A Multi-Locus Genetic Risk Score for Primary Open-Angle Glaucoma (POAG) Variants Is Associated with POAG Risk in a Mediterranean Population: Inverse Correlations with Plasma Vitamin C and E Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Vitamins C and E in Plasma
2.2. Analysis of Genetic Polymorphisms
2.3. Statistical Analyses
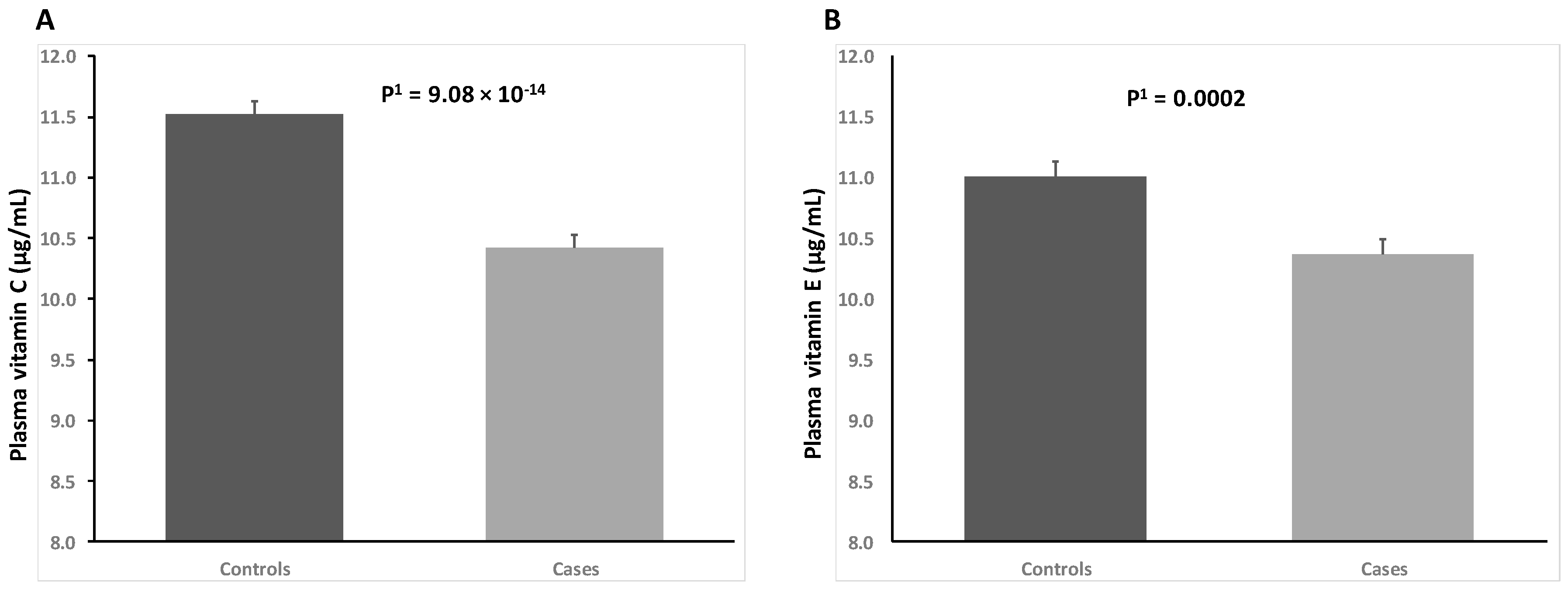
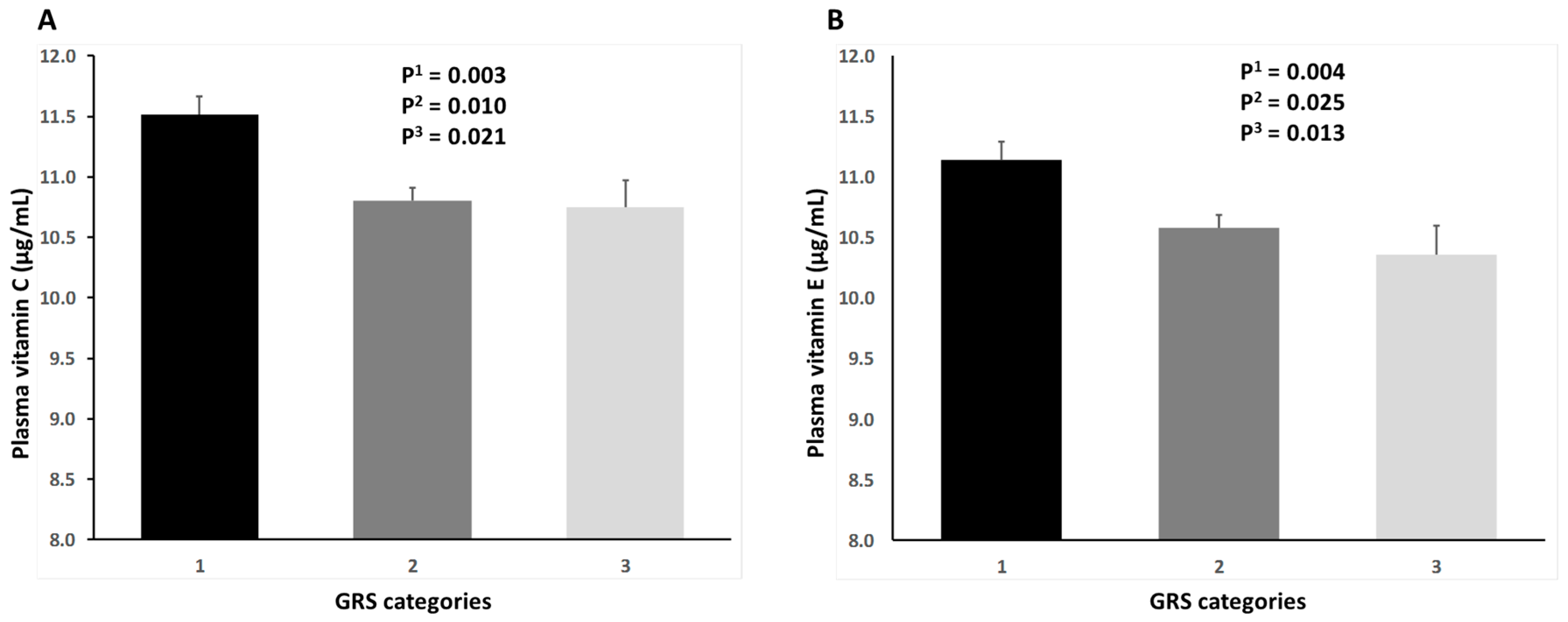
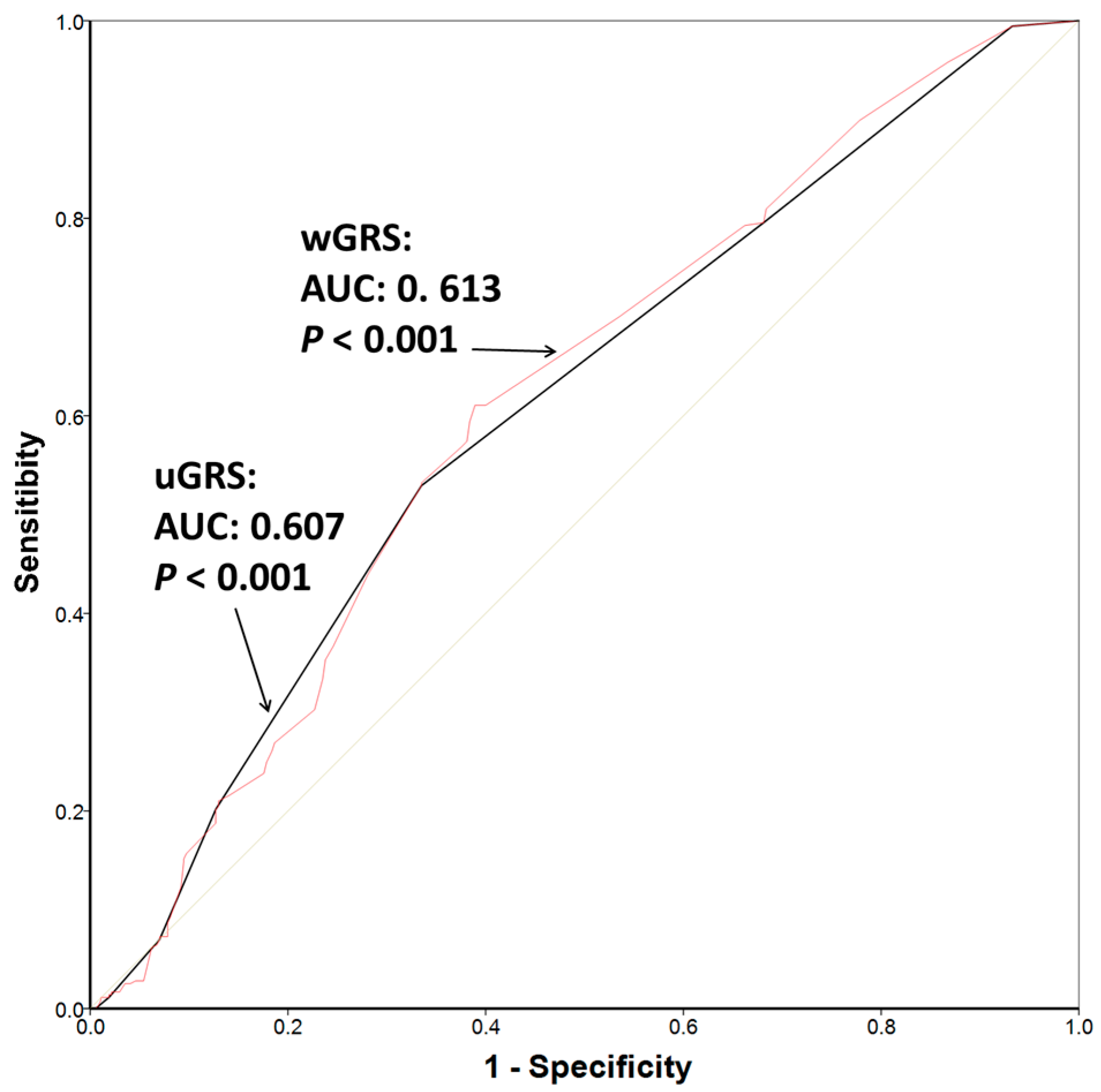
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| POAG | primary open-angle glaucoma |
| BMI | body mass index |
| IOP | intraocular pressure |
| GWAS | genome-wide association study |
| GRS | genetic risk score |
| uGRS | unweighted genetic risk score |
| wGRS | weighted genetic risk score |
References
- Weinreb, R.N.; Leung, C.K.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Prim. 2016, 2, 16067. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017. [Google Scholar] [CrossRef]
- Renard, J.P.; Rouland, J.F.; Bron, A.; Sellem, E.; Nordmann, J.P.; Baudouin, C.; Denis, P.; Villain, M.; Chaine, G.; Colin, J.; et al. Nutritional, lifestyle and environmental factors in ocular hypertension and primary open-angle glaucoma: An exploratory case-control study. Acta Ophthalmol. 2013, 91, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Vaghefi, E.; Braakhuis, A.J. Food components and ocular pathophysiology: A critical appraisal of the role of oxidative mechanisms. Asia Pac. J. Clin. Nutr. 2017, 26, 572–585. [Google Scholar] [PubMed]
- Abu-Amero, K.; Kondkar, A.A.; Chalam, K.V. An Updated Review on the Genetics of Primary Open Angle Glaucoma. Int. J. Mol. Sci. 2015, 16, 28886–28911. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Yin, Y.; Fan, N.; Cheng, H.B.; Li, X.H.; Wang, Y.; Yu, W.H.; Cai, S.P.; Liu, X.Y. Single nucleotide polymorphism of MYOC affected the severity of primary open angle glaucoma. Int. J. Ophthalmol. 2013, 6, 264–268. [Google Scholar] [PubMed]
- Markiewicz, L.; Majsterek, I.; Przybylowska, K.; Dziki, L.; Waszczyk, M.; Gacek, M.; Kaminska, A.; Szaflik, J.; Szaflik, J.P. Gene polymorphisms of the MMP1, MMP9, MMP12, IL-1β and TIMP1 and the risk of primary open-angle glaucoma. Acta Ophthalmol. 2013, 91, e516–e523. [Google Scholar] [CrossRef] [PubMed]
- Szaflik, J.P.; Cuchra, M.; Przybylowska-Sygut, K.; Dziki, L.; Kurowska, A.K.; Gacek, M.; Drzewoski, J.; Szaflik, J.; Majsterek, I. Association of the 399 Arg/Gln XRCC1, the 194 Arg/Trp XRCC1, the 326 Ser/Cys OGG1, and the 324 Gln/His MUTYH gene polymorphisms with clinical parameters and the risk for development of primary open-angle glaucoma. Mutat. Res. 2013, 753, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Burdon, K.P.; Chidlow, G.; Klebe, S.; Crawford, A.; Dimasi, D.P.; Dave, A.; Martin, S.; Javadiyan, S.; Wood, J.P.; et al. Association of genetic variants in the TMCO1 gene with clinical parameters related to glaucoma and characterization of the protein in the eye. