Geranylgeranylacetone Ameliorates Intestinal Radiation Toxicity by Preventing Endothelial Cell Dysfunction
Abstract
:1. Introduction
2. Results
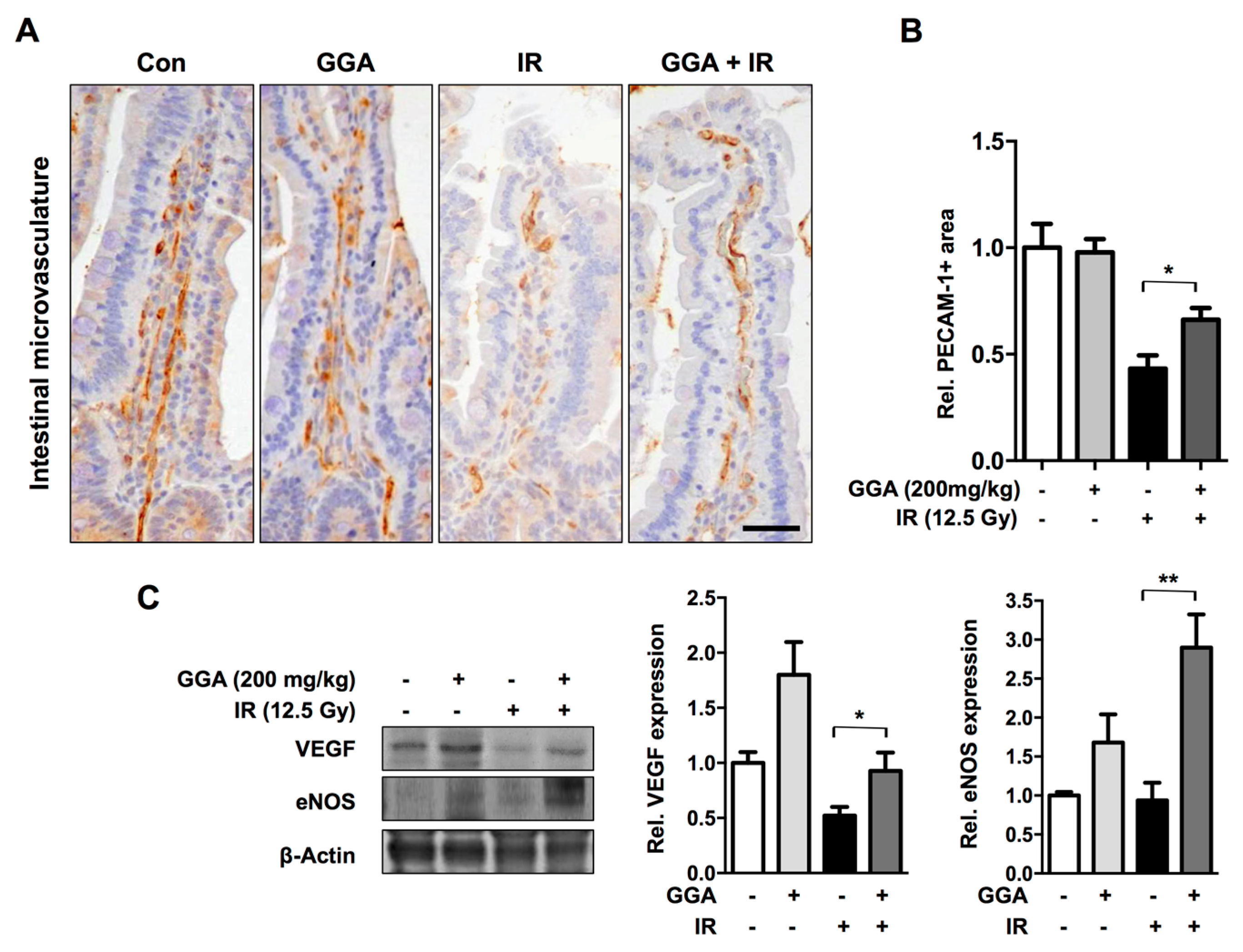
2.1. GGA Protected Against Radiation-Induced Intestinal Injury
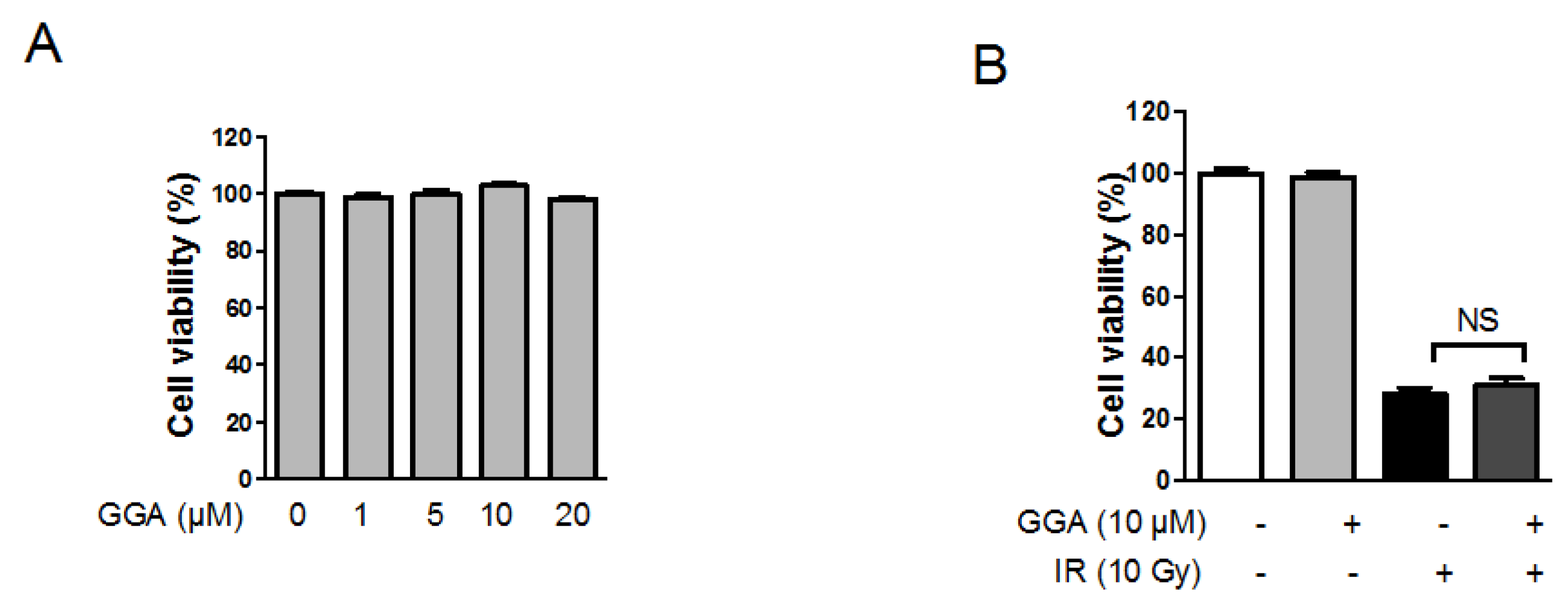
2.2. Effect of GGA on Endothelial Cell Viability Following IR
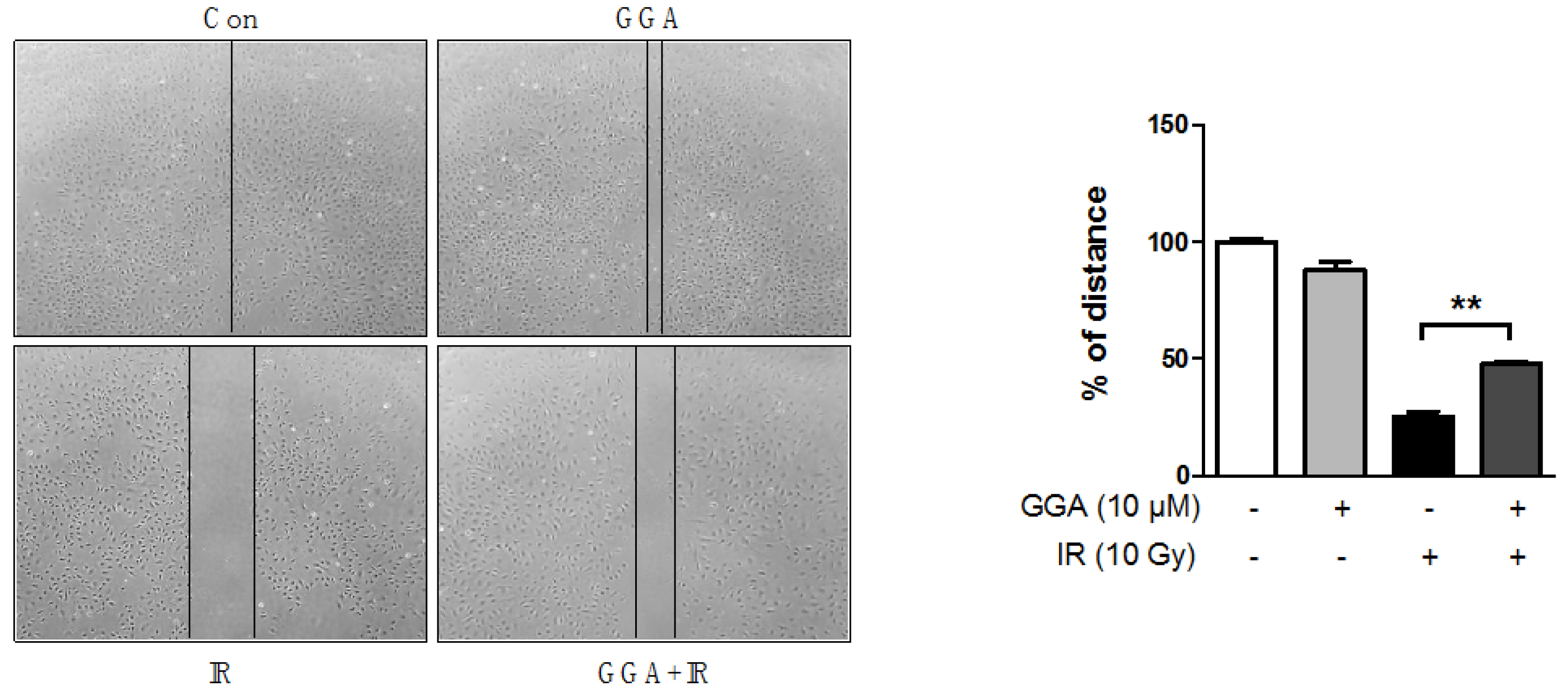
2.3. Effect of GGA on Cell Mobility and Angiogenesis
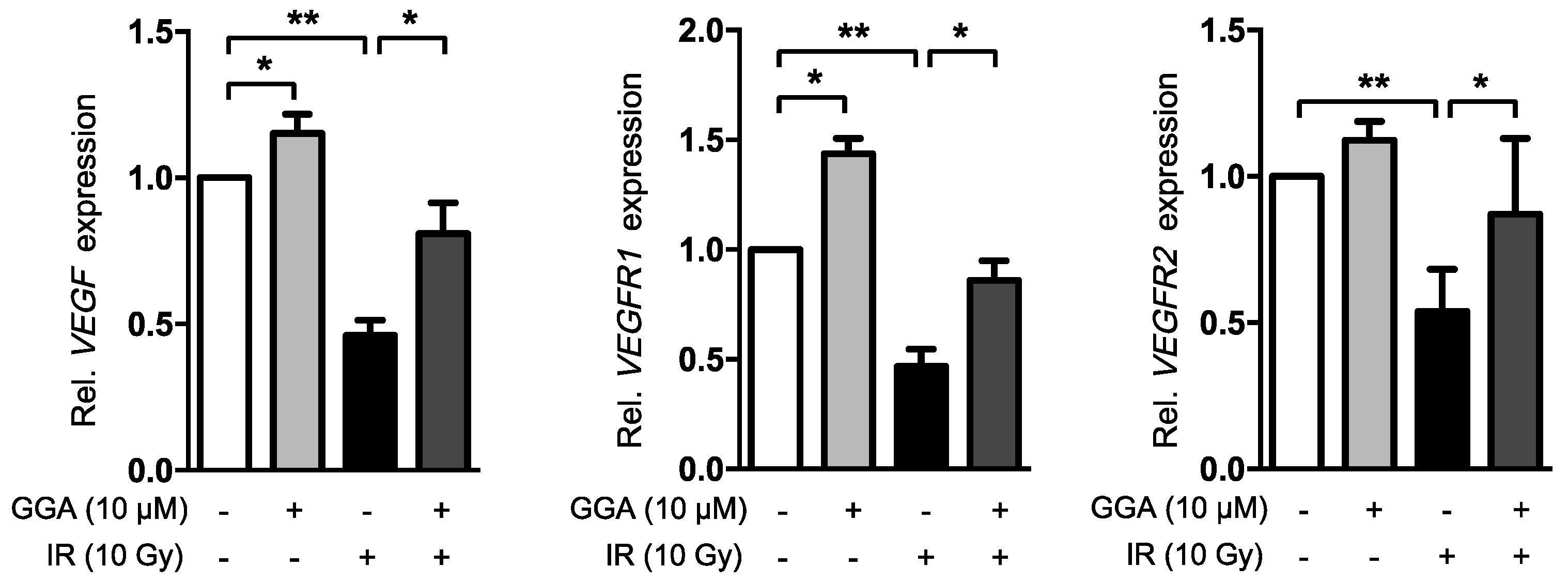
2.4. GGA Promotes Angiogenesis by Inducing VEGF and eNOS Expression
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Histopathological Analysis
4.3. Western Blot Analysis
4.4. Cells and Treatments
4.5. RT-qPCR
4.6. Cell Proliferation Assay
4.7. Wound-Healing Assay
4.8. Tube Formation Assay
4.9. Invasion and Migration Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Korpela, E.; Liu, S.K. Endothelial perturbations and therapeutic strategies in normal tissue radiation damage. Radiat. Oncol. 2014, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J.N. Radiation enteropathy—Pathogenesis, treatment and prevention. Nat. Rev. 2014, 11, 470–479. [Google Scholar]
- Peach, M.S.; Showalter, T.N.; Ohri, N. Systematic review of the relationship between acute and late gastrointestinal toxicity after radiotherapy for prostate cancer. Prostate Cancer 2015, 2015, 624736. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.W.; Hauer-Jensen, M. The radiotherapeutic injury—A complex ‘wound’. Radiother. Oncol. 