F-Box Protein FBXO22 Mediates Polyubiquitination and Degradation of CD147 to Reverse Cisplatin Resistance of Tumor Cells
Abstract
:1. Introduction
2. Results
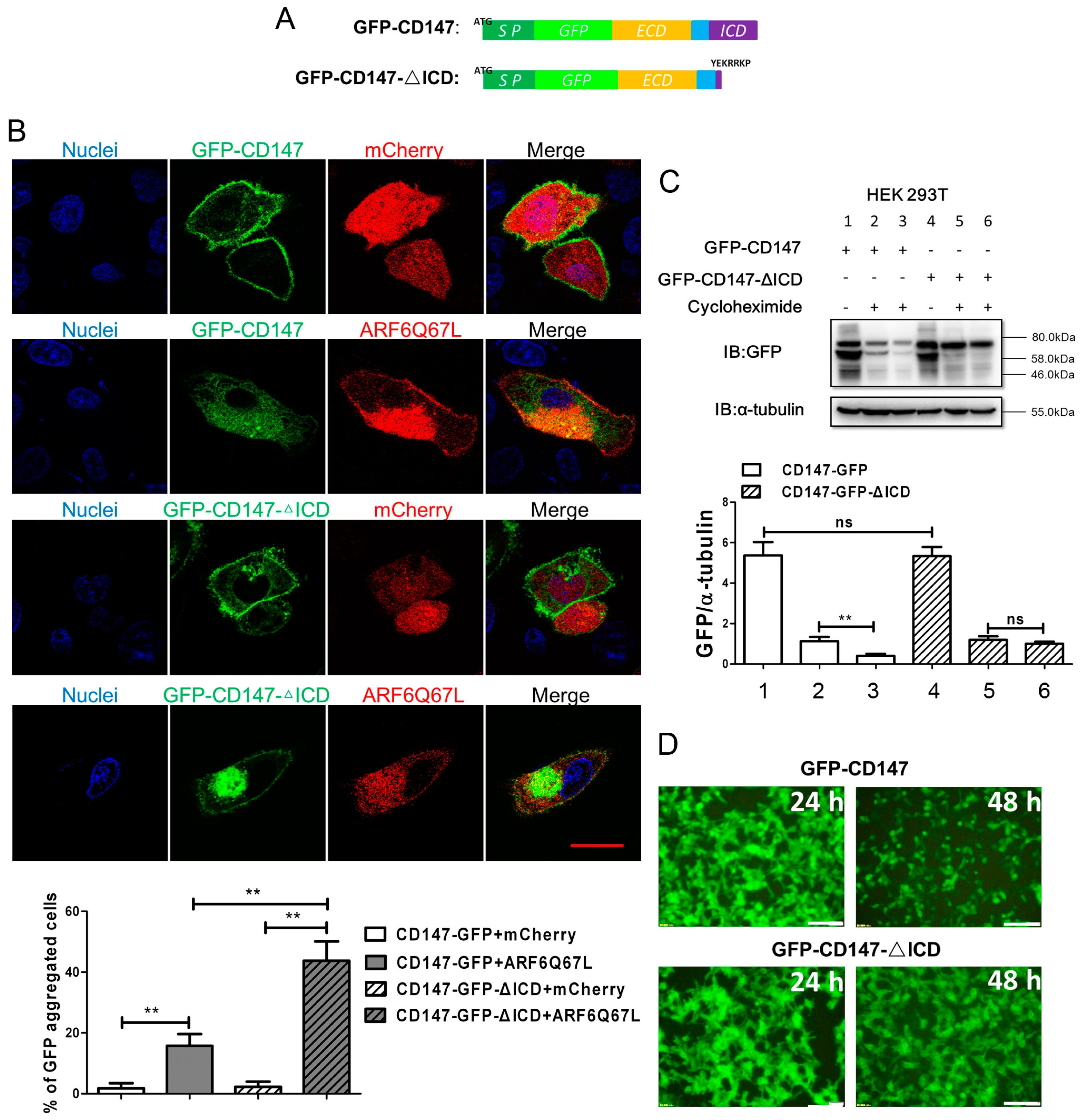
2.1. Ectopic CD147 Avoids Degradation after Intracellular Domain Deletion
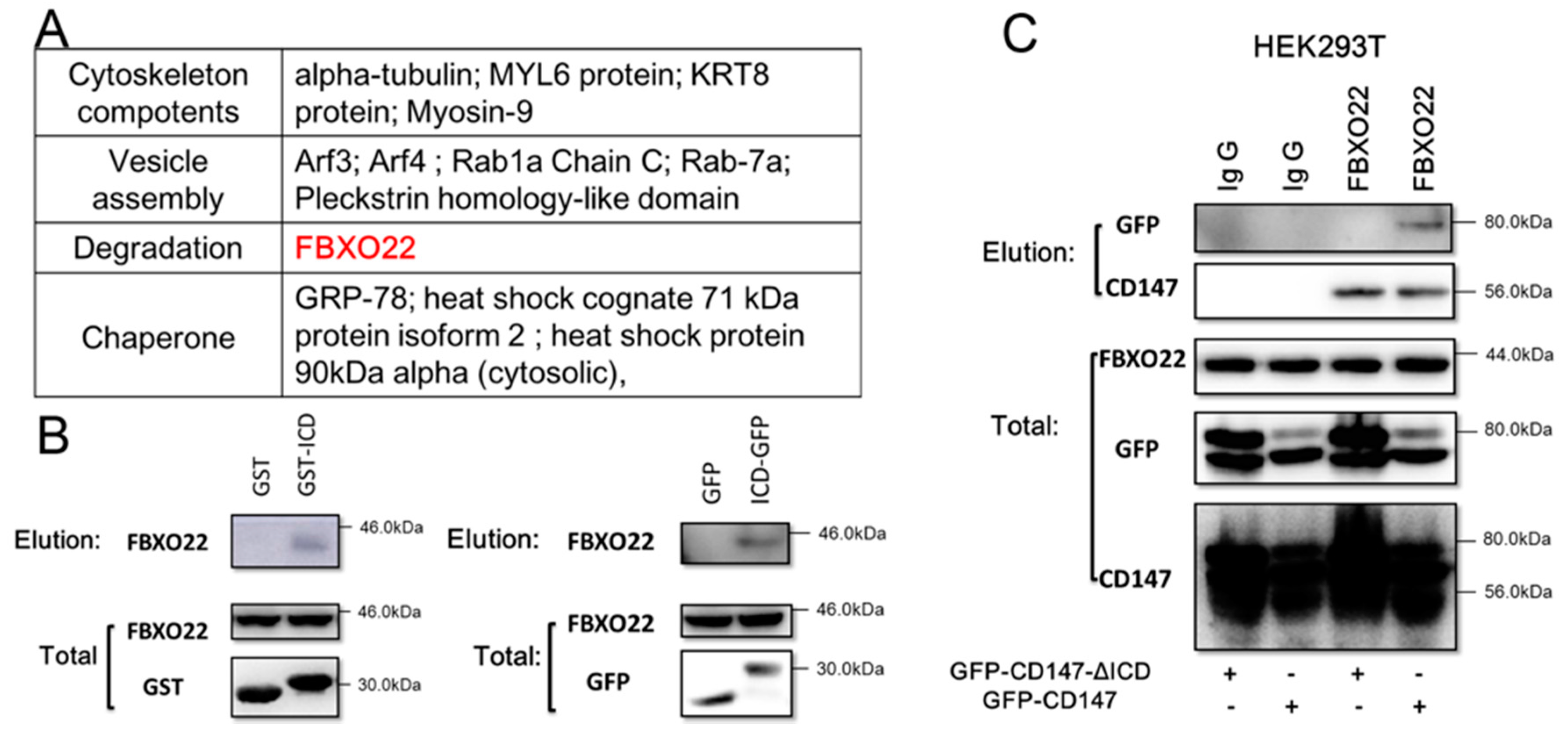
2.2. CD147-ICD Interacts with FBXO22
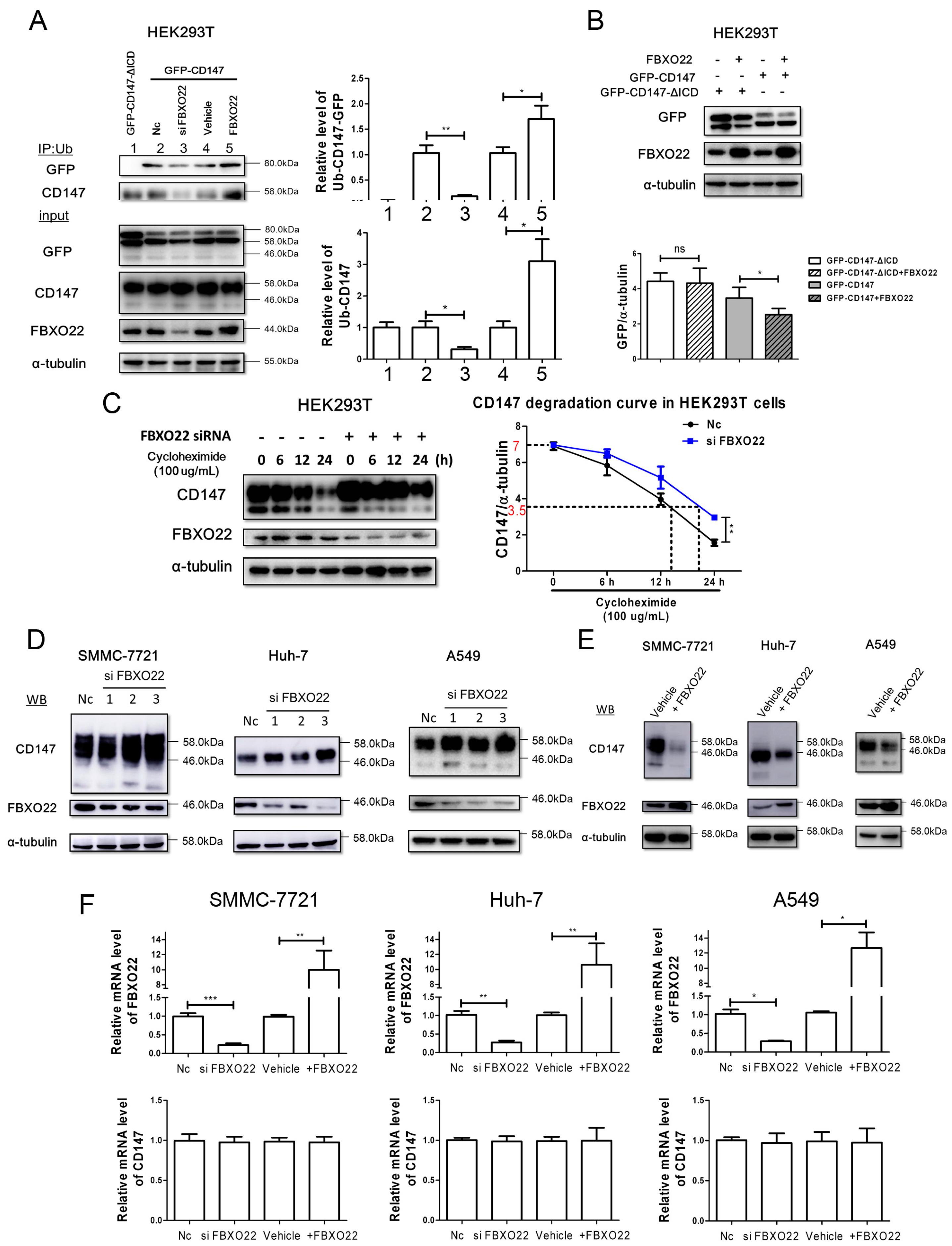
2.3. FBXO22 Mediates Poly-Ubiquitination and Degradation of CD147
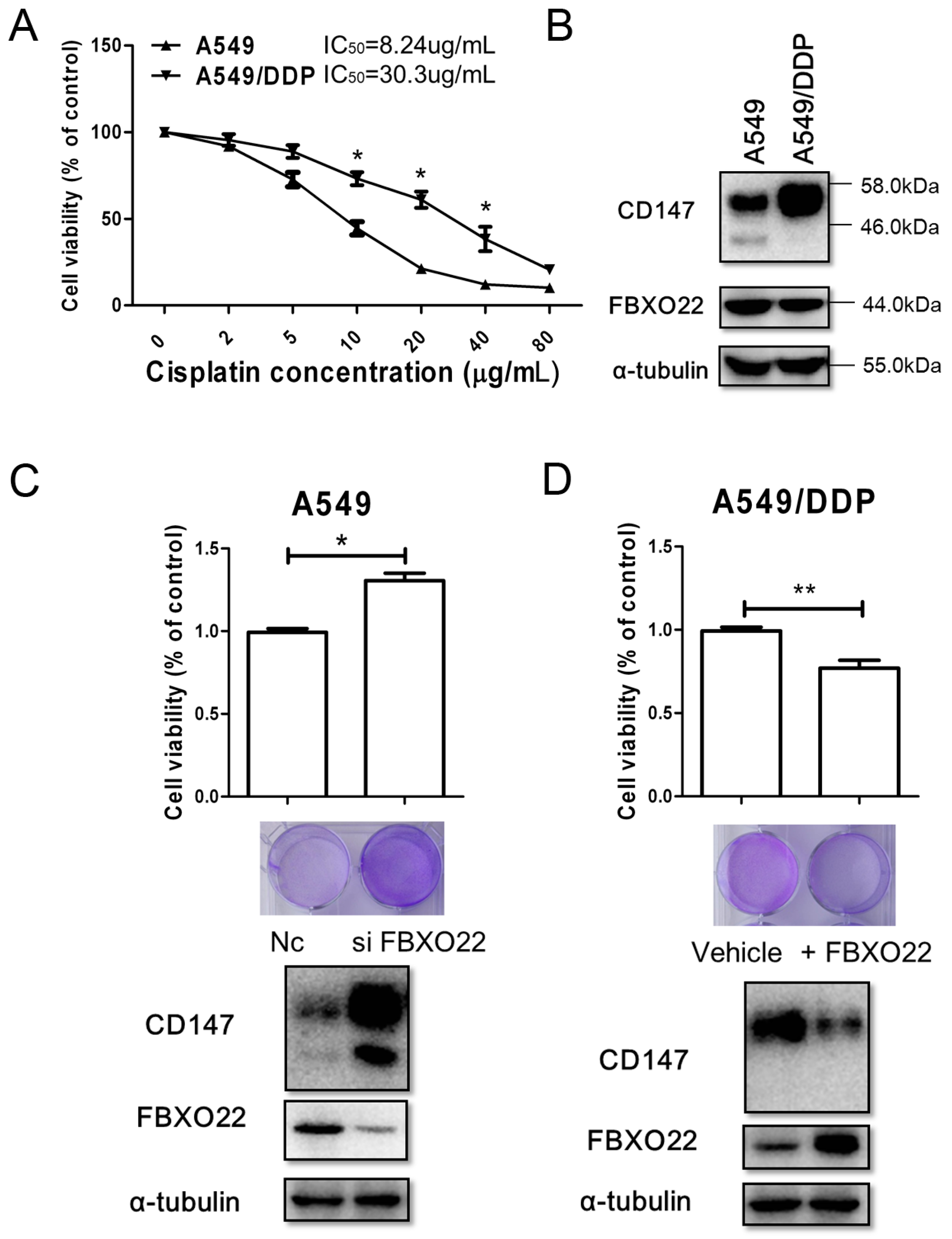
2.4. FBXO22-CD147 Axis Is Involved in A549/DDP Chemo-Resistance
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Plasmids
4.3. Immunofluorescence
4.4. RNA Interference and Transfection
4.5. GST Fusion Protein Pull-Down Assay
4.6. Mass Spectrometry and Western Blot
4.7. Ubiquitination Assay
4.8. qRT-PCR
4.9. Cell Viability Assay
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holohan, C.; van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Obenauf, A.C.; Zou, Y.; Ji, A.L.; Vanharanta, S.; Shu, W.; Shi, H.; Kong, X.; Bosenberg, M.C.; Wiesner, T.; Rosen, N.; et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 2015, 520, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Lv, H.; Wu, F.; Wang, C.; Li, T.; Lv, G.; Tang, L.; Guo, L.; Tang, S.; Cao, D.; et al. Musashi 2 contributes to the stemness and chemoresistance of liver cancer stem cells via LIN28A activation. Cancer Lett. 2016, 384, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Weidle, U.H.; Scheuer, W.; Eggle, D.; Klostermann, S.; Stockinger, H. Cancer-related issues of CD147. Cancer Genom. Proteom. 