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Burdon, K.P.; Macgregor, S.; Hewitt, A.W.; Sharma, S.; Chidlow, G.; Mills, R.A.; Danoy, P.; Casson, R.; Viswanathan, A.C.; Liu, J.Z.; et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat. Genet. 2011, 43, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Puffenberger, E.G.; Turben, S.; Tan, H.; Zhou, A.; Wang, H. Homozygous frameshift mutation in TMCO1 causes a syndrome with craniofacial dysmorphism, skeletal anomalies, and mental retardation. Proc. Natl. Acad. Sci. USA 2010, 107, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Thorleifsson, G.; Walters, G.B.; Hewitt, A.W.; Masson, G.; Helgason, A.; De Wan, A.; Sigurdsson, A.; Jonasdottir, A.; Gudjonsson, S.A.; Magnusson, K.P.; et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat. Genet. 2010, 42, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Berta, A.I.; Kiss, A.L.; Kemeny-Beke, A.; Lukats, A.; Szabó, A.; Szél, A. Different caveolin isoforms in the retina of melanoma malignum affected human eye. Mol. Vis. 2007, 13, 881–886. [Google Scholar] [PubMed]
- Surgucheva, I.; Surguchov, A. Expression of caveolin in trabecular meshwork cells and its possible implication in pathogenesis of primary open angle glaucoma. Mol. Vis. 2011, 17, 2878–2888. [Google Scholar] [PubMed]
- Zhao, J.; Zhou, H.; Sun, L.; Yang, B.; Zhang, L.; Shi, H.; Zheng, Y. Selection of suitable reference genes for quantitative real-time PCR in trabecular meshwork cells under oxidative stress. Free Radic. Res. 2017, 51, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Fuchshofer, R.; Kampik, A.; Welge-Lüssen, U. Effects of oxidative stress in trabecular meshwork cells are reduced by prostaglandin analogues. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: Challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundam. Clin. Pharmacol. 2012, 26, 86–117. [Google Scholar] [PubMed]
- Chen, Z.; Bakhshi, F.R.; Shajahan, A.N.; Sharma, T.; Mao, M.; Trane, A.; Bernatchez, P.; van Nieuw Amerongen, G.P.; Bonini, M.G.; Skidgel, R.A.; et al. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol. Biol. Cell 2012, 23, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, H.; Zhou, J.S.; Cao, J.F.; Zhou, X.B.; Choi, A.M.; Chen, Z.H.; Shen, H.H. Caveolin-1 inhibits expression of antioxidant enzymes through direct interaction with nuclear erythroid 2 p45-related factor-2 (Nrf2). J. Biol. Chem. 2012, 287, 20922–20930. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, N.; Zheng, Y.; Zhang, J.; Zhang, F.; Wang, Z. Caveolin-1: An Oxidative Stress-Related Target for Cancer Prevention. Oxid. Med. Cell. Longev. 2017, 2017, 7454031. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, W.D.; van Koolwijk, L.M.; Lemij, H.G.; Pasutto, F.; Cree, A.J.; Thorleifsson, G.; Janssen, S.F.; Jacoline, T.B.; Amin, N.; Rivadeneira, F.; et al. Common genetic variants associated with open-angle glaucoma. Hum. Mol. Genet. 2011, 20, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, H.M.; Peden, J.F.; Lorkowski, S.; Goel, A.; Ongen, H.; Green, F.; Clarke, R.; Collins, R.; Franzosi, M.G.; Tognoni, G.; et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum. Mol. Genet. 2008, 17, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gius, D.; Onyango, P.; Muldoon-Jacobs, K.; Karp, J.; Feinberg, A.P.; Cui, H. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature 2008, 451, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaf, H.H.; Colak, D.; Al-Saif, M.; Al-Bakheet, A.; Hendrayani, S.F.; Al-Yousef, N.; Kaya, N.; Khabar, K.S.; Aboussekhra, A. p16 (INK4a) positively regulates cyclin D1 and E2F1 through negative control of AUF1. PLoS ONE 2011, 6, e21111. [Google Scholar] [CrossRef] [PubMed]
- Guney, S.; Bertrand, P.; Jardin, F.; Ruminy, P.; Kerckaert, J.P.; Tilly, H.; Bastard, C. Molecular characterization of 9p21 deletions shows a minimal common deleted region removing CDKN2A exon 1 and CDKN2B exon 2 in diffuse large B-cell lymphomas. Genes Chromosomes Cancer 2011, 50, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Willett, W.C.; Rosner, B.A.; Buys, E.; Wiggs, J.L.; Pasquale, L.R. Association of Dietary Nitrate Intake With Primary Open-Angle Glaucoma: A Prospective Analysis From the Nurses’ Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol. 2016, 134, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; Korobelnik, J.F.; Barberger-Gateau, P.; Delyfer, M.N.; Rougier, M.B.; Le Goff, M.; Malet, F.; Colin, J.; Dartigues, J.F. Nutrition and age-related eye diseases: The Alienor (Antioxydants, Lipides Essentiels, Nutrition et maladies OculaiRes) Study. J. Nutr. Health Aging 2010, 14, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Costabile, G.; Di Marino, L.; Rivellese, A.A. Nutrition and oxidative stress: A systematic review of human studies. Int. J. Food Sci. Nutr. 2013, 64, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, S.; Sakai, Y.; Hoseki, J. Screening of dietary antioxidants against mitocondria-mediated oxidative stress by visualization of intracellular redox state. Biosci. Biotechnol. Biochem. 