2002, 63, 129–145. [Google Scholar] [CrossRef]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Boerma, M.; Fu, Q.; Hauer-Jensen, M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J. Gastroenterol. 2007, 13, 3047–3055. [Google Scholar]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Rannou, E.; François, A.; Toullec, A.; Guipaud, O.; Buard, V.; Tarlet, G.; Mintet, E.; Jaillet, C.; Iruela-Arispe, M.L.; Benderitter, M.; et al. In vivo evidence for an endothelium-dependent mechanism in radiation-induced normal tissue injury. Sci. Rep. 2015, 5, 15738. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Jung, M.G.; Son, Y.; Jang, J.-H.; Lee, Y.-J.; Kim, S.-H.; Ko, Y.-G.; Lee, Y.-S.; Lee, H.-J. Coniferyl aldehyde attenuates radiation enteropathy by inhibiting cell death and promoting endothelial cell function. PLoS ONE 2015, 10, e0128552. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.Q.; Wu, Y.Q. Update on the cardioprotective role of heat shock proteins inducer, geranylgeranylacetone. Zhonghua Xin Xue Guan Bing Za Zhi 2016, 44, 1059–1063. (in Chinese). [Google Scholar] [PubMed]
- Tanaka, K.-I.; Tanaka, Y.; Namba, T.; Azuma, A.; Mizushima, T. Heat shock protein 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem. Pharmacol. 2010, 80, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Son, Y.; Jung, M.G.; Jeong, Y.J.; Kim, S.-H.; Lee, S.-J.; Lee, Y.-J.; Lee, H.-J. Geranylgeranylacetone alleviates radiation-induced lung injury by inhibiting epithelial-to-mesenchymal transition signaling. Mol. Med. Rep. 2016, 13, 4666–4670. