Chemical Composition and Antioxidant Properties of Powders Obtained from Different Plum Juice Formulations
Abstract
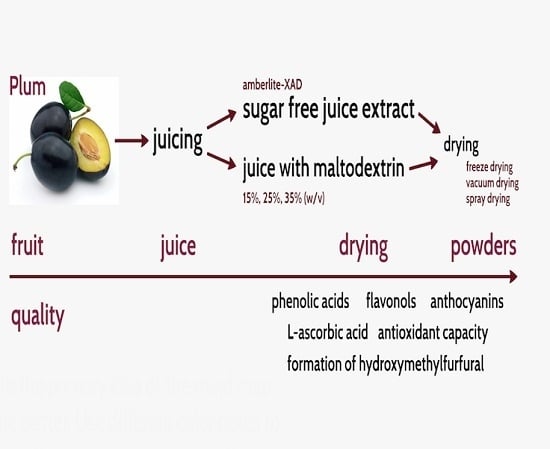
:1. Introduction
2. Results and Discussion
2.1. Polyphenolic Compounds
2.2. l-Ascorbic Acid (AA)
2.3. 5-Hydroxymethylfurfural (HMF)
2.4. Antioxidant Capacity
3. Material and Methods
3.1. Reagents
3.2. Material
3.3. Drying Methods
3.4. Moisture Content
3.5. Identification of Polyphenols by the LC-MS QTof Method
3.6. Quantification of Polyphenols Using the UPLC-PDA System
3.7. l-Ascorbic Acid
3.8. Hydroxymethylfurfural (HMF)
3.9. Trolox Equivalent Antioxidant Capacity (TEAC) and Ferric Reducing Antioxidant Potential (FRAP)
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- FAOSTAT Statistics Division, Food and Agriculture Organization of the United Nations. Available online: http://faostat3.fao.org/home/E (accessed on 11 January 2017).
- Walkowiak-Tomczak, D.; Reguła, J.; Łysiak, G. Physico-chemical properties and antioxidant activity of selected plum cultivars fruit. Acta Sci. Pol. Technol. Aliment. 2008, 7, 15–22. [Google Scholar]
- Michalska, A.; Honke, J.; Łysiak, G.; Andlauer, W. Effect of drying parameters on the formation of early and intermediate stage products of the Maillard reaction in different plum (Prunus domestica L.) cultivars. LWT 2016, 65, 932–938. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.; Bu, S.Y.; Lerner, M.R.; Lancaster, E.A.; Bellmer, D.; Marlow, D.; Lightfoot, S.A.; Arjmandi, B.H.; Brackett, D.J.; Lucas, E.A.; et al. Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone 2006, 39, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Lucas, E.A.; Franklin, M.; Marlow, D.; Brackett, D.J.; Boldrin, E.A.; Devareddy, L.; Arjmandi, B.H.; Smith, B.J. Comparison of dried plum supplementation and intermittent PTH in restoring bone in osteopenic orchidectomized rats. Osteoporos. Int. 2007, 18, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.; Martino, H.S.D.; Simbo, S.; Byrne, D.; Mertens-Talcott, S.U. Consumption of polyphenol-rich peach and plum juice prevents risk factors for obesity-related metabolic disorders and cardiovascular disease in Zucker rats. J. Nutr. Biochem. 2015, 26, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Kalt, W.; Carey, A.N.; Vinqvist-Tymchuk, M.; McDonald, J.; Joseph, J.A. Plum juice, but not dried plum powder, is effective in mitigating cognitive deficits in aged rats. Nutrition 2009, 25, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC−DAD−ESIMS analysis of phenolic compounds in nectarines, peaches and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Karaköse, H.; Rühmann, S.; Goldner, K.; Neumüller, M.; Treutter, D.; Kuhnert, N. Identification of phenolic compounds in plum fruits (Prunus salicina L. and Prunus domestica L.) by high-performance liquid chromatography/tandem mass spectrometry and characterization of varieties by quantitative phenolic fingerprints. J. Agric. Food Chem. 2013, 61, 12020–12031. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Bansal, N.; Zhang, M.; Schuck, P. Introduction to food powders. In Handbook of Food Powders; Woodhead Publishing: Sawston, UK, 2013; pp. 1–27. [Google Scholar]