2016, 80, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Zanon-Moreno, V.; Marco-Ventura, P.; Lleo-Perez, A.; Pons-Vazquez, S.; Garcia-Medina, J.J.; Vinuesa-Silva, I.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Oxidative stress in primary open-angle glaucoma. J. Glaucoma 2008, 17, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci. Rep. 2016, 6, 25792. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, W.D.; Wolfs, R.C.; Kiefte-de Jong, J.C.; Hofman, A.; de Jong, P.T.; Vingerling, J.R.; Jansonius, N.M. Nutrient intake and risk of open-angle glaucoma: The Rotterdam Study. Eur. J. Epidemiol. 2012, 27, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Zanon-Moreno, V.; Ciancotti-Olivares, L.; Asencio, J.; Sanz, P.; Ortega-Azorin, C.; Pinazo-Duran, M.D.; Corella, D. Association between a SLC23A2 gene variation, plasma vitamin C levels and risk of glaucoma in a Mediterranean population. Mol. Vis. 2011, 17, 2997–3004. [Google Scholar] [PubMed]
- Zanon-Moreno, V.; Asensio-Marquez, E.M.; Ciancotti-Oliver, L.; Garcia-Medina, J.J.; Sanz, P.; Ortega-Azorin, C.; Pinazo-Duran, M.D.; Ordovás, J.M.; Corella, D. Effects of polymorphisms in vitamin E-, vitamin C-, and glutathione peroxidase-related genes on serum biomarkers and associations with glaucoma. Mol. Vis. 2013, 19, 231–242. [Google Scholar] [PubMed]
- Li, X.; Franke, A.A. Fast HPLC–ECD analysis of ascorbic acid, dehydroascorbic acid and uric acid. J. Chromatogr. 2009, 877, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, J.; Fortis, I.; Blachier, S.; Kia, D.; Favier, A. Simultaneous determination of retinol, alpha-tocopherol and beta-carotene in serum by isocratic high-performance liquid chromatography. J. Chromatogr. 1991, 572, 103–116. [Google Scholar] [CrossRef]
- Liu, Y.; Allingham, R.R. Major review: Molecular genetics of primary open-angle glaucoma. Exp. Eye Res. 2017, 160, 62–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; He, W.; Zhao, J.; Zeng, Y.; Cheng, H. Association of WDR36 polymorphisms with primary open angle glaucoma: A systematic review and meta-analysis. Medicine 2017, 96, e7291. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Pasquale, L.R. Genetics of Glaucoma. Hum. Mol. Genet. 2017; in press. [Google Scholar]
- Rong, S.S.; Chen, L.J.; Leung, C.K.S.; Matsushita, K.; Jia, L.; Miki, A.; Chiang, S.W.; Tam, P.O.; Hashida, N.; Young, A.L.; et al. Ethnic specific association of the CAV1/CAV2 locus with primary open-angle glaucoma. Sci. Rep. 2016, 6, 27837. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Duran, M.D.; Zanón-Moreno, V.; García-Medina, J.J.; Gallego-Pinazo, R. Evaluation of presumptive biomarkers of oxidative stress, immune response and apoptosis in primary open-angle glaucoma. Curr. Opin. Pharmacol. 2013, 13, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zanon-Moreno, V.; Garcia-Medina, J.J.; Zanon-Viguer, V.; Moreno Nadal, M.A.; Pinazo-Duran, M.D. Smoking, an additional risk factor in elder women with primary open-angle glaucoma. Mol. Vis. 2009, 15, 2953–2959. [Google Scholar] [PubMed]
