1. Introduction
Microorganisms of the genus
Candida are associated with shallow, deep and systemic infections, particularly in patients with underlying diseases such as diabetes and in immunosuppressed patients, patients with neutropenia and transplant patients, due to the opportunistic profile presented by the members of this genus [
1]. The
Candida albicans (
C. albicans) species is presented as the main agent of opportunistic fungal diseases that affect women of all ages, such as vulvovaginal candidiasis (VVC). The pathogenicity of
C. albicans species involves mechanisms of aggression attributed to virulence factors. These mechanisms include the ability to defend itself against the host immune system, hyphae proliferation, biofilm formation in tissue or on medical devices, and production of harmful hydrolytic enzymes including proteases, phospholipases and hemolysin [
2,
3,
4].
Drug therapy targeting fungal infections has limitations such as the high cost of drugs available in the clinic, high toxicity, drug interactions, insufficient bioavailability of the active ingredient and the emergence of resistant strains [
5]. Several antifungals have been indicated for the treatment of these infections, including those belonging to the polyenic, azole, and echinocandin classes; however, due to the indiscriminate use of these antimicrobials targeting genetic and physiological characteristics of the fungus, there has been a significant increase in profile resistance to drug members of these classes [
6,
7]. The health sciences have been increasingly concerned with the exaggerated growth of multidrug-resistant strains to antibiotics currently in use in clinical practice. Therefore, medicinal plants have emerged in the search for new antifungal drugs due to their demonstration of important bioactive properties and the presence of secondary metabolites such as tannins, flavonoids and phenols [
8].
Several plants have been studied to investigate their antimicrobial potential. Some families of plant species have been featured in science, such as Eriocaulaceae. Eriocaulaceae are popularly known as “evergreens” and are used in the manufacture of decorative ornaments and accessories [
9]. Among these is the genus
Syngonanthus, which is found in the states of Goiás and Tocantins, Brazil, where it is known as “golden grass” [
10,
11]. The species
Syngonanthus nitens (Bong.) Ruhland has received substantial attention recently. Pacifico et al. [
12] analyzed the scapes characteristics and found approximately 17 compounds derived from flavones and apigenin plus six new novel molecules. Araújo et al. [
13] detected a significant potential antifungal present in the scapes of
Syngonanthus nitens (
S. nitens) that exhibited activity against four species of the genus
Candida, which is a characteristic therapeutic profile of plant species when subjected to an experimental model of VVC. Although the use of natural products in screening for new drugs is very important, certain limitations have been observed in the development of clinical and pharmacological investigations. These include the difficulty of solubilizing the sample to be analyzed, which may reduce its bioavailability. In this context, nanotechnological tools are commonly used to establish secure platforms for the placement of drugs to develop drug delivery systems. Microemulsions, polymer nanoparticles, solid lipid nanoparticles, liposomes, and, more recently, liquid crystals have been used to enhance therapeutic action and drug selectivity in the vaginal environment [
14].
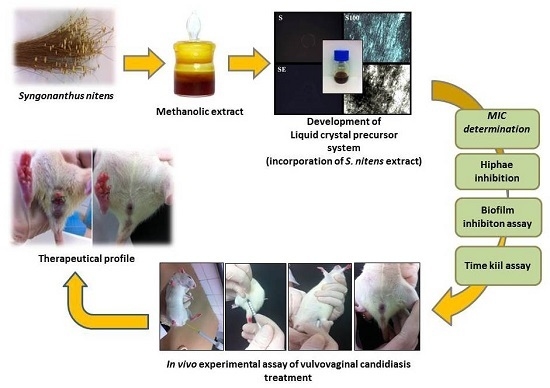
The liquid crystal precursor systems (LCPS) are classified as promising alternatives for the treatment of vulvovaginal candidiasis. Their features are interesting for vaginal administration because they may include liquid forms that facilitate the administration of the formulation. However, upon contact with the vaginal mucosa, LCPS has the ability to incorporate water from the vaginal mucus, thereby becoming a liquid-crystalline mesophase that can promote more viscous controlled release and result in greater applicability of the components of the vegetal extract [
15,
16].
Recently, the biological activity of
S. nitens was evaluated by our research group in an investigation of prophylactic potential against vaginal infection caused by
Candida krusei [
17]. After incorporation in a similar system, it was found that the system is able to enhance the action of the plant extract in all in vitro biological assays as well as promoting prevention of infection by this fungal species according to the model of in vivo infections. In this sense, we propose in this study to investigate the antifungal potential of the methanolic extract of scapes of
S. nitens loaded (or not) into a new liquid crystal precursor system, to highlight their biological applicability for the treatment of vulvovaginal candidiasis caused by