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhou, Y.; Li, Z.; Zhuang, S.; An, X.; Zhang, B.; Chen, W.; Nie, J.; Wang, Z.; Borkan, S.C.; et al. HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Renal Physiol. 2008, 295, F202–F214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Faden, A.I.; Loane, D.J.; Lipinski, M.M.; Sabirzhanov, B.; Stoica, B.A. Neuroprotective effects of geranylgeranylacetone in experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2013, 33, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Liauw, S.L.; Connell, P.P.; Weichselbaum, R.R. New paradigms and future challenges in radiation oncology: An update of biological targets and technology. Sci. Transl. Med. 2013, 5, 173sr2. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, T.; Takeda, H.; Nishiwaki, M.; Nishihira, J.; Asaka, M. Protective effects of heat shock protein 70 induced by geranylgeranylacetone on oxidative injury in rat intestinal epithelial cells. Scand. J. Gastroenterol. 2006, 41, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Isoir, M.; Roque, T.; Squiban, C.; Milliat, F.; Mondon, P.; Mas-Chamberlin, C.; Benderitter, M.; Guipaud, O.; Tamarat, R. Protective effect of geranylgeranylacetone against radiation-induced delayed effects on human keratinocytes. Radiat. Res. 2013, 179, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Sanbe, A.; Daicho, T.; Mizutani, R.; Endo, T.; Miyauchi, N.; Yamauchi, J.; Tanonaka, K.; Glabe, C.; Tanoue, A. Protective effect of geranylgeranylacetone via enhancement of HSPB8 induction in desmin-related cardiomyopathy. PLoS ONE 2009, 4, e5351. [Google Scholar] [CrossRef] [PubMed]