- Jiang, H.; Adhikari, B. Fruit and vegetable powders. In Handbook of Food Powders; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 532–552. [Google Scholar]
- Tsami, E.; Krokida, M.K.; Drouzas, A.E. Effect of drying method on the sorption characteristics of model fruit powders. J. Food Eng. 1998, 38, 381–392. [Google Scholar] [CrossRef]
- Motevali, A.; Minaei, S.; Khoshtagaza, M.H. Evaluation of energy consumption in different drying methods. Energy Convers. Manag. 2011, 52, 1192–1199. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Mujumdar, A.S.; Wang, S. Trends in microwave-related drying of fruits and vegetables. Trends Food Sci. Technol. 2006, 17, 524–534. [Google Scholar] [CrossRef]
- Alamilla-Beltrán, L.; Chanona-Pérez, J.J.; Jiménez-Aparicio, A.R.; Gutiérrez-López, G.F. Description of morphological changes of particles along spray drying. J. Food Eng. 2005, 67, 179–184. [Google Scholar] [CrossRef]
- Hammami, C.; René, F. Determination of freeze-drying process variables for strawberries. J. Food Eng. 1997, 32, 133–154. [Google Scholar] [CrossRef]
- Ajandouz, E.; Desseaux, V.; Tazi, S.; Puigserver, A. Effects of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem. 2008, 107, 1244–1252. [Google Scholar] [CrossRef]
- Vvedenskaya, I.O.; Rosen, R.T.; Guido, J.E.; Russell, D.J.; Mills, K.A.; Vorsa, N. Characterization of flavonols in cranberry (Vaccinium macrocarpon) powder. J. Agric. Food Chem. 2004, 52, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhu, H.; Zhang, G.; Tang, W. Effect of superfine grinding on the physicochemical properties and antioxidant activity of red grape pomace powders. Powder Technol. 2015, 286, 838–844. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Ćetković, G.; Čanadanović-Brunet, J.; Pajin, B.; Djilas, S.; Petrović, J.; Lončarević, I.; Stajčić, S.; Vulić, J. Sour cherry pomace extract encapsulated in whey and soy proteins: Incorporation in cookies. Food Chem. 2016, 207, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Horszwald, A.; Andlauer, W.; Heritier, J. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013, 5, 2858–2863. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.R.; Senoussi, A.; Dumoulin, E.D.; Lebert, A. Spray drying of concentrated fruit juices. Dry. Technol. 1993, 11, 1081–1092. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Piga, A.; del Caro, A.; Corda, G. From plums to prunes: Influence of drying parameters on polyphenols and antioxidant activity. J. Agric. Food Chem. 2003, 51, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kim, D.-O.; Moon, H.Y.; Kang, H.G.; Lee, C.Y. Contribution of individual polyphenolics to total antioxidant capacity of plums. J. Agric. Food Chem. 2003, 51, 7240–7245. [Google Scholar] [CrossRef] [PubMed]
- Functional Foods: Biochemical and Processing Aspects, Volume 2. Available online: https://www.crcpress.com/Functional-Foods-Biochemical-and-Processing-Aspects-Volume-2/Shi-Mazza-Maguer/p/book/9781566769020 (accessed on 11 January 2017).
- United States Department of Agriculture, Agricultural Research Service. Available online: https://www.ars.usda.gov (accessed on 16 January 2017).
