Clinical Usefulness of the Platelet-to Lymphocyte Ratio in Patients with Angiosarcoma of the Face and Scalp
Abstract
:1. Introduction
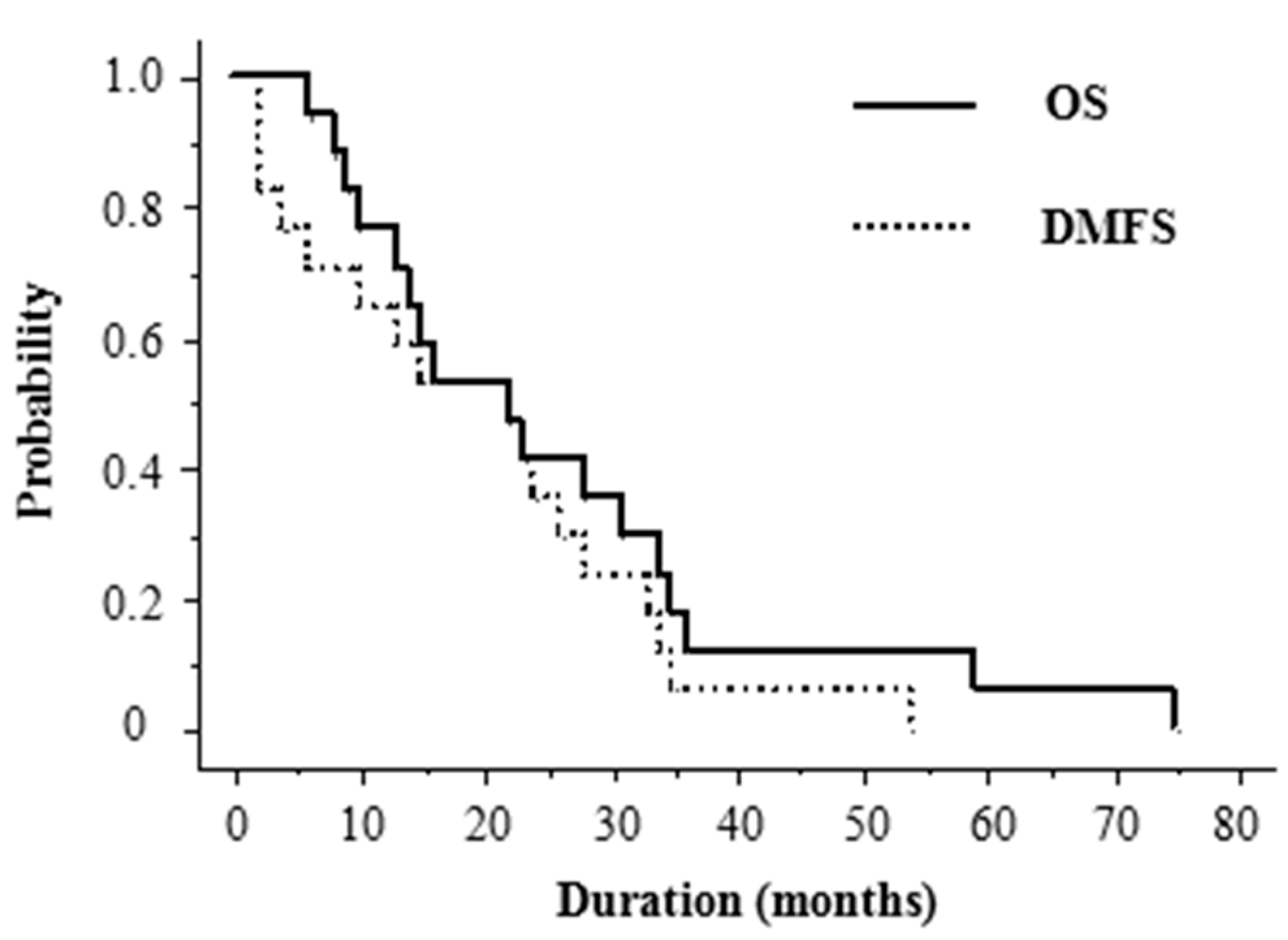
2. Results
2.1. Basic Characteristics of the Study Sample
2.2. Patient Characteristics
2.3. Optimal Cut-Off Values for NLR, PLR, and LMR
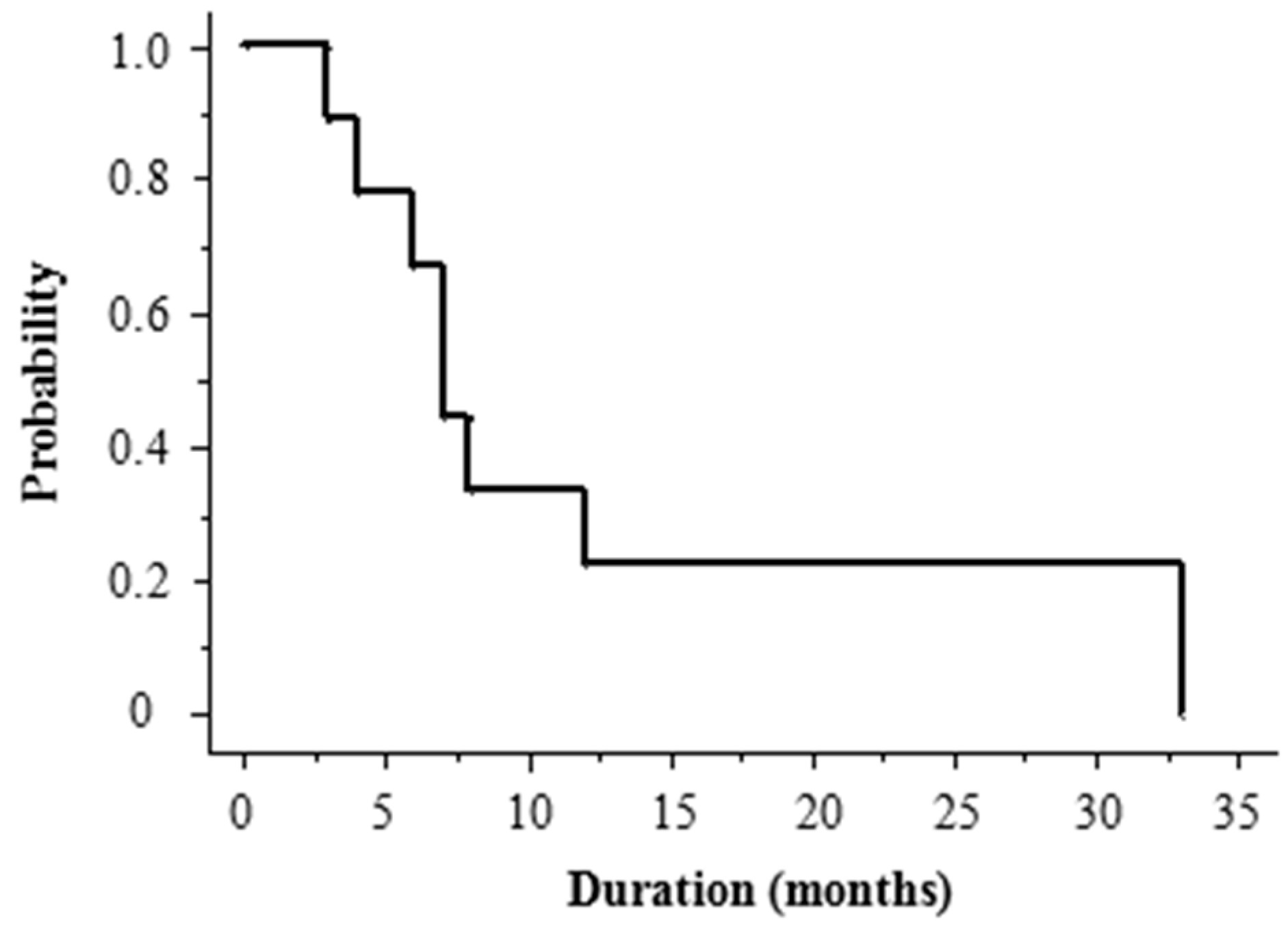
2.4. Recurrence Patterns
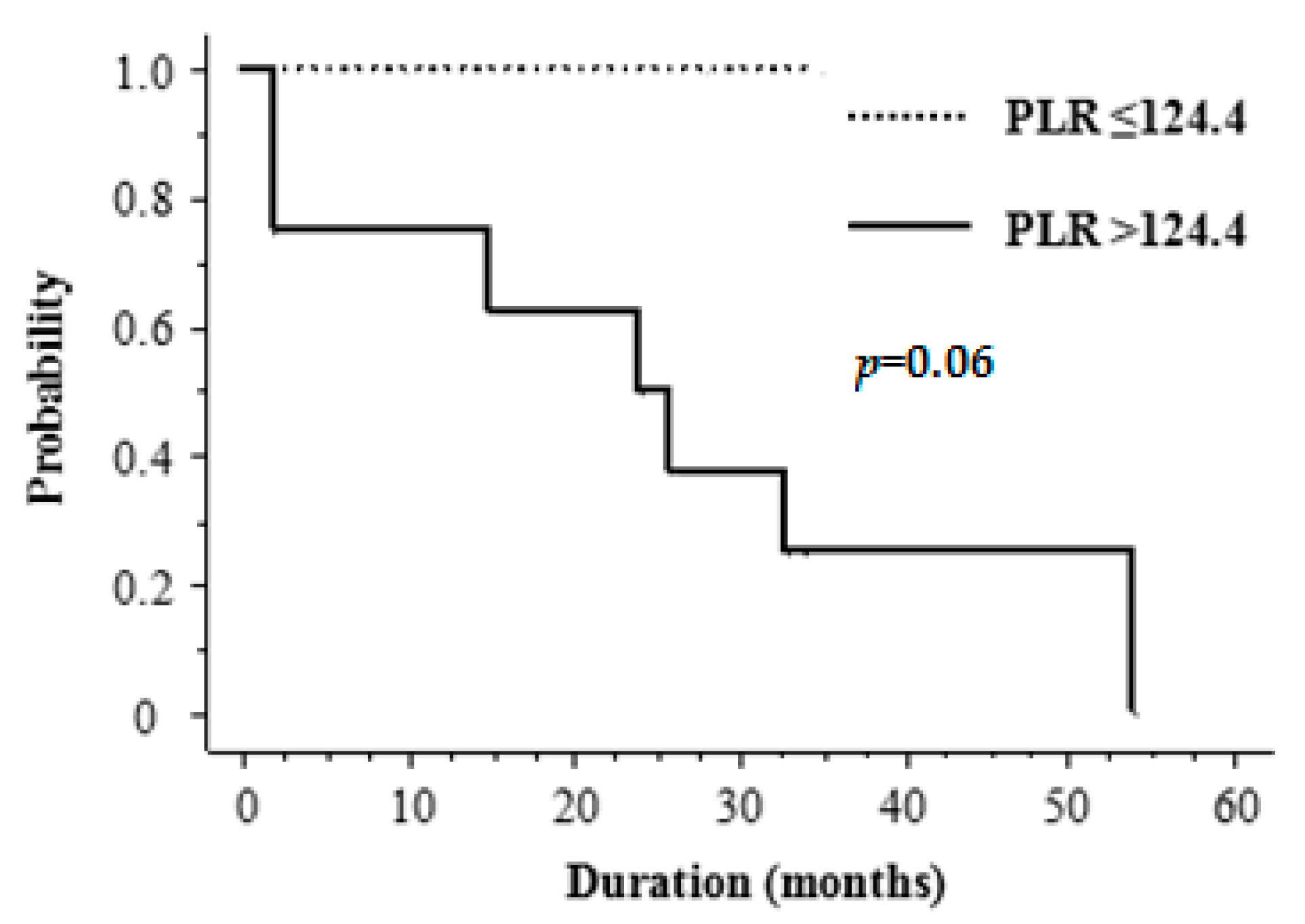
2.5. Treatment Outcome
3. Discussion
4. Patients and Methods
4.1. Study Design and Population
4.2. Treatment
4.3. Hematologic Parameters Measured
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ASFS | angiosarcoma of the face and scalp |
| NLR | neutrophil-to-lymphocyte ratio |
| PLR | platelet-to-lymphocyte ratio |
| LMR | lymphocyte-to-monocyte ratio |
| ASFS | angiosarcoma of the face and scalp |
| DMFS | distant metastasis-free survival |
| ROC | receiver operating curve |
| AUC | area under the curve |
| OS | overall survival |
| PET | positron emission tomography |
| CT | computed tomography |
References
- Fury, M.G.; Antonescu, C.R.; Van Zee, K.J.; Brennan, M.F.; Maki, R.G. A 14-year retrospective review of angiosarcoma: Clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005, 11, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, D.J.; Soule, E.H.; Woods, J.E. Cutaneous angiosarcomas of the head and neck. Cancer 1979, 44, 1106–1113. [Google Scholar] [CrossRef]
- Shin, J.Y.; Roh, S.G.; Lee, N.H.; Yang, K.M. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: Systematic review and meta-analysis. Head Neck 2017, 39, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Paulino, A.F.; McGinn, C.J.; Baker, L.H.; Cohen, D.S.; Morris, J.S.; Rees, R.; Sondak, V.K. Cutaneous angiosarcoma of the scalp: A multidisciplinary approach. Cancer 2003, 98, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Soejima, T.; Kishi, K.; Imajo, Y.; Hirota, S.; Kamikonya, N.; Murakami, M.; Kawabe, T.; Ejima, Y.; Matsumoto, A.; et al. Angiosarcoma treated with radiotherapy: Impact of tumor type and size on outcome. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1032–1040. [Google Scholar] [CrossRef]
