Time Response of Oxidative/Nitrosative Stress and Inflammation in LPS-Induced Endotoxaemia—A Comparative Study of Mice and Rats
Abstract
1. Introduction
2. Results
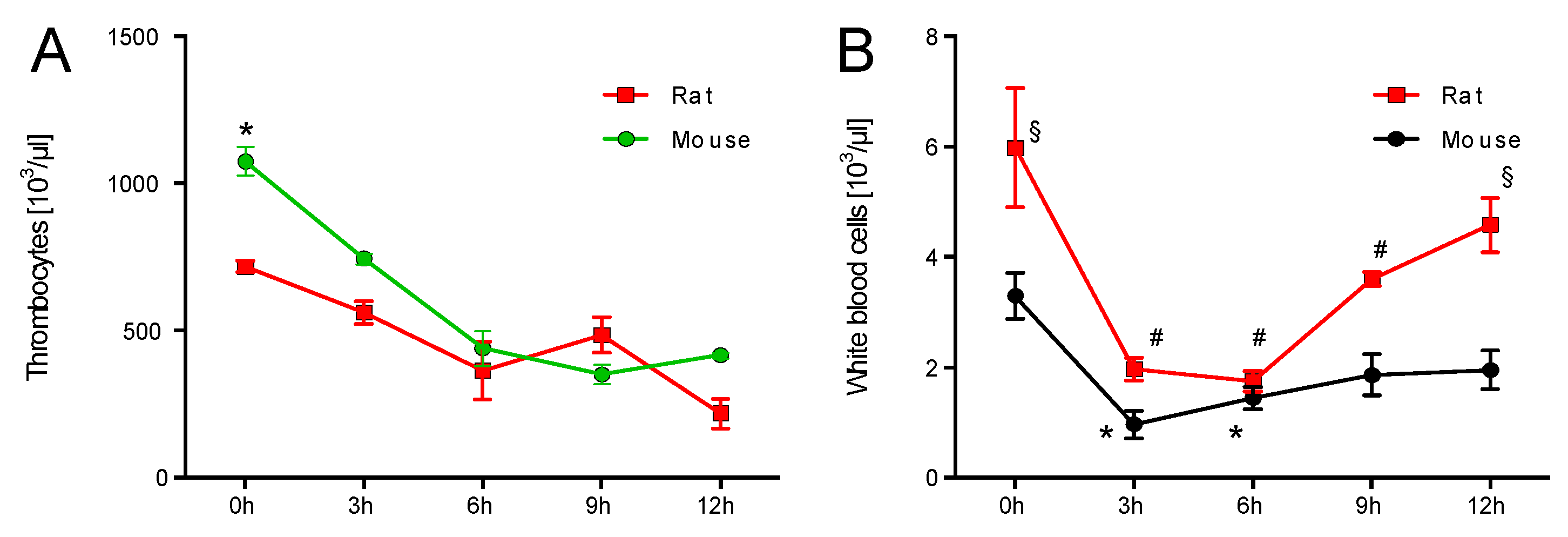
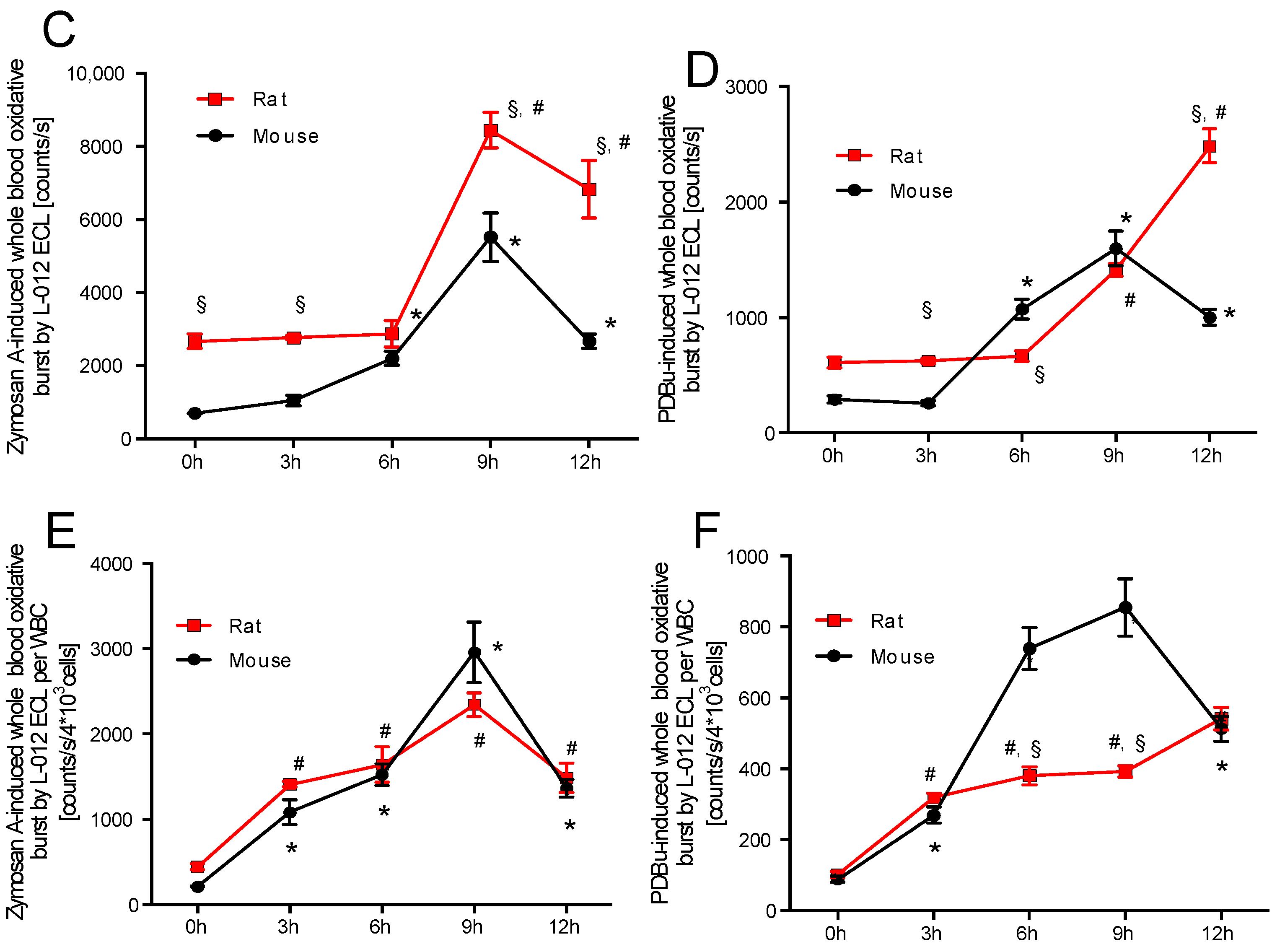
2.1. Time Response of Thrombocyte Count and White Blood Cell Derived Oxidative Burst in Mice and Rats
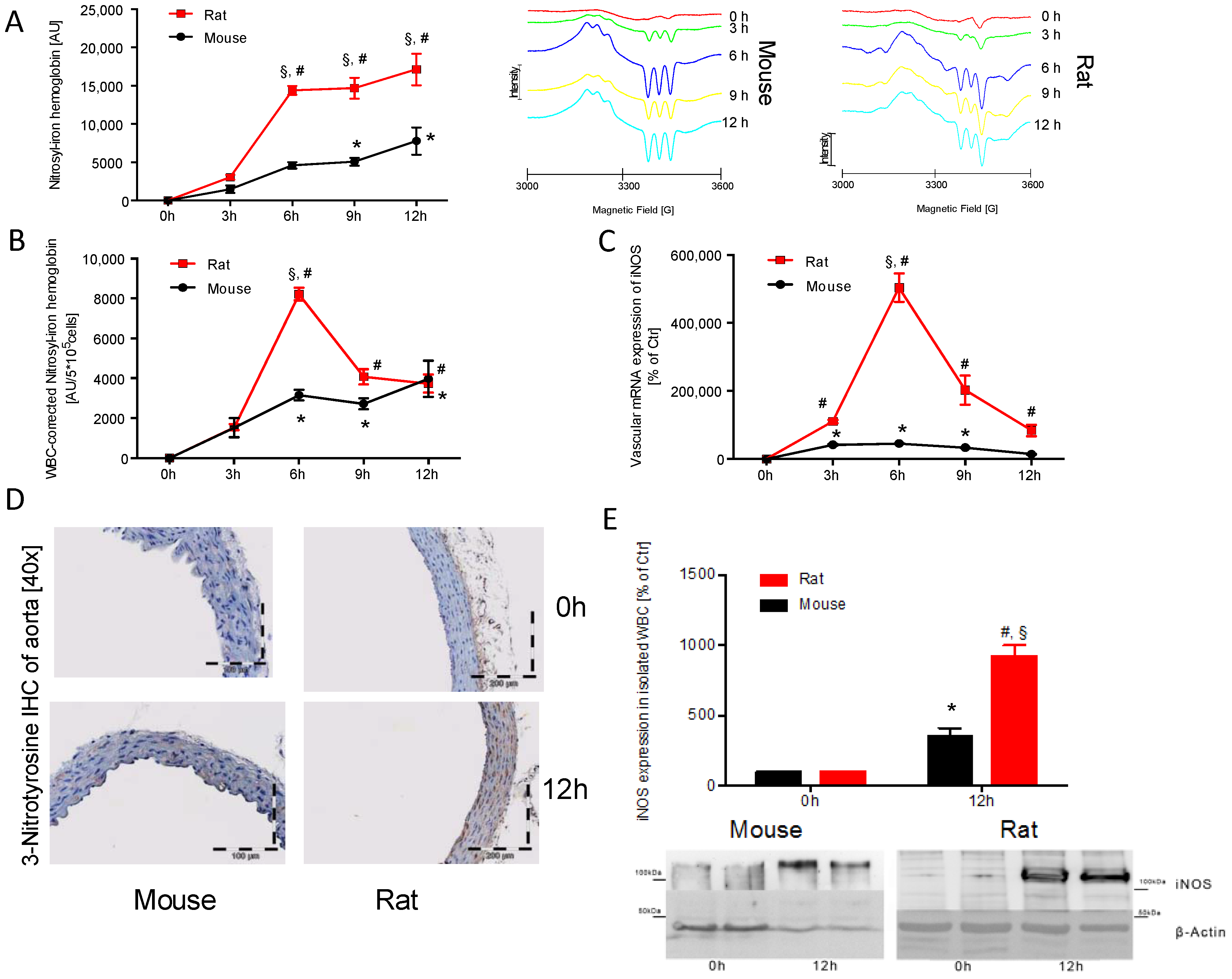
2.2. Time Response of Nitrosyl-Iron Hemoglobin and Inos Expression of Isolated WBC in Mice and Rats
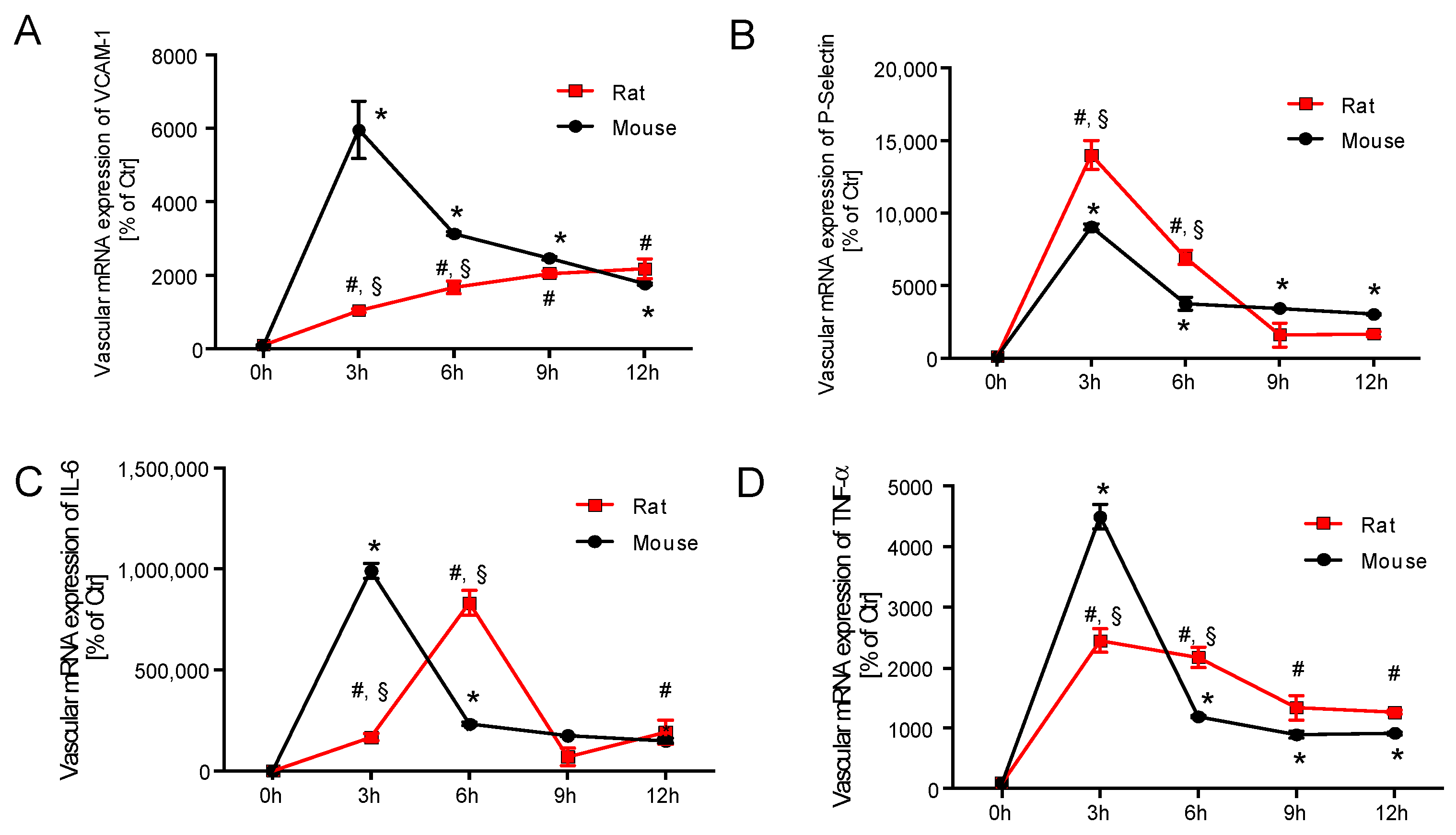
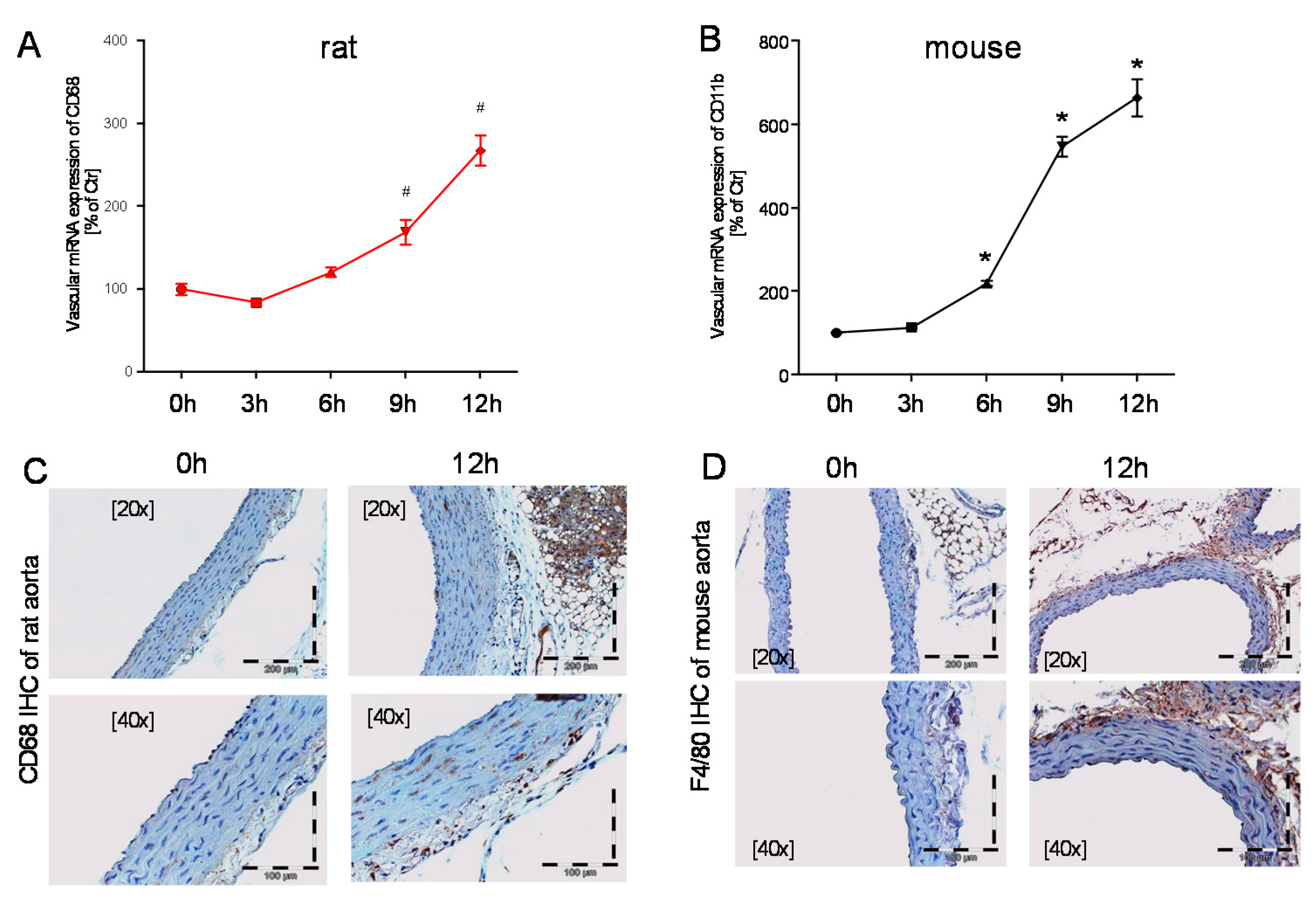
2.3. Time Response of Vascular Inflammation in Mice and Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and In Vivo Treatment
4.3. Chemiluminescence-Based Detection of Oxidative Stress in Whole Blood
4.4. Quantification of Nitrosyl-Iron Hemoglobin in Whole Blood by Electron Paramagnetic Resonance (EPR) Spectroscopy
4.5. Isolation of White Blood Cells
4.6. Western Blot Analysis
4.7. Reverse Transcription Real-Time PCR (qRT-PCR)
4.8. Immunohistochemistry and Fluorescence Microscopy
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| WBC | White blood cell |
| HbNO | nitrosyl-haemoglobin |
| iNOS | inducible NOS (type 2) |
| IL-6 | Interleukin-6 |
| TNF-α | tumor necrosis factor-α |
| VCAM-1 | vascular adhesion molecule-1 |
| PDBu | Phorbol 12,13-dibutyrate |
References