- Ramesh, M.N.; Wolf, W.; Tevini, D.; Jung, G. Studies on inert gas processing of vegetables. J. Food Eng. 1999, 40, 199–205. [Google Scholar] [CrossRef]
- Kaya, A.; Aydın, O.; Kolaylı, S. Effect of different drying conditions on the vitamin C (ascorbic acid) content of Hayward kiwifruits (Actinidia deliciosa Planch). Food Bioprod. Process. 2010, 88, 165–173. [Google Scholar] [CrossRef]
- Rada-Mendoza, M.; Sanz, M.L.; Olano, A.; Villamiel, M. Formation of hydroxymethylfurfural and furosine during the storage of jams and fruit-based infant foods. Food Chem. 2004, 85, 605–609. [Google Scholar] [CrossRef]
- Murkovic, M.; Pichler, N. Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine. Mol. Nutr. Food Res. 2006, 50, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Villamiel, M.; Castillo, M.D.D.; Martín, C.S.; Corzo, N. Assessment of the thermal treatment of orange juice during continuous microwave and conventional heating. J. Sci. Food Agric. 1998, 78, 196–200. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, Y.; Wu, T.; Huang, C.; Pei, K.; Zhang, G.; Lin, X.; Bai, W.; Ou, S. Chlorogenic acid increased 5-hydroxymethylfurfural formation when heating fructose alone or with aspartic acid at two pH levels. Food Chem. 2016, 190, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Del Caro, A.; Piga, A.; Pinna, I.; Fenu, P.M.; Agabbio, M. Effect of drying conditions and storage period on polyphenolic content, antioxidant capacity, and ascorbic acid of prunes. J. Agric. Food Chem. 2004, 52, 4780–4784. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Kljusuric, J.G.; Carle, R.; Schieber, A. Recovery of anthocyanins from grape pomace extracts (Vitis vinifera L. cv. Cabernet Mitos). Eur. Food Res. Technol. 2005, 220, 431–437. [Google Scholar] [CrossRef]
- Figiel, A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Polski Komitet Normalizacyjny. Fruit and Vegetable Products—Preparation of Samples and Testing Methods—Determination of Ascorbic Acid Content; PN-90/A-75101/11; Polski Komitet Normalizacyjny: Warszawa, Poland, 1990. [Google Scholar]
- Gökmen, V.; Senyuva, H.Z. Improved method for the determination of hydroxymethylfurfural in baby foods using liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2006, 54, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay—Electron-transfer reactions with organic compounds in solutions containing nitrite or nitrate. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
| Compound | Rt (min) | λmax (nm) | MS [M − H]− (m/z) | MS/MS [M − H]− (m/z) |
|---|---|---|---|---|
| Phenolic acids | ||||
| Neochlorogenic acid | 3.09 | 326 | 353.08 | 191.05 |
| 3-O-p-coumaroylquinic acid | 3.86 | 312 | 337.09 | 163.05 |
| Chlorogenic acid | 4.16 | 326 | 353.08 | 191.05 |
| 3-Feruloylquinic acid | 4.30 | 324 | 367.10 | 193.05 |
