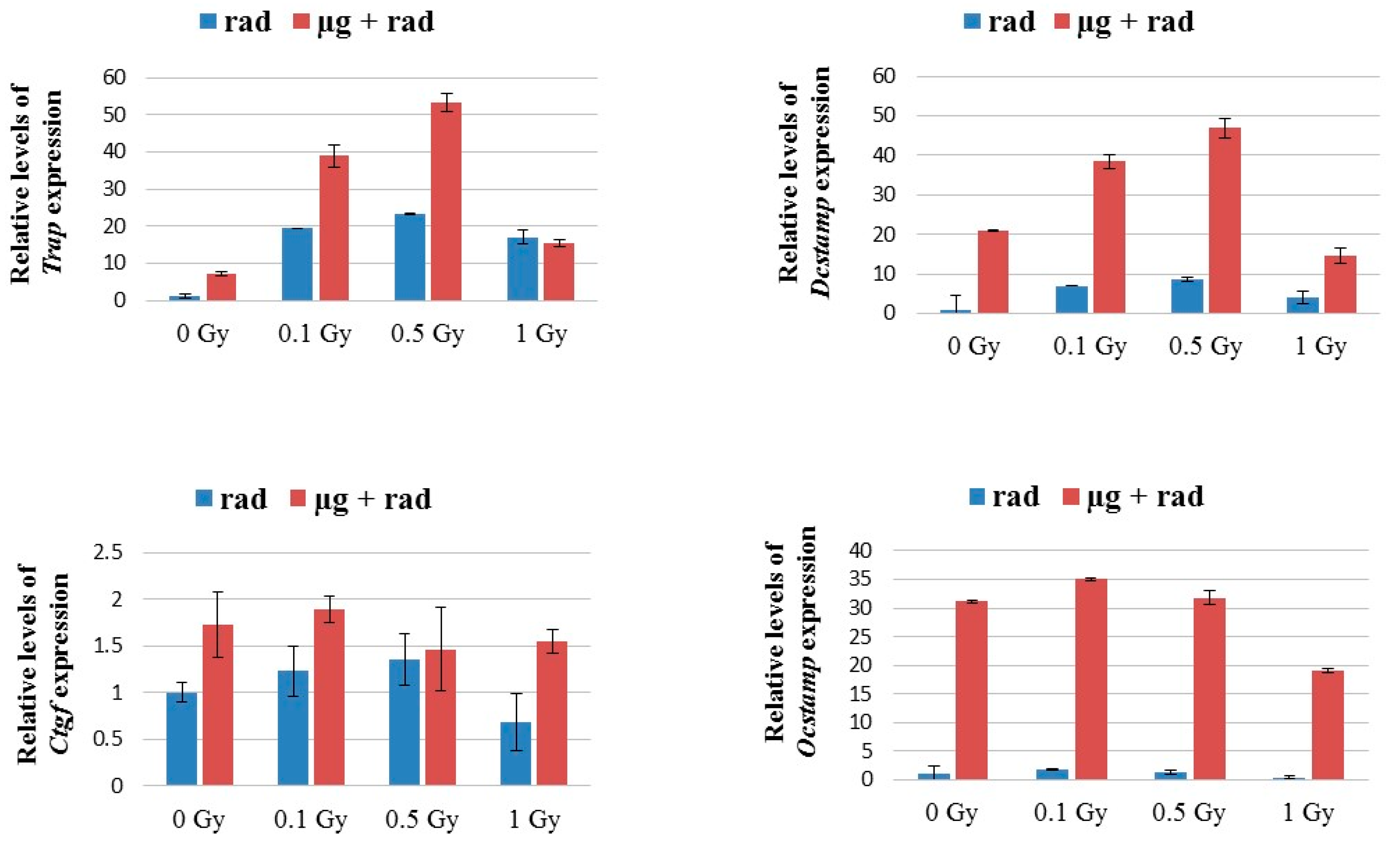
2.5. Osteoclast Fusion Genes Up-Regulated in Microgravity
3. Discussion
4. Materials and Methods
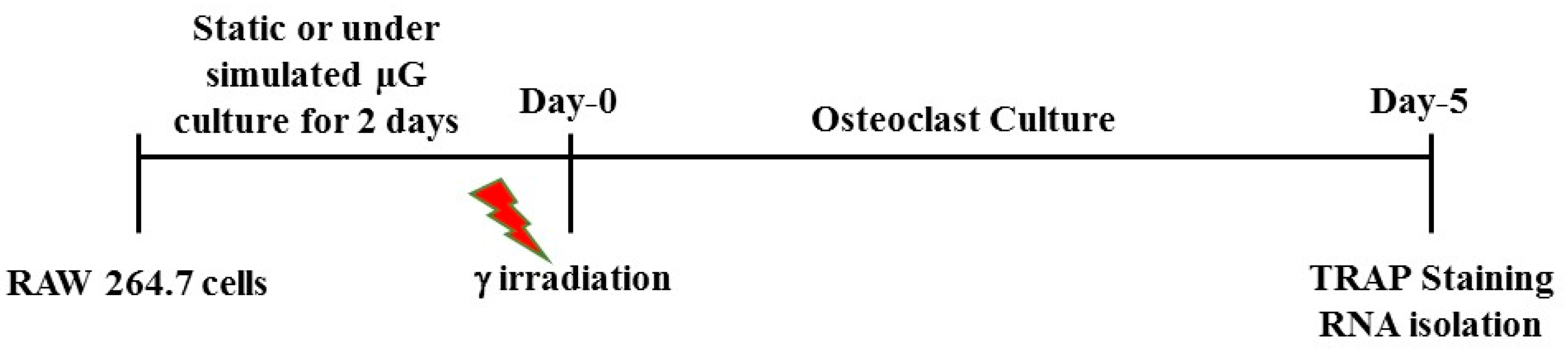
4.1. Cell Culture in Simulated Microgravity and γ Irradiation
4.2. Cell Concentration and Viability
4.3. Osteoclast Culture
4.4. Real-Time Polymerase Chain Reaction (RT-PCR) Analysis
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alwood, J.S.; Ronca, A.E.; Mains, R.C.; Shelhamer, M.J.; Smith, J.D.; Goodwin, T.J. From the bench to exploration medicine: NASA life sciences translational research for human exploration and habitation missions. NPJ Microgravity 2017, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Moreno-Villanueva, M.; Krieger, S.; Ramesh, G.T.; Neelam, S.; Wu, H. Transcriptomics, NF-κB Pathway, and Their Potential Spaceflight-Related Health Consequences. Int. J. Mol. Sci. 2017, 18, 1166. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; LeBlanc, A.; Evans, H.; Lu, Y.; Genant, H.; Yu, A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J. Bone Miner. Res. 2004, 19, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Sibonga, J.D. Spaceflight-induce? Bone loss: Is there an osteoporosis risk? Curr. Osteoporos. Rep. 2013, 11, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Fulford, M.; Tjandrawinata, R.; Fitzgerald, J.; Gasuad, K.; Gilbertson, V. Effects of microgravity on osteoblast growth. Gravit. Space Biol. Bull. 1998, 11, 51–60. [Google Scholar] [PubMed]
- Chatani, M.; Mantoku, A.; Takeyama, K.; Abduweli, D.; Sugamori, Y.; Aoki, K.; Ohya, K.; Suzuki, H.; Uchida, S.; Sakimura, T.; et al. Microgravity promotes osteoclast activity in medaka fish reared at the international space station. Sci. Rep. 2015, 5, 14172. [Google Scholar] [CrossRef] [PubMed]
- Sambandam, Y.; Baird, K.L.; Stroebel, M.; Kowal, E.; Balasubramanian, S.; Reddy, S.V. Microgravity Induction of TRAIL Expression in Preosteoclast Cells Enhances Osteoclast Differentiation. Sci. Rep. 2016, 6, 25143. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Khandani, A.; Camirand, A.; Harrison, R.E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 2011, 49, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; Collet, P.; Guignandon, A.; Lafage-Proust, M.H.; Thomas, T.; Rehaillia, M.; Alexandre, C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 2000, 355, 1607–1611. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.S.; Lloyd, S.A.J.; Nelson, G.A.; Bateman, T.A. Ionizing Radiation and Bone Loss: Space Exploration and Clinical Therapy Applications. Clin. Rev. Bone Miner. Metab. 2011, 9, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.A.; Pecaut, M.J.; Gridley, D.S.; Travis, N.D.; Bandstra, E.R.; Willey, J.S.; Nelson, G.A.; Bateman, T.A. A murine model for bone loss from therapeutic and space-relevant sources of radiation. J. Appl. Physiol. 2006, 101, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.E.; Buijs, J.T.; Kim, H.S.; Coats, L.E.; Scheidler, A.M.; John, S.K.; She, Y.; Murthy, S.; Ma, N.; Chin-Sinex, H.J.; et al. Single-Limb Irradiation Induces Local and Systemic Bone Loss in a Murine Model. J. Bone Miner. Res. 2015, 30, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.S.; Lloyd, S.A.J.; Nelson, G.A.; Bateman, T.A. Space Radiation and Bone Loss. Gravit. Space Biol. Bull. 2011, 25, 14–21. [Google Scholar] [PubMed]
- Moreno-Villanueva, M.; Wong, M.; Lu, T.; Zhang, Y.; Wu, H. Interplay of space radiation and microgravity in DNA damage and DNA damage response. NPJ Microgravity 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Macias, B.R.; Lima, F.; Swift, J.M.; Shirazi-Fard, Y.; Greene, E.S.; Allen, M.R.; Fluckey, J.; Hogan, H.A.; Braby, L.; Wang, S.; et al. Simulating the Lunar Environment: Partial Weightbearing and High-LET Radiation-Induce Bone Loss and Increase Sclerostin-Positive Osteocytes. Radiat. Res. 2016, 186, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Yumoto, K.; Alwood, J.S.; Mojarrab, R.; Wang, A.; Almeida, E.A.; Searby, N.D.; Limoli, C.L.; Globus, R.K. Oxidative stress and gamma radiation-induced cancellous bone loss with musculoskeletal disuse. J. Appl. Physiol. 2010, 108, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kukita, A.; Li, Y.J.; Zhang, J.Q.; Nomiyama, H.; Yamaza, T.; Ayukawa, Y.; Koyano, K.; Kukita, T. Tunneling nanotube formation is essential for the regulation of osteoclastogenesis. J. Cell. Biochem. 2013, 114, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Mensah, K.A.; Ritchlin, C.T.; Schwarz, E.M. RANKL induces heterogeneous DC-STAMP(lo) and DC-STAMP(hi) osteoclast precursors of which the DC-STAMP(lo) precursors are the master fusogens. J. Cell. Physiol. 