2010, 7, 157–169. [Google Scholar]
- Xiong, L.; Edwards, C.K.; Zhou, L. The biological function and clinical utilization of CD147 in human diseases: A review of the current scientific literature. Int. J. Mol. Sci. 2014, 15, 17411–17441. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Kim, H.P.; Lee, K.S.; Yoo, Y.D.; Kwon, Y.T.; Kim, K.M.; Kim, T.Y.; Yi, E.C. Proteomic analysis reveals that CD147/emmprin confers chemoresistance in cancer stem cell-like cells. Proteomics 2013, 13, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.D.; Dai, L.; Qin, Z.; Parsons, C.; Toole, B.P. CD147: Regulator of hyaluronan signaling in invasiveness and chemoresistance. Adv. Cancer Res. 2014, 123, 351–373. [Google Scholar] [PubMed]
- Huang, W.; Luo, W.J.; Zhu, P.; Tang, J.; Yu, X.L.; Cui, H.Y.; Wang, B.; Zhang, Y.; Jiang, J.L.; Chen, Z.N. Modulation of CD147-induced matrix metalloproteinase activity: Role of CD147 N-glycosylation. Biochem. J. 2013, 449, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, X.; Zeng, W.; Su, J.; Yang, K.; Lu, L.; Lim, C.B.; Tang, W.; Wu, L.; Zhao, S.; et al. TRAF6 regulates melanoma invasion and metastasis through ubiquitination of basigin. Oncotarget 2016, 7, 7179–7192. [Google Scholar] [PubMed]