- Abraham, J.A.; Hornicek, F.J.; Kaufman, A.M.; Harmon, D.C.; Springfield, D.S.; Raskin, K.A.; Mankin, H.J.; Kirsch, D.G.; Rosenberg, A.E.; Nielsen, G.P.; et al. Treatment and outcome of 82 patients with angiosarcoma. Ann. Surg. Oncol. 2007, 14, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- Kotilingam, D.; Lev, D.C.; Lazar, A.J.F.; Pollock, R.E. Staging soft tissue sarcoma: Evolution and change. CA Cancer J. Clin. 2006, 56, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Yamazaki, H.; Takenaka, H.; Aibe, N.; Masui, K.; Kimoto, T.; Tatekawa, K.; Nakashima, A.; Takenaka, T.; Asai, J.; et al. Definitive radiation therapy for angiosarcoma of the face and scalp. Vivo 2016, 30, 921–926. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Yoshino, K.; Kadono, T.; Miyagawa, T.; Nakamura, Y.; Fujimoto, M. Chemoradiotherapy with taxane is superior to conventional surgery and radiotherapy in the management of cutaneous angiosarcoma: A multicentre, retrospective study. Br. J. Dermatol. 2014, 171, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Uchi, H.; Nakahara, T.; Tsuji, G.; Oda, Y.; Hagihara, A.; Furue, M. Cutaneous angiosarcoma of the head and face: A single-center analysis of treatment outcomes in 43 patients in Japan. J. Cancer Res. Clin. Oncol. 2016, 142, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Nakaya, Y.; Saito, K.; Miyoshi, H.; Kishi, F.; Hibino, S.; Saijyo, T.; Ito, S.; Nakagawa, K.; Nakanishi, H.; et al. Hemopneumothorax secondary to multiple cavitary metastasis in angiosarcoma of the scalp. Respiration 1994, 61, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Tsuta, K.; Ando, M.; Hirakawa, A.; Hatanaka, Y.; Matsuno, Y.; Chuman, H.; Yamazaki, N.; Fujiwara, Y.; Hasegawa, T. Contrasting prognostic implications of platelet-derived growth factor receptor-β and vascular endothelial growth factor receptor-2 in patients with angiosarcoma. Ann. Surg. Oncol. 