C. albicans azole-resistant derivatives.
3. Discussion
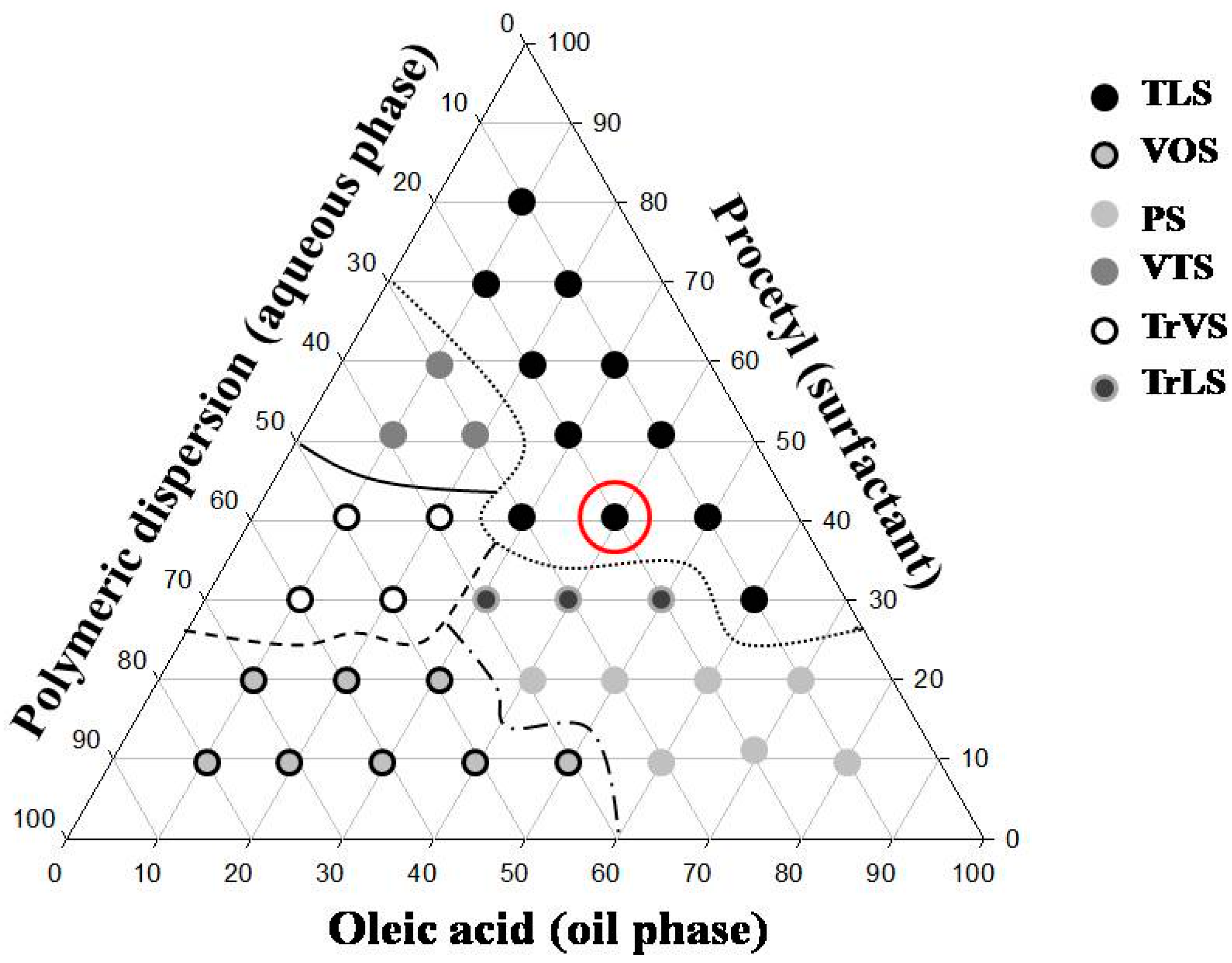
The results obtained from the characterization of the system showed the applicability of the formulation S to be a precursor system of liquid crystal.
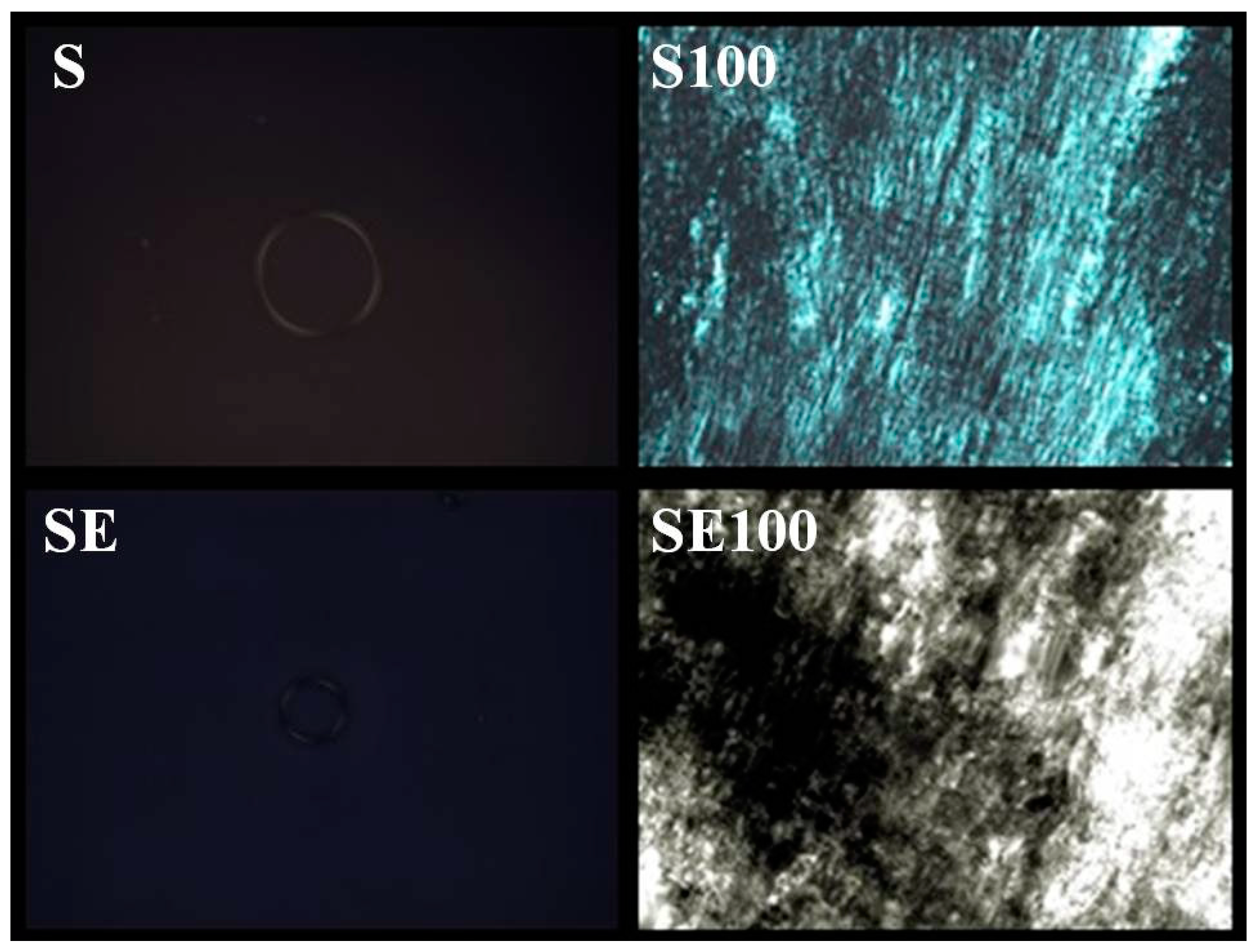
The formulations containing 5% and 10% AVM showed both a liquid and dark field, which was characteristic of microemulsion. The 30% and 50% AVM preparations showed Malta crosses (30%) and striae (50%), which were characteristic of the lamellar and hexagonal phases, respectively. The formulation with 100% AVM was more viscous and showed a hexagonal structure (striae) [
19]. Then, a phase transition was observed for the region of the translucent viscous system when AVM was added to S (100%), indicating that the conformation was changed.