- Maldonado-Baez, L.; Cole, N.B.; Kramer, H.; Donaldson, J.G. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. J. Cell Biol. 2013, 201, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, Y.; Yang, X.M.; Xu, B.Q.; Feng, F.; Wang, B.; Liang, Q.; Li, Y.; Zhou, Y.; Jiang, J.L.; et al. Basigin-mediated redistribution of CD98 promotes cell spreading and tumorigenicity in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 110. [Google Scholar] [CrossRef] [PubMed]
- Eyster, C.A.; Higginson, J.D.; Huebner, R.; Porat-Shliom, N.; Weigert, R.; Wu, W.W.; Shen, R.-F.; Donaldson, J.G. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic 2009, 10, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.; Martin-Belmonte, F. Cargo sorting in the endocytic pathway: A key regulator of cell polarity and tissue dynamics. Cold Spring Harb. Perspect. Biol. 2014, 6, a016899. [Google Scholar] [CrossRef] [PubMed]
- Clague, M.J.; Liu, H.; Urbe, S. Governance of endocytic trafficking and signaling by reversible ubiquitylation. Dev. Cell 2012, 23, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.K.; Lim, H.J.; Harper, J.W. SCFFBXO22 regulates histone H3 lysine 9 and 36 methylation levels by targeting histone demethylase KDM4A for ubiquitin-mediated proteasomal degradation. Mol. Cell. Biol. 2011, 31, 3687–3699. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Dai, S.; Sun, J.; Jin, G.; Jiang, S.; Meng, F.; Li, Y.; Wu, D.; Jiang, Y. F-box protein FBXO22 mediates polyubiquitination and degradation of KLF4 to promote hepatocellular carcinoma progression. Oncotarget 2015, 6, 22767–22775. [Google Scholar] [CrossRef] [PubMed]
- Johmura, Y.; Sun, J.; Kitagawa, K.; Nakanishi, K.; Kuno, T.; Naiki-Ito, A.; Sawada, Y.; Miyamoto, T.; Okabe, A.; Aburatani, H.; et al. SCFFBXO22-KDM4A targets methylated p53 for degradation and regulates senescence. Nat. Commun. 2016, 7, 10574. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Baez, L.; Donaldson, J.G. Hook1, microtubules, and Rab22: Mediators of selective sorting of clathrin-independent endocytic cargo proteins on endosomes. Bioarchitecture 2013, 3, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Wandinger-Ness, A.; Zerial, M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 2014, 6, a022616. [Google Scholar] [CrossRef] [PubMed]
- Eyster, C.A.; Cole, N.B.; Petersen, S.; Viswanathan, K.; Fruh, K.; Donaldson, J.G. March ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation. Mol. Biol. Cell 2011, 22, 3218–3230. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Báez, L.; Williamson, C.; Donaldson, J.G. Clathrin-independent endocytosis: A cargo-centric view. Exp. Cell Res. 2013, 319, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Ruggiano, A.; Foresti, O.; Carvalho, P. Quality control: ER-associated degradation: Protein quality control and beyond. J. Cell Biol. 2014, 204, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Huang, W.; Ma, L.T.; Jiang, J.L.; Chen, Z.N. Importance of N-glycosylation on CD147 for its biological functions. Int. J. Mol. Sci. 2014, 15, 6356–6377. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.; Santos, L.L.; Miranda-Goncalves, V.; Morais, A.; Amaro, T.; Longatto-Filho, A.; Baltazar, F. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol. Carcinog. 2015, 54, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.D.; Wang, M.; Ding, H.H.; Qiu, Z.J. FBXL5 attenuates RhogDI2-induced cisplatin resistance in gastric cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2551–2557. [Google Scholar] [PubMed]
- Ku, X.M.; Liao, C.G.; Li, Y.; Yang, X.M.; Yang, B.; Yao, X.Y.; Wang, L.; Kong, L.M.; Zhao, P.; Chen, Z.N. Epitope mapping of series of monoclonal antibodies against the hepatocellular carcinoma-associated antigen HAb18G/CD147. Scand. J. Immunol. 2007, 65, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xiao, M.; Gao, H.; Chen, Y.; Li, Y.; Liu, Y.; Zhang, N. Identification of a novel mitochondrial interacting protein of C1QBP using subcellular fractionation coupled with CoIP-MS. Anal. Bioanal. Chem. 2016, 408, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, Y.; Qin, R.; Liu, D.; Feng, Q. Silence of fibronectin 1 increases cisplatin sensitivity of non-small cell lung cancer cell line. Biochem. Biophys. Res. Commun. 