Renal Protective Effects of Low Molecular Weight of Inonotus obliquus Polysaccharide (LIOP) on HFD/STZ-Induced Nephropathy in Mice
Abstract
:1. Introduction
2. Results
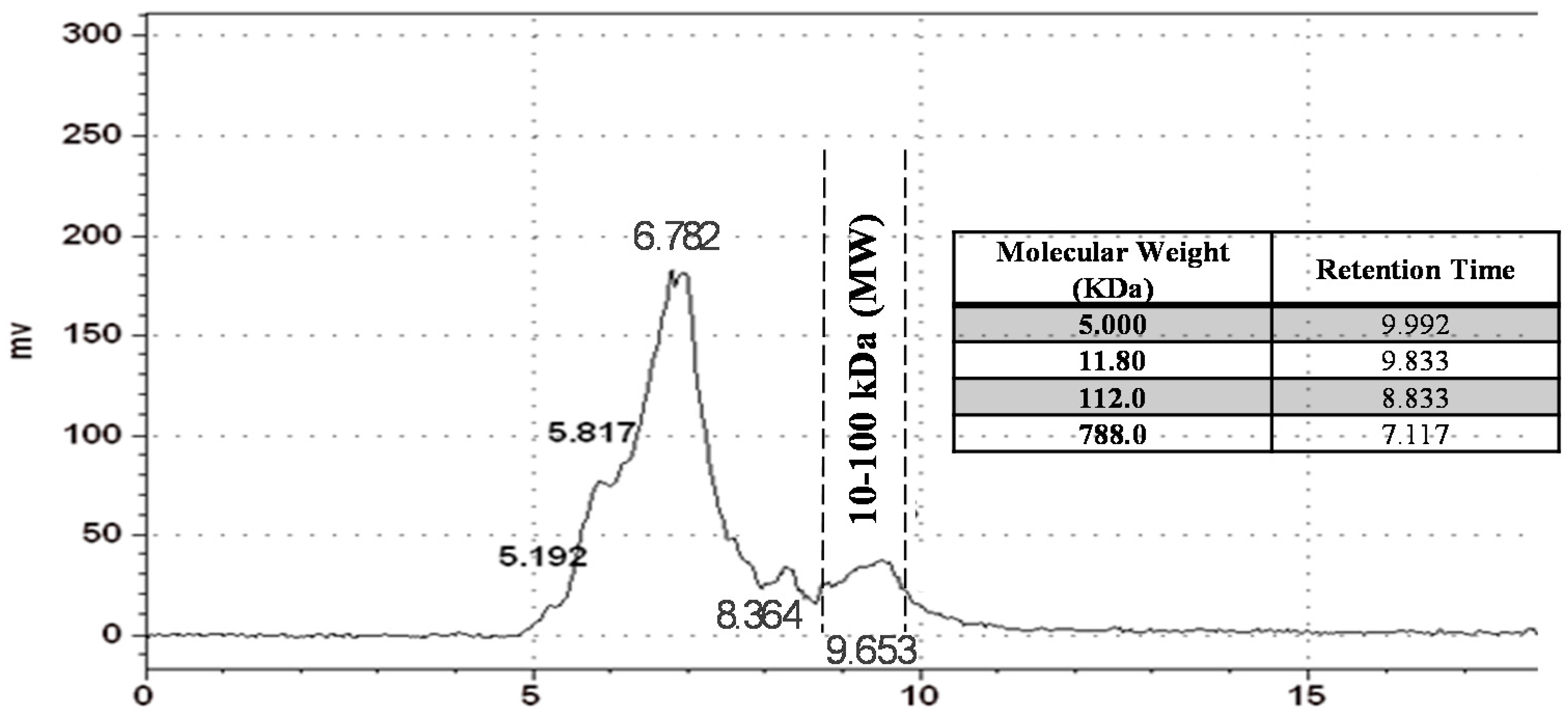
2.1. Inonotus obliquus (IO) Polysaccharide Analysis by Gel Permeation Chromatography (GPC)
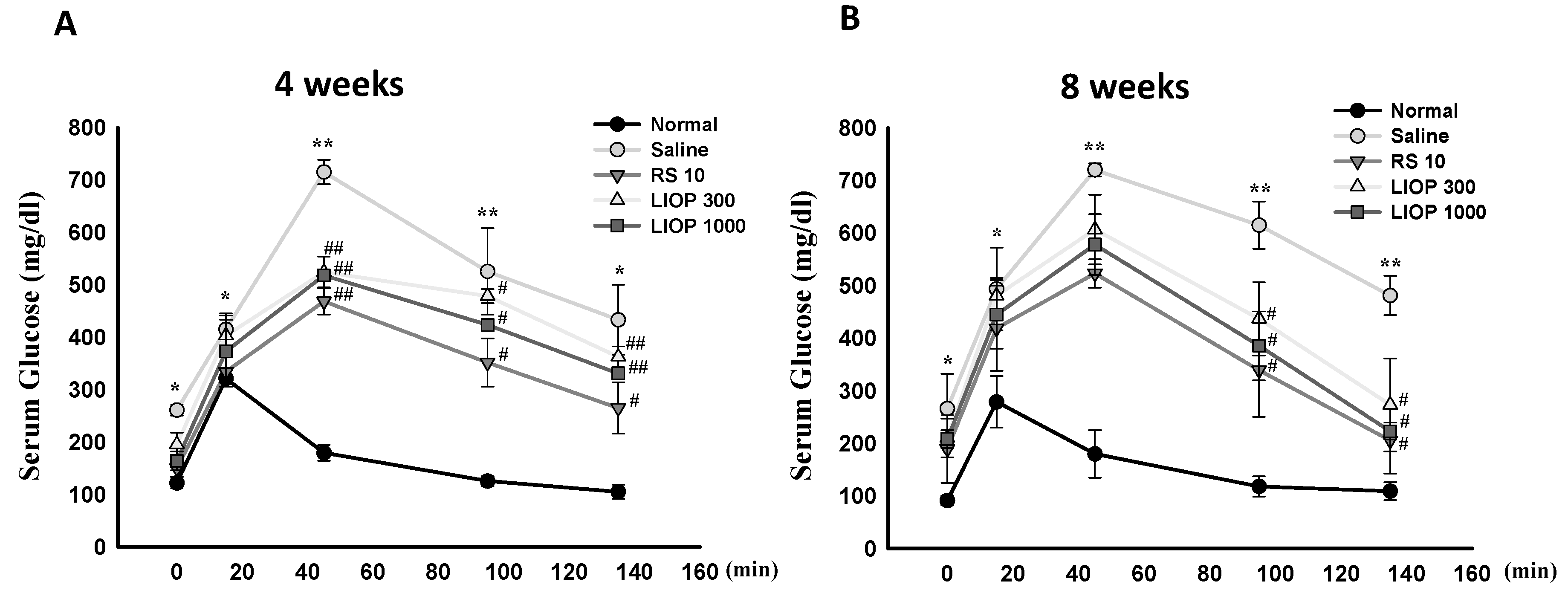
2.2. Effect of Inonotus obliquus Polysaccharides (LIOP) on the Glucose Tolerance Test (OGTT) in C57BL/6 Mice
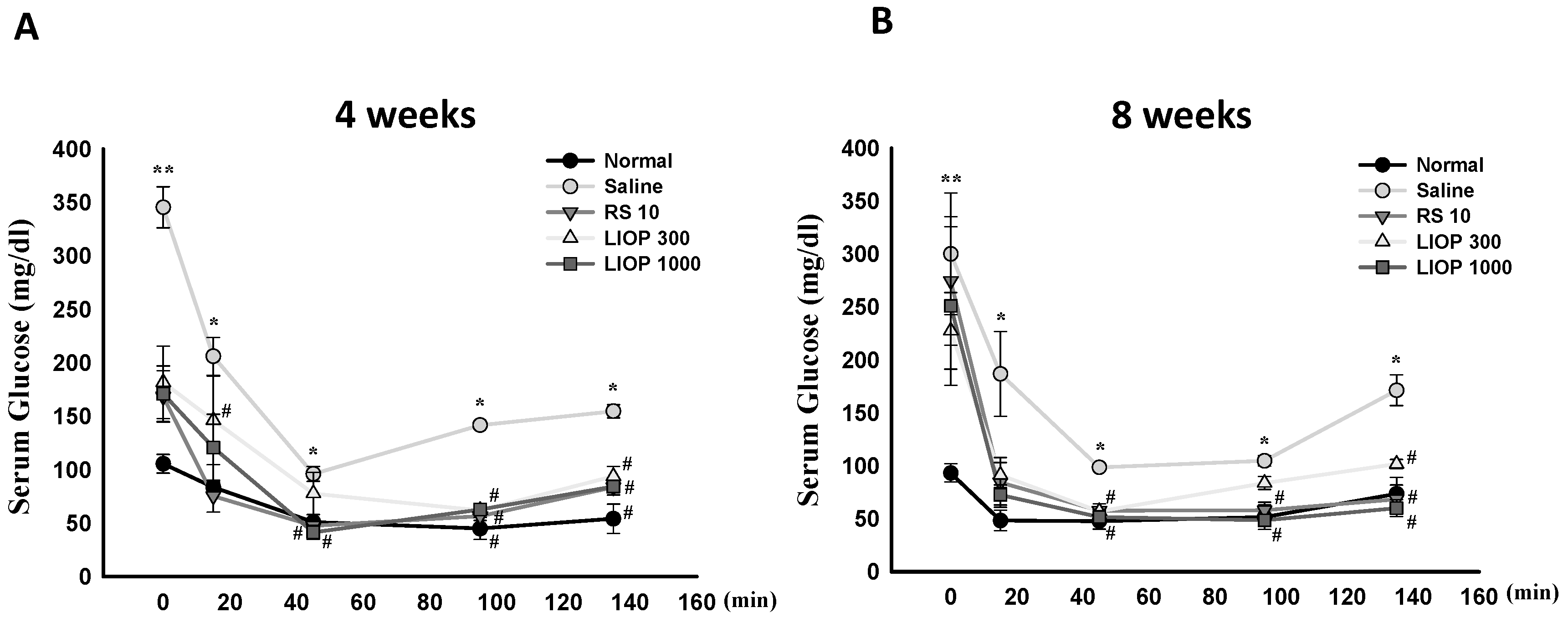
2.3. Effect of LIOP on the Insulin Tolerance Test (ITT) in C57BL/6 Mice
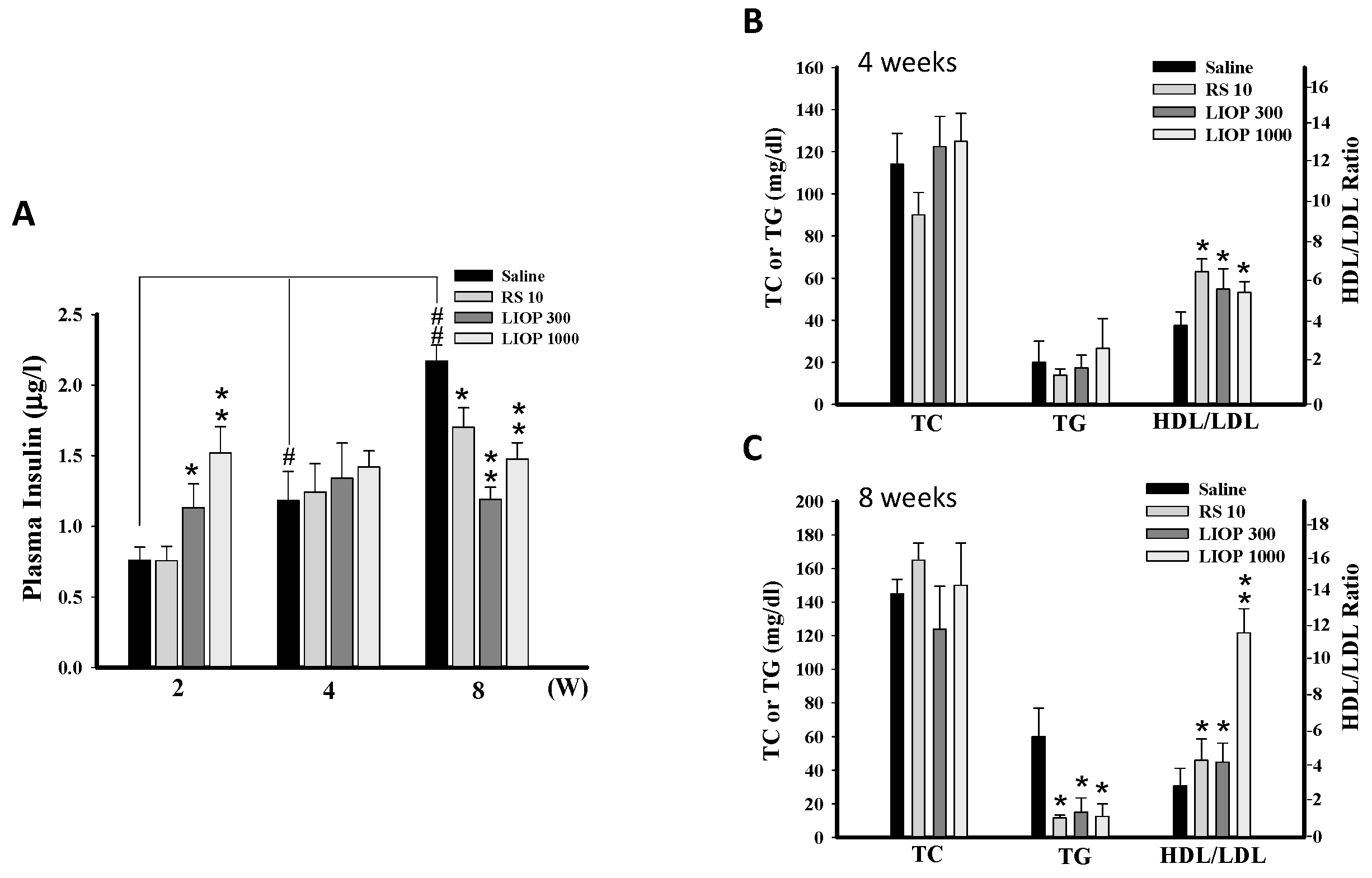
2.4. Effects of LIOP on the Insulin Concentration and Triglyceride, Total Cholesterol and HDL/LDL Ratio in C57BL/6 Mice
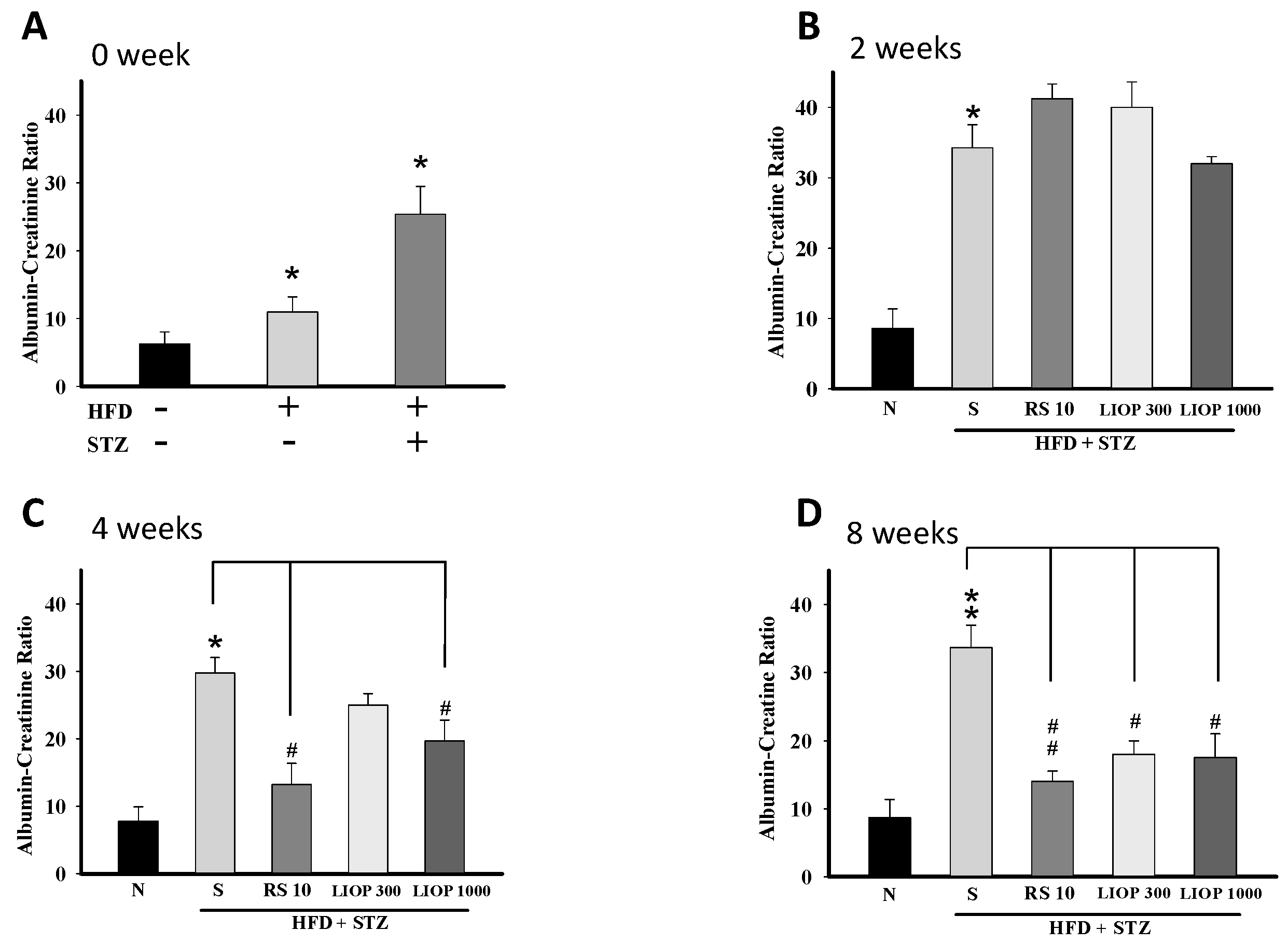
2.5. Effect of LIOP on Kidney Function in C57BL/6 Mice
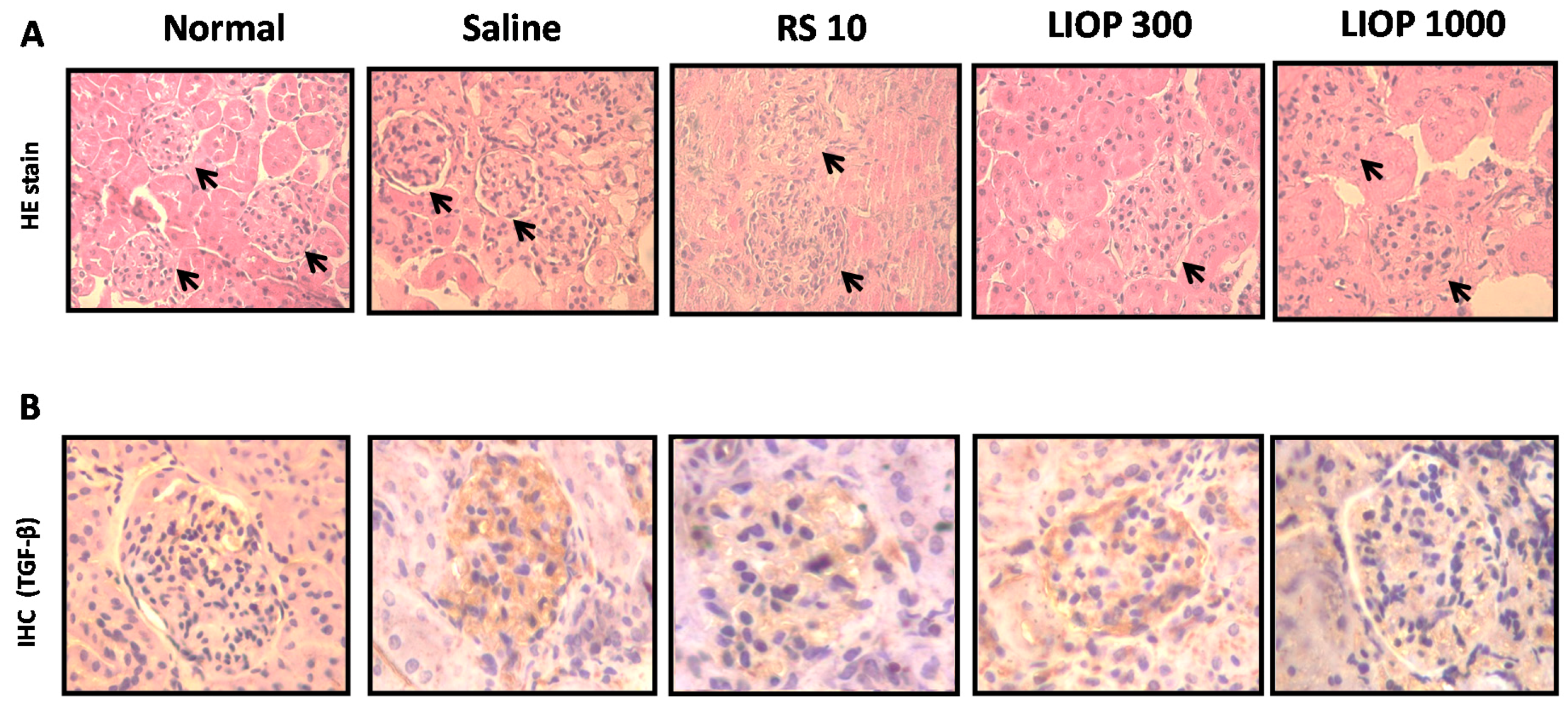
2.6. Renal Hematoxylin and Eosin and Immunohistochemical Staining in the C57BL/6 Mice
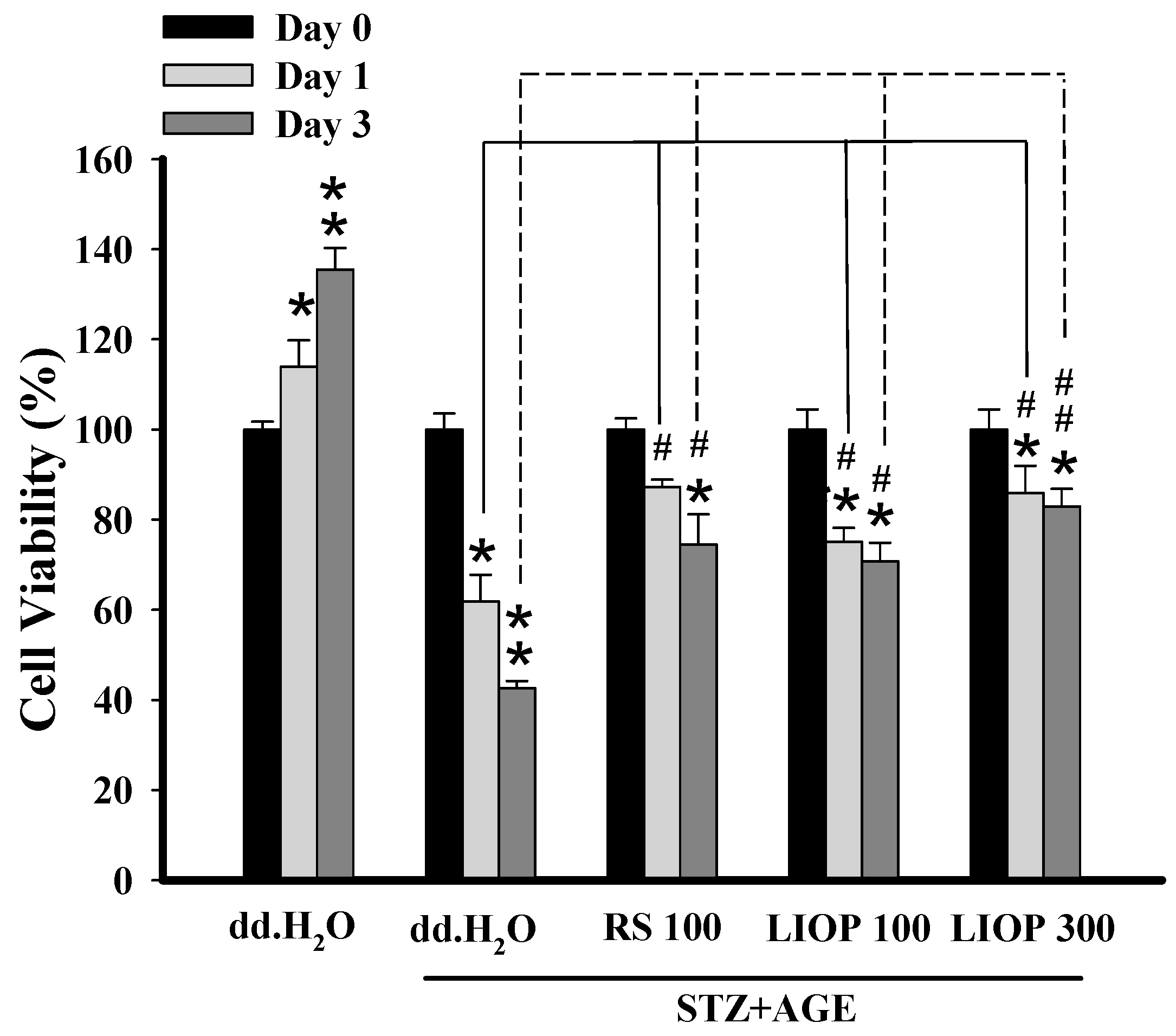
2.7. The Protective Effect of LIOP in STZ + AGEs-Induced Renal Cell Toxicity
2.8. The Effects of LIOP in the NF-κB and TGF-β Expression on Renal Tubular Cell
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparations for Polysaccharide Extracts of Inonotus obliquus
4.3. Molecular Weight Determination by GPC
4.4. Animal Preparation
4.5 Oral Glucose Tolerance Test (OGTT)
4.6. Insulin Tolerance Test (ITT)
4.7. Determination of Biochemistry Indexes
4.8. The Hematoxylin and Eosin (H & E)
4.9. Immunohistochemical Stain
4.10. Anti-Glucotoxicity Assessment (in Vitro)
4.11. Western Blotting Analysis
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, P.C.; Zhao, S.; Yang, B.Y.; Wang, Q.H.; Kuang, H.X. Anti-diabetic polysaccharides from natural sources: A review. Carbohydr. Polym. 2016, 148, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhang, D.; Wu, J.; Chen, C.; Xu, Z.; Yang, H.; Zhou, P. A novel proteoglycan from Ganoderma lucidum fruiting bodies protects kidney function and ameliorates diabetic nephropathy via its antioxidant activity in C57BL/6 db/db mice. Food Chem. Toxicol. 