- Rhodes, A.; Phillips, G.; Beale, R.; Cecconi, M.; Chiche, J.D.; De Backer, D.; Divatia, J.; Du, B.; Evans, L.; Ferrer, R.; et al. The surviving sepsis campaign bundles and outcome: Results from the international multicentre prevalence study on sepsis (the impress study). Intensive Care Med. 2015, 41, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.J.; Williams, S.N.; DeFrances, C.J.; Golosinskiy, A. Inpatient care for septicemia or sepsis: A challenge for patients and hospitals. NCHS Data Brief 2011, 62, 1–8. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C. Sir isaac newton, sepsis, sirs, and cars. Crit. Care Med. 1996, 24, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Miyakawa, T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed]
- Kroller-Schon, S.; Knorr, M.; Hausding, M.; Oelze, M.; Schuff, A.; Schell, R.; Sudowe, S.; Scholz, A.; Daub, S.; Karbach, S.; et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc. Res. 2012, 96, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Munzel, T.; Daiber, A. Exploiting the pleiotropic antioxidant effects of established drugs in cardiovascular disease. Int. J. Mol. Sci. 2015, 16, 18185–18223. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Jurk, K.; Kopp, M.; Kroller-Schon, S.; Mikhed, Y.; Schwierczek, K.; Roohani, S.; Kashani, F.; Oelze, M.; Klein, T.; et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 2017, 174, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Hausding, M.; Kroller-Schon, S.; Mader, M.; Mikhed, Y.; Stamm, P.; Zinssius, E.; Pfeffer, A.; Welschof, P.; Agdauletova, S.; et al. Gliptin and glp-1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide-induced endotoxemia. Basic Res. Cardiol. 