2010, 223, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wisitrasameewong, W.; Kajiya, M.; Movila, A.; Rittling, S.; Ishii, T.; Suzuki, M.; Matsuda, S.; Mazda, Y.; Torruella, M.R.; Azuma, M.M.; et al. DC-STAMP Is an Osteoclast Fusogen Engaged in Periodontal Bone Resorption. J. Dent. Res. 2017, 96, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Witwicka, H.; Hwang, S.Y.; Reyes-Gutierrez, P.; Jia, H.; Odgren, P.E.; Donahue, L.R.; Birnbaum, M.J.; Odgren, P.R. Studies of OC-STAMP in Osteoclast Fusion: A New Knockout Mouse Model, Rescue of Cell Fusion, and Transmembrane Topology. PLoS ONE 2015, 10, e0128275. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lau, L.F. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 2009, 41, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Emura, K.; Kubota, S.; Lyons, K.M.; Takigawa, M. CCN family 2/connective tissue growth factor (CCN2/CTGF) promotes osteoclastogenesis via induction of and interaction with dendritic cell-specific transmembrane protein (DC-STAMP). J. Bone Miner. Res. 2011, 26, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. New immune connections in osteoclast formation. Ann. N. Y. Acad. Sci. 2010, 1192, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Collin-Osdoby, P.; Osdoby, P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol. Biol. 2012, 816, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Sambandam, Y.; Blanchard, J.J.; Daughtridge, G.; Kolb, R.J.; Shanmugarajan, S.; Pandruvada, S.N.; Bateman, T.A.; Reddy, S.V. Microarray profile of gene expression during osteoclast differentiation in modelled microgravity. J. Cell. Biochem. 2010, 111, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Pellis, N.R.; Goodwin, T.J.; Risin, D.; McIntyre, B.W.; Pizzini, R.P.; Cooper, D.; Baker, T.L.; Spaulding, G.F. Changes in gravity inhibit lymphocyte locomotion through type I collagen. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, G. Bone Cell Biology in Microgravity. 1992. Avaliable online: https://lirias.kuleuven.be/handle/123456789/216494 (accessed on 7 October 2017).
- Saxena, R.; Pan, G.; Dohm, E.D.; McDonald, J.M. Modeled microgravity and hindlimb unloading sensitize osteoclast precursors to RANKL-mediated osteoclastogenesis. J. Bone Miner. Metab. 2011, 29, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.A.; Bandstra, E.R.; Willey, J.S.; Riffle, S.E.; Tirado-Lee, L.; Nelson, G.A.; Pecaut, M.J.; Bateman, T.A. Effect of proton irradiation followed by hindlimb unloading on bone in mature mice: A model of long-duration spaceflight. Bone 2012, 51, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Iwaniec, U.T.; Wong, C.P.; Lindenmaier, L.B.; Wagner, L.A.; Branscum, A.J.; Menn, S.A.; Taylor, J.; Zhang, Y.; Wu, H.; et