| Methyl-3-caffeoylquinate | 4.74 | 326 | 367.10 | 135.00/193.01 |
| Flavonols | ||||
| Quercetin-3-O-rutinoside | 6.50 | 264/350 | 609.14 | 301.03 |
| Quercetin-3-O-galactoside | 6.66 | 346 | 463.08 | 301.02 |
| Quercetin-3-O-glucoside | 6.75 | 346 | 463.18 | 301.02 |
| Quercetin-3-O-(6′′acetylgalactoside) | 8.18 | 363 | 505.09 | 301.02 |
| Anthocyanins | ||||
| Cyanidin-3-O-glucoside | 3.72 | 278/515 | 449.10 | 287.05 |
| Cyanidin-3-O-rutinoside | 3.93 | 279/515 | 595.16 | 287.05 |
| Peonidin-3-O-rutinoside | 4.62 | 286/517 | 609.17 | 301.07 |
| Sample | Drying Method | Phenolic Acids | Flavonols | Anthocyanins | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-Feruloylquinic Acid | Neochlorogenic Acid | 3-O-p-Coumaroyl Quinic Acid | Chlorogenic Acid | Methyl 3-Caffeoyl Quinicate | Quercetin-3-O-glucoside | Quercetin-3-O-galactoside | Quercetin-3-O-rutinoside | Quercetin-3-O-(6′′Acetyl galactoside) | Cyanidin-3-O-glucoside | Cyanidin-3-O-rutinoside | Peonidin-3-O-rutinoside | ||
| 15% M | FD | 20.92 ± 0.11 g | 17.46 ± 0.08 g | 6.56 ± 0.06 g | 9.65 ± 0.69 g | 0.58 ± 0.01 a | 8.57 ± 0.24 g | 8.51 ± 0.26 j | 2.04 ± 0.21 f | 1.67 ± 0.02 d | 0.21± 0.01 d,e | 0.58 ± 0.01 f,g | LOD |
| VD 40 °C | 21.89 ± 0.15 h | 20.32 ± 0.04 h | 9.89 ± 0.01 i | 10.29 ± 0.37 g | 0.77 ± 0.01 a | 9.89 ± 0.07 h | 2.43 ± 0.01 g | 0.11 ± 0.01 a | 1.31 ± 0.01 c | 0.27 ± 0.01 f | 0.72 ± 0.03 h | LOD | |
| VD 60 °C | 15.91 ± 0.12 f | 15.25 ± 0.23 f | 7.49 ± 0.26 h | 5.41 ± 0.4 b–e | 0.71 ± 0.03 a | 7.55 ± 0.09 e | 1.84 ± 0.02 f | 0.42 ± 0.03 b | 1.35 ± 0.03 c | 0.11 ± 0.02 b | 0.31 ± 0.01 c,d | LOD | |
| VD 80 °C | 8.75 ± 0.27 a | 11.76 ± 0.05 d | 5.11 ± 0.04 e | 3.96 ± 0.05 a,b | 3.81 ± 0.15 c,d | 7.62 ± 0.02 e | 0.74 ± 0.02 b–d | 2.48 ± 0.02 g | 2.84 ± 0.06 f | 0.03 ± 0.01 a | 0.13 ± 0.02 a | LOD | |
| SD | 21.46 ± 0.11 g,h | 17.93 ± 0.19 g | 5.74 ± 0.11 f | 9.41 ± 0.1 g | 5.98 ± 0.36 e | 8.22 ± 0.02 f | 0.87 ± 0.05 c,d | 2.34 ± 0.05 d | 1.29 ± 0.02 c | 0.39 ± 0.02 g | 0.99 ± 0.02 i | LOD | |
| 25% M | FD | 14.46 ± 0.08 e | 11.45 ± 0.33 d | 5.74 ± 0.17 f | 6.39 ± 0.43 e,f | 0.31 ± 0.08 a | 0.88 ± 0.01 a | 6.03 ± 0.03 i | 1.48 ± 0.02 d | 1.11 ± 0.01 b | 0.28 ± 0.01 f | 0.68 ± 0.01 h | LOD |
| VD 40 °C | 13.52 ± 0.07 d | 11.71 ± 0.01 d | 5.91 ± 0.05 f | 6.11 ± 0.41 d–f | 0.43 ± 0.03 a | 5.93 ± 0.04 d | 1.46 ± 0.02 e | 0.04 ± 0.01 a | 1.08 ± 0.01 b | 0.24 ± 0.01 e,f | 0.61 ± 0.02 g | LOD | |
| VD 60 °C | 13.51 ± 0.05 d | 11.83 ± 0.09 d | 5.31 ± 0.02 e | 6.46 ± 0.04 e,f | 0.52 ± 0.01 a | 5.84 ± 0.02 d | 1.43 ± 0.01 e | 0.21 ± 0.02 a,b | 1.06 ± 0.01 b | 0.11 ± 0.03 b | 0.29 ± 0.01 b,c | LOD | |
| VD 80 °C | 10.65 ± 0.18 b | 10.62 ± 0.11 c | 3.92 ± 0.07 b,c | 4.13 ± 0.11 a–c | 3.33 ± 0.09 b,c | 5.28 ± 0.07 c | 0.48 ± 0.01 a,b | 1.53 ± 0.03 d,e | 2.57 ± 0.09 e | 0.04 ± 0.01 a | 0.12 ± 0.01 a | LOD | |