2015, 110, 6. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Petterino, C.; Argentino-Storino, A. Clinical chemistry and haematology historical data in control sprague-dawley rats from pre-clinical toxicity studies. Exp. Toxicol. Pathol. 2006, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.S.; Clifford, C.B.; Treuting, P.M.; Brayton, C. Immunological variation between inbred laboratory mouse strains: Points to consider in phenotyping genetically immunomodified mice. Vet. Pathol. 2012, 49, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.; Fulton, W.B.; Wilson, C.; Monitto, C.L.; Paidas, C.N.; Reeves, R.H.; De Maio, A. Genetic contribution to the septic response in a mouse model. Shock 2002, 18, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.A.; Wiersinga, W.J.; de Vos, A.F.; van der Poll, T. Recent insights into the pathogenesis of bacterial sepsis. Neth. J. Med. 2010, 68, 147–152. [Google Scholar] [PubMed]
- National Heart, L.; Blood Institute, A.C.T.N.; Truwit, J.D.; Bernard, G.R.; Steingrub, J.; Matthay, M.A.; Liu, K.D.; Albertson, T.E.; Brower, R.G.; Shanholtz, C.; et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N. Engl. J. Med. 2014, 370, 2191–2200. [Google Scholar]
- Bachschmid, M.; Ullrich, V. Redox signalling in endothelial cells—Novel mechanisms explaining endothelium function and dysfunction in health, disease and aging. BIF futura 2003, 18, 223–230. [Google Scholar]