2011, 18, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.D.; McMillan, D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010, 6, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Tamura, K.; Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015, 41, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Eruga, B.; Ocana, A.; Tannock, I.F.; Amir, E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Kano, S.; Homma, A.; Hatakeyama, H.; Mizumachi, T.; Sakashita, T.; Kakizaki, T.; Fukuda, S. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck 2017, 39, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Piciucchi, M.; Stigliano, S.; Archibugi, L.; Zerboni, G.; Signoretti, M.; Barucca, V.; Valente, R.; Fave, G.D.; Capurso, G. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. Int. J. Mol. Sci. 2017, 18, 730. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Kim, H.S.; Han, I. ElevatedpPreoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann. Surg. Oncol. 2014, 21, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Qiu, H.; Li, Y.; Chen, Y.; Xiao, W.; Zhou, Z.; Zhang, X. Preoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC Cancer 2015, 15, 648. [Google Scholar] [CrossRef] [PubMed]
- Szkandera, J.; Gerger, A.; Liegl-Atzwanger, B.; Absenger, G.; Stotz, M.; Friesenbichler, J.; Trajanoski, S.; Stojakovic, T.; Eberhard, K.; Leithner, A.; et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int. J. Cancer 2014, 135, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Review hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Mantovani, A.; Allavena, P.; Allavena, P.; Sica, A.; Sica, A.; Balkwill, F.