| SD | 15.32 ± 0.49 f | 12.87 ± 0.17 e | 7.31 ± 0.08 h | 6.91 ± 0.56 f | 4.42 ± 0.55 d | 6.08 ± 0.05 d | 0.67 ± 0.01 a–c | 1.75 ± 0.01 e | 1.07 ± 0.01 b | 0.62 ± 0.01 h | 1.51 ± 0.03 j | LOD | |
| 35% M | FD | 10.08 ± 0.03 b | 8.04 ± 0.01 a | 3.72 ± 0.04 b | 4.78 ± 0.12 a–d | 0.25 ± 0.01 a | 0.64 ± 0.01 a | 4.31 ± 0.01 h | 1.05 ± 0.01 c | 0.76 ± 0.01 a | 0.16 ± 0.01 c,d | 0.44 ± 0.05 e | LOD |
| VD 40 °C | 9.25 ± 0.01 a | 7.93 ± 0.03 a | 3.05 ± 0.04 a | 4.09 ± 0.28 a–c | 0.23 ± 0.07 a | 3.98 ± 0.03 b | 1.01 ± 0.01 d | 0.03 ± 0.01 a | 0.73 ± 0.01 a | 0.1 ± 0.01 b | 0.26 ± 0.01 b,c | LOD | |
| VD 60 °C | 9.22 ± 0.04 a | 8.21 ± 0.05 a | 3.08 ± 0.07 a | 5.22 ± 0.43 c–e | 0.33 ± 0.01 a | 4.07 ± 0.01 b | 0.98 ± 0.01 d | 0.14 ± 0.01 a | 0.74 ± 0.02 a | 0.08 ± 0.01 b | 0.22 ± 0.01 b | LOD | |
| VD 80 °C | 9.30 ± 0.16 a | 8.28 ± 0.2 a,b | 4.17 ± 0.08 c,d | 3.76 ± 0.08 a | 2.74 ± 0.16 b | 4.07 ± 0.03 b | 0.43 ± 0.02 a | 1.18 ± 0.05 c | 0.77 ± 0.01 a | 0.13 ± 0.01 b,c | 0.38 ± 0.02 d,e | LOD | |
| SD | 11.44 ± 0.25 c | 8.84 ± 0.01 b | 4.43 ± 0.02 d | 4.51 ± 0.01 a–c | 3.27 ± 0.01 b,c | 4.12 ± 0.03 b | 0.46 ± 0.01 a,b | 1.19 ± 0.02 c | 0.68 ± 0.01 a | 0.21 ± 0.01 d,e | 0.52 ± 0.01 f | LOD | |
| Extract | FD | 1051.41 ± 6.37 C | 1025.23 ± 12.21 C | 439.94 ± 8.73 A,B | 414.31 ± 1.11 B | 24.61 ± 0.87 A | 73.06 ± 1.12 A | 500.52 ± 4.87 D | 125.2 ± 0.35 B | 102.89 ± 0.15 B,C | 26.78 ± 0.4 B | 70.31 ± 0.93 A,B | 0.58 ± 0.01 A,B |
| VD 40 °C | 996.32 ± 10.69 C | 1017.86 ± 2.09 C | 472.78 ± 9.93 B | 395.82 ± 0.08 B | 36.68 ± 1.43 A,B | 524.46 ± 1.91 C | 129.35 ± 0.54 B | 9.21 ± 0.46 A | 104.18 ± 0.37 B,C | 25.76±0.25 B | 69.62 ± 0.37 A,B | 0.58 ± 0.02 A,B | |
| VD 60 °C | 851.27 ± 35.55 B | 885.16 ± 10.81 B | 458.76 ± 14.99 A,B | 358.45 ± 2.46 A | 54.74 ± 2.41 B | 514.07 ± 6.79 C | 141.26 ± 0.57 B,C | 24.28 ± 3.87 A | 88.89 ± 9.94 B | 24.37 ± 3.71 A,B | 66.42 ± 10.6 A,B | 0.55 ± 0.09 A,B | |
| VD 80 °C | 541.81 ± 15.22 A | 645.22 ± 2.48 A | 405.2 ± 20.67 A | 335.76 ± 12.09 A | 708.46 ± 10.24 D | 458.41 ± 9.48 B | 147.09 ± 5.61 C | 108.53 ± 16.46 B | 60.57 ± 1.45 A | 17.18 ± 2.75 A | 52.54 ± 3.13 A | 0.44 ± 0.07 A | |
| SD | 1169.09 ± 27.83 D | 1104.03 ± 33.02 D | 529.99 ± 13.61 C | 469.95 ± 4.49 C | 392.14 ± 5.64 C | 610.68 ± 13.8 D | 72.61 ± 0.75 A | 188.22 ± 16.59 C | 119.82 ± 9.5 C | 28.05 ± 0.88 B | 78.34 ± 3.56 B | 0.65 ± 0.03 B | |
| Sample | Drying Method | Dry Matter | Ascorbic Acid | Hydroxymethylfurfural | Antioxidant Capacity | |
|---|---|---|---|---|---|---|
| TEAC ABTS | FRAP | |||||
| 15% M | FD | 93.32 ± 0.17 | 12.41 ± 0.41 a–c | 0.32 ± 0.07 a,b | 2.66 ± 0.23 c | 2.13 ± 0.06 e |
| VD 40 °C | 97.20 ± 0.03 | 9.93 ± 0.72 a | 0.77 ± 0.01 a,b,c | 3.06 ± 0.01 d | 2.75 ± 0.07 e | |