- Yamaguchi, N.; Jesmin, S.; Zaedi, S.; Shimojo, N.; Maeda, S.; Gando, S.; Koyama, A.; Miyauchi, T. Time-dependent expression of renal vaso-regulatory molecules in lps-induced endotoxemia in rat. Peptides 2006, 27, 2258–2270. [Google Scholar] [CrossRef] [PubMed]
- Hein, O.V.; Misterek, K.; Tessmann, J.P.; van Dossow, V.; Krimphove, M.; Spies, C. Time course of endothelial damage in septic shock: Prediction of outcome. Crit. Care 2005, 9, R323–R330. [Google Scholar] [CrossRef] [PubMed]
- Alves, B.E.; Montalvao, S.A.; Aranha, F.J.; Lorand-Metze, I.; De Souza, C.A.; Annichino-Bizzacchi, J.M.; De Paula, E.V. Time-course of sFlt-1 and VEGF-A release in neutropenic patients with sepsis and septic shock: A prospective study. J. Transl. Med. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Connelly, R.; Traber, D.L.; Hamahata, A.; Nakano, Y.; Esechie, A.; Jonkam, C.; von Borzyskowski, S.; Traber, L.D.; Schmalstieg, F.C.; et al. Time course of nitric oxide synthases, nitrosative stress, and poly(adp ribosylation) in an ovine sepsis model. Crit. Care 2010, 14, R129. [Google Scholar] [CrossRef] [PubMed]
- Nakae, H.; Endo, S.; Inada, K.; Watanabe, M.; Baba, N.; Yoshida, M. Relationship between leukotriene b4 and prostaglandin i2 in patients with sepsis. Res. Commun. Mol. Pathol. Pharmacol. 1994, 86, 37–42. [Google Scholar] [PubMed]
- Yellin, S.A.; Nguyen, D.; Quinn, J.V.; Burchard, K.W.; Crowley, J.P.; Slotman, G.J. Prostacyclin and thromboxane a2 in septic shock: Species differences. Circ. Shock 1986, 20, 291–297. [Google Scholar] [PubMed]