2014, 63, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Lu, X.; Cong, W.; Xing, X.; Tan, Y.; Li, Y.; Li, X.; Jin, L.; Wang, X.; Dong, J.; et al. Multiple low-dose radiation prevents type 2 diabetes-induced renal damage through attenuation of dyslipidemia and insulin resistance and subsequent renal inflammation and oxidative stress. PLoS ONE 2014, 9, e92574. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Choi, B.H.; Ku, S.K.; Park, J.H.; Oh, E.; Kwak, M.K. Beneficial effects of sarpogrelate and rosuvastatin in high fat diet/streptozotocin-induced nephropathy in mice. PLoS ONE 2016, 11, e0153965. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Song, D.; Mu, H.; Zhang, W.; Sun, F.; Duan, J. Investigation of three lignin complexes with antioxidant and immunological capacities from Inonotus obliquus. Int. J. Biol. Macromol. 2016, 86, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Kang, J.H.; Kim, D.K.; Oh, S.H.; Kim, M.K. Orally administered aqueous extract of Inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. J. Ethnopharmacol. 2012, 143, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Hyun, C.K. Insulin-sensitizing and beneficial lipid-metabolic effects of the water-soluble melanin complex extracted from Inonotus obliquus. Phytother. Res. 2014, 28, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, H.; Dong, P.; Fu, L.; Zhang, X. Phytochemical characteristics and hypoglycaemic activity of fraction from mushroom Inonotus obliquus. J. Sci. Food Agric. 2010, 90, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Sun, J.E.; Lu, Z.M.; Zhang, X.M.; Dou, W.F.; Xu, Z.H. Beneficial effects of the ethanol extract from the dry matter of a culture broth of Inonotus obliquus in submerged culture on the antioxidant defence system and regeneration of pancreatic β-cells in experimental diabetes in mice. Nat. Prod. Res. 2010, 24, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Van, Q.; Nayak, B.N.; Reimer, M.; Jones, P.J.; Fulcher, R.G.; Rempel, C.B. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell screening assay. J. Ethnopharmacol. 2009, 125, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Q.; Ding, S.; Fan, L. Antioxidant activities of five polysaccharides from Inonotus obliquus. Int. J. Biol. Macromol. 2012, 50, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Chen, H.; Dong, P.; Zhang, X.; Zhang, M. Effects of ultrasonic treatment on the physicochemical properties and DPPH radical scavenging activity of polysaccharides from mushroom Inonotus obliquus. J. Food Sci. 2010, 75, C322–C327. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xu, X.; Li, J. Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn stover medium. Food Chem. 2012, 134, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Han, S.B.; Lee, H.W.; Ahnb, H.J.; Yoonb, Y.D.; Jungb, J.K. Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sci. 2005, 77, 2438–2456. [Google Scholar] [PubMed]
- Chen, Y.; Huang, Y.; Cui, Z.; Liu, J. Purification, characterization and biological activity of a novel polysaccharide from Inonotus obliquus. Int. J. Biol. Macromol. 2015, 79, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.Z.; Jin, W.R.; Yu, X.J. Protective effect of polysaccharides from Inonotus obliquus on streptozotocin-induced diabetic symptoms and their potential mechanisms in rats. Evid. Based Complement. Altern. Med. 2014, 2014, 841496. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sheng, Y.; Yu, M.; Li, K.; Ren, G.; Xu, X.; Qu, J. Antioxidant activity of Inonotus obliquus polysaccharide and its amelioration for chronic pancreatitis in mice. Int. J. Biol. Macromol. 2016, 87, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, H.; Lv, X.; Wang, Y.; Gao, H.; Wang, M. Protection of chronic renal failure by a polysaccharide from Cordyceps sinensis. Fitoterapia 2010, 81, 397–402. [Google Scholar] [CrossRef] [PubMed]