- Pasquale, L.R. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr. Opin. Ophthalmol. 2016, 27, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Levin, L.A. Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annu. Rev. Vis. Sci. 2017, 3, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.; Izzotti, A. Oxidative stress and glaucoma: Injury in the anterior segment of the eye. Prog. Brain Res. 2008, 173, 385–407. [Google Scholar] [PubMed]
- Benoist d’Azy, C.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e016691. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.L.; Peng, P.H.; Hsu, S.Y.; Chen, C.F. Dietary deficiency of vitamin E aggravates retinal ganglion cell death in experimental glaucoma of rats. Curr. Eye Res. 2010, 35, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.M.; Fingert, J.H.; Alward, W.L.; Nguyen, T.D.; Polansky, J.R.; Sunden, S.L.; Nishimura, D.; Clark, A.F.; Nystuen, A.; Nichols, B.E.; et al. Identification of a gene that causes primary open angle glaucoma. Science 1997, 275, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Nickells, R.W. The cell and molecular biology of glaucoma: Mechanisms of retinal ganglion cell death. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Yaspan, B.L.; Hauser, M.A.; Kang, J.H.; Allingham, R.R.; Olson, L.M.; Abdrabou, W.; Fan, B.J.; Wang, D.Y.; Brodeur, W.; et al. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 2012, 8, e1002654. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, L.R.; Loomis, S.J.; Kang, J.H.; Yaspan, B.L.; Abdrabou, W.; Budenz, D.L.; Chen, T.C.; Delbono, E.; Friedman, D.S.; Gaasterland, D.; et al. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. Am. J. Ophthalmol. 2013, 155, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hughes, G.; Chen, X.; Qian, S.; Cao, W.; Wang, L.; Wang, M.; Sun, X. Genetic Variants Associated With Different Risks for High Tension Glaucoma and Normal Tension Glaucoma in a Chinese Population. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; McClellan, M.E.; Tanito, M.; Garteiser, P.; Towner, R.; Bissig, D.; Berkowitz, B.A.; Fliesler, S.J.; Woodruff, M.L.; Fain, G.L.; et al. Loss of caveolin-1 impairs retinal function due to disturbance of subretinal microenvironment. J. Biol. Chem. 2012, 287, 16424–16434. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.H.; Wang, K.; Roos, B.; Stone, E.M.; Kwon, Y.H.; Alward, W.L.; Mullins, R.F.; Fingert, J.H. Chromosome 7q31 POAG locus: Ocular expression of caveolins and lack of association with POAG in a US cohort. Mol. Vis. 2011, 17, 430–435. [Google Scholar] [PubMed]