- Xuan, A.; Long, D.; Li, J.; Ji, W.; Hong, L.; Zhang, M.; Zhang, W. Neuroprotective effects of valproic acid following transient global ischemia in rats. Life Sci. 2012, 90, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Ohta, Y.; Ishiguro, I. Teprenone, an anti-ulcer agent, increases gastric mucosal mucus level via nitric oxide in rats. Jpn. J. Pharmacol. 1998, 78, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Aron, Y.; Vayssier-Taussat, M.; Bachelet, M.; Polla, B.S. Geranylgeranylacetone protects human monocytes from mitochondrial membrane depolarization independently of Hsp70 expression. Cell. Mol. Life Sci. 2001, 58, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, J.A.; Kolesnick, R.; Fuks, Z. Timing of lethality from gastrointestinal syndrome in mice revisited. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Boerma, M.; Zhou, D. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat. Res. 2016, 186, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Fujiki, M.; Uchida, S.; Morishige, M.; Momii, Y.; Ishii, K. A single oral dose of geranylgeranylacetone upregulates vascular endothelial growth factor and protects against kainic acid-induced neuronal cell death: Involvement of the phosphatidylinositol-3 kinase/AKT pathway. Pathobiology 2017, 84, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Gene. Canc. 2012, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, F.M. Portrait of inflammatory response to ionizing radiation treatment. J. Inflamm. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Morshige, K.; Sawada, K.; Tahara, M.; Shimizu, S.; Sakata, M.; Tasaka, K.; Murata, Y. Geranylgeranylacetone inhibits lysophosphatidic acid-induced invasion of human ovarian carcinoma cells in vitro. Cancer 2005, 103, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Tsuno, N.H.; Okaji, Y.; Kawai, K.; Shuno, Y.; Nagawa, H.; Oshima, N.; Takahashi, K. Isoprenoid geranylgeranylacetone inhibits human colon cancer cells through induction of apoptosis and cell cycle arrest. Anticancer Drugs 2010, 21, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Morishige, K.; Sawada, K.; Ogata, S.; Tahara, M.; Shimizu, S.; Sakata, M.; Tasaka, K.; Kimura, T. Geranylgeranylacetone inhibits ovarian cancer progression in vitro and in vivo. Biochem. Biophys. Res. Commun. 2007, 356, 72–77. [Google Scholar] [CrossRef] [PubMed]




| Gene | GenBank Accession No. | Primer Sequence | |
|---|---|---|---|
| VEGF | NM_001025366.2 | Forward | 5′-CTTGCCTTGCTGCTCTACC-3′ |
| Reverse | 5′-CACACAGGATGGCTTGAAG-3′ | ||
| VEGFR1 | NM_001159920.1 | Forward | 5′-TACTTGGATTTTACTGCGGAC-3′ |
| Reverse | 5′-TTTTGTTGCAGTGCTCACC-3′ | ||
| VEGFR2 | NM_002253.2 | Forward | 5′-AACTGAAGACAGGCTACTTG-3′ |
| Reverse | 5′-GTCGTTCACAATGTTCATCC-3′ | ||
| eNOS | NM_000603.4 | Forward | 5′-TCTTCAGCCCCAAACGGAG-3′ |
| Reverse | 5′-CGGATTGTAGCCTGGAACATC-3′ | ||