- He, C.Y.; Li, W.D.; Guo, S.X.; Lin, S.Q.; Lin, Z.B. Effect of polysaccharides from Ganoderma lucidum on streptozotocin-induced diabetic nephropathy in mice. J. Asian Nat. Prod. Res. 2006, 8, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Schinzel, R.; Simm, A.; Sebekova, K.; Heidland, A. Advanced glycation end products impair protein turnover in LLC-PK1: Amelioration by trypsin. Kidney Int. Suppl. 2001, 78, S53–S57. [Google Scholar] [CrossRef] [PubMed]
- Stopper, H.; Schinzel, R.; Sebekova, K.; Heidland, A. Genotoxicity of advanced glycation end products in mammalian cells. Cancer Lett. 2003, 190, 151–156. [Google Scholar] [CrossRef]
- Sebeková, K.; Schinzel, R.; Ling, H.; Simm, A.; Xiang, G.; Gekle, M.; Münch, G.; Vamvakas, S.; Heidland, A. Advanced glycated albumin impairs protein degradation in the kidney proximal tubules cell line LLC-PK1. Cell Mol. Biol. (Noisy-le-grand) 1998, 44, 1051–1060. [Google Scholar]
- Huang, J.S.; Chuang, L.Y.; Guh, J.Y.; Yang, Y.L.; Hsu, M.S. Effect of taurine on advanced glycation end products-induced hypertrophy in renal tubular epithelial cells. Toxicol. Appl. Pharmacol. 2008, 233, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Schinzel, R.; Simm, A.; Münch, G.; Sebekova, K.; Kasper, M.; Niwa, T.; Schmitz, C.; Heidland, A. Advanced glycation end products (AGEs)-induced expression of TGF-β 1 is suppressed by a protease in the tubule cell line LLC-PK1. Nephrol. Dial. Transpl. 2001, 16, 1562–1569. [Google Scholar] [CrossRef]
- Simm, A.; Münch, G.; Seif, F.; Schenk, O.; Heidland, A.; Richter, H.; Vamvakas, S.; Schinzel, R. Advanced glycation endproducts stimulate the MAP-kinase pathway in tubulus cell line LLC-PK1. FEBS Lett. 1997, 410, 481–484. [Google Scholar] [CrossRef]