Therefore, based on these results, we proposed that the formulation could behave as a precursor system of liquid crystals because formation of the liquid crystalline system occurred following the addition of increasing amounts of mucus.
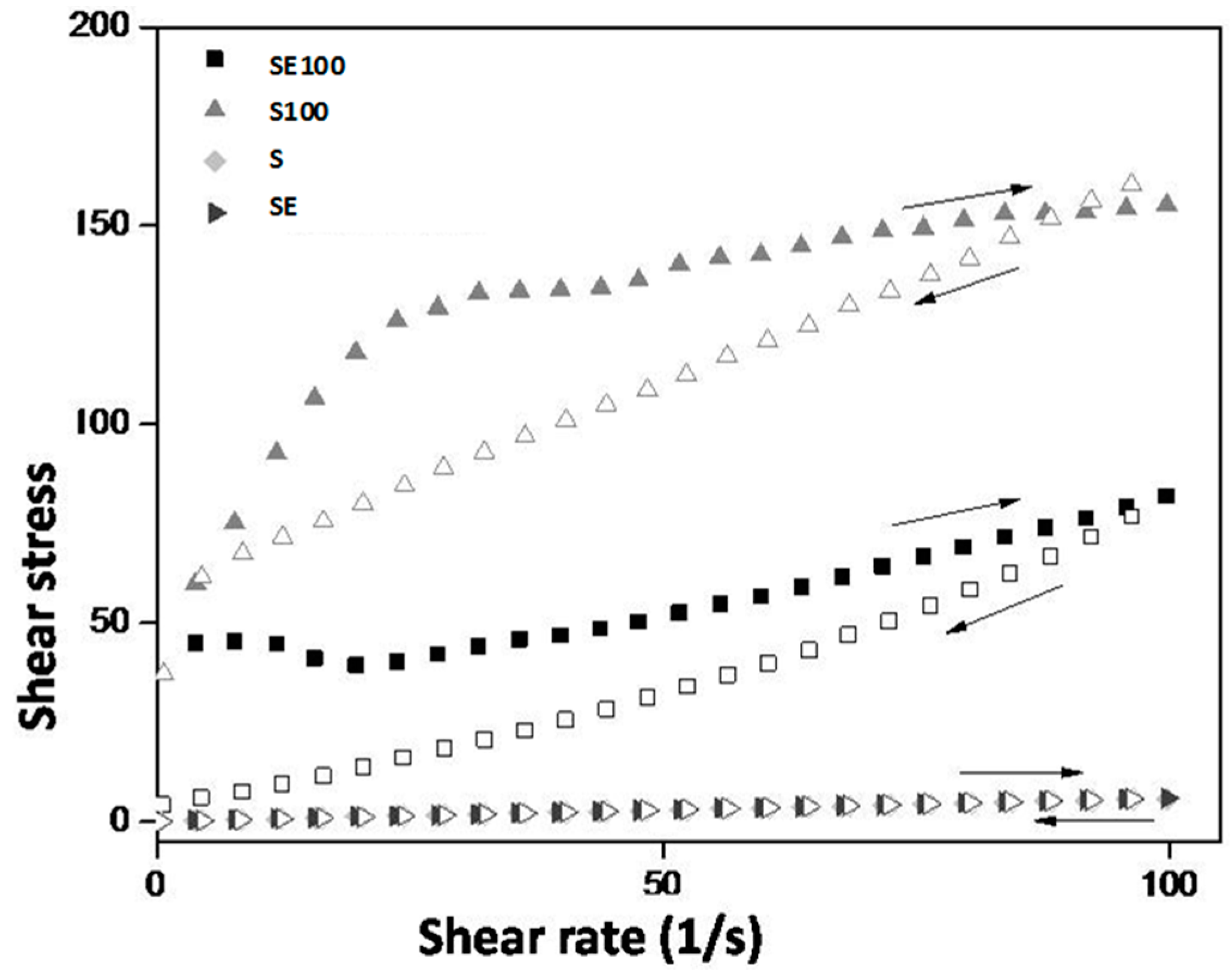
The rheological behavior of the formulations is desirable because, at the moment of the administration, the SE formulation will flow easily from the syringe, thereby facilitating its administration. Contact with vaginal mucus will increase the formulation viscosity, which allows it to stay in contact with the vaginal mucosa so it can release the extract for a longer time, thereby improving the clinical performance of the treatment.