| VD 60 °C | 98.74 ± 0.06 | 10.63 ± 0.78 a | 1.36 ± 0.03 c | 2.77 ± 0.09 c | 2.28 ± 0.05 e | |
| VD 80 °C | 99.31 ± 0.01 | 20.23 ± 0.48 e | 11.14 ± 0.88 e | 3.37 ± 0.02 d | 3.01 ± 0.13 f | |
| SD | 97.71 ± 0.01 | 14.41 ± 1.36 b–d | 0.42 ± 0.02 a–c | 2.42 ± 0.26 c | 2.01 ± 0.16 e | |
| 25% M | FD | 94.67 ± 0.06 | 11.41 ± 0.01 a,b | 0.05 ± 0.005 a | 2.06 ± 0.03 b | 1.56 ± 0.07 c |
| VD 40 °C | 96.20 ± 0.04 | 10.21 ± 1.29 a | 0.12 ± 0.01 a | 1.71 ± 0.02 a | 1.56 ± 0.1 c | |
| VD 60 °C | 98.61 ± 0.03 | 11.58 ± 0.13 a,b | 0.44 ± 0.02 a–c | 2.06 ± 0.1 b | 1.53 ± 0.1 c | |
| VD 80 °C | 99.13 ± 0.01 | 15.37 ± 1.21 c,d | 4.05 ± 0.29 d | 2.31 ± 0.02 b | 1.71 ± 0.06 d | |
| SD | 98.78 ± 0.01 | 11.25 ± 1.08 a,b | 0.48 ± 0.04 a–c | 1.81 ± 0.11 a | 1.57 ± 0.06 c | |
| 35% M | FD | 95.62 ± 0.03 | 11.35 ± 0.06 a,b | 0.05 ± 0.01 a | 1.63 ± 0.12 a | 1.22 ± 0.12 b |
| VD 40 °C | 95.48 ± 0.01 | 10.99 ± 0.18 a | 0.10 ± 0.01 a | 1.47 ± 0.01 a | 1.01 ± 0.14 a | |
| VD 60 °C | 98.07 ± 0.10 | 9.22 ± 0.65 a | 0.21 ± 0.005 a | 1.45 ± 0.03 a | 0.97 ± 0.18 a | |
| VD 80 °C | 98.98 ± 0.03 | 15.92 ± 0.86 d | 1.26 ± 0.19b c | 1.65 ± 0.11 a | 1.21 ± 0.07 b | |
| SD | 98.98 ± 0.01 | 10.72 ± 0.65 a | 0.41 ± 0.03 a,b,c | 1.6 ± 0.13 a | 1.07 ± 0.09 a | |
| Extract | FD | 92.57 ± 0.14 | 14.61 ± 1.69 A | 0.01 ± 0.001 A | 98.9 ± 3.24 C | 85.44 ± 4.14 C |
| VD 40 °C | 96.25 ± 0.17 | 14.55 ± 0.27 A | 0.02 ± 0.001 A,B | 95.15 ± 1.33 B | 80.92 ± 2.5 C | |
| VD 60 °C | 91.83 ± 1.46 | 16.08 ± 1.21 A | 0.03 ± 0.004 C | 98.03 ± 0.26 C | 87.15 ± 2.53 D | |
| VD 80 °C | 96.71 ± 0.08 | 15.28 ± 1.21 A | 0.02 ± 0.001 A,B | 80.86 ± 0.82 A | 75.92 ± 0.78 A | |
| SD | 97.56 ± 0.06 | 33.78 ± 1.96 B | LOD | 81.18 ± 3.84 A | 78.3 ± 1.81 B | |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska, A.; Wojdyło, A.; Łysiak, G.P.; Figiel, A. Chemical Composition and Antioxidant Properties of Powders Obtained from Different Plum Juice Formulations. Int. J. Mol. Sci. 2017, 18, 176. https://doi.org/10.3390/ijms18010176
Michalska A, Wojdyło A, Łysiak GP, Figiel A. Chemical Composition and Antioxidant Properties of Powders Obtained from Different Plum Juice Formulations. International Journal of Molecular Sciences. 2017; 18(1):176. https://doi.org/10.3390/ijms18010176
Chicago/Turabian StyleMichalska, Anna, Aneta Wojdyło, Grzegorz P. Łysiak, and Adam Figiel. 2017. "Chemical Composition and Antioxidant Properties of Powders Obtained from Different Plum Juice Formulations" International Journal of Molecular Sciences 18, no. 1: 176. https://doi.org/10.3390/ijms18010176
APA StyleMichalska, A., Wojdyło, A., Łysiak, G. P., & Figiel, A. (2017). Chemical Composition and Antioxidant Properties of Powders Obtained from Different Plum Juice Formulations. International Journal of Molecular Sciences, 18(1), 176. https://doi.org/10.3390/ijms18010176