- Samra, Y.A.; Said, H.S.; Elsherbiny, N.M.; Liou, G.I.; El-Shishtawy, M.M.; Eissa, L.A. Cepharanthine and piperine ameliorate diabetic nephropathy in rats: Role of NF-κB and NLRP3 inflammasome. Life Sci. 2016, 157, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, J.; Wang, D.; Zhou, F.; Cai, X.; Lu, W.; Hu, C.; Gu, Z.; Qian, S.; Guan, X.; et al. The aqueous extract of Lycopus lucidus Turcz ameliorates streptozotocin-induced diabetic renal damage via inhibiting TGF-β1 signaling pathway. Phytomedicine 2013, 20, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Ellagic acid, an NF-κB inhibitor, ameliorates renal function in experimental diabetic nephropathy. Chem. Biol. Interact. 2014, 219, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhang, Z.; Wang, H. Antioxidant activities of extracts and subfractions from Inonotus obliquus. Int. J. Food Sci. Nutr. 2009, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R. Immunomodulatory Activity of the water extract from medicinal mushroom Inonotus obliquus. Mycobiology 2005, 33, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kolati, S.R.; Kasala, E.R.; Bodduluru, L.N.; Mahareddy, J.R.; Uppulapu, S.K.; Gogoi, R.; Barua, C.C.; Lahkar, M. BAY 11–7082 ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress and renal inflammation via NF-κB pathway. Environ. Toxicol. Pharmacol. 2015, 39, 690–699. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef]
- Hwang, H.J.; Baek, Y.M.; Kim, S.W.; Kumar, G.S.; Cho, E.J.; Oh, J.Y.; Yun, J.W. Differential expression of kidney proteins in streptozotocin-induced diabetic rats in response to hypoglycemic fungal polysaccharides. J. Microbiol. Biotechnol. 2007, 17, 2005–2017. [Google Scholar] [PubMed]
- Xu, X.; Li, J.; Hu, Y. Polysaccharides from Inonotus obliquus sclerotia and cultured mycelia stimulate cytokine production of human peripheral blood mononuclear cells in vitro and their chemical characterization. Int. Immunopharmacol. 2014, 21, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Balandaykin, M.E.; Zmitrovich, I.V. Review on Chaga medicinal mushroom, Inonotus obliquus (Higher Basidiomycetes): Realm of medicinal applications and approaches on estimating its resource potential. Int. J. Med. Mushrooms. 