- Abu-Amero, K.K.; Kondkar, A.A.; Mousa, A.; Osman, E.A.; Al-Obeidan, S.A. Lack of association of SNP rs4236601 near CAV1 and CAV2 with POAG in a Saudi cohort. Mol. Vis. 2012, 18, 1960–1965. [Google Scholar] [PubMed]
- Huang, W.; Wang, W.; Zhou, M.; Zhang, X. Association of single-nucleotide polymorphism rs4236601 near caveolin 1 and 2 with primary open-angle glaucoma: A meta-analysis. Clin. Exp. Ophthalmol. 2014, 42, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.K.; Casson, R.J.; Burdon, K.P.; Craig, J.E. Chromosome 9p21 primary open-angle glaucoma susceptibility locus: A review. Clin. Exp. Ophthalmol. 2014, 42, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Osman, W.; Low, SK.; Takahashi, A.; Kubo, M.; Nakamura, Y. A genome-wide association study in the Japanese population confirms 9p21 and 14q23 as susceptibility loci for primary open angle glaucoma. Hum. Mol. Genet. 2012, 21, 2836–2842. [Google Scholar] [CrossRef] [PubMed]
- Vishal, M.; Sharma, A.; Kaurani, L.; Chakraborty, S.; Ray, J.; Sen, A.; Mukhopadhyay, A.; Ray, K. Evaluation of genetic association of the INK4 locus with primary open angle glaucoma in East Indian population. Sci. Rep. 2014, 4, 5115. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Liao, J.; Vithana, E.N.; Khor, C.C.; Teo, Y.Y.; Tai, E.S.; Wong, T.Y.; Aung, T.; Cheng, C.Y. International Glaucoma Genetics Consortium. Aggregate Effects of Intraocular Pressure and Cup-to-Disc Ratio Genetic Variants on Glaucoma in a Multiethnic Asian Population. Ophthalmology 2015, 122, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, F.; Mabuchi, N.; Sakurada, Y.; Yoneyama, S.; Kashiwagi, K.; Iijima, H.; Yamagata, Z.; Takamoto, M.; Aihara, M.; Iwata, T.; et al. Additive effects of genetic variants associated with intraocular pressure in primary open-angle glaucoma. PLoS ONE 2017, 12, e0183709. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.T.; Prata, T.S.; Kera, C.Z.; Miranda, D.V.; de Moraes Barros, S.B.; Melo, L.A., Jr. Ascorbic acid concentration is reduced in the secondary aqueous humour of glaucomatous patients. Clin. Exp. Ophthalmol. 2009, 37, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Hannou, S.A.; Wouters, K.; Paumelle, R.; Staels, B. Functional genomics of the CDKN2A/B locus in cardiovascular and metabolic disease: What have we learned from GWASs? Trends Endocrinol. Metab. 