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2011, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Scapini, P.; Pizzolo, G.; Cassatella, M.A. On the cytokines produced by human neutrophils in tumors. Semin. Cancer Biol. 2013, 23, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Z.M.; Going, J.J.; Edwards, J.; Elsberger, B.; Doughty, J.C.; McMillan, D.C. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br. J. Cancer 2012, 107, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, H.; Cohen, R.; Bruderman, I.; Steiner, Z.; Klajman, A. Functional analysis of mononuclear cells infiltrating into tumors: Lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res. 1987, 47, 173–177. [Google Scholar] [PubMed]
- Wagner, D.D. New links between inflammation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupaimoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Harris, J.; Ware, J. Platelets: Linking hemostasis and cancer. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Hafner, M.; Echtenacher, B.; Männel, D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999, 59, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Buergy, D.; Wenz, F.; Groden, C.; Brockmann, M.A. Tumor-platelet interaction in solid tumors. Int. J. Cancer 2012, 130, 2747–2760. [Google Scholar] [CrossRef] [PubMed]
- Bain, B.; Seed, M.; Godsland, I. Normal values for peripheral blood white cell counts in women of four different ethnic origins. J. Clin. Pathol. 1984, 37, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Asmane, I.; Litique, V.; Heymann, S.; Marcellin, L.; Métivier, A.C.; Duclos, B.; Bergerat, J.P.; Kurtz, J.E. Adriamycin, cisplatin, ifosfamide and paclitaxel combination as front-line chemotherapy for locally advanced and metastatic angiosarcoma. Analysis of three case reports and review of the literature. Anticancer Res. 2008, 28, 3041–3045. [Google Scholar] [PubMed]
- Skubitz, K.M.; Haddad, P.A. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer 2005, 104, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