- Butler, R.R., Jr.; Wise, W.C.; Halushka, P.V.; Cook, J.A. Thromboxane and prostacyclin production during septic shock. Adv. Shock Res. 1982, 7, 133–145. [Google Scholar] [PubMed]
- Cook, J.A.; Wise, W.C.; Butler, R.R.; Reines, H.D.; Rambo, W.; Halushka, P.V. The potential role of thromboxane and prostacyclin in endotoxic and septic shock. Am. J. Emerg. Med. 1984, 2, 28–37. [Google Scholar] [CrossRef]
- Schildknecht, S.; Heinz, K.; Daiber, A.; Hamacher, J.; Kavakli, C.; Ullrich, V.; Bachschmid, M. Autocatalytic tyrosine nitration of prostaglandin endoperoxide synthase-2 in lps-stimulated raw 264.7 macrophages. Biochem. Biophys. Res. Commun. 2006, 340, 318–325. [Google Scholar] [CrossRef] [PubMed]
- August, M.; Wingerter, O.; Oelze, M.; Wenzel, P.; Kleschyov, A.L.; Daiber, A.; Mulsch, A.; Munzel, T.; Tsilimingas, N. Mechanisms underlying dysfunction of carotid arteries in genetically hyperlipidemic rabbits. Nitric Oxide 2006, 15, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Kessler, P.; Bauersachs, J.; Busse, R.; Schini-Kerth, V.B. Inhibition of inducible nitric oxide synthase restores endothelium-dependent relaxations in proinflammatory mediator-induced blood vessels. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Holub, M. Thromboembolic complications of sepsis: What is the incidence and pathophysiological mechanisms involved? Thromb. Haemost. 