al. Acute exposure to high dose gamma-radiation results in transient activation of bone lining cells. Bone 2013, 57, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, K.; Globus, R.K.; Mojarrab, R.; Arakaki, J.; Wang, A.; Searby, N.D.; Almeida, E.A.; Limoli, C.L. Short-term effects of whole-body exposure to (56)fe ions in combination with musculoskeletal disuse on bone cells. Radiat. Res. 2010, 173, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.S.; Livingston, E.W.; Robbins, M.E.; Bourland, J.D.; Tirado-Lee, L.; Smith-Sielicki, H.; Bateman, T.A. Risedronate prevents early radiation-induced osteoporosis in mice at multiple skeletal locations. Bone 2010, 46, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Searby, N.D.; Mojarrab, R.; Phillips, J.; Alwood, J.; Yumoto, K.; Almeida, E.A.; Limoli, C.L.; Globus, R.K. Total-body irradiation of postpubertal mice with (137)Cs acutely compromises the microarchitecture of cancellous bone and increases osteoclasts. Radiat. Res. 2009, 171, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Wu, A.; Nie, J.; Pei, H.; Hu, W.; Wang, B.; Shang, P.; Li, B.; Zhou, G. Differences in responses to X-ray exposure between osteoclast and osteoblast cells. J. Radiat. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, H.; Zhang, X.D.; Liu, Z.; Fan, F.Y.; Sun, Y.M. Effect of radiation on the expression of osteoclast marker genes in RAW264.7 cells. Mol. Med. Rep. 2012, 5, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Ellman, R.; Spatz, J.; Cloutier, A.; Palme, R.; Christiansen, B.A.; Bouxsein, M.L. Partial reductions in mechanical loading yield proportional changes in bone density, bone architecture, and muscle mass. J. Bone Miner. Res. 2013, 28, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.M.; Lima, F.; Macias, B.R.; Allen, M.R.; Greene, E.S.; Shirazi-Fard, Y.; Kupke, J.S.; Hogan, H.A.; Bloomfield, S.A. Partial weight bearing does not prevent musculoskeletal losses associated with disuse. Med. Sci. Sports Exerc. 2013, 45, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, Y.; Kidane, Y.; Feiveson, A.; Stodieck, L.; Karouia, F.; Ramesh, G.; Rohde, L.; Wu, H. Cellular responses and gene expression profile changes due to bleomycin-induced DNA damage in human fibroblasts in space. PLoS ONE 2017, 12, e0170358. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Behnke, B.J.; Stabley, J.N.; Kilar, C.R.; Park, Y.; Narayanan, A.; Alwood, J.S.; Shirazi-Fard, Y.; Schreurs, A.S.; Globus, R.K.; et al. Effects of High-LET Radiation Exposure and Hindlimb Unloading on Skeletal Muscle Resistance Artery Vasomotor Properties and Cancellous Bone Microarchitecture in Mice. Radiat. Res. 2016, 185, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.R.; Speacht, T.L.; Zhang, Y.; Lang, C.H.; Donahue, H.J. Simulated space radiation sensitizes bone but not muscle to the catabolic effects of mechanical unloading. PLoS ONE 2017, 12, e0182403. [Google Scholar] [CrossRef] [PubMed]
- Makihira, S.; Kawahara, Y.; Yuge, L.; Mine, Y.; Nikawa, H. Impact of the microgravity environment in a 3-dimensional clinostat on osteoblast- and osteoclast-like cells. Cell Biol. Int. 2008, 32, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, B.; Chambers, T.J.; Fuller, K. Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J. Cell. Biochem. 2006, 98, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, N.; Suda, T.; Miura, Y.; Nakauchi, H.; Kodama, H.; Hiura, K.; Hakeda, Y.; Kumegawa, M. Generation of osteoclasts from isolated hematopoietic progenitor cells. Blood 1989, 74, 1295–1302. [Google Scholar] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugarajan, S.; Zhang, Y.; Moreno-Villanueva, M.; Clanton, R.; Rohde, L.H.; Ramesh, G.T.; Sibonga, J.D.; Wu, H. Combined Effects of Simulated Microgravity and Radiation Exposure on Osteoclast Cell Fusion. Int. J. Mol. Sci. 2017, 18, 2443. https://doi.org/10.3390/ijms18112443
Shanmugarajan S, Zhang Y, Moreno-Villanueva M, Clanton R, Rohde LH, Ramesh GT, Sibonga JD, Wu H. Combined Effects of Simulated Microgravity and Radiation Exposure on Osteoclast Cell Fusion. International Journal of Molecular Sciences. 2017; 18(11):2443. https://doi.org/10.3390/ijms18112443
Chicago/Turabian StyleShanmugarajan, Srinivasan, Ye Zhang, Maria Moreno-Villanueva, Ryan Clanton, Larry H. Rohde, Govindarajan T. Ramesh, Jean D. Sibonga, and Honglu Wu. 2017. "Combined Effects of Simulated Microgravity and Radiation Exposure on Osteoclast Cell Fusion" International Journal of Molecular Sciences 18, no. 11: 2443. https://doi.org/10.3390/ijms18112443
APA StyleShanmugarajan, S., Zhang, Y., Moreno-Villanueva, M., Clanton, R., Rohde, L. H., Ramesh, G. T., Sibonga, J. D., & Wu, H. (2017). Combined Effects of Simulated Microgravity and Radiation Exposure on Osteoclast Cell Fusion. International Journal of Molecular Sciences, 18(11), 2443. https://doi.org/10.3390/ijms18112443