2015, 17, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Quan, L.; Shen, M. Effect of chemicals on production, composition and antioxidant activity of polysaccharides of Inonotus obliquus. Int. J. Biol. Macromol. 2015, 77, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Glamočlija, J.; Ćirić, A.; Nikolić, M.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.; Soković, M.; van Griensven, L.J. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015, 162, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, K.H.; Choi, H.J.; Lee, D.S. Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol. Lett. 2005, 27, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.E.; Ao, Z.H.; Lu, Z.M.; Xu, H.Y.; Zhang, X.M.; Dou, W.F.; Xu, Z.H. Antihyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. J. Ethnopharmacol. 2008, 118, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jazi, M.F.; Biglari, A.; Mazloomzadeh, S.; Kingston, P.; Ramazani, A.; Bazzaz, J.T.; Eskandari, M. Recombinant fibromodulin has therapeutic effects on diabetic nephropathy by down-regulating transforming growth factor-β1 in streptozotocin-induced diabetic rat model. Iran J. Basic Med. Sci. 2016, 19, 265–271. [Google Scholar] [PubMed]
- Kanagasabapathy, G.; Kuppusamy, U.R.; Abd Malek, S.N.; Abdulla, M.A.; Chua, K.H.; Sabaratnam, V. Glucan-rich polysaccharides from Pleurotus sajor-caju (Fr.) Singer prevents glucose intolerance, insulin resistance and inflammation in C57BL/6J mice fed a high-fat diet. BMC Complement. Altern. Med. 2012, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.C.; Wasser, S.P. Medicinal mushrooms for glycemic control in diabetes mellitus: History, current status, future perspectives, and unsolved problems (review). Int. J. Med. Mushrooms 2011, 13, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Zhang, Y.; Zhang, N.; Fu, L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohydr. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.B.; Zhang, L.; Yang, L.Y. Quercetin ameliorates diabetic nephropathy by reducing the expressions of transforming growth factor-β1 and connective tissue growth factor in streptozotocin-induced diabetic rats. Ren. Fail. 2012, 34, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, Y.; Bian, Y.; Wong, J.H.; Ng, T.B.; Wang, H. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats. Appl. Microbiol. Biotechnol. 2006, 72, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan-Sahu, A.; Lane, B.; Sliva, D. ReishiMax, mushroom based dietary supplement, inhibits adipocyte differentiation, stimulates glucose uptake and activates AMPK. BMC Complement. Altern. Med. 2011, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Yaghobian, D.; Don, A.S.; Yaghobian, S.; Chen, X.; Pollock, C.A.; Saad, S. Increased sphingosine 1-phosphate mediates inflammation and fibrosis in tubular injury in diabetic nephropathy. Clin. Exp. Pharmacol. Physiol. 2016, 43, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; Cooper, M.E. The tubulointerstitium in progressive diabetic kidney disease: More than an aftermath of glomerular injury? Kidney Int. 1999, 56, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Burns, W.C.; Cooper, M.E. Tubular changes in early diabetic nephropathy. Adv. Chronic Kidney Dis. 2005, 12, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.L. Diabetes and renal tubular cell apoptosis. World J. Diabetes 2013, 4, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Peng, J.; Chang, X.; Huang, K.; Huang, J.; Wang, S.; Shen, X.; Liu, P.; Huang, H. Activation of RhoA/ROCK regulates NF-κB signaling pathway in experimental diabetic nephropathy. Mol. Cell. Endocrinol. 2013, 369, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Shi, R.; Lu, X.; Ma, Y.M.; Cheng, N.N. Combination of active components of Xiexin decoction ameliorates renal fibrosis through the inhibition of NF-κB and TGF-β1/Smad pathways in db/db diabetic mice. PLoS ONE 2015, 10, e0122661. [Google Scholar] [CrossRef] [PubMed]