2008, 99, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Tridente, A.; Clarke, G.M.; Walden, A.; Gordon, A.C.; Hutton, P.; Chiche, J.D.; Holloway, P.A.; Mills, G.H.; Bion, J.; Stuber, F.; et al. Association between trends in clinical variables and outcome in intensive care patients with faecal peritonitis: Analysis of the genosept cohort. Crit. Care 2015, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Hocherl, K.; Schmidt, C.; Kurt, B.; Bucher, M. Activation of the pgi(2)/ip system contributes to the development of circulatory failure in a rat model of endotoxic shock. Hypertension 2008, 52, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.C.; Chen, W.P.; Su, M.J. Glp-1 signaling preserves cardiac function in endotoxemic fischer 344 and dpp4-deficient rats. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 382, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Modis, K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock 2010, 34 (Suppl. S1), 4–14. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; August, M.; Baldus, S.; Wendt, M.; Oelze, M.; Sydow, K.; Kleschyov, A.L.; Munzel, T. Measurement of nad(p)h oxidase-derived superoxide with the luminol analogue l-012. Free Radic. Biol. Med. 2004, 36, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, P.; Schulz, E.; Gori, T.; Ostad, M.A.; Mathner, F.; Schildknecht, S.; Gobel, S.; Oelze, M.; Stalleicken, D.; Warnholtz, A.; et al. Monitoring white blood cell mitochondrial aldehyde dehydrogenase activity: Implications for nitrate therapy in humans. J. Pharmacol. Exp. Ther. 2009, 330, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hausding, M.; Jurk, K.; Daub, S.; Kroller-Schon, S.; Stein, J.; Schwenk, M.; Oelze, M.; Mikhed, Y.; Kerahrodi, J.G.; Kossmann, S.; et al. Cd40l contributes to angiotensin ii-induced pro-thrombotic state, vascular inflammation, oxidative stress and endothelial dysfunction. Basic Res. Cardiol. 