| Blood Components | Patients Group (n = 17) | Control Group (n = 56) | p Value |
|---|---|---|---|
| Total white blood cells (×109/L) | 6.22 ± 1.22 | 5.64 ± 1.38 | 0.21 |
| Absolute neutrophil count (×109/L) | 3.90 ± 1.06 | 3.16 ± 0.95 | 0.013 |
| Absolute lymphocyte count (×109/L) | 1.58 ± 0.36 | 1.77 ± 0.55 | 0.20 |
| Absolute monocyte count (×109/L) | 0.45 ± 0.14 | 0.47 ± 0.16 | 0.32 |
| Total platelets (×109/L) | 201.38 ± 42.72 | 205.32 ± 39.55 | 0.49 |
| NLR | 2.63 ± 0.94 | 1.93 ± 0.81 | 0.0063 |
| PLR | 131.91 ± 36.62 | 125.33 ± 44.77 | 0.59 |
| LMR | 3.83 ± 0.99 | 3.81 ± 1.72 | 0.96 |
| Variable | Number of Patients | DMFS | |
|---|---|---|---|
| 2-Year Rate | p Value | ||
| (%) | |||
| Age (years) | |||
| <78 | 9 | 44 | |
| ≥78 | 8 | 25 | 0.2 |
| Sex | |||
| Male | 11 | 36 | |
| Female | 6 | 33 | 0.2 |
| Tumor size (cm) | |||
| <10 cm | 13 | 46 | |
| ≥10 cm | 4 | 0 | 0.03 |
| Number of tumors | |||
| Solitary | 6 | 20 | |
| Multiple | 11 | 42 | 0.1 |
| PLR | |||
| ≤124.4 | 8 | 25 | |
| >124.4 | 9 | 44 | 0.5 |
| NLR | |||
| ≤2.05 | 11 | 57 | |
| >2.05 | 6 | 33 | 0.8 |
| LMR | |||
| ≤3.24 | 4 | 40 | |
| >3.24 | 13 | 33 | 0.9 |
| Variable | Number of Patients | DMFS | |
|---|---|---|---|
| 2-Year Rate | p Value | ||
| (%) | |||
| PLR | |||
| ≤124.4 | 5 | 100 | |
| >124.4 | 8 | 50 | 0.06 |
| NLR | |||
| ≤2.05 | 5 | 80 | |
| >2.05 | 8 | 55 | 0.7 |
| LMR | |||
| ≤3.24 | 4 | 75 | |
| >3.24 | 9 | 61 | 0.4 |
| Patient | Age (Year) | Gender | Tumor Size (cm) | Sites of Metastases | PLR | NLR | LMR |
|---|---|---|---|---|---|---|---|
| 1 | 76 | male | 1 | Lung and Liver | 157.28 | 2.51 | 4.89 |
| 2 | 57 | male | 1 | Lung | 228.70 | 2.06 | 4.30 |
| 3 | 78 | male | 1.5 | - | 142.00 | 3.21 | 2.33 |
| 4 | 76 | male | 2.3 | - | 118.03 | 3.14 | 7.06 |
| 5 | 67 | male | 2.8 | - | 100.23 | 1.71 | 3.01 |
| 6 | 84 | female | 3 | Lung | 151.74 | 3.33 | 2.59 |
| 7 | 73 | female | 3 | Lung | 142.38 | 4.02 | 5.40 |