| TNFα | NM_000594.3 | Forward | 5′-GGAAGACCCCTCCCAGATAG-3′ |
| Reverse | 5′-CAGAGGGCCTGTACCTCATC-3′ | ||
| Hsp70 | NM_005345.5 | Forward | 5′-ACATCAGCCAGAACAAGCGA-3′ |
| Reverse | 5′-AGTCGATGCCCTCAAACAGG-3′ | ||
| GAPDH | NM_008084.2 | Forward | 5′-TCCATGACAACTTTGGCATT-3′ |
| Reverse | 5′-GTTGCTGTTGAAGTCGCAGG-3′ | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.-K.; Jeong, Y.J.; Pyun, B.-J.; Lee, Y.-J.; Kim, S.-H.; Lee, H.-J. Geranylgeranylacetone Ameliorates Intestinal Radiation Toxicity by Preventing Endothelial Cell Dysfunction. Int. J. Mol. Sci. 2017, 18, 2103. https://doi.org/10.3390/ijms18102103
Han N-K, Jeong YJ, Pyun B-J, Lee Y-J, Kim S-H, Lee H-J. Geranylgeranylacetone Ameliorates Intestinal Radiation Toxicity by Preventing Endothelial Cell Dysfunction. International Journal of Molecular Sciences. 2017; 18(10):2103. https://doi.org/10.3390/ijms18102103
Chicago/Turabian StyleHan, Na-Kyung, Ye Ji Jeong, Bo-Jeong Pyun, Yoon-Jin Lee, Sung-Ho Kim, and Hae-June Lee. 2017. "Geranylgeranylacetone Ameliorates Intestinal Radiation Toxicity by Preventing Endothelial Cell Dysfunction" International Journal of Molecular Sciences 18, no. 10: 2103. https://doi.org/10.3390/ijms18102103
APA StyleHan, N.-K., Jeong, Y. J., Pyun, B.-J., Lee, Y.-J., Kim, S.-H., & Lee, H.-J. (2017). Geranylgeranylacetone Ameliorates Intestinal Radiation Toxicity by Preventing Endothelial Cell Dysfunction. International Journal of Molecular Sciences, 18(10), 2103. https://doi.org/10.3390/ijms18102103