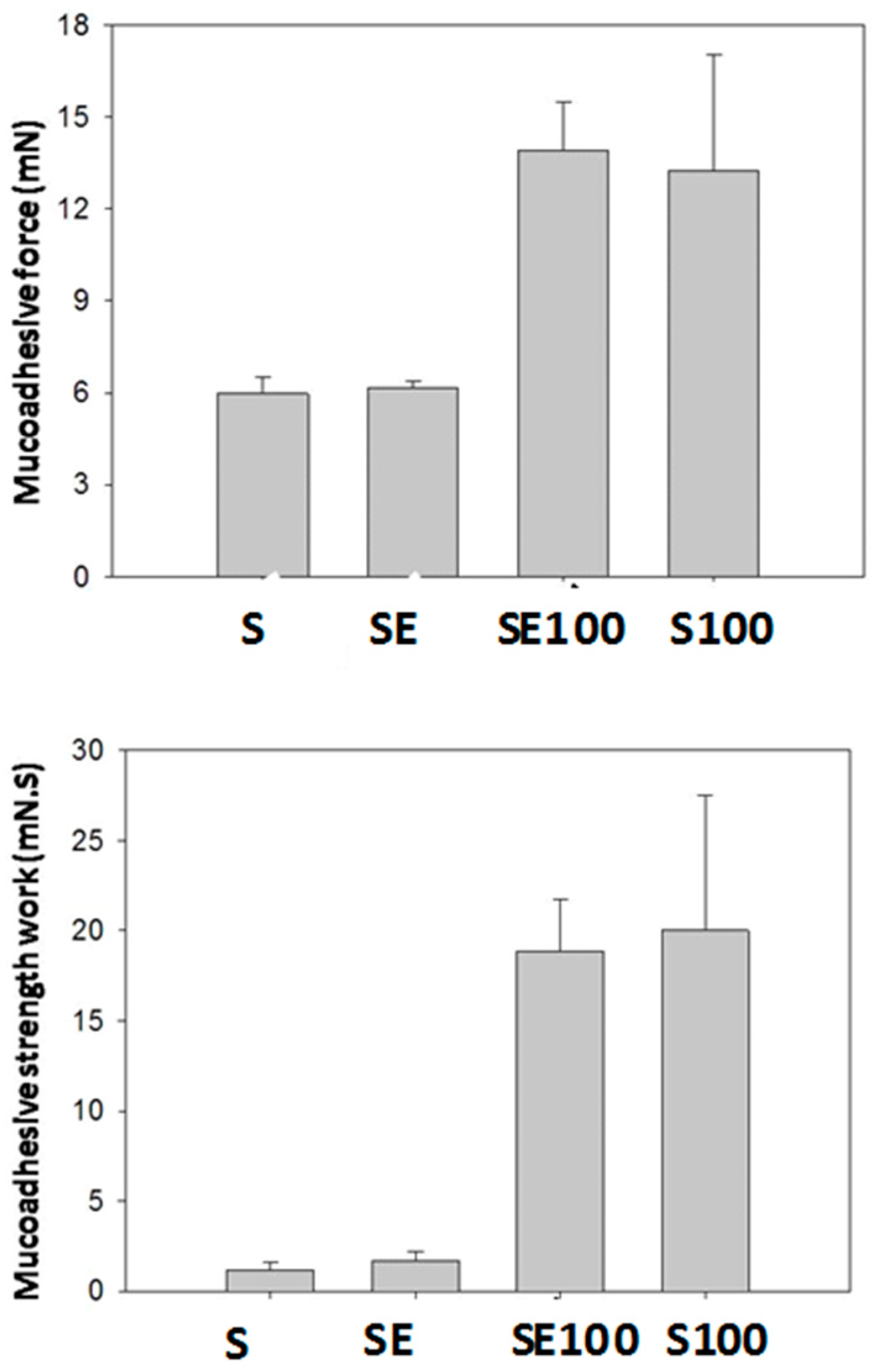
Liquid crystalline hexagonal phases have been widely reported to be mucoadhesive systems due to their high viscosity; however, this property makes their applicability for vaginal administration difficult. Therefore, the system administration of a precursor Newtonian liquid crystal that will form a liquid crystalline mesophase that is mucoadhesive in situ represents one way to overcome this difficulty.
The S100 and SE100 formulations were able to interact more strongly with the vaginal mucosa and consequently remained for a longer time in the vaginal environment.
Thus, the system designed here represents a novel system for vaginal release because it combines the advantage of forming a strong matrix liquid crystal in situ with high vaginal mucoadhesive strength [
20].
The results of the in vitro determination of antifungal potential showed the antifungal effect exerted by the plant extract, which was shown to be active against all of the strains used in this study. According to the data presented in
Table 3, we concluded that the incorporation of the extract in S showed promise because there were decreases in the MICs of the extract for all tested strains. These results may be related to the interaction characteristics with the fungal cell membranes exerted by S (i.e., the use of oleic acid as the oil phase of the formulation). Because oleic acid is a constituent of the cell plasma membrane, it can facilitate the passage of the active compound through the fungal membranes [
21]. The polymer dispersion used as the aqueous phase of the formulation may also be related to the increased biological activity of E by promoting the controlled release of the active principle (i.e., the extract is deposited on the polymer chain and is thus released in a more gradual and controlled manner) [
22].
The literature does not provide a consensus in terms of the MIC values obtained for natural products. Aligiannis and co-workers [
23] considered MICs with values lower than 500 μg/mL to be potent inhibitors, MICs between 600 and 1500 μg/mL to be moderate inhibitors and MICs above 1600 μg/mL to be weak inhibitors. Webster and co-workers [
24] established a satisfactory MIC as an MIC with a value equal to or less than 1000 μg/mL. In this sense, the results reported in this investigation are extremely important.
Secondary metabolites are the major molecules able to develop action against several pathogenic microorganisms by different action mechanisms. The cytoplasmic membrane is classified as the most common site of action of secondary metabolites, with action on this structure triggering with extravasation of cellular contents and, consequently, death of fungal. The interaction with genetic material and protein synthesis is also a predisposing factor in the promotion of therapeutic action. In this case, the contact of the genetic material with secondary metabolites promotes changes in DNA, results in ineffective transcripts promoting disorganization of vital functions of the cell [
9].