| 8 | 80 | female | 3.5 | - | 182.37 | 5.11 | 3.52 |
| 9 | 74 | male | 4 | Lung | 124.55 | 1.66 | 3.11 |
| 10 | 78 | male | 5.5 | - | 83.83 | 1.36 | 3.59 |
| 11 | 82 | male | 5.5 | - | 79.66 | 1.57 | 2.19 |
| 12 | 76 | female | 7 | Lung | 185.48 | 1.78 | 3.22 |
| 13 | 75 | male | 8 | - | 105.63 | 2.26 | 4.53 |
| 14 | 78 | male | 10 | - | 106.24 | 2.67 | 4.56 |
| 15 | 83 | female | 10 | Lung | 176.63 | 4.88 | 3.08 |
| 16 | 74 | female | 11 | Bone | 55.20 | 1.63 | 4.47 |
| 17 | 79 | male | 12 | Lung | 102.53 | 1.75 | 3.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, G.; Yamazaki, H.; Aibe, N.; Masui, K.; Sasaki, N.; Shimizu, D.; Kimoto, T.; Asai, J.; Wada, M.; Komori, S.; et al. Clinical Usefulness of the Platelet-to Lymphocyte Ratio in Patients with Angiosarcoma of the Face and Scalp. Int. J. Mol. Sci. 2017, 18, 2402. https://doi.org/10.3390/ijms18112402
Suzuki G, Yamazaki H, Aibe N, Masui K, Sasaki N, Shimizu D, Kimoto T, Asai J, Wada M, Komori S, et al. Clinical Usefulness of the Platelet-to Lymphocyte Ratio in Patients with Angiosarcoma of the Face and Scalp. International Journal of Molecular Sciences. 2017; 18(11):2402. https://doi.org/10.3390/ijms18112402
Chicago/Turabian StyleSuzuki, Gen, Hideya Yamazaki, Norihiro Aibe, Koji Masui, Naomi Sasaki, Daisuke Shimizu, Takuya Kimoto, Jun Asai, Makoto Wada, Satoshi Komori, and et al. 2017. "Clinical Usefulness of the Platelet-to Lymphocyte Ratio in Patients with Angiosarcoma of the Face and Scalp" International Journal of Molecular Sciences 18, no. 11: 2402. https://doi.org/10.3390/ijms18112402
APA StyleSuzuki, G., Yamazaki, H., Aibe, N., Masui, K., Sasaki, N., Shimizu, D., Kimoto, T., Asai, J., Wada, M., Komori, S., Katoh, N., & Yamada, K. (2017). Clinical Usefulness of the Platelet-to Lymphocyte Ratio in Patients with Angiosarcoma of the Face and Scalp. International Journal of Molecular Sciences, 18(11), 2402. https://doi.org/10.3390/ijms18112402