2013, 108, 386. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steven, S.; Dib, M.; Roohani, S.; Kashani, F.; Münzel, T.; Daiber, A. Time Response of Oxidative/Nitrosative Stress and Inflammation in LPS-Induced Endotoxaemia—A Comparative Study of Mice and Rats. Int. J. Mol. Sci. 2017, 18, 2176. https://doi.org/10.3390/ijms18102176
Steven S, Dib M, Roohani S, Kashani F, Münzel T, Daiber A. Time Response of Oxidative/Nitrosative Stress and Inflammation in LPS-Induced Endotoxaemia—A Comparative Study of Mice and Rats. International Journal of Molecular Sciences. 2017; 18(10):2176. https://doi.org/10.3390/ijms18102176
Chicago/Turabian StyleSteven, Sebastian, Mobin Dib, Siyer Roohani, Fatemeh Kashani, Thomas Münzel, and Andreas Daiber. 2017. "Time Response of Oxidative/Nitrosative Stress and Inflammation in LPS-Induced Endotoxaemia—A Comparative Study of Mice and Rats" International Journal of Molecular Sciences 18, no. 10: 2176. https://doi.org/10.3390/ijms18102176
APA StyleSteven, S., Dib, M., Roohani, S., Kashani, F., Münzel, T., & Daiber, A. (2017). Time Response of Oxidative/Nitrosative Stress and Inflammation in LPS-Induced Endotoxaemia—A Comparative Study of Mice and Rats. International Journal of Molecular Sciences, 18(10), 2176. https://doi.org/10.3390/ijms18102176