The chemical characterization developed by Pacífico [
12] and co-workers showed the presence of secondary metabolites in
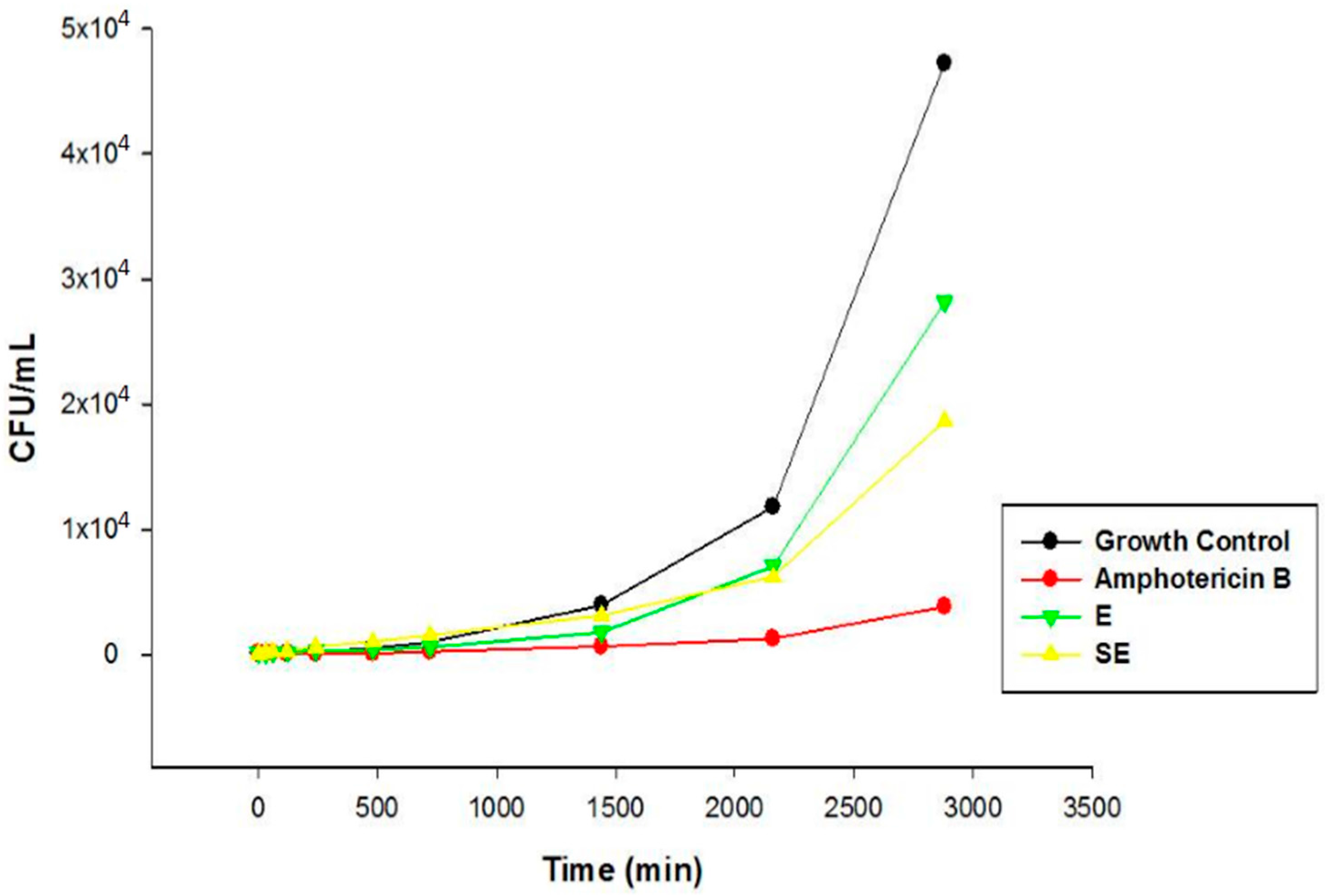
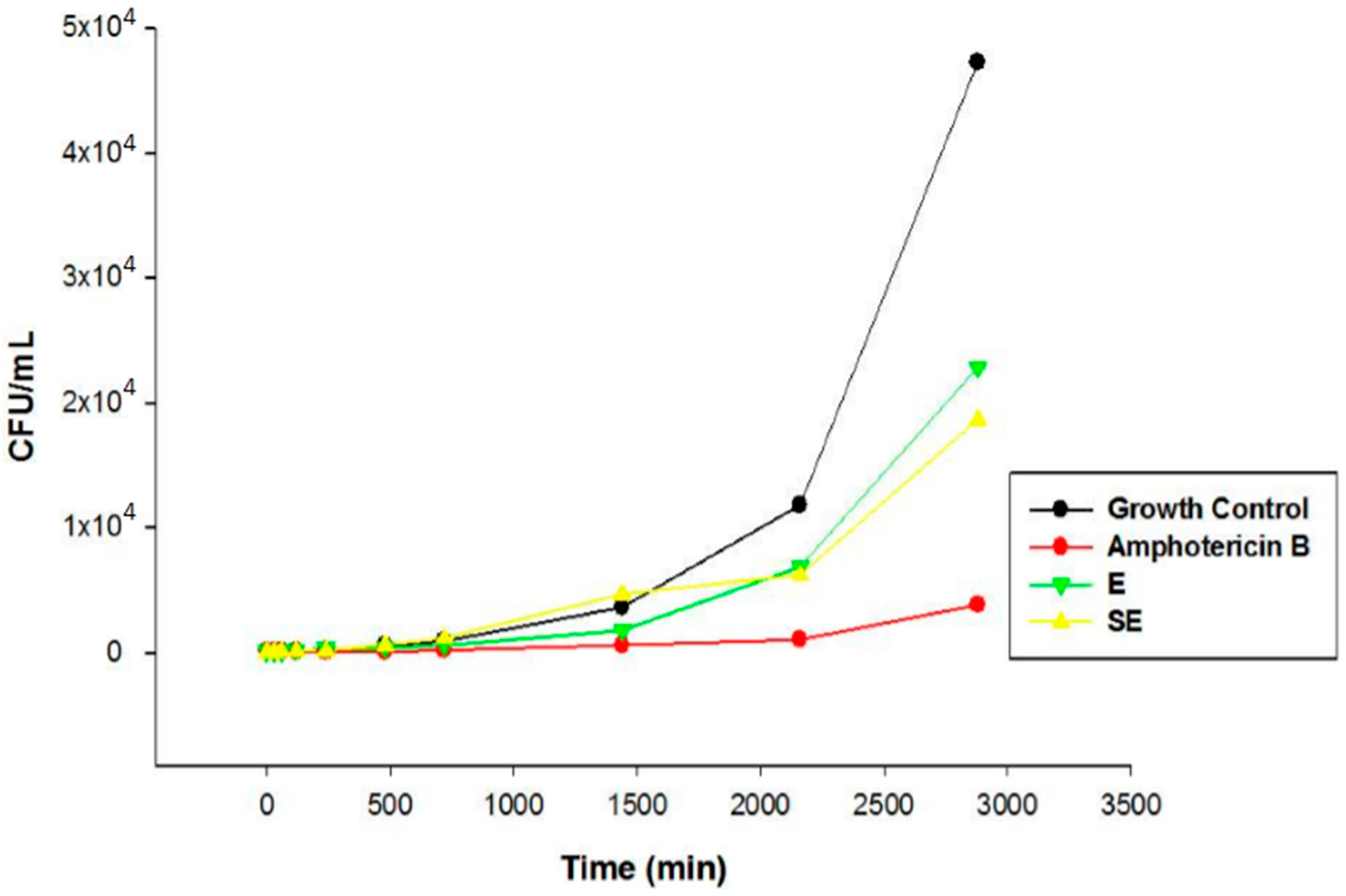
S. nitens extract such as flavones and xanthones. In this sense, the promising antifungal activity presented by extract in this study can be attributed by presence of these compounds. The MIC results were confirmed by Time kill assay, and showed that E and SE are able to control the microbial growth of the strains. Comparing the results between E and S in the assay revealed that the growth inhibitory action exerted by SE was higher than E, where the growth was controlled. After this period, the growth increased at a higher intensity but remained lower compared to the growth control at the end of 48 h, indicating a possible fungistatic mechanism for E and SE.