2016, 476, 35–41. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Liu, Z.-Y.; Cui, J.; Yang, X.-M.; Jing, L.; Zhou, Y.; Chen, Z.-N.; Jiang, J.-L. F-Box Protein FBXO22 Mediates Polyubiquitination and Degradation of CD147 to Reverse Cisplatin Resistance of Tumor Cells. Int. J. Mol. Sci. 2017, 18, 212. https://doi.org/10.3390/ijms18010212
Wu B, Liu Z-Y, Cui J, Yang X-M, Jing L, Zhou Y, Chen Z-N, Jiang J-L. F-Box Protein FBXO22 Mediates Polyubiquitination and Degradation of CD147 to Reverse Cisplatin Resistance of Tumor Cells. International Journal of Molecular Sciences. 2017; 18(1):212. https://doi.org/10.3390/ijms18010212
Chicago/Turabian StyleWu, Bo, Zhen-Yu Liu, Jian Cui, Xiang-Min Yang, Lin Jing, Yang Zhou, Zhi-Nan Chen, and Jian-Li Jiang. 2017. "F-Box Protein FBXO22 Mediates Polyubiquitination and Degradation of CD147 to Reverse Cisplatin Resistance of Tumor Cells" International Journal of Molecular Sciences 18, no. 1: 212. https://doi.org/10.3390/ijms18010212
APA StyleWu, B., Liu, Z.-Y., Cui, J., Yang, X.-M., Jing, L., Zhou, Y., Chen, Z.-N., & Jiang, J.-L. (2017). F-Box Protein FBXO22 Mediates Polyubiquitination and Degradation of CD147 to Reverse Cisplatin Resistance of Tumor Cells. International Journal of Molecular Sciences, 18(1), 212. https://doi.org/10.3390/ijms18010212