- Sabbir, K.; Gopabandhu, J. Sodium butyrate, a HDAC inhibitor ameliorates eNOS, iNOS and TGF-β1-induced fibrogenesis, apoptosis and DNA damage in the kidney of juvenile diabetic rats. Food Chem. Toxicol. 2014, 73, 127–139. [Google Scholar]
- Yang, J.H.; Du, Y.M.; Huang, R.H.; Wan, Y.Y.; Li, T.Y. Chemical modification, characterization and structure–anticoagulant activity relationships of Chinese lacquer polysaccharides. Int. J. Biol. Macromol. 2002, 31, 55–62. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, Y.-J.; Kan, W.-C.; Chang, C.-M.; Peng, Y.-J.; Wang, H.-Y.; Yu, W.-C.; Cheng, Y.-H.; Jhang, Y.-R.; Liu, H.-W.; Chuu, J.-J. Renal Protective Effects of Low Molecular Weight of Inonotus obliquus Polysaccharide (LIOP) on HFD/STZ-Induced Nephropathy in Mice. Int. J. Mol. Sci. 2016, 17, 1535. https://doi.org/10.3390/ijms17091535
Chou Y-J, Kan W-C, Chang C-M, Peng Y-J, Wang H-Y, Yu W-C, Cheng Y-H, Jhang Y-R, Liu H-W, Chuu J-J. Renal Protective Effects of Low Molecular Weight of Inonotus obliquus Polysaccharide (LIOP) on HFD/STZ-Induced Nephropathy in Mice. International Journal of Molecular Sciences. 2016; 17(9):1535. https://doi.org/10.3390/ijms17091535
Chicago/Turabian StyleChou, Yen-Jung, Wei-Chih Kan, Chieh-Min Chang, Yi-Jen Peng, Hsien-Yi Wang, Wen-Chun Yu, Yu-Hsuan Cheng, Yu-Rou Jhang, Hsia-Wei Liu, and Jiunn-Jye Chuu. 2016. "Renal Protective Effects of Low Molecular Weight of Inonotus obliquus Polysaccharide (LIOP) on HFD/STZ-Induced Nephropathy in Mice" International Journal of Molecular Sciences 17, no. 9: 1535. https://doi.org/10.3390/ijms17091535
APA StyleChou, Y.-J., Kan, W.-C., Chang, C.-M., Peng, Y.-J., Wang, H.-Y., Yu, W.-C., Cheng, Y.-H., Jhang, Y.-R., Liu, H.-W., & Chuu, J.-J. (2016). Renal Protective Effects of Low Molecular Weight of Inonotus obliquus Polysaccharide (LIOP) on HFD/STZ-Induced Nephropathy in Mice. International Journal of Molecular Sciences, 17(9), 1535. https://doi.org/10.3390/ijms17091535