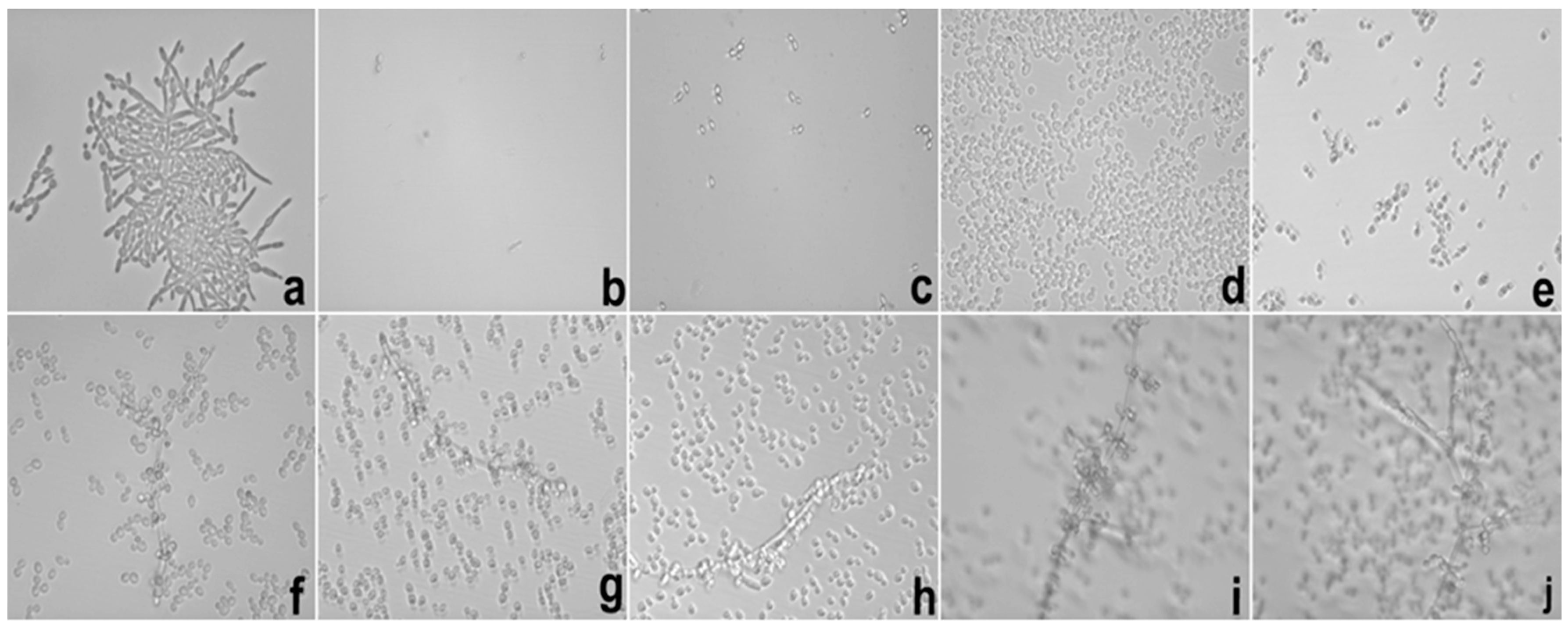
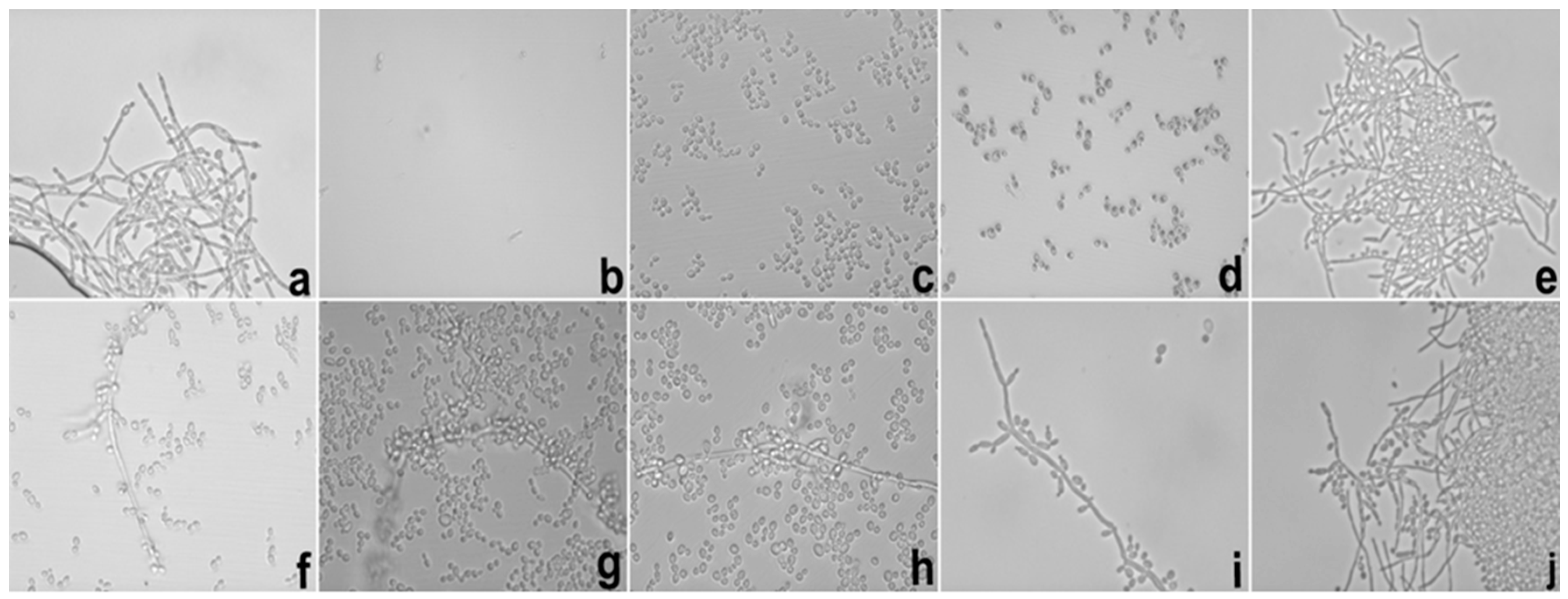
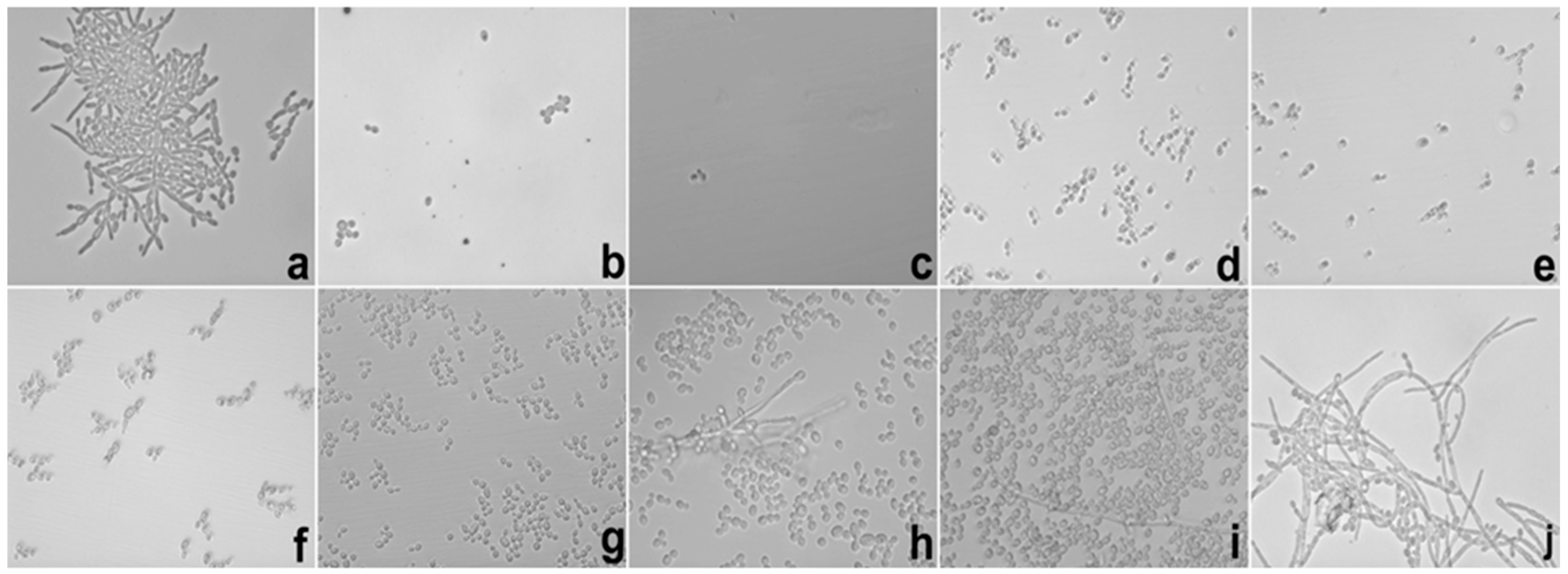
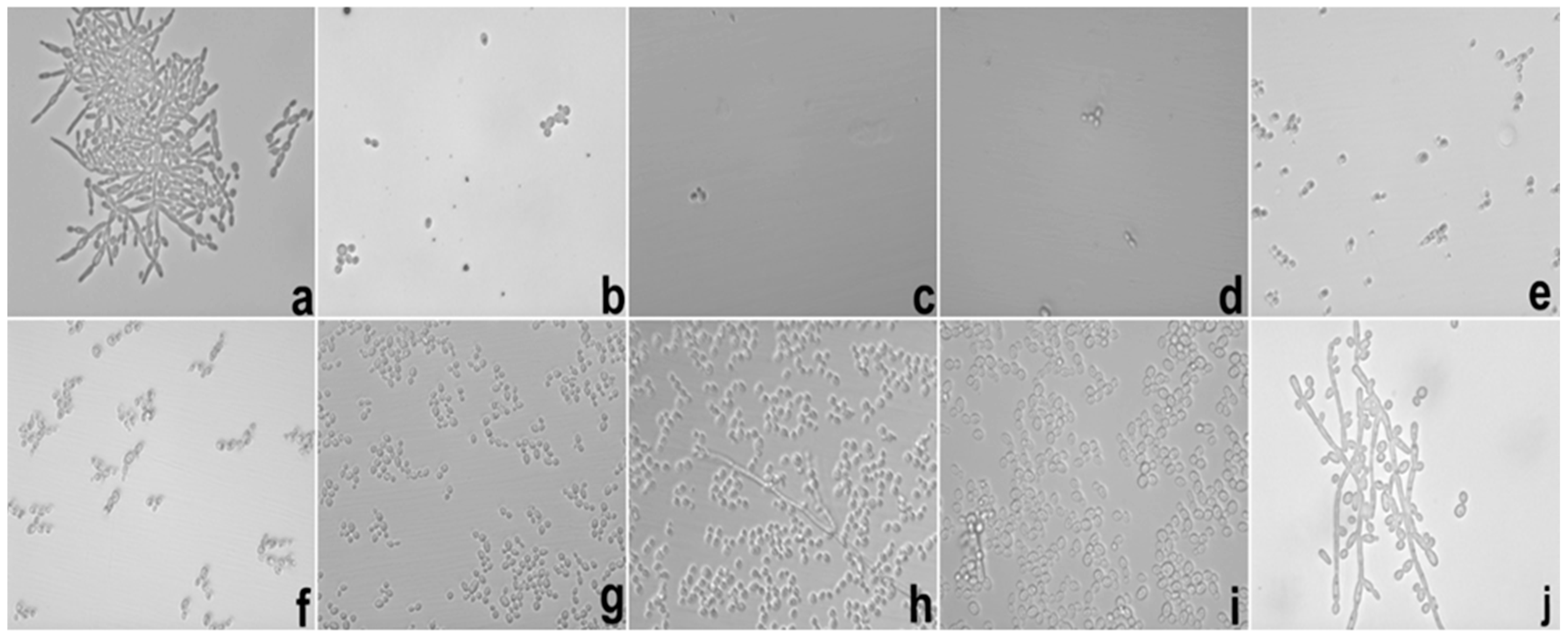
Besides of these important results, the potential hyphae inhibition exerted by E against
C. albicans was superior when it was loaded into S, which could be explained using the same parameters reported in the MIC results. These data are important because the mechanism of cell infestation exercised by
C. albicans in the host mucosa occurs mainly through the hyphae of the fungi in epithelial cells [
25].