2015, 26, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Congrains, A.; Kamide, K.; Ohishi, M.; Rakugi, H. ANRIL: Molecular mechanisms and implications in human health. Int. J. Mol. Sci. 2013, 14, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Sharma, R.B.; Nwosu, B.U.; Alonso, L.C. Islet biology, the CDKN2A/B locus and type 2 diabetes risk. Diabetologia 2016, 59, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, J.; Li, Q. CDKN2A methylation in esophageal cancer: A meta-analysis. Oncotarget 2017, 8, 50071–50083. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Cortesão, E.; Oliveiros, B.; Alves, V.; Espadana, A.I.; Rito, L.; Magalhães, E.; Pereira, S.; Pereira, A.; Costa, J.M.; et al. Oxidative stress levels are correlated with P15 and P16 gene promoter methylation in myelodysplastic syndrome patients. Clin. Exp. Med. 2016, 16, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.C.; Zheng, Q.; Tan, H.; Zhang, B.; Li, X.; Yang, Y.; Yu, J.; Liu, Y.; Chai, H.; Wang, X.; et al. TMCO1 Is an ER Ca(2+) Load-Activated Ca(2+) Channel. Cell 2016, 165, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Wu, W.R.; Chan, T.C.; Wang, Y.H.; Chen, L.R.; Wu, W.J.; Yeh, B.W.; Liang, S.S.; Shiue, Y.L. Transmembrane and coiled-coil domain 1 impairs the AKT signaling pathway in urinary bladder urothelial carcinoma: A characterization of a tumor suppressor. Clin. Cancer Res. 2017; in press. [Google Scholar]
| Characteristic | Cases (n = 391) | Controls (n = 383) | p |
|---|---|---|---|
| Females (%) | 58.5 | 53.5 | 0.191 |
| Age (years) | 69.1 (9.0) | 67.7 (11.1) | 0.460 |
| BMI 3 (kg/m2) | 26.6 (3.8) | 27.0 (4.3) | 0.263 |
| Cup disk ratio | 0.69 (0.01) | 0.31 (0.01) | <0.001 |
| IOP 4 (mmHg) | 25.3 (3.6) | 16.8 (2.5) | <0.001 |
| Smokers (%) | 27.2 | 24.4 | 0.191 |
| Alcohol consumers (%) | 66.0 | 58.0 | 0.080 |
| Genes | SNPs | Alleles 1 | Genotype Frequencies (%) | Genotypes | OR 3 (95% CI) | OR 4 (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR 2 (95% CI) | ||||||||||||
| (1/2) | 1/1 | 1/2 | 2/2 | 1/1 | 1/2 | 2/2 | p-Trend | 1/1 (Ref) | 1/2 | 2/2 | ||||
| TMCO1 | rs4656461 | A/G | 52.4 | 37.2 | 10.3 | 62.9 | 31.0 | 6.1 | 0.002 | 1 | 1.44 (1.05–1.96) | 2.03 (1.17–3.52) | 1.43 (1.14–1.80) | 1.47 (1.16–1.86) |
| CAV1/CAV2 | rs4236601 | G/A | 39.7 | 43.1 | 17.2 | 45.3 | 43.9 | 10.8 | 0.018 | 1 | 1.12 (0.82–1.52) | 1.82 (1.16–2.85) | 1.28 (1.04–1.58) | 1.42 (1.14–1.76) |
| CDKN2B-AS1 | rs2157719 | A/G | 29.7 | 45.6 | 24.7 | 37.9 | 43.4 | 18.7 | 0.007 | 1 | 1.34 (0.97–1.85) | 1.69 (1.14–2.51) | 1.30 (1.07–1.58) | 1.30 (1.07–1.59) |
| CDKN2A | rs3088440 | G/A | 71.1 | 26.8 | 2.1 | 74.3 | 23.8 | 1.9 | 0.059 | 1 | 1.37 (0.99–1.89) | 1.41 (0.52–3.84) | 1.31 (0.99–1.75) | 1.56 (1.15–2.12) |
| Categories 1 uGRS | Cases: n (%) | Controls: n (%) | OR 2 (95% CI) | p 2 | OR 3 (95% CI) | p 3 |
|---|---|---|---|---|---|---|
| Low (0 or 1) | 73 (20.4) | 118 (31.9) | 1 (Ref) | 1 (Ref) | ||
| Medium (2 or 3) | 212 (59.4) | 205 (55.4) | 1.67 (1.18–2.37) | 0.004 | 1.73 (1.21–2.46) | 0.002 |
| High (4,5,6,7 or 8) | 72 (20.4) | 47 (12.7) | 2.48 (1.54–3.96) | <0.001 | 2.92 (1.79–4.77) | <0.001 |
| Models | Regression Coefficient (B) (μg/mL per Allele) | SE | p |
|---|---|---|---|
| Vitamin C (μg/mL) | |||
| Model 1 unadjusted 2 | −0.294 | 0.172 | <0.001 |
| Model 2 adjusted 3 | −0.204 | 0.062 | 0.001 |
| Model 3 adjusted 4 | −0.186 | 0.065 | 0.002 |
| Vitamin E (μg/mL) | |||
| Model 1 unadjusted 2 | −0.248 | 0.180 | <0.001 |
| Model 2 adjusted 3 | −0.205 | 0.067 | 0.002 |
| Model 3 adjusted 4 | −0.233 | 0.070 | 0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanon-Moreno, V.; Ortega-Azorin, C.; Asensio-Marquez, E.M.; Garcia-Medina, J.J.; Pinazo-Duran, M.D.; Coltell, O.; Ordovas, J.M.; Corella, D. A Multi-Locus Genetic Risk Score for Primary Open-Angle Glaucoma (POAG) Variants Is Associated with POAG Risk in a Mediterranean Population: Inverse Correlations with Plasma Vitamin C and E Concentrations. Int. J. Mol. Sci. 2017, 18, 2302. https://doi.org/10.3390/ijms18112302
Zanon-Moreno V, Ortega-Azorin C, Asensio-Marquez EM, Garcia-Medina JJ, Pinazo-Duran MD, Coltell O, Ordovas JM, Corella D. A Multi-Locus Genetic Risk Score for Primary Open-Angle Glaucoma (POAG) Variants Is Associated with POAG Risk in a Mediterranean Population: Inverse Correlations with Plasma Vitamin C and E Concentrations. International Journal of Molecular Sciences. 2017; 18(11):2302. https://doi.org/10.3390/ijms18112302
Chicago/Turabian StyleZanon-Moreno, Vicente, Carolina Ortega-Azorin, Eva M. Asensio-Marquez, Jose J. Garcia-Medina, Maria D. Pinazo-Duran, Oscar Coltell, Jose M. Ordovas, and Dolores Corella. 2017. "A Multi-Locus Genetic Risk Score for Primary Open-Angle Glaucoma (POAG) Variants Is Associated with POAG Risk in a Mediterranean Population: Inverse Correlations with Plasma Vitamin C and E Concentrations" International Journal of Molecular Sciences 18, no. 11: 2302. https://doi.org/10.3390/ijms18112302
APA StyleZanon-Moreno, V., Ortega-Azorin, C., Asensio-Marquez, E. M., Garcia-Medina, J. J., Pinazo-Duran, M. D., Coltell, O., Ordovas, J. M., & Corella, D. (2017). A Multi-Locus Genetic Risk Score for Primary Open-Angle Glaucoma (POAG) Variants Is Associated with POAG Risk in a Mediterranean Population: Inverse Correlations with Plasma Vitamin C and E Concentrations. International Journal of Molecular Sciences, 18(11), 2302. https://doi.org/10.3390/ijms18112302