In the biofilm inhibition assay, it was observed that the adhesiveness of S increased its contact with the
C. albicans biofilm, causing the direct and more intense release of SE with the microbial surface; this finding may explain the inhibition observed in relation to E. It is possible that the observed action is linked to the increased permeability of the fungal membrane that promoted an increase in the substantivity of E. Likewise, it is also possible that the mucoadhesive property interfered directly with the inhibition of the biofilm because it maintained direct contact with the system containing E in a uniform manner with more intense delivery and was fixed in place. The mucoadhesive components (PD and PRO) may have been the main action behind this result because we were able to fix the formulation containing the extract directly onto the biofilms formed in the well of the microplate, which in turn were presented as fixed and uniform biofilms. The search for new compounds that promote the inhibition of fungal biofilms is a focus of research in several countries to stimulate the improvement of drug administration [
2]. The use of mucoadhesive drug delivery systems on biofilms is more suitable when employing polymers that promote direct adhesion with the biofilm as the most promising and effective route for this treatment [
26].
The development of a drug delivery system that is reliable, effective, and safe for treatments against diseases is a goal for various researchers. Furthermore, drug delivery systems should enable the distribution of the drug through the intended route of administration and that prioritize the best drug–receptor interaction and the reduction of harmful effects [
14]. So, an ideal drug delivery system should provide a targeted therapy that will allow effective concentrations of a drug to reach the disease site without exposing other tissues to toxicity. In this way, therapeutic treatment employing liquid crystals as drug release from vaginal diseases caused by pathogenic or opportunistic microorganisms is classified as potentially promising, especially because they can incorporate water from the vaginal mucus to promote structural changes in the system, resulting in a liquid crystalline phase and thereby optimizing the efficiency of the drug [
27].
The use of
S. nitens in the treatment of VVC caused by
C. albicans was studied by Araújo and co-workers [
13]. The authors reported the therapeutic action of the plant extract when it was incorporated in a conventional formulation. The present study showed that the incorporation of the extract in a nanotechnology-based drug delivery system showed superior results compared to the previous study, thereby indicating that S substantially increased the action of the bioactive agent.
In recent years, the use of nanotechnology for drug delivery systems for the incorporation of plant extracts has been shown to be important for vaginal applications [
14]. Bonifácio and co-workers [
28] showed that the antifungal potential of the ethanolic extract of the leaves of
Astronium urundeuva was improved with the incorporation into a nanostructured lipid system (lipid microemulsion) in the treatment of VVC. The results showed by these authors proved that the therapeutic profile in vivo model using the system cured the animals with only six days of treatment. However, the unloaded extract was not effective during the eight days of treatment.
In this study, SE exerted a cured profile on the animals of experimental groups (7 and 13) after only 2 days of treatment, which may be due to the interaction with the fungal membranes described in this work. Therefore, the inhibitory profile in relation to the mucoadhesive properties was presented by S. Infected animals produced excess vaginal discharge that came into contact with the system and promoted membership in the vaginal mucosa, there by stimulating a direct interaction of the active principle with the mucous membranes and triggering a direct release at the desired site of action [
29,
30,
31].
The activity observed with E was significant because it promoted healing of the animals infected with the ATCC strain four days after administration. The animals infected with CAV3 were healed after six days of treatment. The late action compared to the incorporated extract can be explained based on the low viscosity of the vehicle (DMSO 20%) used to solubilize E because, at the time of administration, the animals cast out the contents deposited in the vaginal canal after the application, which led to a low concentration of E in the intravaginal environment.
The therapeutic action with the standard drug (amphotericin B) was inferior to the action shown by the non-incorporated and incorporated extracts, thereby demonstrating that the use of E in both forms is more promising. Reports of the importance of this result concerning the use of amphotericin B are limited due to its nephrotoxicity-related reactions [
32].
We highlighted the results of the therapeutic profile shown by the extract loaded into S because it may be related to the adhesion property.
The objective of the present system was based on the fixation characteristics of the vaginal epithelium; thus, materials with adhesive properties were used. The aqueous phase of the system (polymeric dispersion) was also developed to promote adhesion in the vaginal environment. The adhesive behavior is due to physical and chemical processes (i.e., hydrophobic interactions, hydrogen bonds and van der Waals forces) [
17]. In addition to this characteristic, the use of polymers in the aqueous phase was intended to promote slower liberation and a controlled manner of the plant extract.
The adhesive materials in the pharmaceutical formulations may be hydrophilic molecules of natural or synthetic origin that contain numerous organic components (i.e., carboxyl groups, hydroxyl groups and amines) that form chemical bonds with the biological surface and promote the increase of permeability (i.e., membrane cells) [
33].
The choice of surfactant (PEG-5 Ceteth-20 Procetyl®, Wickliffe, OH, USA) and the constituent of aqueous phase was based on adhesive characteristics because they can come into contact with substances that has water to promote the adhesiveness. Therefore, we aimed to trigger adhesion when the system came into contact with vaginal mucus.
Systems that have polymeric network compositions, the drug may be homogenously dispersed in the polymer matrix or adsorbed on their surface or within a reservoir. This phenomenon involves the liberation of the same physical and chemical processes, such as water penetration into the matrix, diffusion of the drug through the pores of the matrix, polymer degradation or a combination of the last two mechanisms [
14].
In another study, the use of polymeric dispersion (chitosan) as aqueous phase in liquid crystalline system for incorporation of curcumin showed antifungal activity against
C. albicans according to in vitro tests. The authors proved that adhesive property exercised by this polymer was important to promote the intense contact of the system with the fungal membrane cells. This contact may be responsible for the increase of membrane permeability that facilitated the entrance of active constituents within the intracellular environment [
27].